Abstract
Mouse models have become increasingly popular in the field of behavioral neuroscience, and specifically in studies of experimental stroke. As models advance, it is important to develop sensitive behavioral measures specific to the mouse. The present protocol describes a skilled motor task for use in mouse models of stroke. The Pasta Matrix Reaching Task functions as a versatile and sensitive behavioral assay that permits experimenters to collect accurate outcome data and manipulate limb use to mimic human clinical phenomena including compensatory strategies (i.e., learned non-use) and focused rehabilitative training. When combined with neuroanatomical tools, this task also permits researchers to explore the mechanisms that support behavioral recovery of function (or lack thereof) following stroke. The task is both simple and affordable to set up and conduct, offering a variety of training and testing options for numerous research questions concerning functional outcome following injury. Though the task has been applied to mouse models of stroke, it may also be beneficial in studies of functional outcome in other upper extremity injury models.
Keywords: Behavior, Issue 89, Upper extremity impairment, Murine model, Rehabilitation, Reaching, Non-paretic limb training, Good limb training, Less-affected limb training, Learned non-use, Pasta matrix reaching task
Introduction
Mouse models have become increasingly popular for experimental stroke research due in part to their convenience and affordability, as well as the availability of transgenic lines that are suitable for in vivo imaging among other applications. With this increased popularity in experimental models, the interest in developing sensitive behavioral assessments of functional outcome following injury has also increased 1-7. The development of animal training protocols that mimic both rehabilitation and compensatory strategies used by human stroke survivors improves the ability to successfully translate findings from experimental animal studies to use in the clinic 8. Motor skill training on the Pasta Matrix Reaching Task (PMRT) has been previously established as a sensitive behavioral assessment of motor skill outcome following ischemic insult of the sensorimotor cortex 3.
One of the primary interests in stroke research concerns rehabilitation and the development and understanding of behavioral strategies that promote improved recovery of function following insult. Currently, rehabilitation strategies in humans result in incomplete recovery 8. In addition, rehabilitation therapists must combat compensatory strategies that stroke survivors develop during recovery that may undermine their ability to fully regain function of their affected limb(s). For example, following a unilateral stroke that affects upper extremity function, humans tend to develop a reliance on their less-affected limb 9, 10. While improving a person’s ability to function in the short term, this learned non-use of the affected limb may impede its ultimate recovery potential, as demonstrated in animal models 11-13. These findings in animals have helped to inform the development and use of constraint-induced movement therapy in humans 14. Animal models are beneficial for improving rehabilitation strategies by allowing researchers to explore the neurobiological mechanisms that subserve and promote recovery of function. In addition to being an effective behavioral assessment of post-stroke function, the PMRT has been established as an effective rehabilitative strategy to promote improved functional outcome following sensorimotor stroke 15. The PMRT can also be used to effectively mimic learned non-use of the affected limb and therefore offer insight into behavioral manipulations that may improve functional recovery despite initial over-reliance on the less-affected limb 13.
Construction of the PMRT has been described previously 3. Briefly, the reaching chamber is composed of four Plexiglas walls (20 cm tall, 15 cm long, and 8.5 cm wide) with an open top and bottom. There is a center slit (13 cm tall and 5 mm wide) extending from the bottom base of the front wall of the chamber that serves as the reaching aperture (Figure 1A). The pasta matrix is a heavy-duty plastic block (8.5 cm long, 5 cm wide, and 1.5 cm tall) with 1 mm diameter holes drilled completely through the depth of the block. There are a total of 260 holes, beginning 2 mm from the reaching window with 2 mm between each hole (Figure 1B). The pasta matrix is designed such that dry, vertically oriented pasta pieces extend through the entire depth of the matrix stage with approximately half of the pasta piece exposed. A removable piece of overhead plastic or cardstock should be cut to size and taped securely to the underside of the matrix. This prevents the pasta pieces from falling out of the matrix during transport and allows for easy removal of broken pasta pieces.
