Abstract
Obesity is now epidemic worldwide. Beyond associated diseases such as diabetes, obesity is linked to neuropsychiatric disorders such as depression. Alarmingly maternal obesity and high-fat diet consumption during gestation/lactation may “program” offspring longterm for increased obesity themselves, along with increased vulnerability to mood disorders. We review the evidence that programming of brain and behavior by perinatal diet is propagated by inflammatory mechanisms, as obesity and high-fat diets are independently associated with exaggerated systemic levels of inflammatory mediators. Due to the recognized dual role of these immune molecules (eg, interleukin [IL]-6, 11-1β) in placental function and brain development, any disruption of their delicate balance with growth factors or neurotransmitters (eg, serotonin) by inflammation early in life can permanently alter the trajectory of fetal brain development. Finally, epigenetic regulation of inflammatory pathways is a likely candidate for persistent changes in metabolic and brain function as a consequence of the perinatal environment.
Keywords: anxiety, cognition, epigenetics, maternal high-fat diet, maternal obesity, microglia, neuroinflammation, perinatal programming, placenta
Abstract
La obesidad constituye actualmente una epidemia mundial. Más allá de las enfermedades asociadas, como la diabetes, la obesidad se relaciona con trastornos neuropsiquiátricos como la depresión. Es alarmante que la obesidad materna y el consumo de una dieta rica en grasa durante la gestación/lactancia pueda “programar” en los hijos un aumento a largo plazo de la obesidad y un incremento en la vulnerabilidad para los trastornos del ánimo. En este artículo se revisa la evidencia que plantea que la programación cerebral y conductual por la dieta perinatal se difunde a través de mecanismos inflamatorios, y que la obesidad y las dietas ricas en grasa se asocian en forma independiente con los excesivos niveles sistémicos de mediadores inflamatorios. Dado el reconocido papel dual de estas moléculas inmunes (como la interleuquina [IL]-6), IL-1β) en la función placentaria y en el desarrollo cerebral, cualquier desorganización de su delicado balance con factores de crecimiento o neurotrasmisores (como serotonina) debida a inflamaciones precoces en la vida pueden alterar en forma permanente la trayectoria del desarrollo cerebral fetal. Por último, la regulación epigenética de las vías inflamatorias es una probable candidata para los cambios persistentes en la función metabólica y cerebral como consecuencia del ambiente perinatal.
Abstract
L'obésité est maintenant une épidémie mondiale. Au-delà des maladies associées comme le diabète, l'obésité est liée à des troubles neuropsychiatriques comme la dépression. De façon inquiétante, l'obésité maternelle et une alimentation riche en graisses pendant la grossesse et l'allaitement «programmeraient» à long terme la descendance pour une obésité ainsi qu'une vulnérabilité aux troubles de l'humeur augmentées. Nous examinons ici les données selon lesquelles la programmation du cerveau et du comportement par le régime périnatal repose sur des mécanismes inflammatoires, l'obésité et les régimes riches en graisses étant indépendamment associés à des taux systémiques élevés de médiateurs inflammatoires. Le double rôle de ces molécules immunologiques (p. ex., interleukines IL-6, IL-1β) dans la fonction placentaire et le développement du cerveau étant reconnu, toute perturbation de leur équilibre délicat avec les facteurs de croissance ou les neurotransmetteurs (p, ex, la sérotonine) par une inflammation survenant tôt dans la vie peut modifier de façon permanente la trajectoire du développement foetal du cerveau. Enfin, la régulation épigénétique des voies inflammatoires est probablement un des mécanismes induisant des changements persistants des fonctions cérébrale et métabolique en fonction de l'environnement périnatal.
Introduction
Noncommunicable diseases such as heart disease and diabetes now account for the majority of morbidity and mortality worldwide.1 Obesity and overweight are at epidemic proportions and contribute to most, if not all, of these diseases.1 Increasing evidence suggests that the perinatal environment may be especially important in shaping lifelong health and disease outcomes. Maternal obesity is associated with gestational diabetes, stillbirth, and preeclampsia leading to preterm birth.2 Beyond these acute consequences, and perhaps most troubling, maternal obesity may also “program” offspring for lifelong obesity and associated metabolic disorders, setting in motion a vicious cycle of propagating health problems.3,4 For instance, children of obese women exhibit increased body mass index,5 body fat percentage, and insulin resistance,6 and high-fat-fed rodent dams produce offspring with increased fat, leptin, and body length at birth,7,8 and insulin resistance and obesity later in life.9
Though this has received less attention, obesity, metabolic syndrome, and insulin resistance are also increasingly linked to mental health dysfunction, including impaired cognition and increased anxiety.10,11 Multiple animal studies demonstrate links between high-fat diet exposure and/or obesity and increased anxiety-like behavior. Notably, this is true for direct exposure during adulthood as well as in development—ie, the offspring of mothers fed a high-fat diet during pregnancy and lactation show increased anxiety-like behavior, across multiple species including nonhuman primates,12 even in the absence of direct high-fat feeding. The latter case is a classic example of perinatal programming—ie, the capacity for events occurring during the perinatal period to alter or “program” the normal course of development, with the result that adult outcomes, including behavior, are significantly and often permanently altered.13
The mechanisms linking maternal obesity to later-life mental and physical health problems in offspring are undoubtedly complex. However, obesity has been consistently associated with systemic inflammation, which is implicated in numerous disease processes. For instance, increased adipose tissue in obese individuals is associated with elevated inflammatory mediators, such as interleukin [IL]-6, IL-1β, monocyte-chemoattractant protein (MCP)-1, and C-reactive protein (CRP), which are implicated in insulin resistance, type 2 diabetes, and hypertension.14 Moreover, excess proinflammatory cytokine expression within the brain is independently linked to cognitive and emotional disruption.15 Finally, developmental programming of metabolic, neural, and behavioral outcomes by immune activation (eg, in response to infection or trauma) during gestation or the early postnatal period has been linked to specific changes in peripheral and central cytokine expression in multiple studies.16-19
Epigenetic regulation of multiple physiological systems that contribute to disease risk is a likely candidate for persistent changes in metabolic and brain function as a consequence of the perinatal environment. Indeed, multiple studies have examined the impact of perinatal nutrition or obesity on later-life outcomes via epigenetic modulation of metabolic pathways.20-24 Fewer studies have assessed the impact of perinatal nutrition or obesity on mental health, and its potential causal link with epigenetic regulation of inflammatory pathways. Thus, the goal of this review is twofold: (i) to review the literature linking perinatal diet and/or maternal obesity to later-life mental health dysfunction via its impact on inflammatory mechanisms; and (ii) to consider the evidence that such persistent changes may occur via epigenetic mechanisms, which if true has striking implications for transmission of both metabolic and mental health disease vulnerabilities in future generations.
Developmental programming by diet
David Barker and colleagues formalized an association between the intrauterine environment and later-life disease risk in the 1980s, in which they documented a striking correlation between low birth weight (due to maternal famine, and/or fetal growth restriction) and risk for obesity/overweight and its associated health problems (eg, heart disease, diabetes) in adulthood. The Dutch “Hunger Winter” famine of 1944 provided a stark example of this association in humans, with the surviving children of mothers exposed to starvation exhibiting increased incidence of obesity, diabetes, and cardiovascular disease.25 The association between prenatal undernutrition and later-life metabolic disorders has since been overwhelmingly confirmed in animal models.20,26,27 For instance, mild-to-moderate food restriction (30%) in pregnant mouse dams led to reduced birth weight, catch-up growth, and obesity upon exposure to a high-fat diet in adulthood.28 More severe restriction leads to more severe phenotypes (eg, obesity even with normal chow feeding of offspring).29,30 The “Fetal Origins of Adult Disease” or “Barker Hypothesis” has thus been nicknamed the “thrifty phenotype,”31 because in the absence of sufficient calories in the fetal environment, the neonatal metabolism prepares or “programs” the individual to conserve calories, even in adulthood. In our modern world of excess food, this becomes maladaptive.
