Abstract
Surgical site infection (SSI) is one of the most common surgical complications in the world, particularly in developing countries. This study aimed to estimate the incidence and distribution of SSI in mainland China. Eighty-four prospective observational studies (82 surveillance studies, 1 nested case control study, and 1 cohort study) were selected for inclusion in this meta-analysis. The average incidence of SSI in mainland China was 4.5% (95% CI: 3.1–5.8) from 2001 to 2012 and has decreased significantly in recent years. The remote western regions had a higher incidence of 4.6% (95% CI: 4.0–5.3). The most common surgical procedure was abdominal surgery (8.3%, 95% CI: 6.5–10.0). SSI occurred frequently in the elderly (5.1%, 95% CI: 2.2–8.0), patients confined to hospital for over 2 weeks (5.7%, 95% CI: 0.9–10.0), superficial incision wounds (5.6%, 95% CI: 4.4–6.8), dirty wounds (8.7%, 95% CI: 6.9–10.6), operations lasting for over 2 hours (7.3%, 95% CI: 4.9–9.7), general anaesthesia operations (4.7%, 95% CI: 2.7–6.6), emergency surgeries (5.9%, 95% CI: 4.2–7.7), and non-intra-medication operations (7.4%, 95% CI: 1.0–13.7).
Healthcare-associated infections are deemed the most common and deadliest events threatening the health of patients. They prolong the length of hospital stays and increase healthcare costs worldwide1,2,3. A surgical site infection (SSI) is a wound infection that occurs following an invasive procedure4. SSI accounts for over 20% of all healthcare-associated infections in surgical patients5,6. Approximately 2–5% of surgical patients worldwide have developed an SSI7. Moreover, SSI contributes to surgery-related mortality, despite occurring frequently in superficial incisions8. It has been reported that more than one-third of postoperative deaths worldwide are related to SSI9,10.
The additional economic burden caused by extended hospital stays due to SSI is large. Several studies have estimated that the burdens that hospitals or national health trusts face increases in costs ranging from £814 to £6,626 ($1,341–10,922) per patient in England, depending on the type of surgery involved and the severity of infection11. Other studies have reported an average cost of [euro]325 ($4,544) per day in Europe and $25,546 per infection in the United States12,13. In China, the additional cost varies between ¥2,400 and ¥31,700 ($396–5,237)14.
The risk of SSI is higher in developing countries relative to developed nations15. A large cross-sectional survey of healthcare-associated infections, conducted in mainland China in 2010, reported that E.coli (25.9%), S.aureus (14.3%), and P.aeruginosa (11.9%) were the three most common pathogens associated with SSI16. Furthermore, almost half of these pathogens were drug-resistant, similar to drug-resistant S.aureus, and accounted for more than 70% of SSIs16. Risk factors such as age, surgical procedure, wound contamination, and anaesthetic method have been widely recognised to increase risk of SSI17,18,19,20, but findings regarding risk factors specific to China, such as Chinese geographical region and hospital ranks, have been inconsistent in different studies.
Surveillance, which records infection prospectively and actively, is an essential method for understanding the incidence and distribution of healthcare-associated infections. Site-oriented target surveillance, which is usually undertaken for selected high-risk infections and specialties, provides more accurate data21. However, surveillance in developing countries remains inadequate and inaccurate15. In China, public reports of SSI, obtained via ongoing national surveillance activities, have been rare over the past decades. However, many articles have reported that SSI surveillance at hospital level varies widely. These findings have provided us with an opportunity to summarize the profile of SSI at a national level by conducting a meta-analysis.
We collected prospective studies, including target surveillance of SSI, cohort studies, and nested case control studies. The aim of this study was to understand the average incidence of SSI and the overall distribution of its characteristics in mainland China. We expected to generate information on SSI in China for use by policy makers and other researchers.
Results
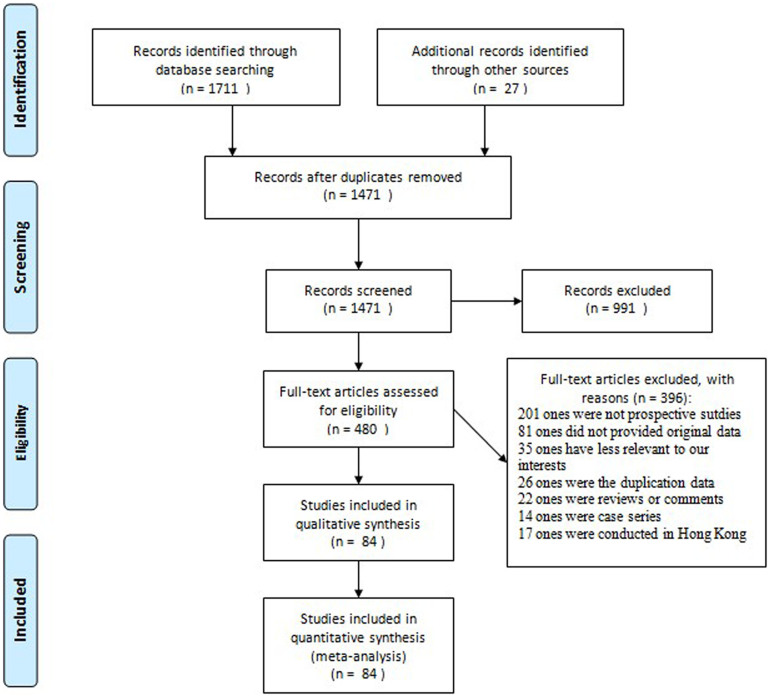
Our search generated 1,471 articles. A flow diagram of the selection process is presented in Figure 1. Of the original articles, 991 publications were excluded because they were irrelevant to the study's objectives. The remaining 480 articles were reviewed by two of the authors, and articles that did not meet the selection criteria were excluded. Consequently, 84 prospective observational studies (82 surveillance, 1 nested case control, and 1 cohort study) were selected for final analysis. Supplementary Table S1 and Supplementary Dataset 1 present a list of the studies included and a summary of their characteristics respectively. This meta-analysis covered approximately 0.49 million patients in 20 provinces, municipalities, and autonomous regions in mainland China.