The PMRT is a versatile and sensitive behavioral assay that permits experimenters to collect accurate outcome data and manipulate limb use to mimic clinical phenomena. As a behavioral outcome measure, the PMRT allows experimenters to collect behavioral data that more accurately reflect the effectiveness of a rehabilitative strategy than does the traditional measure of infarct size 3, 16. As a behavioral manipulation, the PMRT allows experimenters to control upper limb use in mice in order to mimic clinical experiences of rehabilitation (i.e. affected limb training) or learned non-use (i.e. less-affected limb training). When combined with neuroanatomical methods, the PMRT provides researchers with an opportunity to explore the mechanisms that support behavioral recovery of function or maladaptive plasticity following compensatory limb use after stroke. The PMRT could be further applied to other murine models of brain injury and upper extremity impairment, such as traumatic brain injury. Another advantage of the PMRT is its affordability. The equipment required for the task can be constructed fairly reasonably in house, data collection does not require a large amount of space or financial resources, and the task is simple enough for undergraduate students to reliably collect data. Further, the PMRT is sensitive to even small behavioral deficits 3, 13. This protocol provides a simple and effective way to assess motor skill learning, promote behavioral recovery following injury, and mimic learned non-use phenomena in an established murine model of stroke.
Protocol
The following methods are in accordance with protocols approved by the University of Texas at Austin and Illinois Wesleyan Animal Care and Use Committees. It is recommended that researchers either wear gloves or take appropriate precautions (washing hands before and after) when engaging in behavioral training with any laboratory animal. Gloves must be worn when handling animals in preparation for and during surgery.
1. Habituation and Food Restriction
NOTE: As the PMRT is an appetitive task, it is beneficial to restrict the amount and timing of feeding prior to the onset of training in order to motivate the reaching response and prevent satiety during training sessions.
- Place mice on a restricted feeding schedule beginning at least 1 week prior to the onset of task training. Weigh each mouse prior to the onset of food restriction to establish free-feeding weights, which are determined as the weights of each animal on the first day of restricted feeding.
- Feed mice once daily, with each mouse receiving ~2.5 g of standard rodent chow per day (e.g., if there are 4 mice housed in a cage, the cage will receive 10 g of food daily). Ensure feeding occurs at the same time each day, preferably at the time daily training sessions are expected to conclude.
- Weigh animals regularly to ensure that no mouse loses more than 10% of their free-feeding weight. NOTE: Adjust daily food amounts accordingly to promote appropriate weight maintenance. Mice typically gain weight as they mature, even with constant food restriction Mice remain on food restriction for the duration of PMRT training/assessment.
Expose animals to smaller pieces of pasta (cut to 1.6 cm) in their home cage several times prior to training in order to overcome neophobic responses. NOTE: Typically 5-10 days of exposure are sufficient, with about 4 pieces per mouse provided at each feeding.
Cut pasta pieces to size (3.2 cm) using a razor blade. NOTE: It is recommended that an excess of pasta pieces are cut prior to beginning the day’s training and unused pieces stored in an airtight container. Capellini pasta is used to fill the matrix. As different brands of pasta vary in texture and diameter, which could influence the stability of pasta in the matrix or the amount of force necessary for the mice to break the pasta, it is recommended that DeCecco brand pasta be used throughout training and testing.
Place uncooked capellini pasta pieces in the pasta matrix, filling all available holes in the matrix (Figure 1A).
Habituate mice to the testing chamber with a full pasta matrix placed in front (Figure 1A) and several pieces of pasta to eat at the bottom of the testing chamber. Habituate mice for 3 sessions of 5-10 min. NOTE: Mice should not be encouraged to reach for pasta during habituation training. Mice usually begin to show interest in the pasta located outside of the reaching chamber by sniffing at it through the aperture. It is possible to habituate two cage mates in a single chamber if desired, but mice should be shaped and trained individually. All surfaces should be cleaned with 70% ethanol or a similar, safe disinfecting solution before and after each animal is placed in the behavioral apparatus.
2. Shaping the Reach
- Determine each animal’s preferred limb by shaping the reach over 3-5 once-daily shaping trials. Permit mice to reach for 10 min or a total of 10 times, which occurs first.
- Place mice individually in the testing chamber with a full pasta matrix in front of the chamber as in habituation training (Figure 1A).
- Record the number of reaches with each limb, denoting the number of reaches with the right and left limbs separately. Define a reach as extension of the limb through the reaching aperture such that the wrist breaks the plane of the chamber; it is not necessary for the reach to be directed toward or make contact with the pasta.
Hold several pieces of pasta just inside the chamber through the reaching aperture to encourage mice that are reluctant to reach. Draw the pasta pieces out when the mouse shows interest (e.g. sniffing, biting), thus encouraging them to reach after the retreating pasta pieces. If the mice do not initially show interest in the dry pasta, then wet the tips of the pasta pieces in a small cup of warm water to make them more palatable.
