Abstract
The lung is an interface where host cells are routinely exposed to microbes and microbial products. Alveolar macrophages are the first-line phagocytic cells that encounter inhaled fungi and other microbes. Macrophages and other immune cells recognize Aspergillus motifs by pathogen recognition receptors and initiate downstream inflammatory responses. The phagocyte NADPH oxidase generates reactive oxygen intermediates (ROIs) and is critical for host defense. Although NADPH oxidase is critical for neutrophil-mediated host defense1-3, the importance of NADPH oxidase in macrophages is not well defined. The goal of this study was to delineate the specific role of NADPH oxidase in macrophages in mediating host defense against A. fumigatus. We found that NADPH oxidase in alveolar macrophages controls the growth of phagocytosed A. fumigatus spores4. Here, we describe a method for assessing the ability of mouse alveolar macrophages (AMs) to control the growth of phagocytosed Aspergillus spores (conidia). Alveolar macrophages are stained in vivo and ten days later isolated from mice by bronchoalveolar lavage (BAL). Macrophages are plated onto glass coverslips, then seeded with green fluorescent protein (GFP)-expressing A. fumigatus spores. At specified times, cells are fixed and the number of intact macrophages with phagocytosed spores is assessed by confocal microscopy.
Keywords: Immunology, Issue 89, macrophage, bronchoalveolar lavage, Aspergillus, confocal microscopy, phagocytosis, anti-fungal activity, NADPH oxidase
Introduction
Alveolar macrophages are the first-line phagocytic cells that encounter inhaled microbes. Macrophages recognize Aspergillus motifs by pathogen recognition receptors, ingest and limit the growth of inhaled spores (conidia), and initiate inflammatory responses. The phagocyte NADPH oxidase converts molecular oxygen to superoxide anion and downstream reactive oxygen intermediates (ROIs). Chronic granulomatous disease (CGD) is an inherited disorder of the NADPH oxidase characterized by severe bacterial and fungal infections and by excessive inflammatory responses.
Although NADPH oxidase is critical for neutrophil-mediated host defense1-3, the importance of NADPH oxidase in macrophages is not well defined. Prior studies have shown that alveolar macrophages ingest and kill Aspergillus spores, whereas neutrophils principally target the hyphal stage5. However, there have been conflicting results as to the role of NADPH oxidase in macrophage in controlling the growth of A. fumigatus spores6,7.
The goal of this method was to delineate the specific role of NADPH oxidase in macrophages in mediating host defense against A. fumigatus spores. We found that NADPH oxidase in alveolar macrophages controls the growth of phagocytosed A. fumigatus spores4. To most closely model the host response to inhaled fungi, we used alveolar macrophages harvested by bronchoalveolar lavage from unstimulated mice immediately following sacrifice. The use of isolated alveolar macrophages enabled us to focus on their antifungal activity in the absence of other immune cells (e.g., recruited neutrophils) that defend against Aspergillus in vivo. In this method, alveolar macrophages were stained in vivo prior to harvest so as to minimize the amount of post-harvest manipulation.
Another advantage to this protocol is the use of a GFP-expressing A. fumigatus strain. This fungus expresses GFP from the conidial stage through the hyphal stage and has no need for further staining. To obtain images with greater detail, we chose confocal microscopy which enables the reconstruction of three-dimensional structures from the obtained images. Three-dimensional reconstruction gives the ability to differentiate between hyphae growing around rather than in or through a macrophage. Conidia can also be precisely distinguished as intracellular versus extracellular.
Protocol
All procedures performed on animals in this study were approved by the Animal Care and Use Committee at Roswell Park Cancer Institute and complied with all state, federal, and National Institutes of Health regulations.
1. In vivo Macrophage Labeling
Note: PHK26 is a lipophilic dye that will be taken up by phagocytic cells both in circulation and in the tissues when administered intravenously (i.v.). PKH26 was used for in vivo labeling to minimize the amount of post-harvest manipulation of the macrophage. PKH-26 has the advantage of stably accumulating in alveolar macrophages over prolonged periods. Waiting 10 days after administration allows for clearance of all labeled cells from circulation leaving only alveolar macrophage stably labeled with PKH268,9. PKH26 is a red fluorochrome that has excitation (551 nm) and emission (567 nm) characteristics compatible with rhodamine or phycoerythrin detection systems. Any labeling procedure can introduce artifact, including loss of viability. However, since we use the same approach for labeling of macrophages in different mouse genotypes (e.g., WT vs. NADPH oxidase-deficient), the effects of these artifacts would be uniform.