Figure 1. Flow diagram for selection process.
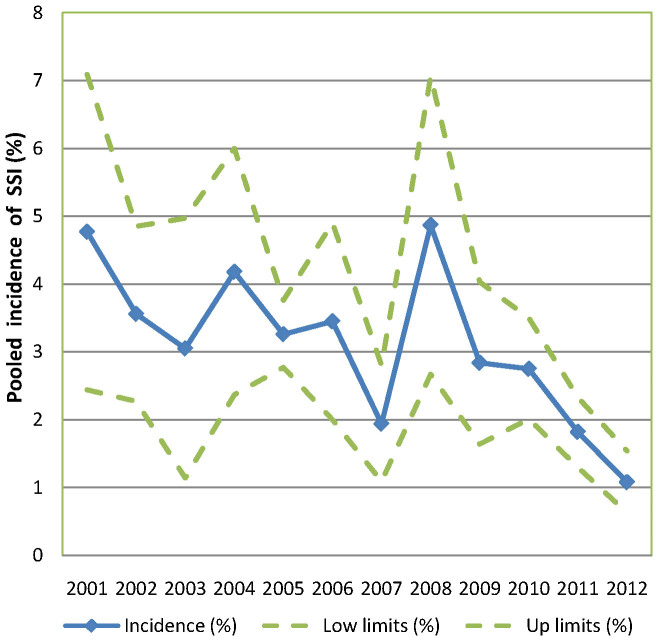
SSI incidence rates were extracted from the 84 included publications, and we found that the incidence rate ranged from 0.2% to 33.0%. Table 1 contains the pooled incidence and subgroup analysis results according to study setting. The pooled average incidence rate in mainland China was 4.5% from 2001 to 2012. A subgroup analysis revealed that SSI incidence rates differed across years (P = 0.045), regions (P = 0.017), and surgical procedures (P = 0.004). The incidence was higher in remote western regions (4.6%, 95% CI: 4.0–5.3) relative to the eastern coastal areas (3.0%, 95% CI: 2.4–3.5) and midlands (3.8%, 95% CI: 1.4–6.3). Among the different surgical procedures examined, the incidence rate was highest for abdominal surgery (8.3%, 95% CI: 6.5–10.0) and lowest for orthopaedic surgery (1.0%, 95% CI: 0.5–1.6). We also found that SSI occurred more frequently prior to 2005. The decreasing trend in the incidence of SSI in recent years is depicted in Figure 2. Hospital rank and surveillance duration were not related to our findings regarding the incidence of SSI. The Egger's test indicated that a publication bias existed (P < 0.001).
Table 1. The results of pooled incidence of SSI and subgroup analysis by study setting.
| Subgroups | No. of studies | Incidence of ISS% (95% CI) | I2 (%) | P value |
|---|---|---|---|---|
| All studies | 84 | 4.5 (3.1,5.8) | 98.3 | |
| Year | ||||
| Before 2005 | 30 | 4.5 (2.3,6.6) | 95.3 | 0.045 |
| After 2005 | 54 | 3.1 (2.8,3.5) | 98.2 | |
| Region | ||||
| Eastern coastal | 40 | 3.0 (2.4,3.5) | 98.1 | 0.017 |
| Midlands | 24 | 3.8 (1.4,6.7) | 99.1 | |
| Remote western | 18 | 4.6 (4.0,5.1) | 94.9 | |
| Hospital rank | ||||
| Grade III | 64 | 4.3 (2.7,5.9) | 98.7 | 0.793 |
| Grade II | 11 | 3.5 (2.6,4.5) | 95.8 | |
| Surveillance implementation | ||||
| <1 year | 24 | 3.6 (2.9,4.4) | 96.6 | 0.762 |
| 1–3 years | 21 | 2.7 (2.1,3.3) | 97.6 | |
| >3 years | 39 | 5.2 (2.7,7.7) | 99.0 | |
| Surgical procedure | ||||
| General surgery | 45 | 3.1 (1.0,5.2) | 98.9 | 0.004 |
| Abdominal surgery | 15 | 8.3 (6.5,10.0) | 95.6 | |
| Gynaecology & obstetrics | 8 | 5.7 (0.9,13.9) | 93.2 | |
| Neurosurgery | 6 | 3.6 (1.4,5.8) | 93.3 | |
| Thoracic surgery | 5 | 3.0 (2.7,3.3) | 97.7 | |
| Orthopaedic surgery | 4 | 1.0 (0.5,1.6) | 90.3 | |
| Confounding factors control | ||||
| Adjusted* | 53 | 3.6 (1.9,5.3) | 94.8 | 0.815 |
| Crude | 31 | 5.1 (2.5,7.9) |
*The SSI incidence was adjusted by at least one confounding factor.
P values test homogeneity between strata with the Wilcoxon or Kruskal-Wallis non-parametric rank test according to the numbers of strata.
Random effects model was use to pool incidences.