Determine limb preference when a mouse exhibits a minimum of 70% of their daily reaches with a single limb. Do not to over-shape animals, as this can limit the amount of learning observed during the training portion of reaching tasks 17. Begin PMRT training once mice are shaped. NOTE: Training can be started as soon as the next day, but it is also acceptable to wait several days between shaping and training procedures.
3. Reaching Training
- Train mice once daily prior to insult in order to establish proficiency on the PMRT. NOTE: Each trial consists of a maximum of 15 min or 100 reaches counted by the observer (successful and unsuccessful), whichever occurs first. Mice are trained to reach only with their preferred limb. Mice will tolerate two trials per day if a more intense training paradigm is desired 18.
- Place mice in the testing chamber with a half-full matrix placed in front (Figure 1B). Train mice to reach only with their preferred limb by filling only the side of the matrix contralateral to the preferred limb since mice reach across the midline of their body. Leave the portion of the matrix corresponding to the unpreferred limb unfilled in order to discourage reach attempts with this limb (which would not be successful because they cannot reach the pasta) and encourage reaching only with the preferred limb.
Place a small amount of pressure on the pasta while it is in the matrix to help mice successfully break pieces during early training sessions. Do not break the pasta pieces for the mouse. Achieve this by holding one or two pasta pieces lightly behind and perpendicular to the intended reaching target. This small amount of support helps stabilize the thin pasta, which is quite flexible and difficult for mice to grasp as they are initially learning the task.
Record the total number of reaches, number of successful reaches, and locations of successful reaches. Successful reaches require the mouse to reach through the aperture, grab a piece of pasta, and break the pasta piece to remove it from the matrix. NOTE: Pasta pieces should not be replaced in the matrix until after the trial has terminated. Early in training, mice will not break many pasta pieces.
Train mice on the PMRT to proficiency, which is defined as at least 3 days (and up to 5 days) of consistent performance (breaking at least 9 pieces of pasta with no more than 2 more or fewer pieces over days). It often takes between 15-20 days to train young adult mice to proficiency.
4. Less-affected Limb Training
- Train the less-affected limb (i.e., the pre-operative unpreferred limb) beginning four days after ischemic insult to the motor cortex contralateral to the preferred reaching limb (see Figure 2 for clarification on affected/preferred and less-affected/unpreferred limbs). Administer once daily 15-min or 100-reach training sessions.
- Remove the first column of pasta (i.e., the most medial vertical column extending from the reaching aperture) for the first week of less-affected limb training in order to ensure that early reaches with the affected limb are not successful.
- Place mice in the reaching chamber with the half-filled matrix placed in front. Ensure that the filled half of the matrix is opposite of the pre-operative filled half, which will force the mice to now reach with the less-affected (unpreferred) limb.
Encourage reaching with the less-affected limb by reinforcing extension of this limb through the reaching aperture. When mice extend the less-affected limb in the first few days of training place a small (1/2 piece) of pasta onto the floor of the reaching chamber regardless of whether or not the reach is successful.
Count only those reaches made with the less-affected limb toward to the 100 reach total. Do not count furtive attempts with the affected limb towards the total number of reaches.
Return the most medial vertical column of pasta to the matrix after the first 7 days of less-affected limb training.
Record the number of reaches, number of successful reaches, and locations of successful reaches. NOTE: These data can be analyzed to observe differences in reaching patterns with the less-affected limb, but have not been previously reported.
5. Reaching Analysis
Determine the impact of less-affected limb use on recovery of function of the affected limb by assessing the affected limb on the PMRT task following less-affected limb training. This assessment occurs after the desired number of less-affected limb training days are complete (typically 14 days).
Place the matrix in front of the chamber, oriented identically to training procedures (i.e., with the half of the matrix contralateral to the preferred limb filled). NOTE: This forces mice to reach with their affected (and previously preferred) limb.
Record the total number of reaches, number of successful reaches, and locations of successful reaches to compare with pre-stroke performance levels. Do not probe performance of the affected limb during less-affected limb training as these affected limb probes may function as training sessions that could impact recovery of the affected limb.
