Abstract
Purpose.
Mutations in the RGS9 gene cause the visual disorder bradyopsia, which includes difficulty adapting to changes in light and photophobia. The purpose of this study was to determine whether lack of Rgs9 also caused photophobia-like behavior in Rgs9 knockout (Rgs9−/−) mice and to identify useful diagnostic measures of Rgs9 dysfunction.
Methods.
We measured two behavioral responses to light and the pupillary light reflex to determine the form and basis of photophobia in Rgs9−/− mice.
Results.
Rgs9−/− mice spent less time than wild-type mice in both dim and bright light. The mice also showed increased sensitivity to light in negative masking behavior, with a half maximal response at 0.08 lux (0.01 μW · cm−2) in Rgs9−/− mice compared to 5.0 lux (0.85 μW · cm−2) in wild-type mice. These behaviors were not due to increased anxiety or increased pupil size causing more light to enter the eye. Rather, constriction of the pupil showed that Rgs9−/− mice had an abnormally sustained response to light across multiple irradiance measurement pathways.
Conclusions.
Rgs9−/− mice recapitulate a photophobia phenotype of bradyopsia, and the pupil light reflex identifies a simple means to screen for irradiance measurement abnormalities in bradyopsia and potentially other genetic disorders involving photophobia.
Keywords: bradyopsia, ipRGC, irradiance measurement, light aversion, pupil light reflex, RGS9
Rgs9 knockout (Rgs9−/−) mouse is an animal model of the visual disorder bradyopsia. The Rgs9−/− mice are more sensitive to light, similar to photophobia reported by patients. A prolonged pupillary response implicates the irradiance circuitry in ocular photophobia.
Introduction
Photophobia is an abnormal sensitivity to light that can be caused by multiple pathologies, including eye disease and migraine.1–3 Ocular photophobia is typical in patients with loss of cone photoreceptor function (achromatopsia) or prolonged rod/cone photoreceptor function (bradyopsia). In both ocular and migraine-associated photophobia, increased sensitivity to light could presumably arise from abnormal responses at the level of the retina or downstream pathways in the brain.3 Presently, the mechanisms of photophobia are still largely unexplored.
Recognizing that there are many types of photophobia, we focused on a single gene model of ocular photophobia. Patients with the inherited retinal disease bradyopsia present with mild ocular photophobia, delayed adaptation to changes in light, and reduced ability to detect moving objects.4–6 Bradyopsia is caused by loss of function mutations in the regulator of G protein signaling 9 (RGS9) or the RGS9 anchor protein (R9AP) genes.4,7 RGS proteins normally facilitate inactivation of G proteins to quickly terminate signal transduction.8–11 This means that the rod/cone response to light is prolonged when RGS9 function is lost.12 It seems likely that photophobia in bradyopsia is caused by this prolonged activation of rods and cones, but an effect of prolonged rod/cone activation on irradiance measurement circuits underlying photophobia has not been demonstrated.
The primary objective of our study was to determine whether Rgs9−/− mice exhibit light-sensitive behavior. The first test was a light aversion assay in which mice seek to escape the illuminated area of a light-dark box.13–15 The second test was a negative masking assay in which voluntary nocturnal wheel running is suppressed by light if escape from light is not possible.16 Negative masking is known to be dependent on a combined input from rod/cone photoreceptors transmitted via intrinsically photosensitive retinal ganglion cells (ipRGC).17,18 To date, neither light aversion nor negative masking has been assessed in a retinal disease model with a delayed inactivation of rod/cone responses to light. Because a caveat of the Rgs9−/− mouse model is that the Rgs9 gene is also expressed in the brain,19 we included control tests for anxiety. Finally, we hypothesized that the abnormally prolonged rod and cone activation in bradyopsia would increase irradiance measurement responses to light. To test this hypothesis, we compared pupillary light reflex and behavioral responses to light in Rgs9−/− mice to those of wild-type mice.