Figure 2. Pooled incidence of SSI in mainland China at different study periods, with corresponding 95% confidence interval.
Table 2 presents the pooled incidence of SSI rates found in the subgroup analysis according to the relevant characteristics of the population. The results indicated that SSI occurred frequently in superficial or contaminated wounds. Some factors also tended to induce SSI, such as emergency operations, long-term operations, operations with general anaesthesia, and operations in which antibiotics were administered after (rather than before) surgery. In addition, older patients and patients who stayed in the hospital for more than 2 weeks were at a high risk of developing SSI. Although the incidence of SSI was higher in men and during the summer, sex and season differences were not statistically significant. The Egger's test (P < 0.001 for all subgroups) indicated that a publication bias existed.
Table 2. The results of subgroup analysis by characteristics of the population.
| Subgroups | No. of studies | Incidence of ISS% (95% CI) | I2 (%) | P value |
|---|---|---|---|---|
| Age | ||||
| ≥60 years old | 22 | 5.1 (2.1, 8.0) | 89.2 | 0.043 |
| <60 years old | 21 | 4.4 (2.0, 6.3) | 98.5 | |
| Sex | ||||
| Male | 8 | 4.6 (3.1, 6.1) | 97.1 | 0.294 |
| Female | 10 | 3.2 (1.7, 4.6) | 95.0 | |
| Wound contamination | ||||
| Dirty | 20 | 8.7 (6.9, 10.6) | 90.1 | 0.001 |
| Contamination | 29 | 3.5 (2.8, 4.3) | 97.8 | |
| Clear | 23 | 0.8 (0.6, 1.0) | 93.0 | |
| Wound depth | ||||
| Superficial | 7 | 5.6 (4.4, 6.8) | 97.6 | 0.006 |
| Deep | 7 | 3.4 (2.9, 4.0) | 98.8 | |
| Operational duration | ||||
| ≥2 hours | 20 | 7.3 (4.9, 9.7) | 90.9 | 0.001 |
| <2 hours | 20 | 2.1 (1.4, 2.8) | 95.9 | |
| Anaesthetic method | ||||
| General | 6 | 4.7 (2.7, 6.6) | 88.0 | 0.001 |
| Local | 7 | 3.2 (2.8, 4.5) | 78.6 | |
| Surgical property | ||||
| Emergency | 11 | 5.9 (4.2, 7.7) | 84.6 | 0.004 |
| Elective | 12 | 4.1 (3.2, 5.0) | 89.0 | |
| Medication timing | ||||
| Post-operation | 6 | 7.4 (1.0, 13.7) | 94.2 | 0.001 |
| Intra-operation | 6 | 2.7 (0.4, 5.0) | 86.8 | |
| Stay length | ||||
| ≥2 weeks | 7 | 5.7 (0.9, 10.4) | 85.7 | 0.001 |
| <2 weeks | 7 | 3.6 (0.01, 8.2) | 89.4 | |
| Season | ||||
| Spring | 9 | 4.5 (3.7, 5.3) | 91.1 | 0.714 |
| Summer | 9 | 4.9 (3.9, 5.8) | 95.6 | |
| Autumn | 9 | 4.3 (3.6, 5.1) | 94.6 | |
| Winter | 8 | 4.2 (2.8, 5.1) | 96.1 |
Random effects model was use to pool incidences. P values test homogeneity between strata with the Wilcoxon or Kruskal-Wallis non-parametric rank test according to the numbers of strata.
In general, studies were of acceptable quality (Table 3). Most (n = 51, 60.7%) used an adequate sample size (n = 66, 78.6%) and a representative sampling frame that contained almost every individual in the target patient population. Bias in sample selection was unlikely, because all studies selected samples using census (surveillance studies) or random methods (nested case control and cohort studies), and response rates for almost all studies (n = 82, 97.6%) were above 80%. Although the majority of studies (n = 71, 84.5%) claimed that they defined SSI according to the same diagnosis standard used by Chinese Ministry of Health (MOH) in 2001, only 23 (27.4%) studies reported the details of follow-up cares. Information bias obtained by proxy record (n = 21, 25.0%), rather than direct measurement (n = 63, 75.0%), was limited; moreover, most studies (n = 58, 69.0%) confirmed SSI using laboratory tests. Statistical adjustments were made or stratified analyses performed to control for some confounding factors in most of the included studies (n = 53, 63.1%).
Table 3. Methodological quality of the studies included in the final meta-analysis.
| Quality variable | Quality variable categories | Number of studies | Proportion (%) |
|---|---|---|---|
| External validity | |||
| Representation | The sampling frame is a list of almost every individual within the target population | 51 | 60.7 |
| The sampling frame is a list of just one particular group within the target population (e.g. the elderly, children, or those with underlying diseases such as tumours, hypertension, or obesity) | 33 | 39.3 | |
| Sampling bias | |||
| Sampling method | Census or random selection | 84 | 100.0 |
| Non-random selection or not reported | 0 | 0.0 | |
| Sample size a | The sample size is ≥456 | 66 | 78.6 |
| The sample size is <456 | 18 | 21.4 | |
| Nonresponse bias | |||
| Response rate | Response rate is ≥80% | 82 | 97.6 |
| Response rate is <80% or not reported | 2 | 2.4 | |
| Internal validity | |||
| Information bias | |||
| Definition | Definition from the diagnosis standard by the MOH in 2001 | 71 | 84.5 |
| Other diagnosis standard or not reported | 13 | 15.5 | |
| Follow up | Patients are followed up after discharge | 23 | 27.4 |
| Patients are not followed up after discharge or not reported | 61 | 72.6 | |
| Data collection | Data are collected directly from the subjects | 63 | 75.0 |
| Data are collected from a proxy (e.g. medical records, administrative databases) | 21 | 25.0 | |
| Measurement bias | |||
| Measurement | The identification of SSI is based on laboratory test for pathogens | 58 | 69.0 |
| The identification of SSI is not based on laboratory test for pathogens or not reported | 26 | 31.0 | |
| Precise parameter | Adjusted incidence or stratified incidence by risk factors | 53 | 63.1 |
| Crude incidence in general population | 31 | 38.9 |
aThe sample size is estimated given α = 0.05, allowable error δ = 0.02, and expected incidence p = 5.0%.