Dilute stock solution of PKH26 (1 x 10-3 M in ethanol) 1:5 in diluent C provided by manufacturer.
Load 100 µl of diluted PKH26 into a 1/2 ml insulin syringe (29 G x 1/2”) for each mouse.
Administer to mice by i.v. injection (either tail vein or retro orbital) at least 10 days prior to harvest.
2. Harvesting Alveolar Macrophages by Bronchoalveolar Lavage (BAL)
The number of mice to use for an experiment can vary. A typical yield from an unstimulated mouse is in the range of 3-5 x 105 alveolar macrophages, however this number can vary greatly based on factors such as size of the mouse, experience of the person performing the lavage, and the number of instillations of lavage fluid and the volume withdrawn from lungs. In this protocol lung lavage is performed with a closed thoracic cavity which along with repeated 1 ml lavages helps to increase cell yield.
Euthanize mouse by administering a lethal dose of avertin anesthetic (12.5 mg) intraperitoneally (i.p.) using aseptic conditions to keep the solution sterile.
Spray mouse thoroughly with 70% ethanol.
- Remove skin from the cranial end of animal exposing the thorax (Figure 1A).
- Make a small cut in skin just beneath the diaphragm.
- Insert finger into the cut and pull skin away from the cranial end of the animal (Figure 1A).
- Push up slightly on the head, extending the ventral side of the neck (Figure 1A).
Cut a small hole through the rib cage into the right side of the thorax to deflate the lungs and also allow visualization of lungs during the following steps.
- Locate the trachea, and carefully dissect away surrounding tissue.
- Remove the salivary glands, sternohyoid muscles and larynx, etc. with curved forceps.
- Carefully lift with a curved forceps and cut away with scissors the thin tissue covering the trachea (adventitia) revealing the underlying ringed cartilage structure.
- Insert catheter into trachea.
- Using curved forceps, place a suture behind (dorsal to) the trachea and pull a 10 cm length through the opening.
- Starting above the cricoid (the thickened ring of cartilage) carefully guide a 22 G catheter down the trachea stopping where the trachea disappears behind the sternum.
- Firmly tie suture around trachea and catheter for a snug fit. Remove needle from catheter.
- Assemble 4-way stopcock.
- Fill one 6 ml syringe with DPBS, 1% FBS and attach to 4-way stopcock as indicated in Figure 1B.
- Attach an empty 6 ml syringe at the indicated position on the 4-way stopcock (Figure 1B).
- Attach open port on the 4-way stopcock to catheter (Figure 1B). Be careful to not dislodge catheter from trachea.
- Collect lavage fluid from lungs. Note: Investigators may opt to use lower volumes of lavage fluid to minimize the possibility of damage to the tissue. It is important that the same approach be consistently applied in all mouse groups.
- Turn the handle on the stopcock so the empty syringe is closed and slowly inject ~1 ml DPBS, 1% FBS to inflate lungs. Observe lung inflation through the hole cut in step 2.4.
- Be careful NOT to overinflate the lungs.
- Turn the handle on the stopcock so that the full syringe is closed, and carefully withdraw the fluid from the lungs.
- Observe the lungs deflate through the hole cut in step 1.4. The catheter may need to be moved slightly back or forth to get a good draw. Take special precaution to keep the catheter within the trachea.
- Repeat steps 2.8.1-2.8.4 5-6x until all the DPBS, 1% FBS has been injected and withdrawn from the lungs. Note: recovery of lung fluid will not be 100% of injected fluid.
Empty syringe with lavage fluid into a 50 ml conical tube. Keep cells at room temperature.
Pellet cells by centrifuging at 300 x g for 5 min at room temperature and pour off supernatant. This can be saved for cytokine analysis if desired.
Resuspend cells in 1-5 ml RPMI 1640 with 10% FBS and count on a hemocytometer. In the unstimulated mouse, > 95% of cells harvested by BAL are expected to be macrophages, which can be confirmed by cytology.
3. Culturing and Harvesting Fungus
The Aspergillus fumigatus used in this protocol expresses GFP throughout all stages of its life cycle: spores, germlings and hyphae. This fungal strain was provided by Dr. Margo Moore, Simon Fraser University, Burnaby, BC, Canada.
Plate conidia of an Aspergillus fumigatus strain expressing GFP on Sabouraud brain heart infusion (BHI) agar slants with Chloramphenicol (0.05 g/L) and Gentamicin (0.5 g/L).
Incubate in the dark loosely capped for 7 to 10 days at room temperature.