A relatively more recent problem, perinatal overnutrition paradoxically lends similar metabolic programming and disease risk. Rats exposed in utero to a maternal high-fat diet exhibit increased body size at birth and insulin resistance throughout life,32 in agreement with numerous other rodent studies.20,33-35 Female baboons overfed as infants were heavier throughout life, with increased adiposity compared with controls,36 also similar to rodent studies.37 Interestingly, however, maternal high-fat diet in pigs followed by postnatal high-fat feeding of the offspring is protective in a model of atherosclerosis.38 This is an elegant example of developmental plasticity that is nonetheless consistent with the ”thrifty phenotype“ and suggests that a match between the perinatal and adult environments may be important.
Nutrition vs body weight
A majority of studies do not distinguish between the effects of high-fat diet and obesity per se, eg, due to genetic susceptibility and/or hyperphagia while maintained on an ad libitum low-fat diet. This question is interesting from a mechanistic perspective, but is absolutely critical from a public health perspective. A study in Japanese macaques compared the offspring of lean (with normal insulin sensitivity) and obese (with insulin resistance) mothers fed a high-fat diet prior to and during pregnancy, and found that juveniles from either group were heavier, with increased adiposity, leptin, and fatty liver disease compared with the offspring of mothers fed a low-fat diet.12 These somewhat surprising data suggest that diet composition may be more important than body weight. In contrast, the offspring of obese rat dams exhibited obesity themselves following high-fat feeding in adulthood, whereas the offspring of rat dams fed a high-fat—but isocaloric when compared with low-fat control—diet did not, suggesting fat composition of the diet alone is not sufficient to program obesity in rodents.39 Similarly, a clinical study determined that children born after maternal gastrointestinal bypass surgery are less obese and more insulin sensitive compared with siblings born before maternal surgery, in association with differential epigenetic regulation of glucoregulatory and inflammatory genes (including IL-Iβ) in these offspring.40 Further work must be done to determine whether maternal diet or obesity itself is more relevant to the developmental programming of brain and behavior. A plethora of studies have also examined the impact of specific macro and micronutrients in the perinatal diet on metabolic outcomes in human, primate, and rodent models, but their discussion goes beyond the scope of the current paper and we refer the reader to several excellent reviews.41-45
The evidence that the perinatal nutritional environment can significantly impact or program behavioral outcomes is accumulating. As introduced previously, studies in rats,46 mice,47 and nonhuman primates12 have linked high-fat diet exposure in utero to increased anxiety later in life. Young rats exposed to a high-fat diet prior to weaning show similar increases in anxiety-like behavior in adulthood.48 Importantly, whereas adult exposure to high-fat diet is also linked to increased anxiety-like behavior in rodents,49 a much longer duration of feeding (~8 to 12 weeks or more) is typically required compared with during development. One interesting exception is if a prenatal inflammatory challenge precedes high-fat feeding in adulthood; in this case weight gain and elevated anxiety-like behavior are accelerated compared with controls that did not receive an inflammatory challenge in utero,50,51 further cementing a critical interplay between inflammatory signaling during development and changes in metabolic and behavioral function throughout the lifespan. Changes in central reward processing are also reported; rats exposed prenatally, and mice exposed prior to weaning, to a high-fat diet show increased motivation for fat rewards and increased fat preference, respectively, in adulthood.52,53 In humans, both small for gestational age (SGA) and large for gestational age (LGA) infants (which are often the result of maternal obesity or metabolic dysregulation) have increased risk of attention deficit/hyperactivity disorder, as well as cognitive delays, autism, anxiety, and depression.54-60 Maternal diabetes, hypertension, and obesity are also independently associated with an increased incidence of autism in children.61
Mechanisms of programming brain and behavior
The proximate brain mechanisms underlying such changes in behavior are many, including decreased serotonin levels in primates,12 reduced dopamine transmission in rats,52,53 and increased glucocorticoid receptor expression along with increased stress-induced corticosterone release in rats.48 Inflammatory molecules such as cytokines can interact with all of these mechanisms. Thus, in order to understand the mechanisms by which the maternal nutritional environment leads to long-term changes in offspring brain and behavior and its potential link with inflammatory pathways, we consider the mechanisms of brain-immune communication, the role of immune molecules in normal brain development, and ultimately how epigenetic mechanisms interface with these pathways.
Brain-immune communication
Communication pathways between the brain and immune system have been well characterized and extensively reviewed.62-70 These pathways include the autonomic nervous system (ANS), activation of the hypothalamic-pituitary-adrenal (HPA) axis, and cytokines, chemokines, and leukocytes that travel or signal across the blood-brain barrier (BBB). The capacity for such interactions to impact behavioral outcomes, for instance in the context of sickness behavior, is now firmly established. These changes in food and water intake, activity, exploration, sleep, and social interactions, are not mediated by the infectious pathogens themselves, but rather by the immune system via inflammatory pathways within the CNS,71-74 and are organized, adaptive strategies that are critical to host survival.74,75 However, there are also similarities between sickness behaviors caused by an acute illness and the chronic behavioral changes expressed by individuals with certain neuropsychiatric disorders such as depression.74,76 Thus, some psychiatric disorders may involve a dysregulation of immune function even in the absence of an overt immune challenge such as infection.77-82
Immune molecules and brain development
Many of these neuropsychiatric disorders have a known or suspected developmental origin, suggesting a role for inflammatory mechanisms in both the origin and maintenance of neural dysfunction. Immune molecules play a ubiquitous role in normal brain development.17,83,84 Briefly, microglia and astrocytes, the primary immuno-competent cells of the CNS, are involved in every major aspect of brain development and function, including synaptogenesis and refinement, apoptosis, and angiogenesis. Many cytokines and chemokines are important for progenitor cell maintenance, proliferation, differentiation, and migration.85-88 Other traditionally defined “immune” molecules such as MHC I and complement components C3 and C1q are critical for the activity dependent refinement of synapses within the visual cortex, and likely many other brain regions.89-92 Perhaps not surprising given these interactions, elevated levels of proinflammatory cytokines generated by the maternal or fetal immune system have been associated with abnormal fetal brain development and an increased risk of neurodevelopmental disorders such as autism and schizophrenia.93-96
Epigenetic alterations
Epigenetic processes necessarily play a critical role in fetal development and thus are a likely candidate for developmental programming in response to environmental influences. These mechanisms include DNA methylation; histone modifications including methylation, acetylation, and ubiquitination; noncoding RNAs including micro-RNAs; and genomic imprinting, among others. DNA methylation is initiated very early in embryogenesis, with the silencing of genes from either maternal or paternal origins even prior to implantation.97-99 This process is critical for maintaining the replication of DNA methylation patterns during rapid cell division. A second phase of DNA methylation occurs after implantation and continues throughout early postnatal development. During this time, cell-specific methylation mediates tissue and cell-specific gene expression during the process of cellular differentiation. In general, the end point of these DNA methylation processes includes the long-term silencing of genes from development into adulthood. Many epigenetic mechanisms have been described in relation to maternal diet and/or the modification of immune function.24,100,101 For instance, maternal folic acid (ie, methyl donor) supplementation in mice results in widespread DNA methylation of immune genes, and increases an allergic phenotype in adulthood.102
Perhaps the best characterized example of the perinatal environment impacting later-life behavior via epigenetic mechanisms is the literature describing the impact of maternal care during the early postnatal period—a stable decrease in adult stress-responsiveness as a result of high maternal licking and grooming early in life is directly caused by an increase in glucocorticoid receptor expression in the hippocampus, which is the result of decreased DNA methylation of the glucocorticoid receptor promoter.103 Notably, we have demonstrated that maternal care programming of brain immune responses occurs via epigenetic mechanisms and also has significant consequences for behavior long-term. Specifically, a neonatal handling intervention that increases maternal licking and grooming causes decreased methylation of the promoter for IL-10, an important anti-inflammatory cytokine, leading to a marked increase in its expression levels in adult offspring.104 Remarkably, this stable epigenetic change was present only in microglia, and the augmented levels of IL-10 were protective in a model of opioid addiction. We suspect such a change in immune gene expression and thus long-term behavior by early-life events represents merely the “tip of the iceberg”; and, in the sections that follow, we consider the mechanisms by which maternal diet and/or obesity can modulate offspring behavioral outcomes long-term, and what is known about epigenetic modifications to inflammatory pathways.