Materials and Methods
Animals
Animal care and procedures were approved by the University of Iowa Animal Care and Use Committee and performed in accordance with National Institutes of Health standards and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J;Rgs9tm1Citb (Rgs9−/−) mice were provided by Ching-Kang Chen, PhD (Virginia Commonwealth University),12 and were further back-crossed (3–5 generations) to a C57BL/6J background at the University of Iowa. C57BL/6J wild-type mice (Jackson Laboratory, Bar Harbor, ME, USA) were used for breeding and as controls. As an additional control, heterozygous Rgs9+/− mice were also tested. Because there were no behavioral differences between wild-type and Rgs9+/− mice, the Rgs9−/− mice were carried as a homozygous line, and age- and sex-matched C57BL/6J wild-type mice were used to minimize mouse breeding and genotyping. Genotyping was carried out by PCR; wild-type allele primers spanned part of the deletion in Rgs9−/− (5′-TTGTGCATAAGGCTGCACAGGTCT-′3 and 5′-AAGCAAGGATTTGTGGGTTCAGCC-′3); primers to the Rgs9 knockout were designed to confirm the presence of the Neo cassette used in generating the knockout (5′-TTGCTCCTGCCGAGAAAGTATCCA-′3 and 5′-CCATGATATTCGGCAAGCAGGCAT-′3).
Light Aversion Assay
C57BL/6J wild-type, Rgs9+/−, and Rgs9−/− mice were tested with a light aversion assay. Male and female mice were tested at between 10 and 36 weeks of age. Numbers of animals in each experiment ranged between 11 and 12 per genotype (n is indicated in figure legends).
The light aversion assay was performed using light-dark boxes, as previously described,20 except without drug treatments. Briefly, mice were tested in poly(methyl methacrylate) (Plexiglas) chambers equipped with infrared rays. Chambers were divided into equally sized zones by a dark Plexiglas insert (Med Associates, St. Albans, VT, USA), creating a light and a dark zone. Light was provided by light-emitting diode (LED) panels located above each chamber, containing 36 collimated 5500 K daylight white 1-W LEDs (LED Wholesalers, Hayward, CA, USA), with all intensities reported as photopic lux. These LEDs have peak outputs at 450, 525, and 570 nm, which would effectively activate rod and medium wavelength cone photoreceptors, as well as melanopsin in the intrinsically photosensitive retinal ganglion cells. Light intensity was controlled by a dimmable LED driver (LINEARdrive; eldoLED America, Conyers, GA, USA).
Light intensity was measured with a traceable dual-display light meter (Control Company, Friendswood, TX, USA) placed on the floor of the testing chamber. Four different intensities were used for testing the animals, 27,000, 1000, 55, and 0.05 lux. It should be noted that the 0.05-lux condition was originally designed as a dark control because the light meter indicated 0 lux. However, using a more sensitive instrument (Spectra Pritchard model 1980APL photometer; Photo Research, Inc., Chatsworth, CA, USA), we measured illumination in the middle of each of the 6 testing chambers that ranged between 0.01 and 0.05 lux (0.001–0.007 μW · cm−2). Thus, this condition was subsequently referred to simply as <0.05 lux. Due to the size of the equipment, the amount of light in the dark chamber could not be measured.
Prior to the experiment, mice were acclimated to the testing room for at least one hour with room light on. Mice tested in dark (0.05 lux) were acclimated to the testing room with all lights off. Mice were then placed in the light zone of the light-dark box and tested for 20 minutes. Two different testing protocols were used. In the first protocol, naïve mice with no previous exposure to the light-dark box were tested under highest light condition (27,000 lux, 4000 μW · cm−2). The second protocol was used to allow testing mice with previous exposure to the testing chamber. In that protocol, a new group of naïve mice with no previous exposure to the light-dark box was tested with lights off (0.05 lux, 0.007 μW · cm−2), then tested again up to 3 additional times under different light conditions (55, 1000, 27,000 lux, corresponding to 8.1, 148, and 4000 μW · cm−2, respectively), with at least 3 days recovery in their home cages.
Time spent in each chamber was analyzed using Prism software (GraphPad Software, San Diego, CA, USA) and reported as mean ± standard error of the mean (SEM). Comparison was by two-way repeated measures ANOVA, with Bonferroni multiple comparison test for post hoc analysis.
Motility Measurements
Motility in the light-dark box was measured at the same time as light aversion. Transitions are presented as the total number of times the animal transitioned between the two zones. Resting time was calculated as the percentage of time spent not breaking any new beams. Ambulatory distance (centimeters) is presented as total distance traveled during ambulatory movement. Both parameters were normalized to the time spent in each zone. Data were analyzed using Prism software (GraphPad Software) and reported as mean ± standard error of the mean (SEM). Two-way repeated measures ANOVA was used for comparisons, with Bonferroni multiple comparison test for post hoc analysis.