MOH is the Ministry of Health of China.
Discussion
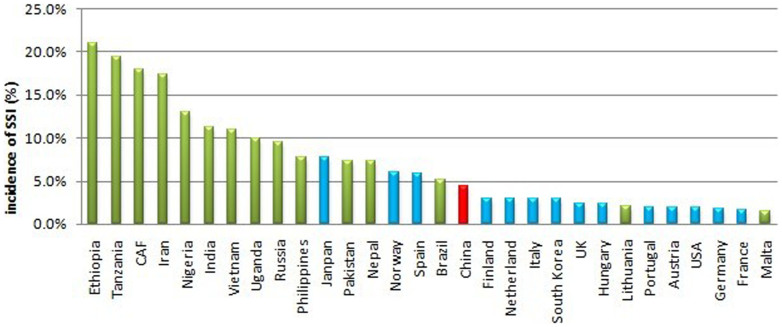
In our meta-analysis, the pooled incidence rate of SSI in mainland China was 4.5%. The incidence rate was substantially lower than the average of 11.8% in developing countries and even lower than those of several developed countries1,22,23. However, the rate was higher than those in most developed countries. For example, incidence rates were 1.9% in the United States, 2.2% in Europe, 1.6% in Germany, 1.4% in England, 1.6% in France, and 2.0% in Portugal. Comparisons of SSI incidence rates between countries is presented in Figure 39,24,25,26,27,28,29,30,31,32,33. The pooled SSI incidence rate was higher (4.6%) in remote western regions relative to those of the midlands (3.8%) and eastern coastal regions (3.0%). In fact, eastern coastal areas enjoy strong economies and abundant healthcare resources, while western areas have limited resources in mainland China. The differences between developing and developed countries and between different geographical regions in mainland China indicated that economic status is a factor that influences the incidence of SSI.
Figure 3. Incidence of SSI in several countries around the world.
The red bar stands for the pooled incidence in mainland China in our meta-analysis, blue bars stand for incidences from published literatures in developed countries, and green bars stand for incidences from published literatures in developing countries. CAF is short for Central African Republic.
It is worth mentioning that a decrease in the incidence of SSI in mainland China has occurred in recent years (Figure 2). Since the SARS epidemic in 2003, numerous resources and finances have been used to control healthcare-associated infection. Currently, Chinese hospitals are duty bound to establish a healthcare-associated infection management committee, which is required by the Chinese Ministry of Health (MOH). Hospitals must survey the prevalence of healthcare-associated infections at least once per year and hire at least one professional per 200–250 beds. At the same time, they must conduct hospital-wide surveillance and antimicrobial drug surveillance involving patients and medical staff. Since 2005, 147 developing countries have been committed to reducing healthcare-associated infections by signing the pledge of the WHO's First Global Patient Safety Challenge with 46 developed countries34. All of these efforts could play a considerable role in the successful control of SSI in mainland China.
The results of this study highlighted some SSI risk factors, such as age, sex, abdominal surgery, wound contamination, duration of surgery, general anaesthesia, emergency surgery, and timing of medication. These results were consistent with those of some previous studies from other countries17,18,19,20. As we know, numerous bacteria, which are the source of the infection, thrive in contaminated/dirty wounds. In addition, a long operation and hospital stay could increase the probability of exposure to pathogens35. Moreover, the elderly and general anaesthesia patients are unable to provide a robust defence against pathogens because of their weak or depressed immune systems18,20. Under normal circumstances, bacteria in the abdomen maintain an ecological balance. However, abdominal surgery could upset this balance and change the proportion of bacteria, thereby inducing an SSI. We also found that intra-medication administration is the key to avoiding SSI in surgical patients. Intra-medication was defined as the prophylactic administration of antibiotics within 1 hour before and 24 hours after an operation; post-medication refers to administration 24 hours postoperatively. In fact, the inappropriate use of antibiotics is a common problem in China. Intra-medication still accounts for a lower proportion of SSI relative to post-medication36. It is necessary to increase the appropriate use of prophylactic antibiotics in Chinese hospitals.
The SSI incidence can be affected by some potential confounding factors. In our meta-analysis, 53 (63.1%) studies reported the risk-adjusted incidences that adjust at least one risk factor. The main adjusted risk factors included age, sex, wound contamination, hospitalization etc. (see Supplementary dataset 1). We also compared the pooled SSI incidences between adjusted and non-adjusted groups. Although there was no significant difference in our founding (P = 0.815), other potential confounding factors can modify the SSI incidence indirectly and should still be considered. For example, patient factors (smoking, BMI, life style, nutritional status) are associated with resistance of body to germs after operation; similarly, surgical complexity would influence operation duration and exposure possibility37,38,39. However these confounding factors have not been concerned in the included studies. Although it is hard to take all risk factors into consideration, researchers should adjust some important factors for accurate SSI incidence estimation; just like the nosocomial infection surveillance system (NNIS) in the USA mainly adjusted hospitalization, catheter-related bloodstream, catheter-related urinary-tract infection, and ventilator-associated pneumonia etc40. However, in our meta-analysis, some studies (36.9%) reported only crude incidence that did not taken any risk factors into consideration. More attention needs to be given to estimate the risk-adjusted SSI incidence in Mainland China in future.