Representative Results
The results from PMRT analysis should include the number of pasta pieces broken and the pattern of successful reaches. Results from mice with sensorimotor cortical lesions indicate that ischemic insult affects both the number of successful reaches as well as physical reach patterns 3, 19, as demonstrated in Figure 3A. Representative pattern changes can be observed in Figure 3B. The ability of the PMRT to mimic learned non-use effects in mice is demonstrated by impaired recovery of the affected limb following less-affected limb training 13, 20. As a result, only successful reaches with the affected limb are analyzed. Results from mice with ischemic lesions to the sensorimotor cortex suggest that two weeks of less-affected limb training following ischemic stroke impairs affected limb use for at least seven days 13. As demonstrated in Figure 4, mice that receive less-affected limb training for 15 days after unilateral ischemic insult exhibit fewer successful reach attempts over seven days of affected limb assessment. Mice that receive no training (control) for 15 days following stroke recover the ability to successfully retrieve pasta pieces with seven days of affected limb assessment. This effect has been found to persist with up to 28 days of subsequent affected limb assessment following less-affected training 20.
The PMRT could also be used to assess qualitative changes in reaching as has been studied in rats with the single pellet reaching task 21. This assessment, which has yet to be conducted in mice, would require the systematic analysis of videotaped reaches in the PRMT.
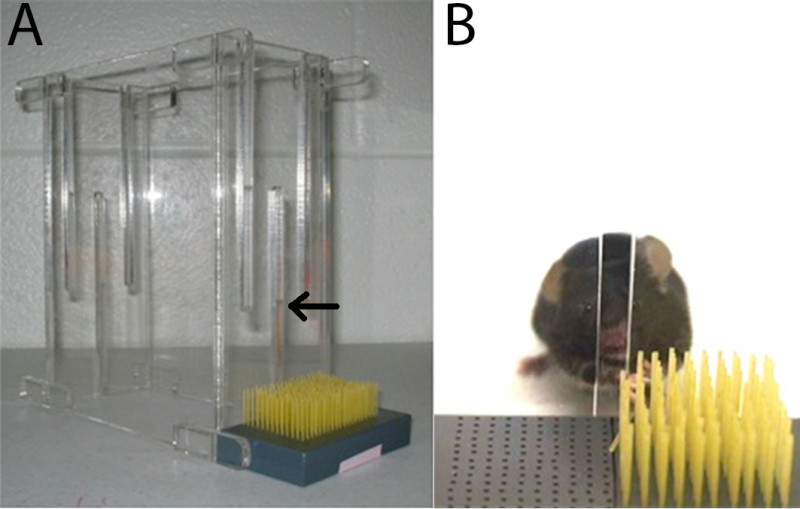 Figure 1. Pasta matrix reaching task materials. The PMRT requires a rectangular box (20 cm tall, 15 cm long, and 8.5 cm wide) with a 13 mm high, 5 mm wide reaching aperture (indicated by arrow) in the center of one wall. The matrix consists of a heavy-duty plastic block (8.5 cm long, 5 cm wide, and 1.5 cm tall) with 260 1 mm holes drilled 2 mm apart. Dry capellini pasta (3.2 cm long) is oriented vertically in the block (A). During shaping, the entire matrix is filled with pasta. However, during skilled reach training of the affected or less-affected limb only half of the matrix is filled. This requires the mouse to reach across the body with a single limb (e.g., the preferred limb during pre-operative training) (B). The present images are reprinted with permission 3.
Figure 1. Pasta matrix reaching task materials. The PMRT requires a rectangular box (20 cm tall, 15 cm long, and 8.5 cm wide) with a 13 mm high, 5 mm wide reaching aperture (indicated by arrow) in the center of one wall. The matrix consists of a heavy-duty plastic block (8.5 cm long, 5 cm wide, and 1.5 cm tall) with 260 1 mm holes drilled 2 mm apart. Dry capellini pasta (3.2 cm long) is oriented vertically in the block (A). During shaping, the entire matrix is filled with pasta. However, during skilled reach training of the affected or less-affected limb only half of the matrix is filled. This requires the mouse to reach across the body with a single limb (e.g., the preferred limb during pre-operative training) (B). The present images are reprinted with permission 3.
