Abstract
Rationale: Risk of subsequent asthma-like symptoms after early-life lower respiratory illness (LRI) caused by respiratory syncytial virus (RSV) is increased during the first decade of childhood and diminished thereafter by adolescence.
Objectives: To determine the relation of early-life RSV-LRI on adult asthma-like symptoms and its interactive role with adult smoking.
Methods: A total of 1,246 nonselected infants were enrolled at birth and prospectively followed. Virologically confirmed RSV-LRIs were assessed during the first 3 years of life. At age 22, 24, 26, and 29 years, current asthma and smoking behavior were evaluated by questionnaire. Peak flow variability was assessed at age 26 and expressed as amplitude % mean. A longitudinal analysis was used to investigate the relation of RSV-LRI and active smoking to adult outcomes.
Measurements and Main Results: Neither RSV-LRI nor active smoking were directly associated with increased current adult asthma or peak flow variability. However, there was a significant interaction between RSV-LRI and active smoking in relation to current asthma (P for interaction = 0.004) and peak flow variability (P for interaction = 0.04). Among subjects with early RSV-LRI, those who actively smoked were 1.7 times more likely to have current asthma (95% confidence interval, 1.2–2.3; P = 0.003) and had greater amplitude % mean (10.0% vs. 6.4%; P = 0.02) than nonsmokers. Among subjects without early RSV-LRI, there was no difference in asthma risk or peak flow variability between active smokers and nonsmokers.
Conclusions: Smoking is associated with increased risk of having asthma in young adults who had RSV-LRI in early life but not among subjects without these illnesses.
Keywords: respiratory syncytial virus, smoking, adult, asthma
At a Glance Commentary
Scientific Knowledge on the Subject
Respiratory syncytial virus (RSV) lower respiratory illness (LRI) in infancy is associated with subsequent asthma and recurrent wheezing during the first decade of life, but the risk tends to diminish by adolescence. Cigarette smoking is a risk factor for the development of asthma symptoms in adulthood. The relation of RSV-LRI with asthma in adult life and the possible role of active smoking in determining this association remain elusive.
What This Study Adds to the Field
Early-life RSV-LRI is not directly associated with increased adult asthma. However, there is an association between RSV-LRI and active smoking as determinants of adult outcomes. Young adults who had RSV-LRI in early life and become active smokers are more likely to have current asthma and increased peak flow variability compared with those who do not smoke. What determines this association is unknown, but either airway abnormalities preexisting RSV-LRI or early-life insult by RSV, or both, may increase susceptibility to active cigarette smoking.
In 1953, Oswald and coworkers (1) first reported that adults with chronic respiratory symptoms were more likely to report a history of “respiratory infections” during childhood than those without such symptoms. Subsequently, Burrows and coworkers (2) observed that a history of “respiratory trouble” during childhood increased susceptibility to the effects of smoking in adults. These retrospective studies could have been biased by preferential recall (3), but a subsequent study reported increased respiratory symptoms and evidence of airflow limitation in 70-year-old subjects living in Derbyshire, England who had a history of pneumonia during the first 2 years of life, as confirmed by their medical records (4). These results suggested the hypothesis that infection during a time of fast somatic growth could impair normal airway development, thus increasing susceptibility to noxious stimuli, such as active smoking (5).
Smokers are more likely to experience respiratory symptoms, have pulmonary function abnormalities, and a greater decline in FEV1 than nonsmokers (6). Some studies have found an association between smoking and asthma in adult life (7). However, controversy exists because other studies have failed to confirm these findings (8). It is plausible that smoking modifies or increases asthma risk only in susceptible individuals, and early-life respiratory illnesses might be one of the factors that determine vulnerability to smoking (5).
The most common etiologic agent for severe lower respiratory illness (LRI) during early life is the respiratory syncytial virus (RSV) (9–12). Longitudinal studies in which young children with confirmed, severe RSV-LRI were followed until adulthood showed increased risk of subsequent asthma-like symptoms; this risk decreased with age until adolescence, but increased again during early adult life (13). In the longest ongoing longitudinal study of young children hospitalized with LRI in the first 2 years of life, 40% of whom were infected with RSV, prevalence of asthma decreased from 25% at age 4.5–6 years to 12–15% at ages 8–15, only to increase again to 30% at age 18–20 and up to 35% at age 30 (14–18). There was no clear explanation for this trend but the authors noted that, surprisingly, adults with a history of LRI were more likely to smoke than control subjects (18).
As part of the Tucson Children’s Respiratory Study (CRS), we previously reported that, similar to other cohorts, the risk of asthma-like symptoms after confirmed RSV-LRI in early life was highest at age 6, and decreased thereafter, becoming statistically nonsignificant by the age of 13 years (19). The purpose of this study was to determine the outcome of RSV-LRI during the third decade of life and its possible interaction with active smoking in determining asthma risk in the CRS cohort. Some of the results of these studies have been previously reported in the form of an abstract (20).