Mechanisms of programming by maternal diet
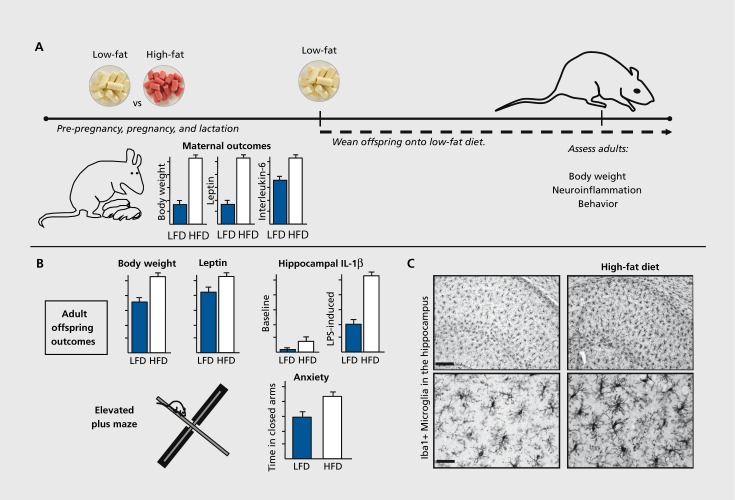
There is strong evidence that systemic inflammation as a consequence of maternal diet or obesity can impact the developing fetal brain. For instance, high-fat diet exposure prior to and during pregnancy in rats increases maternal body weight, and circulating leptin, CRR and IL-6. This activation of the systemic maternal immune system increases cytokine expression within the offspring brain at birth, and results in long-term changes in neuroimmune function and behavior, strikingly similar to that observed following an early-life bacterial infection.105 Specifically, there are increases in both basal and LPS-induced proinflammatory IL-1β expression within the hippocampus, long-term changes in microglial phenotype, and increased anxiety-like behavior in adult offspring of high-fat dams, particularly in males (a topic we return to in the final section of this review), despite the fact that the offspring themselves are maintained on a low-fat diet after weaning (Figure 1.).46 In further support of the link between prenatal inflammation and later-life physical and mental health, prenatal exposure to high levels of air pollution, which induces maternal inflammation, causes a predisposition to obesity and increased anxiety-like behavior in the mouse offspring when they are given access to a high-fat diet in adulthood.50,51 Similarly, prenatal cytokine exposure (ie, TNF-a, IL-6) is sufficient by itself to induce laterlife obesity.106 Overall, it is clear that programming by maternal diet involves inflammatory pathways, which interact with both the intrauterine and postnatal environments.
Figure 1. Maternal high-fat diet programs offspring metabolism, neuroinflammation, and behavior. (A) Dams ate a low-fat (LFD) or high-fat diet (HFD) prior to breeding, and throughout pregnancy and lactation. HFD increased maternal body weight, and circulating leptin and proinflammatory cytokine (interleukin-6) concentrations. Offspring were assessed in adulthood following consumption of a LFD since weaning. (B) Adult offspring of dams fed a HFD had increased body weight and leptin, along with increased basal and LPS-induced proinflammatory cytokine (interleukin-1β) concentrations within the hippocampus. HFD offspring also had increased anxiety-like behavior in the elevated plus maze. (C) Adult offspring of dams fed a HFD showed morphological changes in microglia (Iba1 + cells) within the hippocampus, consistent with greater activation. These changes were apparent at baseline (absent an adult immune challenge), suggesting long-term programming of microglial function. Images are taken from the CA1 region of hippocampus. Scale bar = 50 um (top panels) and 12.5 um (bottom panels). Adapted from ref 46: Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104-2115. Copyright © Federation of American Societies for Experimental Biology 2010.