Negative Masking
Mice are nocturnal animals that avoid bright light. When they cannot escape a brightly lit area, activity is suppressed in a response termed negative masking. In the laboratory, negative masking can be measured as reduction in voluntary wheel running activity in response to light applied during the active phase of the day. The response amplitude is irradiance-dependent. To determine the photosensitivity of negative masking, a dose-response function for the effect of light on wheel running was completed as previously described.21
Male Rgs9−/− and wild-type mice between 16 and 30 weeks of age were tested. Number of animals per genotype ranged between 12 and 15 (indicated in figure legends). Mice were maintained in a 12-hour light, 12-hour dark cycle with fluorescent white light (4100 K, at ~19 μW cm−2 s−1). Eight light levels between 0.00002 and 105 lux (3.0 × 10−6 and 15.4 μW · cm−2 fluorescent white light, 4100 K) were applied in a sequence that distributed bright and dim pulses over the course of testing. Light timing was controlled using a programmable digital timer (Westek, Watsonville, CA, USA), and irradiance was controlled using neutral density gel filters (Rosco, Stamford, CT, USA). Animals were singly housed in customized wheel cages (Thoren Caging Inc., Hazelton, PA, USA). Cages were mounted in customized environment control cabinets (University of Iowa Medical Instruments, Iowa City, IA, USA), and wheel running was continuously recorded using ClockLab (Actimetrics, Inc., Evanston, IL, USA).
Changes in activity over the 1-hour light treatment were calculated as percentage of baseline activity at the corresponding time on the preceding day for each animal. Variable slope sigmoidal dose-response curves were fitted to data in Prism with a fixed constant for the minimum set at 0% and no weighting. The 4-parameter logistic equation was Y = Bottom + [(Top − Bottom)/1 + 10(logEC50 − x) − hill-slope]. The irradiance producing a half maximal response (half maximal effective concentration [EC50]) and hill-slope were calculated in Prism from fitted curves. Features of fitted curves were then compared by using an F-test of a two-fit comparison in Prism. Curves were fitted for two genotypes independently and then to the combined data set for both genotypes; the effect of combining data sets on the quality of fit was then used to calculate whether there was a difference between genotypes for the EC50 response.
Open Field Test
Open field assay was used to measure anxiety and to determine its contribution to the light aversion assay. The assay was conducted as previously described,20 using both naïve mice and mice previously tested in the light-dark assay, as indicated. Testing was performed in the same chambers as light aversion assay but without the dark insert. Mice were acclimated to the testing room for at least 1 hour with room light on. Mice were placed in the center of the open field and tested for 20 minutes at 55 and 27,000 lux (8.1 and 4000 μW · cm−2). The periphery was defined as a 4.22-cm border along the inside wall with a center area of 18.56 cm × 18.56 cm. Number of animals per group ranged between 11 and 12 (indicated in figure legends). Time spent in the center area was analyzed using Kruskal-Wallis test with Dunn's multiple-comparison test.
Predator Odor-Evoked Fear Test
Predator odor fear test was conducted as previously described.22 Mice that were previously tested with the light aversion assay were used after a 1-week recovery. Each mouse was placed in an acrylic glass chamber (18 cm wide × 18 cm long × 18 cm high) with a beaker containing tissue wipes with or without 30 μL of trimethylthiazoline (TMT; ConTech, Delta, British Columbia, Canada) and videotaped for 10 minutes. Freezing behavior, defined as the absence of movement except for respiration, was recorded for each minute by a blinded experienced observer. Number of animals per group ranged between 11 and 12 (indicated in figure legends). Data were analyzed using a two-way repeated measures ANOVA, with Bonferroni multiple comparison test for post hoc analysis.