Methods
Study Design
Participants were a subset of the 1,246 children born in 1980–1984 and enrolled in the Tucson CRS, a longitudinal nonselected birth cohort study (21). The enrollment process and study design are described elsewhere (21). Parents were contacted shortly after their child was born and completed a questionnaire describing their race and ethnicity, current age, years of education, current smoking habits, and history of physician-diagnosed asthma. Informed consent was obtained from the parents for their children, or starting in adolescence, from the subjects themselves if appropriate. The study was approved by the Institutional Review Board of the University of Arizona.
Data Collection
Parents were instructed at enrollment to bring their child to collaborating pediatricians whenever they had signs or symptoms of LRI (deep or wet chest cough, wheezing, hoarseness, stridor, shortness of breath) before age 3 years (11). A detailed clinical history was taken and physical examination performed at each medical visit. Nasopharyngeal swabs were collected at the time of LRI for RSV culture and/or immunofluorescence. An episode was considered to be RSV-positive if culture, immunofluorescence, or both were positive (22). Subjects who tested positive for RSV were assigned to “RSV-LRI” group; those who never tested positive for RSV during a complete 3-year follow-up were assigned to “No RSV-LRI” group.
Current asthma, defined as self-reported physician-diagnosed asthma with active symptoms (asthma attacks, episodes, or wheeze) during the previous year, as previously described (23), was assessed by questionnaires at ages 22, 24, 26, and 29 years. Active smoking at ages 22 and 26 years was defined based on the participant’s answer to the question “Do you now smoke cigarettes?” Those who answered “yes” were classified as active smokers, whereas those who answered “no” were classified as nonsmokers. Active smoking at ages 24 and 29 years was defined based on the participant’s answer to the question “Have you ever smoked cigarettes?” Those who answered “yes, I still smoke” were classified as active smokers, whereas those who answered “yes, but I no longer smoke” or “no” were combined and classified as nonsmokers/exsmokers (nonsmokers hereafter). Data on current wheeze, defined as having had at least one self-reported episode during the past year, were also obtained at ages 22, 24, 26, and 29 years. Current cough without a cold was evaluated by questionnaire at ages 22 and 26 years.
At the Year 26 visit, participants were trained by study nurses on the use of a peak flow meter (PiKo-1; nSpire Health, Inc., Longmont, CO), and asked to perform three PEF measurements twice per day (once in the morning and once in the evening) for approximately 1 week. The first-day data were excluded because of the possibility of a learning effect. Only participants who had data recorded at least twice daily for at least 2 days were included in the analysis. The amplitude % mean (Amp%mean) was chosen as the peak flow variability index and defined as follows (24):
Details on Amp%mean at age 11 were previously described (24) (see online supplement).
Skin prick tests to local aeroallergens (Bermuda grass, Alternaria alternata, careless weed, mesquite, mulberry and olive tree pollens) were performed at age 6 years as previously described (19). Tests were read at 20 minutes and the sum of the largest wheal diameter plus the perpendicular diameter recorded. Wheal sizes greater than 3 mm, after subtracting the negative control, were considered positive.
Statistical Analysis
Proportions were compared with chi-square test. A longitudinal model using generalized estimating equations (GEE) was used to assess the relation of RSV-LRI and active smoking to adult outcomes including current asthma, wheeze, and cough without a cold, with adjustment for age, sex, parental asthma, and maternal smoking at enrollment. Active smoking was entered as a time-dependent covariate in the GEE model. When subjects had missing values for a categorical predictor variable, an additional dummy category was created for these subjects and included in the model. This allowed us to include all subjects with asthma information. Amp%mean values were log-transformed and expressed as geometric mean and 95% confidence interval (CI). Interaction between RSV-LRI and active smoking at age 26 on Amp%mean at age 26 was assessed using a linear regression, adjusted for sex and height. Additionally, log-transformed values of Amp%mean at ages 11 and 26 were divided into quintiles, and entered into an interaction with smoking in a logistic regression model for asthma. Two-sided P less than or equal to 0.05 were considered significant. Statistical analyses were performed using SPSS for Windows 20.0 (IBM Corp., Armonk, NY) and STATA 12.0 (StataCorp LP, College Station, TX).