Intrauterine environment
An adverse intrauterine environment has been implicated in the pathogenesis of multiple chronic health problems in adulthood, including cardiovascular disease, metabolic syndrome, and mental health disorders such as anxiety and depression.107,108 The placenta is the interface between the maternal and intrauterine environments and the fetus and the primary means of nutrient acquisition; thus, it is likely that the placenta plays a critical role in the fetal programming of offspring physiology by maternal diet. Furthermore, in the context of a maternal immune challenge, such as high-fat diet, the placenta is fully immunocompetent and prepared to mount an inflammatory response to defend the fetus, as it possesses macrophages109 with pattern recognition receptors (eg, toll-like-receptor [TLR]4110) that can bind saturated fatty acids.111 However, inflammation during pregnancy is a double-edged sword, in that inflammation can resolve an infection that could damage the developing fetus, but inflammation itself, especially when chronic, as is typically the case for maternal high-fat diet, can also alter the trajectory of fetal development, including the fetal brain, resulting in long-term changes in brain function and behavior.112
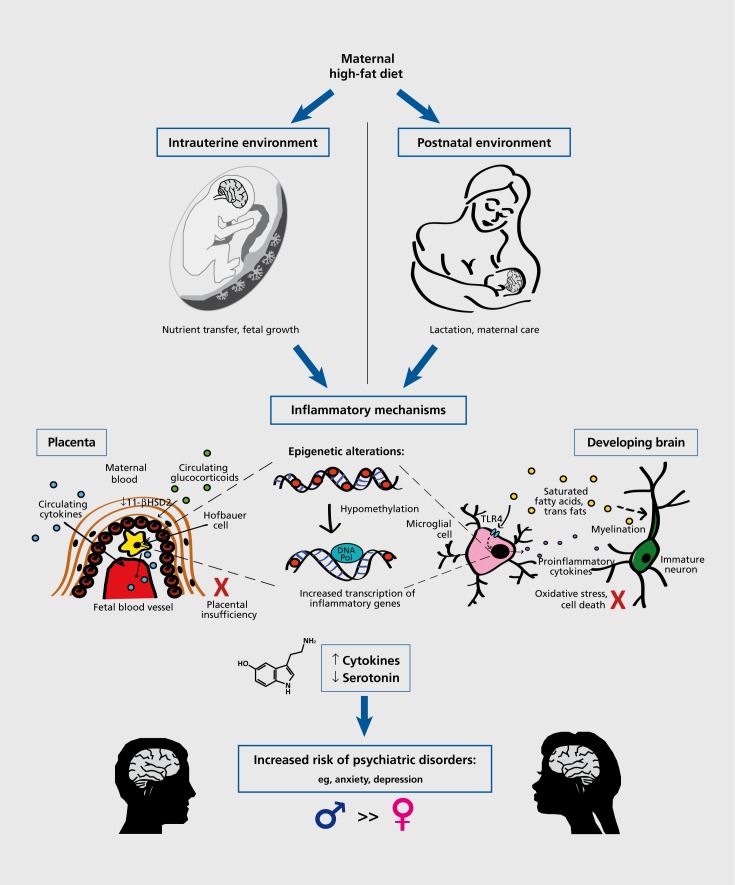
Epidemiological studies in humans have shown that placental dysfunction is associated with later-life psychiatric disorders. For example, low placental weight, in conjunction with low birth weight, is associated with an increased incidence of schizophrenia in adulthood.113 This type of intrauterine growth restriction, commonly associated with maternal obesity as well as undernutrition, is caused by “placental insufficiency,” or inadequate placental blood flow,114 which is accompanied by a placental proinflammatory cytokine response115,116 (Figure 2). Notably, the balance between the fetal demand and placental supply of nutrients, which can be modulated by epigenetic mechanisms, is critical for normal development and can have consequences for laterlife behavior. For example, a placenta-specific knockout of the maternally imprinted gene Igf2, which causes reduced placental supply relative to fetal demand, leads to intrauterine growth restriction, followed in adulthood by increased anxiety-like behavior and a corresponding decrease in GABA and serotonin receptor expression in the hippocampus of adult offspring.117 Considering the role of inflammatory processes in intrauterine growth restriction, it is likely that immune changes also play a role in the long-term behavioral consequences of this manipulation, but this remains to be tested directly.
Figure 2. Programming of brain and behavior by maternal high-fat diet involves inflammatory pathways, which interact with both the intrauterine and postnatal environments. In the placenta (inset depicts a schematic of a chorionic villus), these inflammatory mechanisms include the passage of elevated maternal cytokines through the placenta into the fetal circulation,122 the production of proinflammatory cytokines by fetal placental macrophages (ie, Hofbauer cells),109,123 and the increased passage of circulating maternal glucocorticoids across the placenta, due to decreased 1 1-β-HSD2.121 Collectively, these conditions are associated with placental insufficiency or decreased placental perfusion, as well as intrauterine growth restriction.115,116 In the developing brain, the inflammatory mechanisms include the microglial production of proinflammatory cytokines (as a result of activation of the TLR4-mediated signaling cascade by saturated fatty acids111), and the incorporation of saturated fatty acids and trans fats into the myelin of developing neurons.133 Thus, there is the promotion of a neuroinflammatory microenvironment, which may lead to oxidative stress, cell death, and an overall altered trajectory of brain development. Inflammatory mechanisms in both the placenta and the brain likely involve long-term epigenetic alterations, such as the hypomethylation of inflammatory genes, resulting in their increased transcription. Overall, increased levels of proinflammatory cytokines lead to decreased serotonin synthesis,129 by the placenta during fetal development, and by the brain during postnatal development, which may contribute to the alteration of brain development and an increased risk of psychiatric disorders in adulthood (a risk that has been shown to be greater in males in human and animal models).
Although the placenta was originally assumed to act as a protective barrier between mother and fetus, it is becoming clear that it is actually more of an interface than a barrier, as it mediates the complex interactions of maternal and fetal cells and the exchange of various substances between the two compartments.118,119 The limited “barrier” capacity of the placenta is further disrupted in the case of perturbations in maternal diet. For example, placentas from protein-restricted rats exhibit a marked reduction of 11-β-hydroxysteroid dehydrogenase 2 enzyme (11-β-HSD2), which normally protects the fetus from elevated maternal corticosteroids, thus resulting in fetal exposure to abnormally high glucocorticoid levels during gestation and later hypertension in the adult offspring.120 This finding is representative of fetal programming of the HPA axis, and has been shown in other contexts to be associated with increases in anxiety and the incidence of other neuropsychiatric disorders in adulthood,108 although the ultimate mechanism remains undefined. Proinflammatory cytokines can cause decreased activity of 11-β-HSD2,121 and thus may play a role in programming by maternal diet (Figure 2).
Proinflammatory cytokines found in the placenta may come from two sources: (i) Certain maternal cytokines (eg, IL-6), resulting from the systemic inflammation associated with obesity, may be able to cross the placenta into the fetal compartment122; and/or (ii) the placenta itself is capable of mounting an inflammatory response to maternal immune challenges,109,123 including maternal high-fat diet. During a normal pregnancy, both maternal decidual macrophages and fetal placental macrophages (also known as Hofbauer cells) exhibit an anti-inflammatory phenotype in order to maintain maternal immunotolerance of the fetal allograft. Notably, this state is perpetuated by the hypermethylation of immune response genes and genes encoding classical macrophage activation markers.124 However, during maternal obesity, in parallel with increased maternal monocyte differentiation and inflammatory markers in the maternal circulation, there is also the accumulation of fetal Hofbauer cells in the placenta and the increased placental production of proinflammatory cytokines125,126 (Figure 2). It is likely that this marked divergence from the normal immune state of the placenta is associated with significant epigenetic changes (eg, hypomethylation of inflammatory cytokine genes), as maternal dietary extremes (ie, protein restriction) can cause the altered expression of genes involved in epigenetic modifications.127 Moreover, due to the dual role of innate immune molecules in host defense and developmental processes (as discussed above), the creation of an inflammatory milieu in utero can have significant consequences for later-life brain function and behavior.
In an elegant demonstration of the placenta's direct role in fetal brain development, Bonnin and colleagues128 demonstrated using a novel ex vivo placental perfusion technique that the placenta is capable of synthesizing serotonin from maternal tryptophan. Serotonin is a critical modulator of neurogenesis and axon growth in the developing forebrain, especially during the period of E10.5-E15.5 in the mouse (corresponding to the first and early second trimester in humans). Notably, the neural and immune products of the placenta have the potential for interaction and reciprocal regulation, especially in the case of a maternal immune challenge (Figure 2). The enzyme indoleamine 2,3-diox-ygenase (IDO), which causes tryptophan degradation, is expressed in the normal placenta, but is upregulated by high levels of proinflammatory cytokines, resulting in decreased serotonin synthesis and a potentially damaging by-product, quinolinic acid, which is a helpful defense against a pathogen, but also a potent NMDA receptor agonist that is capable of causing excitotoxicity and oxidative stress in the brain.129 For example, maternal endotoxin exposure decreased serotonin synthesis, increased cell death, and diminished serotonergic innervation of the somatosensory cortex in the brains of newborn rabbits.130 Though yet to be tested, it is possible that maternal high-fat diet disrupts the delicate balance between the cytokine - and serotonin-producing capabilities of the placenta that is critical for normal brain development, which, especially in light of later-life alterations in serotonin receptor expression,12 may underlie the observed association with offspring mood disorders and cognitive alterations.