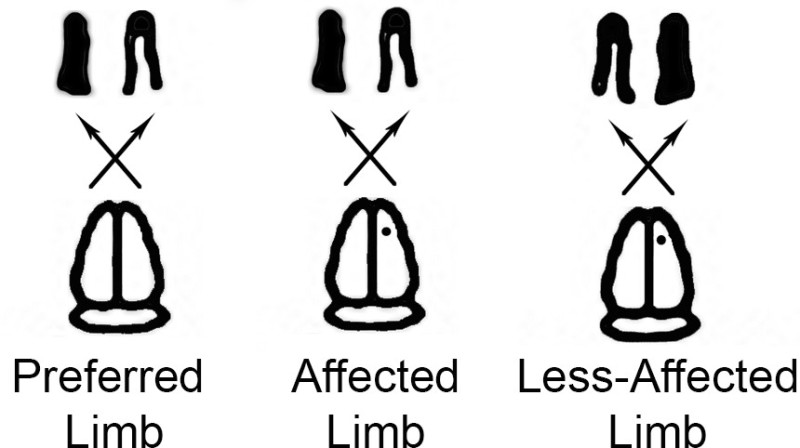 Figure 2. Affected and less-affected limbs. The PMRT can be manipulated to engage either the affected or the less-affected limb. Solid black coloring indicates Preferred/affected limbs in the first two images and the less-affected limb in the third image. This graphic helps to demonstrate the affected and less-affected limbs in reference to the preferred limb.
Figure 2. Affected and less-affected limbs. The PMRT can be manipulated to engage either the affected or the less-affected limb. Solid black coloring indicates Preferred/affected limbs in the first two images and the less-affected limb in the third image. This graphic helps to demonstrate the affected and less-affected limbs in reference to the preferred limb.
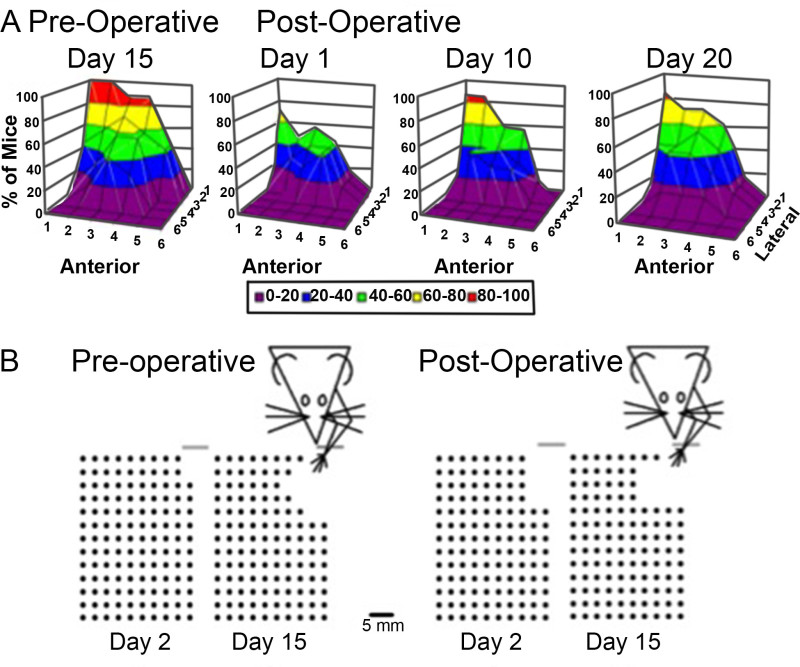 Figure 3. Representative reaching patterns with the affected limb. Reaching patterns of mice both before and after unilateral ischemic insult of the sensorimotor cortex opposite the preferred limb are presented (A). Anterior denotes pasta pieces located increasingly further in front of the mouse, while lateral denotes pasta pieces located increasingly to the side of the mouse. Representative pattern changes can be observed in B. With focused training of the affected limb, reach trajectory and success improve. This figure is reprinted with permission 3.
Figure 3. Representative reaching patterns with the affected limb. Reaching patterns of mice both before and after unilateral ischemic insult of the sensorimotor cortex opposite the preferred limb are presented (A). Anterior denotes pasta pieces located increasingly further in front of the mouse, while lateral denotes pasta pieces located increasingly to the side of the mouse. Representative pattern changes can be observed in B. With focused training of the affected limb, reach trajectory and success improve. This figure is reprinted with permission 3.