Results
Of 918 participants with data for RSV-LRI in early life, 682 (74.3%) participants had adult data for current asthma and active smoking at ages 22, 24, 26, or 29 years, and were included in the present study (see Figure E1 in the online supplement). Relative to those not included, included individuals were more likely to be non-Hispanic white, and to have nonsmoking parents of older age with more years of education (Table 1). There were no differences in sex, skin test positivity at age 6 years, or parental history of asthma between those included and not included in the study. Of the 682 participants, 162 (23.8%) had experienced RSV-LRI in the first 3 years of life. The median (interquartile range) age at first RSV-LRI was 0.7 (0.4–1.4) years. Most RSV-LRI subjects were seen in outpatient settings and did not require hospitalization; only two subjects were hospitalized for RSV. Participants with and without early-life RSV-LRI did not significantly differ in baseline characteristics (Table 2), including sex, ethnicity, skin test positivity at age 6 years, active smoking in adulthood, and parent-related variables (age, years of education, history of asthma, active smoking at enrollment).
Table 1.
Baseline Characteristics of Participants Included in the Study Compared with Those Not Included
| Participant Characteristics | Included; Subjects with RSV and Adult Data (n = 682*) | Not Included; Subjects with RSV but without Adult Data (n = 236*) | Not Included; Subjects without RSV Data (n = 328*) | P Value† |
|---|---|---|---|---|
| Male | 48.5 | 53.0 | 47.9 | 0.43 |
| Ethnicity (non-Hispanic white) | 63.3 | 55.1 | 52.4 | 0.002 |
| Skin test positivity at age 6 yr | 39.0 (n = 561) | 36.4 (n = 107) | 34.0 (n = 94) | 0.61 |
| Current wheeze at age 6 yr | 26.3 (n = 666) | 32.0 (n = 175) | 20.6 (n = 175) | 0.052 |
| Maternal characteristics | ||||
| Asthma | 10.4 (n = 674) | 13.8 (n = 224) | 10.1 (n = 257) | 0.32 |
| Smoking at enrollment | 14.1 | 23.8 (n = 235) | 20.9 (n = 326) | 0.001 |
| Education (>12 yr) | 75.6 (n = 681) | 55.5 | 62.0 (n = 324) | <0.001 |
| Age (>28 yr) | 41.1 | 39.8 | 32.1 (n = 327) | 0.02 |
| Paternal characteristics | ||||
| Asthma | 13.4 (n = 647) | 10.4 (n = 211) | 9.7 (n = 236) | 0.24 |
| Smoking at enrollment | 27.9 (n = 673) | 35.6 (n = 233) | 35.6 (n = 320) | 0.02 |
| Education (>12 yr) | 76.2 (n = 668) | 61.2 (n = 232) | 64.7 (n = 317) | <0.001 |
| Age (>28 yr) | 60.4 (n = 671) | 51.9 (n = 235) | 41.2 (n = 323) | <0.001 |
Definition of abbreviation: RSV = respiratory syncytial virus.
Data are presented as %.
Unless otherwise specified.
P values based on chi-square statistic.
Table 2.
Baseline Characteristics of Participants in RSV-LRI Group Compared with Those in No RSV-LRI Group
| Participant Characteristics | RSV-LRI (n = 162*) | No RSV-LRI (n = 520*) | P Value† |
|---|---|---|---|
| Male | 54.3 | 46.7 | 0.09 |
| Ethnicity (non-Hispanic white) | 63.0 | 63.5 | 0.91 |
| Skin test positivity at age 6 yr | 33.8 (n = 136) | 40.7 (n = 425) | 0.15 |
| Active smoking at ages 22–26 yr | 40.7 (n = 140) | 35.1 (n = 464) | 0.23 |
| Maternal characteristics | |||
| Asthma | 12.5 (n = 160) | 9.7 (n = 514) | 0.32 |
| Smoking at enrollment | 14.2 | 14.0 | 0.96 |
| Education (>12 yr) | 74.1 | 76.1 (n = 519) | 0.60 |
| Age (>28 yr) | 39.5 | 41.5 | 0.65 |
| Paternal characteristics | |||
| Asthma | 13.7 (n = 153) | 13.4 (n = 494) | 0.91 |
| Smoking at enrollment | 29.1 (n = 158) | 27.6 (n = 515) | 0.71 |
| Education (>12 yr) | 76.4 (n = 157) | 76.1 (n = 511) | 0.94 |
| Age (>28 yr) | 62.4 (n = 157) | 59.7 (n = 514) | 0.55 |
Definition of abbreviations: LRI = lower respiratory illness; RSV = respiratory syncytial virus.
Data are presented as %.
Unless otherwise specified.
P values based on chi-square statistic.
Physician-diagnosed current asthma with active symptoms in the past year was reported by 18.6, 19.7, 19.9, and 18.1% of participants at ages 22, 24, 26, and 29 years, respectively. The cumulative prevalence of current asthma from ages 22 to 29 was 26.2%. Maternal asthma, paternal asthma, current wheeze at age 6, and skin test positivity at age 6 were associated with current adult asthma, whereas sex, maternal smoking, and paternal smoking at enrollment were not (see Table E1). In a longitudinal mixed model for adult asthma (GEE; 682 subjects with 2,118 observations, adjusted for age, sex, parental asthma, and maternal smoking at enrollment), there was no association between early-life RSV-LRI and current adult asthma (Table 3). Active smoking was reported by 25.9, 22.5, 22.4, and 17.0% of participants at ages 22, 24, 26, and 29 years, respectively. There was no difference in the prevalence of current adult asthma between active smokers and nonsmokers (Table 3).