Postnatal environment
Most maternal diets are not simply a potential programming factor during gestation, as they are likely initiated prior to pregnancy and continue after birth. Thus, maternal diet can exert influence on offspring during the postnatal period, at least throughout lactation and up until weaning. Most components of the maternal diet, including saturated and trans fats, influence the composition of the breast milk,131 which makes up the sole source of an infant's nutrients, both in animals and in humans that choose to breast feed, during the critical period of postnatal brain development that includes extensive myelination and synaptic pruning.132 As such, maternal dietary fats, such as trans fats, are incorporated into neuronal myelin sheaths, synaptic terminals, and capillaries of an infant's brain.133 Furthermore, the ability of the these dietary fats to traverse the BBB134,135 suggests it is possible that maternal high-fat diet can continue to foster an inflammatory environment in the brains of offspring, potentially disrupting the postnatal period of brain development (as discussed in the previous section for the fetal period; Figure 2). Gorski and colleagues136 demonstrated that cross-fostering the pups born to lean dams to obese dams resulted in impaired insulin sensitivity and a predisposition to obesity, whereas the converse was true for the pups of obese dams cross-fostered to lean dams. Future studies must determine if exposure to maternal high-fat diet during the early postnatal period alone is also sufficient to program behavioral changes in adulthood.
In addition to the direct consumption of maternal diet by offspring, maternal diet can influence maternal behavior and the quality of maternal-offspring interactions after birth. As discussed above, the quality of maternal care during the early postnatal period has significant consequences for offspring neural development and behavior in adulthood, which is linked in part to the epigenetic modification of immune genes within the brain.104 However, thus far, only a small number of studies have examined changes in maternal care due to alterations in maternal diet. Smart and Preece137 showed that rat mothers that were malnourished throughout pregnancy and lactation licked and groomed their pups significantly less than control mothers, but did not examine consequences for the offspring. On the other hand, Purcell and colleagues138 found that dams consuming a high-fat diet throughout gestation and lactation spent more time nursing their pups and more time overall caring for their pups, although no difference in time spent licking and grooming, during the first week of postnatal life. However, even in the absence of the mother, the pups of high-fat diet dams consumed more milk in tests of independent ingestion, suggesting that an increase in pup appetite may be driving the observed change in maternal behavior. It remains to be shown whether alterations in maternal care due to high-fat diet play a role in the changes in offspring behavior that are seen in adulthood, and whether these changes are accompanied by epigenetic modifications in immune genes as we have previously demonstrated in another model.104 However, the strong link between maternal high-fat diet and an increase in anxiety-like behavior in adulthood, in rats as well as other species, at a minimum suggests that the observed increase in nursing (in the absence of increased licking and grooming) may be insufficient to decrease offspring stress-responsiveness in adulthood.103
Sex differences
Sex differences in offspring outcomes due to perinatal events, including maternal diet, are gradually gaining recognition in the field of perinatal programming. For example, male newborns of women with pregestational diabetes suffer worse perinatal outcomes, including preterm birth and birth defects,139 and male babies born to women with gestational diabetes are more likely to be delivered by caesarean section and to have a higher risk of neonatal hypoglycemia than female babies.140 At the same time, there is a well-documented, but poorly understood, male bias in the prevalence of neurodevelopmental disorders, including learning disabilities141 and autism,142 that have been linked to maternal immune challenges. Maternal diet, now recognized to modulate the immune system, has increasingly been found to be the common factor linking certain neonatal outcomes and later-life psychiatric disorders, often with a male bias. For example, SGA male, but not female, infants have a greater risk of depression as adults.143 Furthermore, as mentioned previously, only the male offspring of high-fat diet rat dams exhibit elevated body weight and increased anxiety-like behavior in adulthood.46 However, the mechanisms underlying these intriguing sex differences remain poorly defined and theoretical.
One of the potential factors underlying the greater vulnerability of males to early-life programming of brain and behavior may lie in sex differences in the immune responsiveness of the developing brain and placenta. For example, there are marked sexual dimorphisms in microglial colonization of the rat brain across development, such that males have a greater number of microglia than females shortly after birth, which has been hypothesized to render them more vulnerable to early-life immune challenges.144 This type of microglial “priming,” or long-term alteration of microglial neuroimmune function, has clear consequences for cognition and behavior in adulthood.105 Alternatively, Clifton145 suggests that sex differences in offspring outcome may be due to the sexually dimorphic strategies males and females employ in a compromised intrauterine environment. Male placentae respond to maternal inflammation with few changes in gene expression in order to allow for accelerated or continued growth, but at the cost of increased risk for adverse outcomes. On the other hand, female placentae respond with multiple gene changes that enhance the fetal immune response to the maternal immune challenge, which leads to a minor decrease in growth, but has adaptive value overall in promoting survival, especially in the face of further maternal insults.146-148 In a striking example of the interaction between maternal diet and placental sex, Mao and colleagues149 demonstrated that maternal high-fat diet causes more dramatic alterations in the gene expression of female placentae than male placentae, thus providing experimental support for Clifton's hypothesis in the context of maternal diet. Future studies aimed at exploring the range of sex differences will advance our understanding of the mechanisms by which perinatal programming occurs, particularly how the same maternal immune challenge can lead to disparate brain and behavior outcomes in males and females. Importantly, this knowledge will better inform social or medical interventions that must be tailored to the sex of the at-risk child.
Conclusions and implications for future research
Perinatal programming and epigenetic alterations due to maternal diet are likely culprits underlying the ever-increasing rates of obesity worldwide. Beyond the plethora of cardiovascular and metabolic diseases that are associated with obesity, the increased incidence of psychiatric disorders is another by-product of this global epidemic. Inflammatory processes are known to contribute to all of these conditions, and may be epigenetically programmed by maternal diet to cause later-life physiological and psychological dysfunction in offspring.
When examining the epigenetic mechanisms underlying perinatal programming, it is critical to distinguish between transgenerational epigenetic effects, which include all phenotypes observed in offspring that are not genetically determined (eg, changes in offspring behavior as a result of maternal care, sustained by reversible epigenetic changes within somatic cells), and gametic epigenetic inheritance, which includes only those phenotypes observed in offspring that are the result of epigenetic modifications passed through the germ line.150 Currently, the transgenerational epigenetic perpetuation of traits as a result of maternal diet remains a relatively unexplored area of study, as such experiments must be carried out to the third (F3) generation.150 In one of the only examples of this type of experiment, Dunn and Bale20 demonstrated that increases in body size due to maternal high-fat diet are transmitted epigenetically via the paternal lineage across three generations. Furthermore, paternally expressed genes exhibit more dramatic alterations in the F3 female offspring of the paternal lineage, suggesting that the epigenetic mechanisms of maternal high-fat diet may involve the sex-specific transgenerational programming of imprinted genes.20 Future studies are needed to determine whether the effects of maternal diet on offspring brain function and behavior are propagated by transgenerational epigenetic effects or gametic epigenetic inheritance, in order to determine the best methods for intervention in the multigenerational cycle of obesity.
For instance, it may be possible to deliberately manipulate epigenetic processes via the diet itself. The consumption of a diet supplemented with methyl donors (folic acid, vitamin B12, choline, and betaine) during pregnancy and lactation prevents the transgenerational amplification of body weight observed in the Avy genetic mouse model of obesity.151 Furthermore, the consumption of a methyl-donor-deficient diet following weaning induces loss of imprinting of Igf2 and suppression of postnatal growth in mice,152 suggesting that epigenetic effects may be reversible even after the critical period of perinatal development.