Although a substantial proportion of the literature in the subgroup analyses reported a stratified incidence of SSI associated with various risk factors, the definitions or cut-off points that divided the subsets into the stratifications were inconsistent. This problem made it difficult to combine the stratified incidence of SSI across all studies. Due to this limitation, we selectively pooled subsets that had been reported in at least in 5 studies by using the same definition and the most common cut-off points. It is essential to use standard definitions and cut-off points for future research reports on SSI in China.
A considerable number of prospective observational studies were included in our analyses; while most of them (82/84) were target surveillance studies, the others were a cohort study and a nested case-control study. The variety of study designs that dealt with SSIs in mainland China was relatively uniform according to the results of our literature search. Most of the published reports were random controlled trials (RCTs), retrospective case control studies, or cross-sectional studies. As for prospective observational studies, surveillance of SSI was the most popular type of study in mainland China. This finding may be attributed in part to the compulsory requirement for healthcare-associated surveillance by the Chinese Ministry of Health (MOH) since 2003. Although researchers from mainland China should be commended for paying attention to the surveillance of SSI, other types of research, such as large-scale cohort studies, should also be encouraged in future investigations.
The available tools used to assess the methodological quality of observational studies were generally considered to be unsatisfactory and were not widely used41. Moreover, only five tools relevant to the incidence or prevalence of diseases were identified by a previous systematic review42. Unfortunately, there was no consensus with respect to individual quality criteria. In the course of performing systematic reviews on the incidence of SSI, we were unable to find a tool specifically designed to assess the methodological quality of surveillance studies. Thus, we used a modified checklist that was based on several previous tools43,44,45,46,47,48,49,50 to conduct a descriptive assessment. The modified checklist addressed external and internal validity, and assessed representativeness, sampling bias, nonresponse bias, and measurement bias. Generally speaking, the methodological quality of included studies was acceptable; nevertheless, we recommend that future studies focus on strict follow-up care following patient discharge.
Our meta-analysis has several limitations. First, heterogeneity is a common dilemma in observational studies51, particularly those that involve proportions1,44,45,52,53. A similar situation was observed in our study. We tried to explain heterogeneity by performing subgroup analyses, but after an exploration of the factors that were likely to contribute to the variation, such as study design and population characteristics, the heterogeneity remained unexplained. Although the ‘Standard for Healthcare-associated Infection Surveillance’ was used as a guideline for SSI monitoring, it was implemented independently in different hospitals, regions, and populations. For example, studies were typically undertaken in different hospital wards; however, the classification of the wards in different hospitals may have been inconsistent in Chinese hospitals. Therefore, it is difficult to ensure that all surveillance activities are homogeneous in real circumstances. These constraints and variations in setting may account, at least partly, for the significant heterogeneity observed.
Second, publication bias may have existed, as indicated by the Egger's test P values (<0.001). Taking the quality of surveillance into consideration, we limited our selection to prospective target surveillance studies that were more often than not supported by well-trained investigators with large budgets. A limited number of hospitals in mainland China have sufficient resources to conduct prospective target surveillance for SSI. Such studies were often performed in Grade III hospitals in economically developed regions. Additionally, the Grade III hospitals in eastern developed regions usually have superior research conditions relative to those of Grade I and II hospitals and hospitals in western regions with limited resources. Therefore, higher-grade hospitals in developed regions may publish their studies frequently in various journals, resulting in a degree of publication bias. Although there were no significant differences between hospital rankings in our study, future investigations should focus on lower-grade hospitals in resource-limited regions.
Third, although a drastic decline in the incidence of SSI was revealed in our study, this finding should be interpreted with caution. Because the meta-analysis was based on the data from published studies, it was difficult to avoid some bias; for example, authors of later studies may have been more likely to publish their research if they found that the infection rate was low. Thus, information from ongoing SSI surveillance across the entire country is indispensable. However, until now, public information regarding SSI, published by the national surveillance system, has been scarce and its coverage limited (most national surveillance units are located in developed areas and in Grade III hospitals). Consequently, we believe that our meta-analysis is an acceptable method with which to estimate the incidence of SSI prior to the publication of authoritative data from the national surveillance system. Furthermore, this method can be regarded as a supplemental tool for a national surveillance system in the future.
Methods
Selection of studies
This meta-analysis was conducted according to the Meta-Analysis of Observational Studies in Epidemiology Guidelines54. We searched the prospective studies that reported SSI in mainland China, including target surveillance of SSI, cohort studies, and nested case control studies. Publications in Chinese and English were searched. The literature in Chinese was searched using the China National Knowledge Infrastructure (CNKI), Wan Fang Database, and VIP Database for Chinese Technical Periodicals (VIP). The English literature was searched using PubMed, EMBASE, the Database of Abstracts of Reviews of Effects, and the Cochrane Database. Relevant articles were identified according to the following terms: (surgical site infections [Title/Abstract] OR surgical incision infection [Title/Abstract] OR wound infections [Title/Abstract] OR wound infection [Title/Abstract]) AND prospective studies [MeSH Terms] and China [MeSH Terms]. The publication time was limited to 2001–2013, as the Diagnosis Standard of Nosocomial Infection was issued by the Ministry of Health of China in 2001. Grey literatures, such as surveillance reports, academic dissertations, and conference abstracts, were examined. A reference list of key reviews was also searched for additional studies.