Table 3.
Adjusted Relative Risk for Current Asthma, Current Wheeze, or Current Cough without a Cold in Adult Life by Early-Life RSV-LRI and Active Smoking
| Current Asthma |
Current Wheeze |
Current Cough without a Cold |
||||
|---|---|---|---|---|---|---|
| RR (95% CI) | P Value* | RR (95% CI) | P Value* | RR (95% CI) | P Value* | |
| RSV-LRI | 1.25 (0.92–1.70) | 0.15 | 1.13 (0.95–1.33) | 0.16 | 1.09 (0.87–1.36) | 0.46 |
| Active smoking | 1.09 (0.89–1.32) | 0.41 | 1.54 (1.37–1.74) | <0.001 | 1.49 (1.23–1.81) | <0.001 |
Definition of abbreviations: CI = confidence interval; LRI = lower respiratory illness; RR = relative risk; RSV = respiratory syncytial virus.
P values based on generalized estimating equation adjusted for age, sex, parental asthma, and maternal smoking at enrollment.
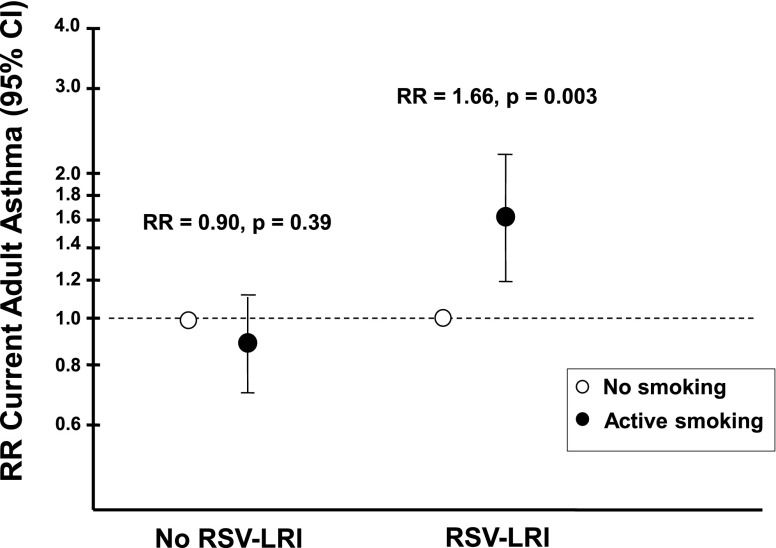
There was a significant interaction between early-life RSV-LRI and active adult smoking in relation to current asthma in adulthood (P for interaction = 0.004). Specifically, among subjects with early RSV-LRI, those who actively smoked were 1.66 (95% CI, 1.19–2.31) times more likely to have current asthma than nonsmokers (Figure 1). In contrast, there was no difference in asthma risk in adult life between active smokers and nonsmokers without early RSV-LRI (relative risk [RR], 0.90; 95% CI, 0.70–1.15; P = 0.39).
Figure 1.
Adjusted relative risk of current adult asthma by smoking status among subjects with and without early-life respiratory syncytial virus (RSV) lower respiratory illness (LRI). Among subjects with early RSV-LRI, active smoking was associated with an increased risk of current asthma (relative risk [RR], 1.66; 95% confidence interval [CI], 1.19–2.31; P = 0.003). The effect of smoking on asthma was not observed among those without an early RSV-LRI (RR, 0.90; 95% CI, 0.70–1.15; P = 0.39). Data were analyzed by generalized estimating equation adjusted for age, sex, parental asthma, and maternal smoking at enrollment.
Current wheeze was reported by 37.8, 40.8, 38.5, and 37.2% of individuals at ages 22, 24, 26, and 29 years, respectively. The prevalence of current cough without a cold was 32.5% at age 22 and 26.5% at age 26 years. Active smoking was found to be a significant risk factor for both current wheeze and cough without a cold (Table 3). In contrast, neither of these respiratory symptoms was significantly associated with RSV-LRI (Table 3). Unlike what was observed for current asthma, there was no interaction between RSV-LRI and active smoking on current wheeze or cough without a cold.