Due to the critical role of inflammatory processes in the programming of brain and behavior by perinatal diet, therapeutic approaches and interventions that target inflammatory pathways in either the obese pregnant woman herself or in the offspring following birth may also be warranted. For example, epidemiological studies in humans suggest that the “Mediterranean diet” during pregnancy is beneficial for the prevention of wheeze and atopy in children,153 which may be due to increased consumption of long-chain polyunsaturated fatty acids and their impact on inflammatory mechanisms (eg, T-cell signaling; prostaglandin synthesis).154-156 More work is needed to identify the specific epigenetic changes in immune genes that underlie the programming of offspring brain and behavior by perinatal diet, and to determine whether overarching changes in diet—either prenatal and/or postnatal—are sufficient to reverse these effects.
Contributor Information
Jessica L. Bolton, Department of Psychology and Neuroscience, Duke Institute for Brain Sciences, Duke University, Durham, North Carolina, USA.
Staci D. Bilbo, Department of Psychology and Neuroscience, Duke Institute for Brain Sciences, Duke University, Durham, North Carolina, USA.
REFERENCES
- 1.Alwan A. Global Status Report on Noncommunicable Diseases 2010. World Health Organization; 2011 [Google Scholar]
- 2.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 3.Grattan DR. Fetal programming from maternal obesity: Eating too much for two? Endocrinology. 2008;149:5345–5347. doi: 10.1210/en.2008-1106. [DOI] [PubMed] [Google Scholar]
- 4.McGuire W., Dyson L., Renfrew M. Maternal obesity: consequences for children, challenges for clinicians and carers. Semin Fetal Neonatal Med. 2010;15:108–112. doi: 10.1016/j.siny.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Simmons R. Perinatal programming of obesity. Semin Perinatol. 2008;32:371–374. doi: 10.1053/j.semperi.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman DJ. Effects of maternal obesity on fetal growth and body composition: implications for programming and future health. Semin Fetal Neonatal Med. 20010;60:1078–1055. doi: 10.1016/j.siny.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Chen H., Simar D., Lambert K., Mercier J., Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348–5356. doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GA., Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armitage JA., Khan IY., Taylor PD., Nathanielsz PW., Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561(Pt 2):355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasinetti GM., Eberstein JA. Metabolic syndrome and the role of dietary lifestyles in Alzheimer's disease. J Neurochem. 2008;106:1503–1514. doi: 10.1111/j.1471-4159.2008.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Monte SM. Insulin resistance and Alzheimer's disease. BMB Rep. 2009;42:475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan EL., Grayson B., Takahashi D., et al Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennet L., Gunn A. The fetal origins of adult mental illness. In: Wintour-Coghlan M, Owens J, eds. Early Life Origins of Health and Disease (Advances in Experimental Medicine and Biology. New York, NY: Springer; 2006:204–211. [Google Scholar]
- 14.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 15.McNaull BB., Todd S., McGuinness B., Passmore AP. Inflammation and anti-inflammatory strategies for Alzheimer's disease - a mini-review. Gerontology. 2010;56:3–14. doi: 10.1159/000237873. [DOI] [PubMed] [Google Scholar]
- 16.Bilbo SD., Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz JM., Bilbo SD. The immune system and the developing brain. 2011;2:1–128. [Google Scholar]
- 18.Walker FR., Owens J., AM S., Hodgson DM. Individual differences in glucose homeostasis: do our early life interactions with bacteria matter? Brain Behav lmmun. 2006;20:401–409. doi: 10.1016/j.bbi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Smith SE., Li J., Garbett K., Mimics K., Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn GA., Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–2236. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vucetic Z., Kimmel J., Totoki K., Hollenbeck E., Reyes TM. Maternal highfat diet alters methylation and gene expression of dopamine and opioidrelated genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlin J., George R., Reyes TM. Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PloS One. 2013;8:e63549. doi: 10.1371/journal.pone.0063549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeisel SH. Nutritional genomics: defining the dietary requirement and effects of choline. J Nutr. 2011;141:531–534. doi: 10.3945/jn.110.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canani RB., Di Costanzo M., Leone L., et al Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011;24:198–205. doi: 10.1017/S0954422411000102. [DOI] [PubMed] [Google Scholar]
- 25.Roseboom T., de Rooij S., Painter R. The Dutch famine and its longterm consequences for adult health. Early Hum Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.de Boo HA., Harding JE. The developmental origins of adult disease (barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 27.Armitage JA., Poston L., Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- 28.Yura S., Itoh H., Sagawa N., et al Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Jones AP., Friedman Ml. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215:1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- 30.Vickers MH., Breier BH., Cutfield WS., Hofman PL., Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 31.Hales CN., Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 32.Dunn GA., Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo F., Jen KC. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q., Suzuki M. Parental obesity and overweight affect the body fat accumulation in the offspring: the possible effect of a high fat diet through epigenetic inheritance. Obes Rev. 2006;7:201–208. doi: 10.1111/j.1467-789X.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 35.Parente LB., Aguila MB., Mandarim-de-Lacerda CA. Deleterious effects of high-fat diet on perinatal and postweaning periods in adult rat offspring. Clin Nutr. 2008;27:623–634. doi: 10.1016/j.clnu.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DS., Bertrand HA., McMahan CA., McGill HC Jr., Carey KD., Masoro EJ. Preweaning food intake influences the adiposity of young adult baboons. J Clin Invest. 1986;78:899–905. doi: 10.1172/JCI112678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin BE. Metabolic imprinting: Critical impact of the perinatal environment on the regulation of energy homeostasis. Philos Trans R Soc Lond B Biol Sci. 2006;361:1107–1121. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norman JF., LeVeen RF. Maternal atherogenic diet in swine is protective against early atherosclerosis development in offspring consuming an atherogenic diet post-natally. Atherosclerosis. 2001;157:41–47. doi: 10.1016/s0021-9150(00)00668-7. [DOI] [PubMed] [Google Scholar]
- 39.White CL., Purpera MN., Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1464–R1472. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guenard F., Deshaies Y., Cianflone K., Krai JG., Marceau P., Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci U S A. 2013;110:11439–11444. doi: 10.1073/pnas.1216959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Symonds ME., Mendez MA., Meltzer HM., et al Early life nutritional programming of obesity: mother-child cohort studies. Ann Nutr Metab. 2013;62:137–145. doi: 10.1159/000345598. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z., Huffman SL. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern Child Nutr. 2013;9(suppl 1):105–119. doi: 10.1111/mcn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fall C. Maternal nutrition: effects on health in the next generation. Indian J Med Res. 2009:130. [PubMed] [Google Scholar]
- 44.Metges CC. Early nutrition and later obesity: animal models provide insights into mechanisms. In: Early Nutrition Programming and Health Outcomes in Later Life. Amsterdam, the Netherlands: Springer; 2009:105–112. doi: 10.1007/978-1-4020-9173-5_11. [DOI] [PubMed] [Google Scholar]
- 45.Demmelmair H., von Rosen J., Koletzko B. Long-term consequences of early nutrition. Early Hum Dev. 2006;82:567–574. doi: 10.1016/j.earlhumdev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Bilbo SD., Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 47.Peleg-Raibstein D., Luca E., Wolfram C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res. 2012;233:398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki A., de Vega W., St-Cyr S., Pan P., McGowan P. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience. 2013;240:1–12. doi: 10.1016/j.neuroscience.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 49.Sharma S., Fernandes M., Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes. 2013;37:1183–1191. doi: 10.1038/ijo.2012.197. [DOI] [PubMed] [Google Scholar]