Selection criteria
We included eligible studies that met the following criteria:
Dealt with healthcare-associated infections defined by WHO as ‘an infection acquired in the hospital by a patient who was admitted for a reason other than that infection’. We included the literature on infections acquired in hospital, including those that were acquired in hospital but occurred after discharge. However, incubating or existing infections at the time of admission, such as community-acquired infections, were excluded.
Reported the incidence of SSI. A considerable number of Chinese publications reported outcomes using the Chinese term ‘infection rate’, which was easily confused with ‘incidence’ and ‘prevalence’. The true meaning of this ‘rate’ was judged according to the study's design. The prospective design can be used to calculate incidence; therefore, we selected prospective studies, including target surveillance, cohort, and nested case control studies. Retrospective case-control, cross-sectional, and non-observational studies, including RCTs, were excluded.
Provided original data, such as the number of SSI cases (n) or total number of respondents (N). These two parameters were necessary to calculate the pooled incidence of SSI in the meta-analysis. Studies containing at least one parameter (n or N) satisfied this criterion.
Conducted in mainland China. Many studies dealt with SSI in Taiwan, Hong Kong, and Macao in China. However, socio-economic status and health policies in these areas are different from those of mainland China; therefore, these studies were excluded.
Additionally, publications such as case series (defined as studies with less than 100 respondents), reviews, and commentaries were excluded. In case involving duplicate studies on the same subject, we chose the most recent one.
Study identification
All titles and abstracts of the citations that were generated by the literature search were screened independently by two reviewers. Relevant publications were reviewed in their entirety, and the reviewers were blind to the author and research institution of each study. Each reviewer made a judgment regarding inclusion or exclusion of the study. In the event of disagreement, a third reviewer served as a consultant to resolve the issue.
Subgroups
We performed pre-specified subgroup analyses of studies according to their quality and setting characteristics (including year, region, hospital rank, duration of surveillance implementation, surgical procedure, and confounding factors control). We classified the Chinese regions into eastern coastal, midlands, and remote western regions, according to geographical location, economic status, and resources. The hospitals were divided into three ranks, grade I (1–100 beds), grade II (101–500 beds), and grade III (>500 beds), according to size, technology, equipment, and management level.
We also conducted subgroup analyses of the attributes of their populations. Among the relevant risk factors for the population characteristics that were mentioned in the included studies, ten subsets, which had been mentioned at least 5 times and stratified by the accepted and comparable cut-off points, were screened to perform the subgroup analyses. The screened subsets with their cut-off points were age (≤60 or >60 years old), sex (male or female), wound contamination (dirty, contaminated or clear), wound depth (superficial or deep), operational duration (≤2 hours or >2 hours), anaesthetic method (general or local), surgical type (emergency or elective), length of stay (≤2 or >2 weeks), season (spring, summer, autumn, or winter), and medication timing (intra or post). Intra-medication was defined as the prophylactic administration of antibiotics within 1 hour before and 24 hours after an operation, and post-medication as administration 24 hours postoperatively.
Data extraction
For each included report, the following data were extracted: publication date, region, type of surgery, population, hospital rank, incidence of SSI with number of infections (n) or corresponding denominators (N), and the stratified incidence, characterized by risk factors (including age, gender, wound depth, length of stay, duration of operation, anaesthetic method, disease history, surgical procedure, infection season, wound contamination, and medication timing). We also contacted corresponding authors (by e-mail) of articles that did not provide details of the study's background and asked them for the relevant information, such as study time, region, or hospital rank.
Quality assessment of the included studies
A descriptive quality assessment of the final studies included in our meta-analysis was conducted using a checklist that was based on previously available tools43,44,45,46,47,48,49,50. The checklist measured two study dimensions: external validity and internal validity.
The external validity included:
Representation: whether the sampling frame was a list of almost every individual or just one particular group (e.g. the elderly, children, or those with underlying diseases such as tumours, hypertension, or obesity) within the target population.
Sampling method: whether a census or random method (e.g. simple random sampling, stratified random sampling, cluster sampling, or systematic sampling) was used to select the sample.
Sample size: whether the sample size was 456 or larger, which is the sample size required to estimate the incidence, given α = 0.05, δ = 0.02, and expected incidence = 5.0%.
Response rate: whether the response rate was 80% or more.The internal validity included:
Definition: whether the SSI was identified according to the Diagnosis Standard of Nosocomial Infection issued by the Ministry of Health of China in 2001.
Follow-up: whether patients were followed up after discharge.
Data collection: whether data were collected from subjects directly or via a proxy (e.g. medical records and administrative databases)
Measurement: whether the identification of SSI was based on a laboratory test for pathogens.
Precise parameter: whether the parameter of interest was the adjusted/stratified incidence of SSI.
Two assessors independently evaluated methodological quality of included studies, and disagreement was resolved through discussion with a third assessor.
Statistical analysis
Random effect model weighted by inverse variance was used to calculate the pooled incidence due to significant heterogeneity which was measured by I2 statistics:
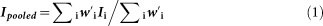 |
where Ipooled is the pooled incidence, Ii is the incidence of study i, 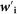 is the adjusted weight of Ii, which can be calculated as:
is the adjusted weight of Ii, which can be calculated as:
 |
where wi is the inverse of sampling variance of study i, and a is the adjusted factor:
 |
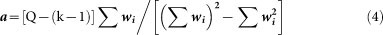 |
where Ni is the total number of patients in hospital in study i, k is the number of studies, Q is the value of Q test of heterogeneity.