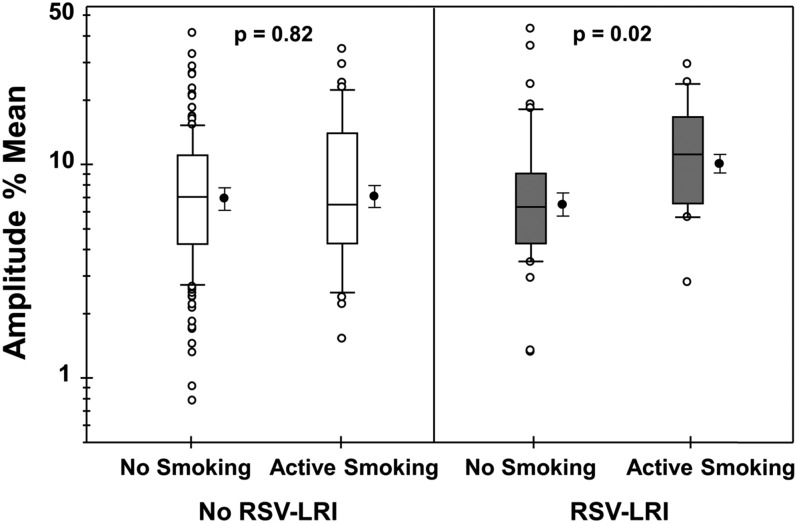
Of the 682 participants, 273 (40.0%) had data on peak flow variability expressed as Amp%mean at age 26 years. Geometric mean for Amp%mean for all participants was 7.0% (95% CI, 6.5–7.6). Using linear regression with adjustment for sex and height, there were no significant differences in Amp%mean among those subjects with or without early RSV-LRI (7.2% [6.1–8.5], n = 69 vs. 7.0% [6.3–7.6], n = 204; P = 0.86), or among those with or without active smoking at age 26 years (7.8% [6.5–9.3], n = 61 vs. 6.8% [6.2–7.4], n = 208; P = 0.29). However, similar to current asthma, there was an interaction between RSV-LRI and active smoking in relation to Amp%mean (P for interaction = 0.04). Participants with early RSV-LRI who actively smoked at age 26 years had greater Amp%mean (10.0% [7.4–13.5]) than those who did not smoke (6.4% [5.2–7.8]; P = 0.02) (Figure 2). Similar to our findings with current asthma, no difference was found between active smokers and nonsmokers without RSV-LRI (7.0% [5.6–8.7] vs. 6.9% [6.2–7.7]; P = 0.82).
Figure 2.
Amplitude % mean (as an index of peak flow variability) among adult participants with and without respiratory syncytial virus (RSV) lower respiratory illness (LRI) in the first 3 years of life by smoking status. The box plots show the median (middle line), 25th and 75th percentiles (box), and 90th and 10th percentiles (whiskers). The figures on the right of each box plot display the mean and 95% confidence interval. n = 157, 43, 51, and 18 from left to right, respectively.
No interaction was observed between Amp%mean at age 26 and active smoking at age 26 on current asthma at age 26 (all P for interaction > 0.05) (see Figure E2A). Similarly, there was no interaction either between Amp%mean at age 11 and parental smoking at age 11 on current asthma at age 11 (all P for interaction > 0.05) (see Figure E2B) or between Amp%mean at age 11 and active smoking at age 26 on current asthma at age 26 (all P for interaction > 0.05).
Discussion
The role of viral respiratory tract illnesses in early life, particularly those caused by RSV, on subsequent development of asthma and wheeze has been a topic of interest for decades. It is now well-established that RSV-LRI in infancy is associated with recurrent wheezing and asthma during the first decade of life, and that the risk tends to diminish by adolescence (19, 25, 26). Although prospective studies of virologically confirmed RSV infection followed to adult life are limited and the link between RSV and adult asthma remains obscure, long-term studies in Finland demonstrated an increase in asthma prevalence in adulthood after severe bronchiolitis in infancy (13). In a group of children younger than 2 years of age hospitalized for bronchiolitis or pneumonia in which RSV was detected in 40% of cases, 25% of children reported asthma at ages 4–6 years as compared with only 12–15% at ages 8–15, but prevalence of asthma rose to 30% at ages 18–20 and up to 35% at age 30 (14–18) . Similar results were reported by this research group when specifically considering subjects hospitalized with bronchiolitis caused by RSV; asthma risk was not elevated during the second decade of life, yet increased in the third decade (27, 28). In addition, current smoking was found to be an independent predictor for adult asthma in the bronchiolitis group (29). An analogous trend was observed in Swedish prospective studies of children aged younger than 2 years hospitalized with wheezy bronchitis, in which 50% of cases were caused by RSV; asthma was reported in 47, 30, and 43% of participants at ages 4–6, age 10, and ages 17–20 years, respectively (30–32).