- 50.Bolton JL., Auten RL., Bilbo SD. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav lmmun. 2014;37:30–44. doi: 10.1016/j.bbi.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Bolton JL., Smith SH., Huff NC., et al Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26:4743–4754. doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- 52.Naef L., Moquin L., Dal Bo G., Giros B., Gratton A., Walker C. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 2011;176:225–236. doi: 10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 53.Teegarden SL., Scott AN., Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162:924–932. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herva A., Pouta A., Hakko H., Laksy K., Joukamaa M., Veijola J. Birth measures and depression at age 31 years: the northern Finland 1966 birth cohort study. Psychiatry Res. 2008;160:263–270. doi: 10.1016/j.psychres.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 55.Halmoy A., Klungsoyr K., Skjaerven R., Haavik J. Pre-and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:474–481. doi: 10.1016/j.biopsych.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Colman I., Ataullahjan A., Naicker K., Van Lieshout RJ. Birth weight, stress, and symptoms of depression in adolescence: evidence of fetal programming in a national Canadian cohort. Can J Psychiatry. 2012;57:422–428. doi: 10.1177/070674371205700705. [DOI] [PubMed] [Google Scholar]
- 57.Moore GS., Kneitel AW., Walker CK., Gilbert WM., Xing G. Autism risk in small-and large-for-gestational-age infants. Obstet Gynecol. 2012;206:314.e1–e9. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 2010;51:134–143. doi: 10.1111/j.1469-7610.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 59.Rofey DL., Kolko RP., losif A., et al A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum Dev. 2009;40:517–526. doi: 10.1007/s10578-009-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grissom NM., Reyes TM. Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int J Dev Neurosci. 2013;31:406–414. doi: 10.1016/j.ijdevneu.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krakowiak P., Walker CK., Bremer AA., et al Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dantzer R., Konsman JP., Bluthe RM., Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- 63.J.I. WM., Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Bellinger DL., Millar BA., Perez S., et al Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nance DM., Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav lmmun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elenkov IJ., Wilder RL., Chrousos GP., Vizi ES. The sympathetic nerve-an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 67.Rivest S. Interactions between the immune and neuroendocrine systems. Prog Brain Res. 2010;181:43–53. doi: 10.1016/S0079-6123(08)81004-7. [DOI] [PubMed] [Google Scholar]
- 68.Costa-Pinto FA., Palermo-Neto J. Neuroimmune interactions in stress. Neuroimmunomodulation. 2010;17:196–199. doi: 10.1159/000258722. [DOI] [PubMed] [Google Scholar]
- 69.Rabin BS., Ganguli R., Lysle DT., Cunnick JE. Interaction between the brain and the immune system. Immunol Ser. 1990;52:125–154. [PubMed] [Google Scholar]
- 70.Turnbull AV., Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 71.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 72.Dantzer R., Bluthe RM., Gheusi G., et al Molecular basis of sickness behavior. Ann NY Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 73.Dantzer R., Bluthe RM., Laye S., Bret-Dibat JL., Parnet P., Kelley KW. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 74.Dantzer R., Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav lmmun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blatteis CM. The onset of fever: new insights into its mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- 76.Dantzer R., O'Connor JC., Freund GG., Johnson RW., Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pace TW., Miller AH. Cytokines and glucocorticoid receptor signaling, relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hornig M., Weissenbock H., Horscroft N., Lipkin Wl. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci U S A. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson KB., Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000;13:133–139. doi: 10.1097/00019052-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Rantakallio P., Jones P., Moring J., Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- 82.Shi L., Smith SE., Malkova N., Tse D., Su Y., Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav lmmun. 2009;23:116–23. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Deverman BE., Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Gaulden J., Reiter JF. Neur-ons and neur-offs: regulators of neural induction in vertebrate embryos and embryonic stem cells. Hum Mol Genet. 2008;17(R1):R606–6. doi: 10.1093/hmg/ddn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Derouet D., Rousseau F., Alfonsi F., et al Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc Natl Acad Sci USA. 2004;101:4827–4832. doi: 10.1073/pnas.0306178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gregg C., Weiss S. CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain. Development. 2005;132:565–578. doi: 10.1242/dev.01592. [DOI] [PubMed] [Google Scholar]
- 88.Mehler MF., Marmur R., Gross R., et al Cytokines regulate the cellular phenotype of developing neural lineage species. Int J Dev Neurosci. 1995;13:213–240. doi: 10.1016/0736-5748(94)00060-g. [DOI] [PubMed] [Google Scholar]
- 89.Corriveau RA., Huh GS., Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 90.Datwani A., McConnell MJ., Kanold PO., et al Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shatz CJ. MHC class I: An unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schafer DP., Lehrman EK., Kautzman AG., et al Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai Z., Pan ZL., Pang Y., Evans OB., Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopoly-saccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 94.Meyer U., Feldon J., Schedlowski M., Yee BK. Immunological stress at the maternal foetal interface: a link between neurodevelopment and adult psychopathology. Stress, Genet Immun. 2006;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Pang Y., Cai Z., Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 96.Urakubo A., Jarskog LF., Lieberman JA., Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 97.Mayer W., Smith A., Fundele R., Haaf T. Spatial separation of parental genomes in preimplantation mouse embryos. J Cell Biol. 2000;148:629–634. doi: 10.1083/jcb.148.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oswald J., Engemann S., Lane N., et al Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–478. doi: 10.1016/s0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- 99.Rougier N., Bourc'his D., Gomes DM., et al Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 1998;12:2108–2113. doi: 10.1101/gad.12.14.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heerwagen MJ., Miller MR., Barbour LA., Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul lntegr Comp Physiol. 2010;299:R711–R722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lillycrop KA., Burdge GC. Epigenetic mechanisms linking early nutrition to long term health. Best Pract Res Clin Endocrinol Metab. 2012;26:667–676. doi: 10.1016/j.beem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 102.Hollingsworth JW., Maruoka S., Boon K., et al In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Weaver IC., Cervoni N., Champagne FA., et al Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 104.Schwarz JM., Hutchinson MR., Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williamson LL., Sholar PW., Mistry RS., Smith SH., Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dahlgren J., Nilsson C., Jennische E., et al Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281:E326–E334. doi: 10.1152/ajpendo.2001.281.2.E326. [DOI] [PubMed] [Google Scholar]
- 107.Godfrey KM. The role of the placenta in fetal programming—a review. Placenta. 2002;23:S20–S27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 108.Seckl JR., Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Wat. Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 109.Wynn RM. Derivation and ultrastructure of the so-called Hofbauer cell. Am J Obstet Gynecol. 1967;97:235–248. doi: 10.1016/0002-9378(67)90546-7. [DOI] [PubMed] [Google Scholar]