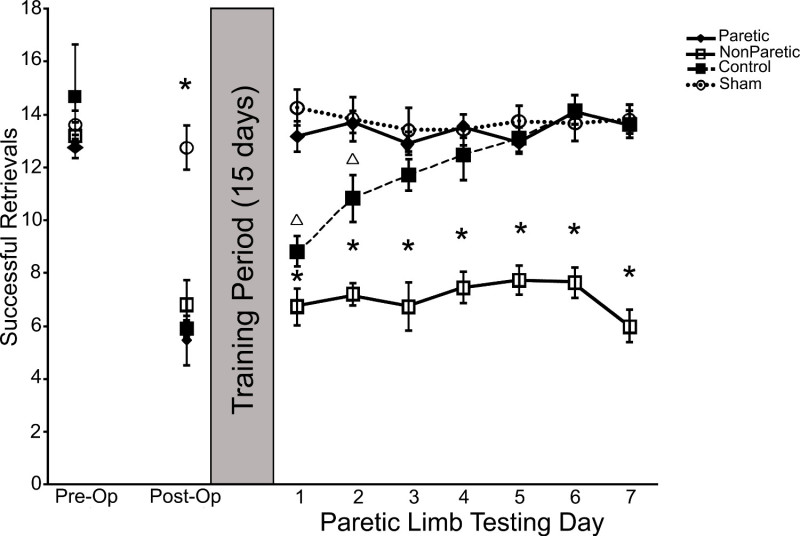 Figure 4. Representative results of less-affected limb training on affected limb outcome. Training of the less-affected limb for 15 days following unilateral ischemic insult of the sensorimotor cortex impairs functional outcome of the affected limb, preventing functional recovery. This figure is reprinted with permission 13.
Figure 4. Representative results of less-affected limb training on affected limb outcome. Training of the less-affected limb for 15 days following unilateral ischemic insult of the sensorimotor cortex impairs functional outcome of the affected limb, preventing functional recovery. This figure is reprinted with permission 13.
Discussion
The PMRT represents a simple, quantitative way to assess skilled reaching performance in mice. Though time consuming, it is possible for previously inexperienced undergraduates to be trained to collect reliable and reproducible data with only a few training sessions. The task is sensitive enough to measure even slight changes in mouse motor skill performance following ischemic insult 3, 13, 15, and a number of studies cite long-term deficits with a variety of training protocols 15, 20, 22. Because it is a unilateral task, it can also be used to dictate the limb with which mice perform the task. This feature of the PMRT allows it to be effective in studies of both adaptive behavioral and neural plasticity following insult as well as maladaptive and compensatory changes that occur with learned reliance on the less-affected limb. The task also lends itself to studies of bimanual limb use, which have been investigated in rat models but not presently in the mouse 23.
The most critical aspects of the current protocol include: accurately determining the preferred limb prior to training, training mice to proficiency on the skilled motor task prior to injury, and switching the mice from preferred/affected limb reaching to unpreferred/less-affected limb reaching in the first week of post-operative training. To accurately determine the preferred limb prior to training it is important to allow mice to reach for pasta in the full matrix during shaping. Mice should be encouraged to reach, but they should not be rewarded for reaches, which may unintentionally bias the limb with which they reach. Shaping sessions are not training sessions, and therefore mice should not be specifically encouraged to break pasta pieces, be rewarded for reaching behavior, or reach an excessive number of times per session 3. It is also important that mice acquire the motor skill prior to ischemic insult so that accurate data can be collected regarding the effects of both affected and less-affected limb training after injury, as well as to permit appropriate quantification of initial deficit following injury. A minimum of 15 days of pre-operative training (excluding shaping) is suggested to ensure acquisition on the task. A good rule of thumb is to make sure that animals are consistent in their performance, thus exhibiting a stable performance across at least 3-5 training of days. This can best be accomplished by graphing the pre-operative reaching data for each animal to visually observe success patterns. If performance appears asymptotic over 3-5 days (i.e., varies by less than 2 successful retrievals across days), it is safe to assume that the animal has sufficiently acquired the task. Mice typically reach an asymptotic success level of ~11 pieces, but with extended (months) training can gradually reach success levels of ~14 pieces 24. Finally, especially with relatively small lesions 13, 20, mice may continue to attempt reaching with the affected limb early in less-affected limb training. It is helpful to remove the first column of pasta from the matrix during the first 7 days of less-affected limb training to discourage this behavior. The mice may be able to break the first several pieces of pasta with their affected limb if the full half matrix used, and this success will further encourage affected limb reaching despite an inability to successfully retrieve pieces beyond these first few. During the first week of less-affected limb training, the total number of affected reaches can be quantified, but the number of successful breaks will not be informative. As mentioned above, only successful affected limb reaches are analyzed for data interpretation. To further encourage less-affected limb reaching, it is helpful to provide small (½ sized) pasta pieces as rewards for attempted reaches with the less-affected limb. This practice is usually necessary for the first 2-3 days of less-affected limb training, at which point mice become proficient at reaching with the less-affected limb and will begin successfully breaking and retrieving pasta pieces.