Furthermore, Wilcoxon and Kruskal-Wallis non-parametric rank tests were conducted to compare the statistical significance of SSI incidence within each subgroup. Publication biases were measured using the Egger's test and we considered P ≤ 0.05 significant. All analyses were conducted using STATA software (version 11.0, Stata corp., College Station, TX, USA).
Author Contributions
Y.F., Z.W. and W.W. conducted the literature search, reviewed the articles, conducted the statistical analyses, and drafted the manuscript. L.T., H.J. and L.T. (another) made substantial contributions to reviewing the articles, interpreting data and drafting or critically revising the manuscript. S.N. and Y.C. conceived and designed the study, and supervised the work in all phases. All of the authors read and approved the final manuscript.
Supplementary Material
Supplementary Dataset-1 (Nie)
Supplementary Table-S1 (Nie)
Acknowledgments
We deeply appreciated the contribution to this thesis made in various ways by all members in the Department of Epidemiology and Biostatistics.
References
- Allegranzi B. et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 377, 228–241 (2011). [DOI] [PubMed] [Google Scholar]
- Bates D. W., Larizgoitia I., Prasopa-Plaizier N. & Jha A. K. Global priorities for patient safety research. BMJ 338, b1775 (2009). [DOI] [PubMed] [Google Scholar]
- Burke J. P. Infection control - a problem for patient safety. N Engl J Med 348, 651–656 (2003). [DOI] [PubMed] [Google Scholar]
- Horan T. C., Gaynes R. P., Martone W. J., Jarvis W. R. & Emori T. G. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13, 606–608 (1992). [PubMed] [Google Scholar]
- Klevens R. M. et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 122, 160–166 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill S. S. et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol 33, 283–291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth E. T. et al. Four country healthcare associated infection prevalence survey 2006: overview of the results. J Hosp Infect 69, 230–248 (2008). [DOI] [PubMed] [Google Scholar]
- Leaper D. J. et al. Surgical site infection - a European perspective of incidence and economic burden. Int Wound J 1, 247–273 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoran O. E., Sogebi A. O. & Fatugase O. M. Rates and Risk Factors Associated with Surgical Site Infections in a Tertiary Care Center in South-Western Nigeria. Int J Trop Dis Health 1, 25–36 (2013). [Google Scholar]
- Awad S. S. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect (Larchmt) 13, 234–237 (2012). [DOI] [PubMed] [Google Scholar]
- Coello R. et al. Adverse impact of surgical site infections in English hospitals. J Hosp Infect 60, 93–103 (2005). [DOI] [PubMed] [Google Scholar]
- Stone P. W. Economic burden of healthcare-associated infections: an American perspective. Expert Rev Pharmacoecon Outcomes Res 9, 417–422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman R. et al. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect 47, 198–209 (2001). [DOI] [PubMed] [Google Scholar]
- Wenying H., Haoli C., Lan Z., Xiaoning W. & Peizhu X. Economic evaluation of the loss resulted by operative site infection. J Clinl Expl Med 9, 655–657 (2010). [Google Scholar]
- Rosenthal V. D., Maki D. G. & Graves N. The International Nosocomial Infection Control Consortium (INICC): goals and objectives, description of surveillance methods, and operational activities. Am J Infect Control 36, e1–12 (2008). [DOI] [PubMed] [Google Scholar]
- Wen X., Ren N. & Wu A. Distibution of pathogens and antimicrobial resistance: an analysis of China healthcare-associated infection cross-sectional survey in 2010 (in Chinese). Chin J Infect Control 11, 1–6 (2012). [Google Scholar]
- Culver D. H. et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med 91, 152S–157S (1991). [DOI] [PubMed] [Google Scholar]
- Kaye K. S., Schmader K. E. & Sawyer R. Surgical site infection in the elderly population. Clin Infect Dis 39, 1835–1841 (2004). [DOI] [PubMed] [Google Scholar]
- Haley R. W. et al. Identifying patients at high risk of surgical wound infection. A simple multivariate index of patient susceptibility and wound contamination. Am J Epidemiol 121, 206–215 (1985). [DOI] [PubMed] [Google Scholar]
- Tsai P. S., Hsu C. S., Fan Y. C. & Huang C. J. General anaesthesia is associated with increased risk of surgical site infection after Caesarean delivery compared with neuraxial anaesthesia: a population-based study. Br J Anaesth 107, 757–761 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M. C. & Perl T. M. Basics of surgical-site infection surveillance. Infect Cont Hosp Ep 18, 659–668 (1997). [DOI] [PubMed] [Google Scholar]
- Harihara Y. & Konishi T. [Surgical site infection (SSI) surveillance]. Nihon Geka Gakkai Zasshi 107, 230–234 (2006). [PubMed] [Google Scholar]
- Sakong P. L. J. L. E. Association between the pattern of prophylactic antibiotic use and surgical site infection rate for major surgeries in Korea. J Prev Med Public Health 12–20 %\ 2013-2007-2031 2018:2004:2000 (2009). [DOI] [PubMed] [Google Scholar]
- Bagheri N. S., Allegranzi B., Syed S. B., Ellis B. & Pittet D. Health-care-associated infection in Africa: a systematic review. B World Health Organ 89, 757–765 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M. et al. Prospective surveillance for surgical site infection in St. Petersburg, Russian Federation. Infect Control Hosp Epidemiol 28, 319–325 (2007). [DOI] [PubMed] [Google Scholar]