Pupillary Light Reflex
Pupillary responses were recorded from both eyes using an A2000 pupillometer (Neuroptics, Irvine, CA, USA). C57BL/6J, Rgs9+/−, and Rgs9−/− mice were dark-adapted for 2 hours and then lightly sedated with a ketamine-xylazine combination (at a ratio of 50:10 mg/kg). Animals were positioned on a platform, and responses to stimuli were recorded. To determine whether Rgs9−/− mice sustained pupil constriction under prolonged exposure to light that mice experienced in behavioral tests, a 20-minute bright blue stimulus protocol was used (1000 μW · cm−2 at 480 ± 3 nm, ~615 lux; n = 3). The light source of the pupillometer is dispersed, with a relatively large aperture (2 cm) placed a short distance from the cornea (1.2 cm) and was deliberately oriented on the axis of pupil center to optic nerve. Therefore, the angle of illumination should have exceeded 100° and be similarly centered in animals. When real-time, ellipse fitting of the pupil in video frames was unreliable, the recording was discarded. When both eyes were successfully recorded, the mean of the two eyes was used. For comparison of redilation kinetics, data were inverted, with dark-adapted baseline at 0 for each animal. A one-phase exponential decay curve was then fitted in Prism by using least squares to the pupil response from 30 seconds post-stimulus to test end at 20 minutes. The equation for the regression was: Y = (Y0 − Plateau) × exp (−k × X) + Plateau. Curves were compared for differences in the kinetics of redilation (K) by exact sum of squares F-test. To investigate how Rgs9−/− was affecting rod, cone, and melanopsin contributions to the pupil response, single 1-second flashes of light were applied to dark-adapted mice. A dim blue stimulus (1.0 μW · cm−2 at 480 ± 3 nm, ~0.6 lux) exploited the low photon threshold for rod photoreceptor activation to selectively measure rod-driven responses. A bright red stimulus (10,000 μW · cm−2 at 622 ± 3 nm, ~25,610 lux) exploited differences in spectral sensitivity of rod, cone, and melanopsin to activate rod and cone photoreceptors while minimizing activation of melanopsin (melanopsin-relative sensitivity is <0.001% at 622 nm). The maximal constriction within 2 seconds of stimulus onset was designated as the initial response. The mean constriction at 30 seconds post-stimulus (average of 1 second) was designated as the post-stimulus residual constriction. Number of animals per group ranged between 7 and 10 (indicated in figure legend). Statistical analysis between genotypes was by two-tailed t-test.
Results
Rgs9−/− Mice Spend Less Time in Light Than Wild-Type Mice
To test whether Rgs9−/− mice have an aversion to light, we compared their behavior with that of mice heterozygous for the Rgs9 knockout (Rgs9+/−) and wild-type C57BL/6J mice in a light-dark exploration assay.
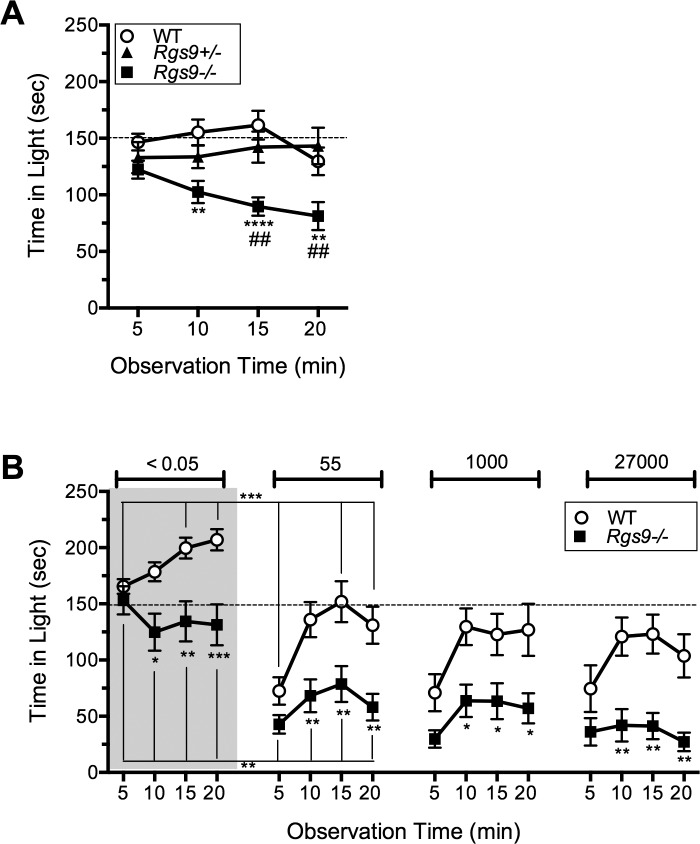
In the first protocol, naïve Rgs9−/−, Rgs9+/−, and wild-type mice were exposed to a very bright light of 27,000 lux, equivalent to bright daylight.23 Following the first interval, Rgs9−/− mice spent significantly less time in the light than either Rgs9+/− or wild-type mice (Fig. 1A). Because Rgs9+/− mice behaved the same as wild-type mice, we used wild-type mice as controls in subsequent experiments.
Figure 1.