Harvest conidia by washing slant with 10 ml of 0.01% Tween 20 in DPBS. Lightly scrape the fungus with the stripette to loosen. Rinse the slant several times to increase yield.
Pass conidial suspension through a 100 µm cell strainer into a 50 ml conical.
Pellet conidia by centrifuging at 400 x g for 10 min.
Resuspend conidia in 50 ml DPBS and count using a hemocytometer.
Wash twice with DPBS and dilute to desired concentration.
4. Fungal Challenge and Confocal Microscopy
To investigate the role of macrophage NADPH oxidase in host defense, the ability of isolated alveolar macrophages from mice of three genotypes to control the growth of phagocytosed Aspergillus spores was evaluated. The genotypes used in this study include; 1) wild-type mice, 2) Ncf1*/* Mn- mice with a naturally occurring Ncf1 mutation (Ncf1 encodes for p47phox, a required component of the phagocyte NADPH oxidase) leaving them NADPH oxidase deficient, and 3) Ncf1*/* Mn+ transgenic mice (MN denotes the transgene harboring wildtype Ncf1 under the control of the human CD68 promoter) where NADPH oxidase activity has been reconstituted in the monocyte/macrophage population10,11.
Confocal microscopy is best suited for this assay due to the presence and growth of non-phagocytosed conidia on the slides. The confocal microscope will allow for visualization of individual planes within the Z-axis, and enable differentiation between fungus growing around rather than inside macrophage. All confocal images are acquired using a 63X oil immersion lens. Z-stacks are acquired with an electronic zoom factor of 2.5. The thickness of a Z-stack is determined using DAPI and bright field images to locate the top and bottom of the field. Z-stacks ranged from 14 to 24 µm with individual slices ~1 µm thick. For quantification of macrophages, single scans of 1X electronic zoom were performed on 10 fields per genotype. Intact macrophages were identified based on PKH26 and DAPI staining of the membrane and nucleus respectively. Conidia were identified based on FITC expression and bright field image.
- Prepare sterile coverslips and plates
- Under a Biological Safety Cabinet, soak 22 x 22 mm glass coverslips in 70% ethanol for 5-10 min.
- Rinse coverslips in sterile PBS.
- Using sterile forceps, gently place a single coverslip in the bottom of each well of a sterile singly packed 6-well plate.
Seed ~1 x 106 harvested PKH26 labeled alveolar macrophages suspended in 300 µl RPMI 1640, 10% FBS onto each coverslip. The exact number may vary depending on the harvest yield.
Allow macrophage to adhere by incubating at 37 °C with 5% CO2 overnight.
The following morning, add ~1 x 107 GFP-expressing A. fumigatus conidia suspended in 100 µl pre-warmed (37 °C) RPMI 1640, 10% FBS.
Return plate to 37 °C incubator with 5% CO2.
Cells should be fixed for a minimum of 2 hr at room temperature or overnight at 4 °C. At 3, 7, and 14 hr fix cells by adding an equal volume of 2% formaldehyde to each well (final concentration 1% formaldehyde). Place plates for the early time points at 4 °C overnight. For the final time point (14 hr), fix cells at room temperature for 2 hr.
After fixation, gently remove coverslips using forceps and rinse in DPBS.
Remove excess fluid by placing the edge of the coverslip on a folded Kimwipe.
Apply 6 µl fluorescence mounting medium with DAPI to a glass microscope slide.
Lay one edge of the coverslip on the slide and gently drop with cell side down into the mounting medium. Mounting medium will spread and form an even layer beneath the coverslip. There is no need to press on the coverslip.
Seal edges of coverslip with clear nail polish and allow to air dry.
Store prepared slides in a slide box (protected from light) at room temperature until ready to analyze by confocal microscopy.
Representative Results
Collecting alveolar macrophage by BAL from unstimulated mice will result in a > 95% pure population based on cytology. The 3 and 7 hr (Figure 2) time points precede the hyphal stage of fungal growth and allow for a comparison of phagocytic efficiency of conidia among genotypes. The 14 hr time point is where genotypes can be compared with regard to the ability to inhibit the transition of phagocytosed conidia to the tissue-invasive hyphal stage (Figure 3). Intact macrophage can be identified by confocal microscopy based on PKH26 and DAPI staining of the membrane and nucleus, respectively (Figures 2 and 3). Conidia and hyphae are identified based on GFP expression (Figures 2 and 3).