- 110.Abrahams VM. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37:427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- 111.Milanski M., Degasperi G., Coope A., et al Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. Science's STKE. 2009;29:359. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsiao EY., Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72:1317–1326. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
- 113.Wahlbeck K., Forsén T., Osmond C., Barker DJ., Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry. 2001;58:48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- 114.Jansson T., Powell T. Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin Sci. 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 115.Wang X., Athayde N., Trudinger B. A proinflammatory cytokine response is present in the fetal placental vasculature in placental insufficiency. Obstet Gynecol. 2003;189:1445–1451. doi: 10.1067/s0002-9378(03)00652-5. [DOI] [PubMed] [Google Scholar]
- 116.Bartha JL., Romero-Carmona R., Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2003;82:1099–1102. doi: 10.1046/j.1600-0412.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 117.Mikaelsson MA., Constancia M., Dent CL., Wilkinson LS., Humby T. Placental programming of anxiety in adulthood revealed by Igf2-null models. Nat Comm. 2013;4:2311. doi: 10.1038/ncomms3311. [DOI] [PubMed] [Google Scholar]
- 118.Mor G., Romero R., Aldo PB., Abrahams VM. Is the trophoblast an immune regulator? the role of toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 119.Robinson N., Atkinson D., Jones C., Sibley C. Permeability of the nearterm rat placenta to hydrophilic solutes. Placenta. 1988;9:361–372. doi: 10.1016/0143-4004(88)90049-5. [DOI] [PubMed] [Google Scholar]
- 120.Langley-Evans S., Phillips G., Benediktsson R., et al Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 121.Kossintseva I., Wong S., Johnstone E., Guilbert L., Olson DM., Mitchell BF. Proinflammatory cytokines inhibit human placental 11beta-hydroxysteroid dehydrogenase type 2 activity through Ca2+ and cAMP pathways. Am J Physiol Endocrinol Metab. 2006;290:E282–E288. doi: 10.1152/ajpendo.00328.2005. [DOI] [PubMed] [Google Scholar]
- 122.Zaretsky MV., Alexander JM., Byrd W., Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- 123.Hsiao EY., Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim SY., Romero R., Tarca AL., et al Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reproduct Immunol. 2012;68:8–27. doi: 10.1111/j.1600-0897.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Challier J., Basu S., Bintein T., et al Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roberts K., Riley S., Reynolds R., et al Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32:247–254. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 127.Gheorghe CP., Goyal R., Holweger JD., Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta. 2009;30:411–417. doi: 10.1016/j.placenta.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonnin A., Goeden N., Chen K., et al A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manuelpillai U., Ligam P., Smythe G., Wallace EM., Hirst J., Walker DW. Identification of kynurenine pathway enzyme mRNAs and metabolites in human placenta: Up-regulation by inflammatory stimuli and with clinical infection. Obstet Gynecol. 2005;192:280–288. doi: 10.1016/j.ajog.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 130.Kannan S., Saadani-Makki F., Balakrishnan B., et al Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero. J Cerebr Blood Flow Metab. 2010;31:738–749. doi: 10.1038/jcbfm.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wolff RL. Trans-18 1 isomers in rat milk fat as effective biomarkers for the determination of individual isomeric trans-18 1 acids in the dams' diet. Lipids. 2003;38:1143–1148. doi: 10.1007/s11745-003-1172-z. [DOI] [PubMed] [Google Scholar]
- 132.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 133.Grandgirard A., Bourre J., Julliard F., et al Incorporation oftrans longchain n- 3 polyunsaturated fatty acids in rat brain structures and retina. Lipids. 1994;29:251–258. doi: 10.1007/BF02536329. [DOI] [PubMed] [Google Scholar]
- 134.Spector R. Fatty acid transport through the blood-brain barrier. J Neurochem. 1988;50:639–643. doi: 10.1111/j.1471-4159.1988.tb02958.x. [DOI] [PubMed] [Google Scholar]
- 135.Strosznajder J., Chalimoniuk M., Strosznajder RP., Albanese V., Alberghina M. Arachidonate transport through the blood-retina and blood-brain barrier of the rat during aging. Neurosci Lett. 1996;209:145–148. doi: 10.1016/0304-3940(96)12624-0. [DOI] [PubMed] [Google Scholar]
- 136.Gorski JN., Dunn-Meynell AA., Hartman TG., Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Ami J Physiol Regul Integr Comp Physiol. 2006;291:R768–78. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- 137.Smart J., Preece J. Maternal behaviour of undernourished mother rats. Anim Behav. 1973;21:613–619. doi: 10.1016/s0003-3472(73)80024-7. [DOI] [PubMed] [Google Scholar]
- 138.Purcell RH., Sun B., Pass LL., Power ML., Moran TH., Tamashiro KL. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav. 2011;104:474–479. doi: 10.1016/j.physbeh.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garcia-Patterson A., Aulinas A., Sojo L., et al Poorer perinatal outcome in male newborns of women with pregestational diabetes mellitus. Diabetic Med. 2011;28:436–439. doi: 10.1111/j.1464-5491.2011.03227.x. [DOI] [PubMed] [Google Scholar]
- 140.Tundidor D., Garcia-Patterson A., Maria MA., et al Perinatal maternal and neonatal outcomes in women with gestational diabetes mellitus according to fetal sex. Gender Med. 2012;9:411–417. doi: 10.1016/j.genm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 141.Flannery KA., Liederman J., Daly L., Schultz J. Male prevalence for reading disability is found in a large sample of black and white children free from ascertainment bias. J Int Neuropsychol Soc. 2000;6:433–442. doi: 10.1017/s1355617700644016. [DOI] [PubMed] [Google Scholar]
- 142.Stone JL., Merriman B., Cantor RM., et al Evidence for sex-specific risk alleles in autism spectrum disorder. Am J Hum Genet. 2004;75:1117–1123. doi: 10.1086/426034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thompson C., Syddall H., Rodin I., Osmond C., Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–455. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 144.Schwarz JM., Sholar PW., Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clifton VL. Review: sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 146.Murphy VE., Gibson PG., Giles WB., et al Maternal asthma is associated with reduced female fetal growth. Am J Resp Crit Care Med. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- 147.Scott NM., Hodyl NA., Murphy VE., et al Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol. 2009;182:1411–1420. doi: 10.4049/jimmunol.182.3.1411. [DOI] [PubMed] [Google Scholar]
- 148.Murphy VE., Gibson P., Talbot PI., Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol. 2005;106:1046. doi: 10.1097/01.AOG.0000185281.21716.02. [DOI] [PubMed] [Google Scholar]
- 149.Mao J., Zhang X., Sieli PT., Falduto MT., Torres KE., Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci U S A. 2010;107: 5557–5562. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Youngson NA., Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–257. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- 151.Waterland R., Travisano M., Tahiliani K., Rached M., Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes. 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Waterland RA., Lin JR., Smith CA., Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet. 2006;15:705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- 153.Chatzi L., Torrent M., Romieu I., et al Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507–513. doi: 10.1136/thx.2007.081745. [DOI] [PubMed] [Google Scholar]
- 154.Stulnig TM., Zeyda M. Immunomodulation by polyunsaturated fatty acids: Impact on T-cell signaling. Lipids. 2004;39:1171–1175. doi: 10.1007/s11745-004-1344-x. [DOI] [PubMed] [Google Scholar]
- 155.Bagga D., Wang L., Farias-Eisner R., Glaspy JA., Reddy ST. Differential effects of prostaglandin derived from co-6 and o>3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Amarasekera M., Prescott SL., Palmer DJ. Nutrition in early life, immune-programming and allergies: the role of epigenetics. Asian Pacific J Allergy Immunol. 2013;31:175–182. [PubMed] [Google Scholar]