Collecting behavioral data with mice can be effective and rewarding, but it is important to remember that mice are not small rats. They have a unique temperament and behavioral repertoire that should be considered for behavioral tasks. Using well-handled, tamed mice increases successful behavioral data collection. Several weeks of regular handling are recommended. In addition, mice sometimes require “focusing” during behavioral training. It is helpful to gently brush pasta pieces in the matrix to make a distinct noise that the mice respond to. It may also be helpful to gently brush the inside of the reaching aperture.
The current protocol describes a sensitive behavioral task for the collection of skilled reaching data in mice following small ischemic strokes. Motor impairments are among the most common and chronic consequences of stroke 25, 26, and are therefore the focus of a number of basic science research laboratories (e.g., 27-32). Skilled reaching tasks are the most commonly used tests of dexterous forelimb use in rodents, and have historically been most often used in rat models of injury (e.g., 11, 19, 28, 33), although their use in mouse models is becoming quite frequent 34, 35. The application of skilled reaching tasks in mice, such as the PMRT, allows for improved mouse models of stroke 3 and thus better translational potential of these basic science experiments. Because of their affordability, the available genetic tools, and their prevalence in neurobiological laboratories, mice are important tools in the study of stroke mechanisms and recovery. The availability of behavioral manipulations that sensitively assess motor performance, can be used for rehabilitative strategies, and can mimic human compensatory responses extends the application of the mouse model. The methods described in this protocol can certainly be further refined and extended to other mouse injury models, including traumatic brain injury, studies of bimanual limb use following insult and studies of neural mechanisms that sub serve and support functional recovery.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors would like to thank Theresa Jones, Ph.D. for her guidance and assistance in adapting the reaching task to mice. Data presented in this manuscript were supported by grants from NIH-NINDS (NS64586 to TAJ and NS076275 to ALK) and a predoctoral NRSA to KAT (F31AG034032). The NIH was not involved in any aspect of study designs or analyses presented in this manuscript nor in the composition of this manuscript.
References
- Branchi I, Ricceri L. Transgenic and knock-out mouse pups: the growing need for behavioral analysis. Genes Brain and Behavior. 2002;1(3):135–141. doi: 10.1034/j.1601-183x.2002.10301.x. [DOI] [PubMed] [Google Scholar]
- Bucan M, Abel T. The mouse: Genetics meets behaviour. Nature Reviews Genetics. 2002;3(2):114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J. Neurosci. Methods. 2009;181(1):18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cerebral Cortex. 2011;21(4):865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J. Neurosci. Methods. 2002;117(2):207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- Li XL, Blizzard KK, Zeng ZY, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp. Neurol. 2004;187(1):94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp. Neurol. 2007;203(2):555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting Neurorehabilitation Right: What Can Be Learned From Animal Models. Neurorehabil. Neural Repair. 2012;26(8):923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DMM. The learned nonuse phenomenon: implications for rehabilitation. Europa Medicophysica. 2006;42(3):241–256. [PubMed] [Google Scholar]
- Taub E. Harnessing brain plasticity through behavioral techniques to produce new treatments in neurorehabilitation. Am. Psychol. 2004;59(8):692–704. doi: 10.1037/0003-066X.59.8.692. [DOI] [PubMed] [Google Scholar]
- Allred RP, Maldonado MA, Hsu JE, Jones TA. Training the "less-affected" forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restorative Neurol. Neurosci. 2005;23(5-6):297–302. [PubMed] [Google Scholar]
- Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp. Neurol. 2008;210(1):172–181. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AL, Wolke ML, Bell JA, Jones TA. Post-stroke protection from maladaptive effects of learning with the non-paretic forelimb by bimanual home cage experience in C57BL/6 mice. Behav. Brain Res. 2013;252:180–187. doi: 10.1016/j.bbr.2013.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, et al. Method for enhancing real-world use of a more affected arm in chronic stroke: transfer package of constraint-induced movement therapy. Stroke. 2013;44(5):1383–1388. doi: 10.1161/STROKEAHA.111.000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, et al. Age-dependent reorganization of peri-infarct "premotor" cortex with task-specific rehabilitative training in mice. Neurorehabilitation and Neural Repair. 2014. [DOI] [PMC free article] [PubMed]
- Binkofski F, Seitz RJ, Hacklander T, Pawelec D, Mau J, Freund HJ. Recovery of motor functions following hemiparetic stroke: A clinical and magnetic resonance-morphometric study. Cerebrovascular Diseases. 2001;11(3):273–281. doi: 10.1159/000047650. [DOI] [PubMed] [Google Scholar]
- Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462(7275):915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA, Wolke ML, Ortez RC, Jones TA, Kerr AL. The effects of training intensity on functinal outcome following unilateral ischemic insult of sensorimotor cortex in C57BL/6 mice. Society for Neuroscience Annual Convention. 2012.