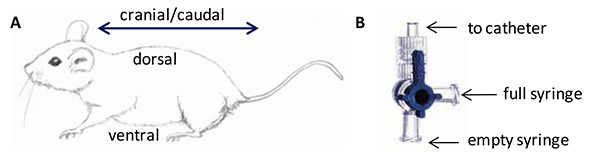 Figure 1. Anatomical Directional References and the 4-Way Stopcock.A) The anatomical directional references for a mouse are shown. Cranial is in the direction of the head, while caudal is in the direction of the tail. The ventral side of a quadruped is toward the belly or the lower surface of the animal, while the dorsal side is toward the back or the upper surface of the animal. B) The 4-way stopcock has three luer connections and a handle that rotates 360° to direct the flow of fluids through the stopcock. Placement of the empty syringe, the full syringe and the catheter are indicated. Click here to view larger image.
Figure 1. Anatomical Directional References and the 4-Way Stopcock.A) The anatomical directional references for a mouse are shown. Cranial is in the direction of the head, while caudal is in the direction of the tail. The ventral side of a quadruped is toward the belly or the lower surface of the animal, while the dorsal side is toward the back or the upper surface of the animal. B) The 4-way stopcock has three luer connections and a handle that rotates 360° to direct the flow of fluids through the stopcock. Placement of the empty syringe, the full syringe and the catheter are indicated. Click here to view larger image.
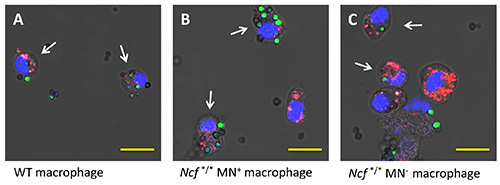 Figure 2. Phagocytosis of A. fumigatus spores is similar between genotypes at 7 hr. To evaluate the ability of isolated alveolar macrophages with NADPH oxidase activity to limit the growth of phagocytosed conidia, (A) wild-type, (B) Ncf1*/*Mn+, and (C) Ncf1*/*Mn- mice were administered i.v. PKH26. Alveolar macrophages were harvested by BAL 10 days later and seeded with conidia of GFP-expressing A. fumigatus. Cells were fixed at 3, 7, and 14 hr after addition of conidia. At 3 (data not shown) and 7 hr both the number of total macrophages and macrophage with ≥ 1 phagocytosed conidia (arrows) were similar among the three genotypes, reflecting similar phagocytosis efficiency. Intact macrophages are identified based on PKH26 (red) and DAPI (blue) staining of membrane and nuclei respectively. The fluorescence images from a single Z-plane are overlaid with the bright field image. The bright field image consists of light transmitted through the entire specimen and so when overlain on the fluorescent Z-plain allows visualization of cells and conidia within the entire Z-stack. Scale bar, 19 µm. Click here to view larger image.
Figure 2. Phagocytosis of A. fumigatus spores is similar between genotypes at 7 hr. To evaluate the ability of isolated alveolar macrophages with NADPH oxidase activity to limit the growth of phagocytosed conidia, (A) wild-type, (B) Ncf1*/*Mn+, and (C) Ncf1*/*Mn- mice were administered i.v. PKH26. Alveolar macrophages were harvested by BAL 10 days later and seeded with conidia of GFP-expressing A. fumigatus. Cells were fixed at 3, 7, and 14 hr after addition of conidia. At 3 (data not shown) and 7 hr both the number of total macrophages and macrophage with ≥ 1 phagocytosed conidia (arrows) were similar among the three genotypes, reflecting similar phagocytosis efficiency. Intact macrophages are identified based on PKH26 (red) and DAPI (blue) staining of membrane and nuclei respectively. The fluorescence images from a single Z-plane are overlaid with the bright field image. The bright field image consists of light transmitted through the entire specimen and so when overlain on the fluorescent Z-plain allows visualization of cells and conidia within the entire Z-stack. Scale bar, 19 µm. Click here to view larger image.