Light aversion behavior in Rgs9−/− mice. (A) Time spent in the light by naïve mice upon exposure to 27,000 lux. The time was calculated over 5-minute intervals following placement of the mouse in the light-dark box. The mean ± SEM of each 5-minute interval is shown for wild-type (C57BL/6J; n = 12; circles), Rgs9+/− (n = 11; triangles), and Rgs9−/− (n = 11; squares). Overall effect of genotype (F(2,124) = 20.27; P < 0.0001); Rgs9−/− versus wild-type, **P < 0.01, ****P < 0.0001; Rgs9−/− versus Rgs9+/−, ##P < 0.01. (B) Effect of previous exposure on time spent in the light by mice sequentially exposed to the indicated light levels of <0.05, 55, 1000, and 27,000 lux. Each exposure was separated by at least 3 days. Mean ± SEM of each 5-minute interval is shown for wild-type (n = 11; circles) and Rgs9−/− (n = 12; squares). Overall effects of genotype F(1,21) = 9.21; P = 0.0063 at <0.05 lux; F(1,21) = 11.04; P = 0.0032 at 55 lux; F(1,21) = 8.84; P = 0.0073 at 1000 lux; and F(1,21) = 13.38; P = 0.0015 at 27,000 lux. Rgs9−/− versus wild-type: *P < 0.05; **P < 0.01; ***P < 0.001. Brackets marked with symbols indicate significant differences between light intensities. For both panels, a dashed line indicates 50% time in the light.
The second protocol was designed to test light aversive behavior at lower light levels, as commonly reported by photophobic patients. The strategy was to use sequential testing that involved previous exposures to the chamber. This approach would potentially reduce anxiety, and we previously found it reduces exploratory drive, which helped unmask calcitonin gene related peptide (CGRP)-induced light aversive behavior in wild-type mice.20 The rationale for this strategy was further supported by the progressively greater light aversion seen with naïve mice over time in the chamber (Fig. 1A).
To avoid the possibility of a learned behavior due to exposure to bright light, the mice were first exposed to the chamber with lights off (<0.05 lux), followed by sequential exposure to brighter lights (55, 1000 and 27,000 lux) (Fig. 1B). As predicted, the time in light for both genotypes was significantly lower following previous experience in the chamber (Fig. 1B, 27,000 lux), compared to naïve mice at 27,000 lux (Fig. 1A) (P < 0.001 for Rgs9−/− mice, P < 0.05 for wild-type). In the first exposure, Rgs9−/− mice still had a preference for the dark zone even though the light was very dim (<0.05 lux) (Fig. 1B). Note that under these conditions, wild-type mice actually preferred the lit zone, which is consistent with our previous studies,13,20 and with the point of entry as a reference for exploration.24 The preference of Rgs9−/− mice for the dark zone even under very low light conditions suggests that the mice are remarkably sensitive to light. Furthermore, Rgs9−/− mice spent more time in the dark zone compared to wild-type at all light intensities, with equal light aversion observed at 55, 1000, and 27,000 lux (Fig. 1B).
In addition to time spent in the light, the number of transitions between the light and dark zones was a secondary measure of light aversion,25 as observed in our previous studies with CGRP-induced light aversion.13,20 In this study, Rgs9−/− mice showed fewer transitions than wild-type mice, but this difference was only significant at the brighter light levels of 1000 and 27,000 lux (Table).
Table.
Motility Measurements During the Light Aversion Assay
| Light, lux |
Genotype |
n |
Mean ± SEM |
P |
| Total no. of transitions | ||||
| 1000 | WT | 11 | 78.7 ± 6.7 | 0.0149 |
| RGS9−/− | 12 | 56.8 ± 8.1 | ||
| 27,000 | WT | 11 | 77.5 ± 6.1 | 0.0015 |
| RGS9−/− | 12 | 38.2 ± 8.0 | ||
| % of resting time in light | ||||
| 0.05 | WT | 11 | 55.7 ± 0.9 | 0.0002 |
| RGS9−/− | 12 | 48.6 ± 1.2 | ||
| 55 | WT | 11 | 55.9 ± 1.1 | 0.0171 |
| RGS9−/− | 12 | 52.1 ± 1.3 | ||
| % of resting time in dark | ||||
| 1000 | WT | 11 | 64.5 ± 2.6 | 0.0144 |
| RGS9−/− | 12 | 73.4 ± 2.4 | ||
| 27,000 | WT | 11 | 65.9 ± 2.6 | 0.0044 |
| RGS9−/− | 12 | 77.1 ± 2.5 | ||
Differences in motility between the light and dark zones were also consistent with aversion to light. Resting time of Rgs9−/− mice was significantly reduced at lower light levels and greater in the dark at higher light levels compared to that of wild-type mice (Table). Likewise, the ambulatory distance of Rgs9−/− mice was significantly greater in light and reduced in dark compared to that of wild-type at all light intensities. Both of these measures demonstrated that Rgs9−/− mice preferred to keep moving in the brighter light and rest once they were in the dark.