- Gastmeier P. et al. Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect 64, 16–22 (2006). [DOI] [PubMed] [Google Scholar]
- Lamarsalle L., Hunt B., Schauf M., Szwarcensztein K. & Valentine W. J. Evaluating the clinical and economic burden of healthcare-associated infections during hospitalization for surgery in France. Epidemiol Infect, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. C. et al. Surgical site infection in a university hospital in northeast Brazil. Braz J Infect Dis 9, 310–314 (2005). [DOI] [PubMed] [Google Scholar]
- Mu Y., Edwards J. R., Horan T. C., Berrios-Torres S. I. & Fridkin S. K. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol 32, 970–986 (2011). [DOI] [PubMed] [Google Scholar]
- Mustafa A. B. A. K. D. Incidence of nosocomial wound infection in postoperative patients at a teaching hospital in Kashmir. JK-practitioner 1, 38–40 (2004). [Google Scholar]
- Nguyen D. et al. Incidence and predictors of surgical-site infections in Vietnam. Infect Control Hosp Epidemiol 22, 485–492 (2001). [DOI] [PubMed] [Google Scholar]
- Razavi S. M., Ibrahimpoor M., Sabouri K. A. & Jafarian A. Abdominal surgical site infections: incidence and risk factors at an Iranian teaching hospital. BMC Surg 5, 2 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safia Bibi G. A. C. T. Frequency and risk factors of surgical site infections in general surgery ward of a tertiary care hospital of Karachi, Pakistan. Int J Infect Control 3 (2011). [Google Scholar]
- Pittet D. & Donaldson L. Clean Care is Safer Care: a worldwide priority. Lancet 366, 1246–1247 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong G., Wilson J. & Charlett A. Duration of operation as a risk factor for surgical site infection: comparison of English and US data. J Hosp Infect 63, 255–262 (2006). [DOI] [PubMed] [Google Scholar]
- Liu S., Gao X., Luo X., Shi A. & He K. Investigation and management of prophylactic antibiotics using during perioperative period (in Chinese). J Regional Anat & Operative Surg 19, 480–483 %\ 2013-2008-2004 2011:2018:2000 (2010). [Google Scholar]
- Cooper R. A. Surgical site infections: epidemiology and microbiological aspects in trauma and orthopaedic surgery. Int Wound J 10 Suppl 1, 3–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Walraven C. & Musselman R. The Surgical Site Infection Risk Score (SSIRS): A Model to Predict the Risk of Surgical Site Infections. Plos One 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons C. et al. Identification of risk factors by systematic review and development of risk-adjusted models for surgical site infection. Health Technol Assess 15, 1–156, iii–iv (2011). [DOI] [PubMed] [Google Scholar]
- System N. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 to June 2002, issued August 2002. Am J Infect Control 30, 458–475 (2002). [DOI] [PubMed] [Google Scholar]
- Mallen C., Peat G. & Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. Journal of Clinical Epidemiology 59, 765–769 (2006). [DOI] [PubMed] [Google Scholar]
- Shamliyan T., Kane R. L. & Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. Journal of Clinical Epidemiology 63, 1061–1070 (2010). [DOI] [PubMed] [Google Scholar]
- Woodbury M. G. & Houghton P. E. Prevalence of pressure ulcers in Canadian healthcare settings. Ostomy Wound Manage 50, 22–24, 26, 28, 30, 32, 34, 36–28 (2004). [PubMed] [Google Scholar]
- Chen J. J., Yu C. B., Du W. B. & Li L. J. Prevalence of hepatitis B and C in HIV-infected patients: a meta-analysis. Hepatobiliary Pancreat Dis Int 10, 122–127 (2011). [DOI] [PubMed] [Google Scholar]
- Li H. M. et al. HIV Incidence among Men Who Have Sex with Men in China: A Meta-Analysis of Published Studies. Plos One 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamliyan T. A. et al. Development quality criteria to evaluate nontherapeutic studies of incidence, prevalence, or risk factors of chronic diseases: pilot study of new checklists. J Clin Epidemiol 64, 637–657 (2011). [DOI] [PubMed] [Google Scholar]
- Loney P. L., Chambers L. W., Bennett K. J., Roberts J. G. & Stratford P. W. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can 19, 170–176 (1998). [PubMed] [Google Scholar]
- Hoy D. et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 65, 934–939 (2012). [DOI] [PubMed] [Google Scholar]
- Francis J. M., Grosskurth H., Changalucha J., Kapiga S. H. & Weiss H. A. Systematic review and meta-analysis: prevalence of alcohol use among young people in eastern Africa. Trop Med Int Health 19, 476–488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane T. V., Glenny A. M. & Worthington H. V. Systematic review of population-based epidemiological studies of oro-facial pain. J Dent 29, 451–467 (2001). [DOI] [PubMed] [Google Scholar]
- Zheng, T., Boffetta, P. & Boyle, P. (eds.). Epidemiology and Biostatistics, (International Prevention Research Institute, Lyon, 2011). [Google Scholar]
- Gao X. et al. Prevalence and trend of hepatitis C virus infection among blood donors in Chinese mainland: a systematic review and meta-analysis. BMC Infect Dis 11, 88 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough E. et al. HIV and hepatitis B and C incidence rates in US correctional populations and high risk groups: a systematic review and meta-analysis. BMC Public Health 10, 777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Dataset-1 (Nie)
Supplementary Table-S1 (Nie)