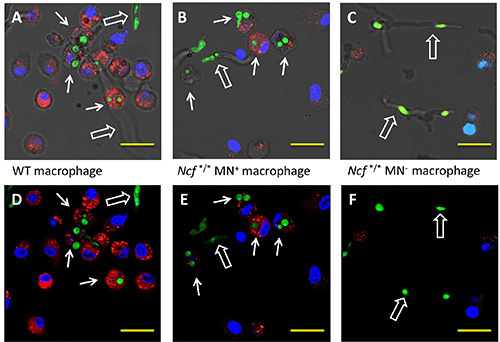 Figure 3. Macrophage NADPH oxidase is required to control the growth of A. fumigatus spores. Confocal microscopy of (A, D) wild-type, (B, E) Ncf1*/*Mn+, and (C, F) Ncf1*/*Mn- alveolar macrophage at 14 hr after seeding with GFP+
A. fumigatus conidia. Intact macrophages are identified based on PKH26 (red) and DAPI (blue) staining of membranes and nuclei, respectively. Solid arrows show intact macrophages with at least one phagocytosed conidia and hollow arrows point to hyphae. Numerous AMs with at least 1 phagocytosed conidia were observed with wild-type and Ncf1*/*Mn+ cells, but not with NADPH oxidase-deficient cells. GFP signal in hyphae was variable based on the plane of the z-stacked image shown. A-C) Fluorescence images from a single Z-plane with the bright field image enable visualization of cells and hyphae within the entire Z-stack. D-F) Fluorescence images from a single Z-plane without the bright field background enable identification of cells, but not the spatial relationship of cells and fungi in different Z-planes. Scale bar, 19 µm. Click here to view larger image.
Figure 3. Macrophage NADPH oxidase is required to control the growth of A. fumigatus spores. Confocal microscopy of (A, D) wild-type, (B, E) Ncf1*/*Mn+, and (C, F) Ncf1*/*Mn- alveolar macrophage at 14 hr after seeding with GFP+
A. fumigatus conidia. Intact macrophages are identified based on PKH26 (red) and DAPI (blue) staining of membranes and nuclei, respectively. Solid arrows show intact macrophages with at least one phagocytosed conidia and hollow arrows point to hyphae. Numerous AMs with at least 1 phagocytosed conidia were observed with wild-type and Ncf1*/*Mn+ cells, but not with NADPH oxidase-deficient cells. GFP signal in hyphae was variable based on the plane of the z-stacked image shown. A-C) Fluorescence images from a single Z-plane with the bright field image enable visualization of cells and hyphae within the entire Z-stack. D-F) Fluorescence images from a single Z-plane without the bright field background enable identification of cells, but not the spatial relationship of cells and fungi in different Z-planes. Scale bar, 19 µm. Click here to view larger image.
Discussion
Using this approach for ex vivo analysis of the antifungal activity of macrophages together with in vivo fungal challenge, we previously demonstrated that NADPH oxidase in macrophages plays an important role in defense against A. fumigatus4. The use of isolated alveolar macrophages enables us to focus on their antifungal activity in the absence of other immune cells (e.g., recruited neutrophils) that defend against Aspergillus in vivo.
Various visualization strategies have been used to evaluate macrophage antifungal activity in vivo and ex vivo. Luther et al., used confocal microscopy to study phagocytosis of Aspergillus conidia by human (harvested from lung transplant recipients) and mouse AMs12. Geunes-Boyer et al. characterized the interaction between Candida krusei and murine macrophages following phagocytosis. They used a macrophage cell line, RAW264.7 and primary macrophage from the peritoneum13. Garcia-Rodas et al. showed that surfactant protein-D binds to and facilitates the phagocytosis and survival of Cryptococcus neoformans14. SP-D binding was also investigated in vivo using SP-D-/- mice intranasally inoculated with Alexa Fluor 488-labeled C. neoformans. Following lung lavage, AMs were immunofluorescently labeled and confocal microscopy was used to analyze phagocytosis.
Macrophages from different anatomic sites (e.g. alveolar, splenic, peritoneal), generated with different stimuli (e.g., thioglycollate elicitation or in vitro differentiation), or from cell lines (e.g., RAW264.7) can have dramatically different antimicrobial and inflammatory responses15-18. Therefore, to most closely model the host response to inhaled fungi, we used alveolar macrophages harvested by bronchoalveolar lavage from unstimulated mice immediately following sacrifice.
Initially we used live-cell imaging to evaluate macrophage antifungal activity4. Alveolar macrophage were plated in chambered glass slides then seeded with GFP expressing Aspergillus. After 3 hr, non-phagocytosed conidia were removed by gentle washing to allow unobstructed visualization of phagocytosed conidia. Conidial germination was then monitored by time-lapse imaging at 30 min intervals using bright light images to visualize macrophage and GFP fluorescence to visualize fungi. This method has the advantage of acquiring sequential images of the same primary cell culture. However, the tendency for the macrophage to migrate across the field of view during the course of acquisition allowed for a qualitative rather than a quantitative assessment of conidiocidal activity. Another disadvantage of live-cell imaging is the lack of clarity and detail as cells move in and out of the plane of focus.