- Ballermann M, Metz GAS, McKenna JE, Klassen F, Whishaw IQ. The pasta matrix reaching task: a simple test for measuring skilled reaching distance, direction, and dexterity in rats. J. Neurosci. Methods. 2001;106(1):39–45. doi: 10.1016/s0165-0270(01)00326-0. [DOI] [PubMed] [Google Scholar]
- Cheffer KA, Kerr AL. Effects of "good" limb training on long-term rehabilitation of motor function following ischemic stroke in C57BL/6 mice. Society for Neuroscience Annual Convention. 2013.
- Alaverdashvili M, Whishaw IQ. A behavioral method for identifying recovery and compensation: hand use in a preclinical stroke model using the single pellet reaching task. Neurosci. Biobehav. Rev. 2013;37(5):950–967. doi: 10.1016/j.neubiorev.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke. 2013;44(9):2579–2586. doi: 10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RP, Cappellini CH, Jones TA. The "good" limb makes the "bad" limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav. Neurosci. 2010;124(1):124–132. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, et al. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol. Learn. Mem. 2012;98(3):291–302. doi: 10.1016/j.nlm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Boerma T, Fat DM. Global and regional causes of death. Br. Med. Bull. 2009;92(1):7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- Go AS, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1) doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J, Mala H, Windle V, Chernenko G, Corbett D. The Effects of Repeated Rehabilitation "Tune-Ups" on Functional Recovery After Focal Ischemia in Rats. Neurorehabil. Neural Repair. 2009;23(9):886–894. doi: 10.1177/1545968309341067. [DOI] [PubMed] [Google Scholar]
- Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128(3):473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Bryant A, Bernier B, Jones TA. Abnormalities in skilled reaching movements are improved by peripheral anesthetization of the less-affected forelimb after sensorimotor cortical infarcts in rats. Behav. Brain Res. 2007;177(2):298–307. doi: 10.1016/j.bbr.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Coles B. Varieties of paw and digit movement during spontaneous food handling in rats: Postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav. Brain Res. 1996;77(1-2):135–148. doi: 10.1016/0166-4328(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Dringenberg HC, Pellis SM. Spontaneous Forelimb Grasping in Free Feeding by Rats - Motor Cortex Aids Limb and Digit Positioning. Behav. Brain Res. 1992;48(2):113–125. doi: 10.1016/s0166-4328(05)80147-0. [DOI] [PubMed] [Google Scholar]
- Horie N, Maag A, Hamilton SA, Shichinohe H, Bliss TM, Steinberg GK. Mouse model of focal cerebral ischemia using endothelin-1. J. Neurosci. Methods. 2008;173(2):286–290. doi: 10.1016/j.jneumeth.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil. Neural Repair. 2008;22(3):250–261. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Carmichael ST. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. Journal of Cerebral Blood Flow and Metabolism. 2013;33(5):716–723. doi: 10.1038/jcbfm.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chopp M, Ding X, Cui Y, Li Y. Axonal remodeling of the corticospinal tract in the spinal cord contributes to voluntary motor recovery after stroke in adult mice. Stroke. 2013;44(7):1951–1956. doi: 10.1161/STROKEAHA.113.001162. [DOI] [PMC free article] [PubMed] [Google Scholar]