What determines this apparent rebound in the risk for asthma in early adulthood among subjects with RSV-LRI during infancy has not been elucidated. Our results strongly suggest that increased susceptibility to tobacco smoke plays a major role in this temporal relation. In our birth cohort, subjects with confirmed RSV-LRI during the first 3 years were more likely to experience recurrent wheezing during childhood up to age 11, but not at age 13 (19). We now followed the cohort until age 29 years and found no significant difference in asthma risk between the groups with and without RSV-LRI. However, when the data were stratified by smoking status, prevalence of current asthma was significantly higher in subjects with early RSV-LRI who became active smokers as adults, as compared with subjects with early RSV-LRI who did not smoke. No such difference in risk was observed between smokers and nonsmokers without early RSV-LRI.
Several previous studies have suggested that active smoking is a risk factor for relapse or persistence of asthma symptoms into adulthood among subjects who had asthma or wheezing in early life. In a population-based longitudinal study of 2,300 subjects in Tucson, remission of asthma was uncommon among adults who actively smoked, and smokers were found to have the lowest remission rates and the highest relapse rates of asthma (33). In the British 1958 birth cohort, relapse of asthma symptoms at age 33 years after prolonged remission among children who had asthma or “wheezy bronchitis” before the age of 7 was significantly more frequent among smokers than among nonsmokers (34). Finally, in a birth cohort in New Zealand prospectively followed up to age 26, smoking at age 21 was a predictor for the persistence or relapse of wheezing in adult life (35). Ours is the first study in which physician-diagnosed confirmed episodes of RSV-LRI before age 3 have been shown to interact with active smoking in determining the risk for asthma in adult life. Interestingly, we found that no such interaction was present when any wheezing or chronic cough were used as outcomes, suggesting that RSV-LRI confers susceptibility to the development of more severe asthma symptoms among smokers. In support of this explanation, we found increased peak flow variability in smokers with early RSV-LRI as compared with those without these illnesses. Increased peak flow variability has been found to identify adults with asthma with propensity to subsequently have acute exacerbations, a marker of disease severity (36).
The mechanisms underlying the interaction between RSV-LRI in early childhood and active smoking as determinants of asthma in early adult life are unknown, and they cannot be definitively established in an epidemiologic study like ours (37). A first possibility is that early life RSV illness may play a direct role by altering immune responses and/or inducing airway injury, which would predispose the host to asthma, impaired lung function, and airway hyperreactivity on exposure to cigarette smoking in adulthood. Findings from retrospective and open label studies have shown that RSV immunoprophylaxis may be efficacious in reducing subsequent RSV-related morbidity in high-risk infants (38–40). Whether these findings can be extended to low-risk infants and to asthma symptoms later in life is currently unknown. Alternatively, RSV infection may simply identify a group of adults particularly susceptible to the effect of smoking on the development of asthma. Studies of the heritability of RSV and asthma in twins have suggested that RSV infection does not cause asthma but is an indicator of the genetic predisposition to asthma (41). Airway hyperresponsiveness early in life has been described in association with subsequent bronchiolitis in infancy (42, 43) and childhood asthma (44), suggesting that preexisting airway hyperreactivity may be a shared predisposition to both viral LRIs and asthma. Furthermore, the interaction between airway hyperresponsiveness and smoking on the progression of airway obstruction has been demonstrated in subjects with early chronic obstructive pulmonary disease (45). It is thus reasonable to hypothesize that airway reactivity may be responsible for the link between RSV, smoking, and asthma (46). However, in the present study, no interaction was found either between peak flow variability and active smoking on the risk of adult asthma or between childhood peak flow variability and parental smoking on the risk of childhood asthma. These data suggest that, to the extent peak flow variability is a good indicator of airway reactivity, it is unlikely to be a major contributor to the interaction between RSV and smoking observed in our cohort.
The major strengths of our study are its prospective nature, which eliminates the potential for recall bias; our nonselected birth cohort with long-term follow-up after RSV illnesses, virologically confirmed in all cases; and that most RSV-LRI subjects were seen in outpatient settings, thereby avoiding the selective nature of a hospitalized population. Our study also has limitations that need to be taken into account when interpreting our findings. Over a fourth of study participants with data for RSV-LRI in early life were no longer followed by age 29, and those remaining were more likely to be non-Hispanic white, to come from families with older, better educated parents, and were less exposed to parental smoking than those who withdrew from the study (Table 1). This subtle population selection may explain the observed high frequency of current wheeze at age 6 in the group with RSV data but without adult asthma data, because this group was more likely to have smoking parents with lower education levels. The prevalence of wheeze (37.2–40.8%) and cough without a cold (26.5–32.5%) in our study was fairly high. This was likely explained by the nonspecific irritant effects of smoking; approximately one out of four participants in our study reported active smoking at each time point.
In conclusion, our findings indicate that subjects with RSV-LRI during the first 3 years of life who actively smoke are at increased risk of having current asthma and increased peak flow variability in adulthood, as compared with those who do not smoke. These findings support the potential interactive role of early-life insult by RSV and subsequent exposure to cigarette smoke on the development of obstructive lung diseases in later life. The question as to whether the interaction between RSV and smoking directly contributes to asthma causation or is simply indicative of at-risk, susceptible individuals requires further study. Further follow-up of our cohort will determine if early-life RSV-LRI also increases the likelihood of developing chronic airflow limitation among active smokers.