To obtain images with greater detail we chose confocal microscopy which enables the reconstruction of three-dimensional structures from the obtained images. Three-dimensional reconstruction gives the ability to differentiate between hyphae growing around rather than in or through a macrophage. With this ability to view the cells and fungus in three-dimensions, removal of non-phagocytosed conidia at 3 hr is no longer a necessary step. Conidia can also be precisely distinguished as intracellular versus extracellular.
For enhanced visualization, alveolar macrophage were labeled in vivo with PKH26. This fluorescent dye has a long aliphatic tail which stably incorporates into the lipid regions of cell membranes. When injected intravenously, this lipophilic dye is taken up by phagocytic cells both in the bloodstream and in tissues. After 10 days, labeled phagocytes are cleared from murine circulation and remain only in the tissues9, allowing for the collection of already labeled alveolar macrophage by BAL. With the use of in vivo labeling, post-harvest manipulation of alveolar macrophages is minimized.
Another advantage to this protocol is the use of a GFP-expressing A. fumigatus strain. This fungus expresses GFP from the conidial stage through the hyphal stage and has no need for further staining. With dual staining of macrophages and Aspergillus, we are able to identify phagocytosed conidia.
A limiting factor in this protocol is the macrophage yield from the BAL. A typical yield from an unstimulated mouse is in the range of 3-5 x 105 alveolar macrophages, however this number can vary greatly based on factors such as size of the mouse, experience of the person performing the lavage, and the number of instillations of lavage fluid and the volume withdrawn from lungs. In this protocol, lung lavage is performed with a closed thoracic cavity which along with repeated 1 ml instillations helps to increase cell yield when compared to an open thoracic cavity and fewer instillations. Mouse numbers can also be increased to increase harvest yield.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Disease Grant R01AI079253 (to B.H.S.), and by the National Cancer Institute Cancer Center Support Grant CA016056 to Roswell Park Cancer Institute.
References
- Reeves EP, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Bianchi M, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethanayagam RR, et al. Role of NADPH oxidase versus neutrophil proteases in antimicrobial host defense. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MJ, et al. Monocyte- and Macrophage-Targeted NADPH Oxidase Mediates Antifungal Host Defense and Regulation of Acute Inflammation in Mice. The Journal of Immunology. 2013;190:4175–4184. doi: 10.4049/jimmunol.1202800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe B, et al. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish EJ, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase-independent resistance to Aspergillus fumigatus in alveolar macrophages. J Immunol. 2008;180:6854–6867. doi: 10.4049/jimmunol.180.10.6854. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Linderman DJ, Hu B, Sonstein J, Curtis JL. Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly. Am J Respir Cell Mol Biol. 2005;32:108–117. doi: 10.1165/rcmb.2004-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BA, et al. DISCRIMINATION OF RESIDENT AND INFILTRATED ALVEOLAR MACROPHAGES BY FLOW CYTOMETRY IN INFLUENZA A VIRUS-INFECTED MICE. Experimental Lung Research. 2005;31:323–339. doi: 10.1080/01902140590918524. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J Clin Invest. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolla A, et al. Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J Immunol. 2012;188:5003–5011. doi: 10.4049/jimmunol.1103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther K, et al. Characterisation of the phagocytic uptake of Aspergillus fumigatus conidia by macrophages. Microbes and Infection. 2008;10:175–184. doi: 10.1016/j.micinf.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Geunes-Boyer S, et al. Surfactant Protein D Increases Phagocytosis of Hypocapsular Cryptococcus neoformans by Murine Macrophages and Enhances Fungal Survival. Infection and Immunity. 2009;77:2783–2794. doi: 10.1128/IAI.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodas R, González-Camacho F, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. The Interaction between Candida krusei and Murine Macrophages Results in Multiple Outcomes, Including Intracellular Survival and Escape from Killing. Infection and Immunity. 2011;79:2136–2144. doi: 10.1128/IAI.00044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LK, Vermeulen MW. Alveolar macrophages from C3H/HeJ mice show sensitivity to endotoxin. Am J Respir Cell Mol Biol. 1995;12:540–546. doi: 10.1165/ajrcmb.12.5.7742017. [DOI] [PubMed] [Google Scholar]
- Redente EF, et al. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation. J Leukoc Biol. 2010;88:159–168. doi: 10.1189/jlb.0609378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A, Douglas H, Braude AI, Davis CE. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983;42:1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge HS, et al. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J Immunol. 2009;182:1146–1154. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]