Acknowledgments
Acknowledgment
The authors gratefully acknowledge the contributions of Lynn Taussig who started the Tucson Children’s Respiratory Study in 1980. They thank Bruce Saul for data management and the study nurses, Marilyn Lindell, Lydia de la Ossa, and Nicole Pargas, for data collection and participant follow-up.
Footnotes
Supported by grant HL-56177 and HL-14136 from the National Heart Lung and Blood Institute.
Author Contributions: F.D.M. designed the study, and N.V. analyzed the data under the direction of F.D.M. All authors contributed to interpretation of the data. F.D.M. and N.V. wrote the report with input from the other authors. All authors made important intellectual contributions and approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201311-2095OC on June 13, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Oswald NC, Harold JT, Martin WJ. Clinical pattern of chronic bronchitis. Lancet. 1953;265:639–643. doi: 10.1016/s0140-6736(53)90369-9. [DOI] [PubMed] [Google Scholar]
- 2.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115:195–205. doi: 10.1164/arrd.1977.115.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Samet JM, Tager IB, Speizer FE. The relationship between respiratory illness in childhood and chronic air-flow obstruction in adulthood. Am Rev Respir Dis. 1983;127:508–523. doi: 10.1164/arrd.1983.127.4.508. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen SO, Barker DJ, Shiell AW, Crocker FJ, Wield GA, Holgate ST. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med. 1994;149:616–619. doi: 10.1164/ajrccm.149.3.8118627. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen S. The beginnings of chronic airflow obstruction. Br Med Bull. 1997;53:58–70. doi: 10.1093/oxfordjournals.bmb.a011606. [DOI] [PubMed] [Google Scholar]
- 6.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 7.Gwynn RC. Risk factors for asthma in US adults: results from the 2000 Behavioral Risk Factor Surveillance System. J Asthma. 2004;41:91–98. doi: 10.1081/jas-120026066. [DOI] [PubMed] [Google Scholar]
- 8.Arif AA, Delclos GL, Lee ES, Tortolero SR, Whitehead LW. Prevalence and risk factors of asthma and wheezing among US adults: an analysis of the NHANES III data. Eur Respir J. 2003;21:827–833. doi: 10.1183/09031936.03.00054103a. [DOI] [PubMed] [Google Scholar]
- 9.Glezen WP, Loda FA, Clyde WA, Jr, Senior RJ, Sheaffer CI, Conley WG, Denny FW. Epidemiologic patterns of acute lower respiratory disease of children in a pediatric group practice. J Pediatr. 1971;78:397–406. doi: 10.1016/s0022-3476(71)80218-4. [DOI] [PubMed] [Google Scholar]
- 10.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 11.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children’s Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 12.Jartti T, Lehtinen P, Vuorinen T, Osterback R, van den Hoogen B, Osterhaus AD, Ruuskanen O. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piippo-Savolainen E, Korppi M. Wheezy babies—wheezy adults? Review on long-term outcome until adulthood after early childhood wheezing. Acta Paediatr. 2008;97:5–11. doi: 10.1111/j.1651-2227.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuikka L, Reijonen T, Remes K, Korppi M. Bronchial asthma after early childhood wheezing: a follow-up until 4.5-6 years of age. Acta Paediatr. 1994;83:744–748. doi: 10.1111/j.1651-2227.1994.tb13131.x. [DOI] [PubMed] [Google Scholar]
- 15.Korppi M, Kuikka L, Reijonen T, Remes K, Juntunen-Backman K, Launiala K. Bronchial asthma and hyperreactivity after early childhood bronchiolitis or pneumonia. An 8-year follow-up study. Arch Pediatr Adolesc Med. 1994;148:1079–1084. doi: 10.1001/archpedi.1994.02170100077015. [DOI] [PubMed] [Google Scholar]
- 16.Hyvärinen M, Piippo-Savolainen E, Korhonen K, Korppi M. Teenage asthma after severe infantile bronchiolitis or pneumonia. Acta Paediatr. 2005;94:1378–1383. doi: 10.1111/j.1651-2227.2005.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 17.Piippo-Savolainen E, Remes S, Kannisto S, Korhonen K, Korppi M. Asthma and lung function 20 years after wheezing in infancy: results from a prospective follow-up study. Arch Pediatr Adolesc Med. 2004;158:1070–1076. doi: 10.1001/archpedi.158.11.1070. [DOI] [PubMed] [Google Scholar]
- 18.Backman K, Piippo-Savolainen E, Ollikainen H, Koskela H, Korppi M. Increased asthma risk and impaired quality of life after bronchiolitis or pneumonia in infancy. Pediatr Pulmonol. 2014;49:318–325. doi: 10.1002/ppul.22842. [DOI] [PubMed] [Google Scholar]
- 19.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 20.Voraphani N, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD.Increased risk of asthma-like symptoms among adult smokers with a history of RSV in early life. Am J Respir Crit Care Med 2013;187:A6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129:1219–1231. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 22.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 23.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, Martinez FD. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946–952. doi: 10.1136/thx.52.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Régnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820–826. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 26.Szabo SM, Levy AR, Gooch KL, Bradt P, Wijaya H, Mitchell I. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev. 2013;13:S9–S15. doi: 10.1016/S1526-0542(12)70161-6. [DOI] [PubMed] [Google Scholar]
- 27.Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38:155–160. doi: 10.1002/ppul.20058. [DOI] [PubMed] [Google Scholar]
- 28.Ruotsalainen M, Piippo-Savolainen E, Hyvärinen MK, Korppi M. Respiratory morbidity in adulthood after respiratory syncytial virus hospitalization in infancy. Pediatr Infect Dis J. 2010;29:872–874. doi: 10.1097/inf.0b013e3181dea5de. [DOI] [PubMed] [Google Scholar]
- 29.Piippo-Savolainen E, Remes S, Kannisto S, Korhonen K, Korppi M. Early predictors for adult asthma and lung function abnormalities in infants hospitalized for bronchiolitis: a prospective 18- to 20-year follow-up. Allergy Asthma Proc. 2006;27:341–349. doi: 10.2500/aap.2006.27.2912. [DOI] [PubMed] [Google Scholar]
- 30.Wennergren G, Hansson S, Engström I, Jodal U, Amark M, Brolin I, Juto P. Characteristics and prognosis of hospital-treated obstructive bronchitis in children aged less than two years. Acta Paediatr. 1992;81:40–45. doi: 10.1111/j.1651-2227.1992.tb12076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennergren G, Amark M, Amark K, Oskarsdóttir S, Sten G, Redfors S. Wheezing bronchitis reinvestigated at the age of 10 years. Acta Paediatr. 1997;86:351–355. doi: 10.1111/j.1651-2227.1997.tb09021.x. [DOI] [PubMed] [Google Scholar]
- 32.Goksör E, Amark M, Alm B, Gustafsson PM, Wennergren G. Asthma symptoms in early childhood—what happens then? Acta Paediatr. 2006;95:471–478. doi: 10.1080/08035250500499440. [DOI] [PubMed] [Google Scholar]
- 33.Bronnimann S, Burrows B. A prospective study of the natural history of asthma. Remission and relapse rates. Chest. 1986;90:480–484. doi: 10.1378/chest.90.4.480. [DOI] [PubMed] [Google Scholar]
- 34.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312:1195–1199. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 36.Frey U, Brodbeck T, Majumdar A, Taylor DR, Town GI, Silverman M, Suki B. Risk of severe asthma episodes predicted from fluctuation analysis of airway function. Nature. 2005;438:667–670. doi: 10.1038/nature04176. [DOI] [PubMed] [Google Scholar]
- 37.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenzel SE, Gibbs RL, Lehr MV, Simoes EA. Respiratory outcomes in high-risk children 7 to 10 years after prophylaxis with respiratory syncytial virus immune globulin. Am J Med. 2002;112:627–633. doi: 10.1016/s0002-9343(02)01095-1. [DOI] [PubMed] [Google Scholar]
- 39.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42, e31. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 40.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, Bont L Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 41.Thomsen SF, van der Sluis S, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, Duffy DL, Backer V, Bisgaard H. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 42.Young S, O’Keeffe PT, Arnott J, Landau LI. Lung function, airway responsiveness, and respiratory symptoms before and after bronchiolitis. Arch Dis Child. 1995;72:16–24. doi: 10.1136/adc.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chawes BL, Poorisrisak P, Johnston SL, Bisgaard H. Neonatal bronchial hyperresponsiveness precedes acute severe viral bronchiolitis in infants. J Allergy Clin Immunol. 2012;130:354–361, e353. doi: 10.1016/j.jaci.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 45.Tashkin DP, Altose MD, Connett JE, Kanner RE, Lee WW, Wise RA The Lung Health Study Research Group. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:1802–1811. doi: 10.1164/ajrccm.153.6.8665038. [DOI] [PubMed] [Google Scholar]
- 46.Postma DS, Boezen HM. Rationale for the Dutch hypothesis. Allergy and airway hyperresponsiveness as genetic factors and their interaction with environment in the development of asthma and COPD. Chest. 2004;126:96S–104S; discussion 159S–161S. doi: 10.1378/chest.126.2_suppl_1.96S. [DOI] [PubMed] [Google Scholar]