Rgs9−/− Mice Showed Suppression of Wheel Running at Dimmer Light Levels Than Wild-Type Mice
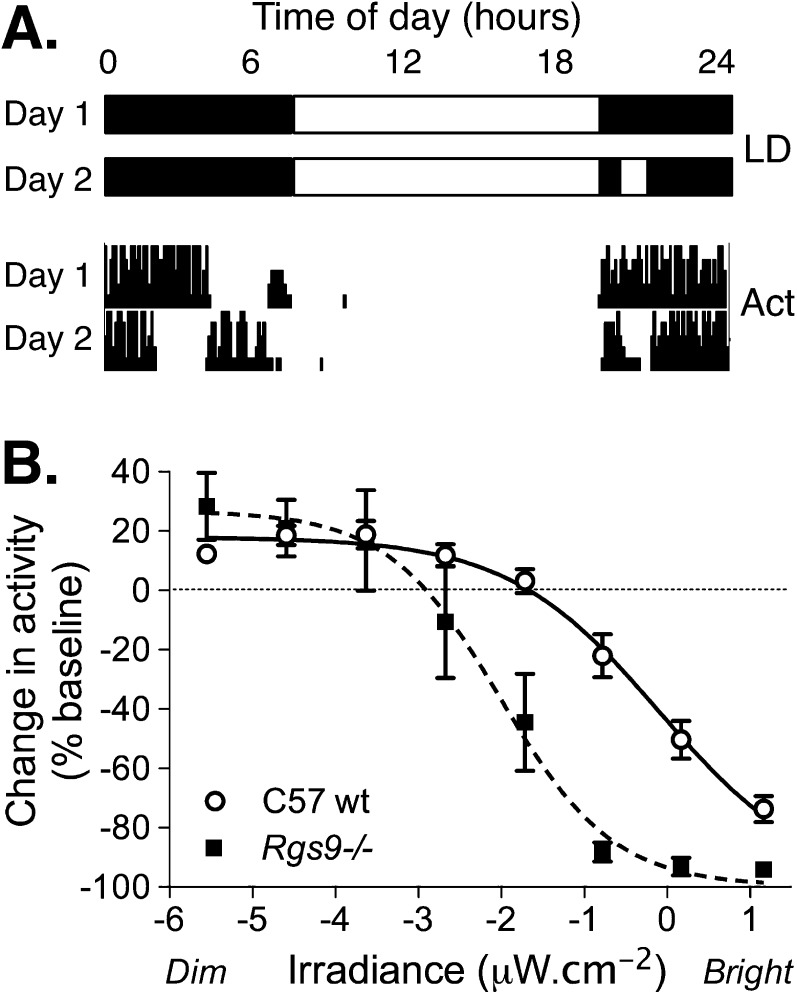
Mice also showed sensitivity to light that could be measured by suppression of wheel-running activity.18 Both wild-type and Rgs9−/− mice showed suppression of wheel running by bright light (negative masking) in a dose dependent manner (Fig. 2). However, Rgs9−/− mice showed negative masking responses at much lower irradiance levels than wild-type mice (EC50 for Rgs9−/− = 0.071 lux or 0.01 μW · cm−2; and 5.8 lux or 0.85 μW · cm−2 for wild-type mice; P < 0.0001). This shows that the threshold for suppression of activity (negative masking of activity and increased sleep propensity) in Rgs9−/− mice occurs at a very low light level, similar to the very dim light conditions that induced light aversion in the light-dark exploration assay.
Figure 2.
Wheel-running activity in Rgs9−/− mice. Mice will voluntarily use a running wheel in their home cage during the night when they are active (dark phase of the day). Bright light acutely suppresses wheel running in a dose-dependent manner. Wheel running activity was quantified against time for singly housed mice maintained under a 12-hour light:12-hour dark cycle. On test days, lights were turned on during the active or dark phase of the day, and change in wheel running activity was calculated against a preceding baseline day for individual animals for a given treatment. (A) Illustration of the light cycle protocol and corresponding recorded activity are shown for a baseline (Day 1) and test day (Day 2). (B) The effect of light on activity is calculated as a percentage of baseline and plotted against irradiance. The mean ± SEM change in activity is shown for wild-type (n = 15; circles) and Rgs9−/− (n = 12; squares) mice at 8 light levels.
Differences in Light Aversion and Negative Masking Are Not Caused by Underlying Changes in Anxiety
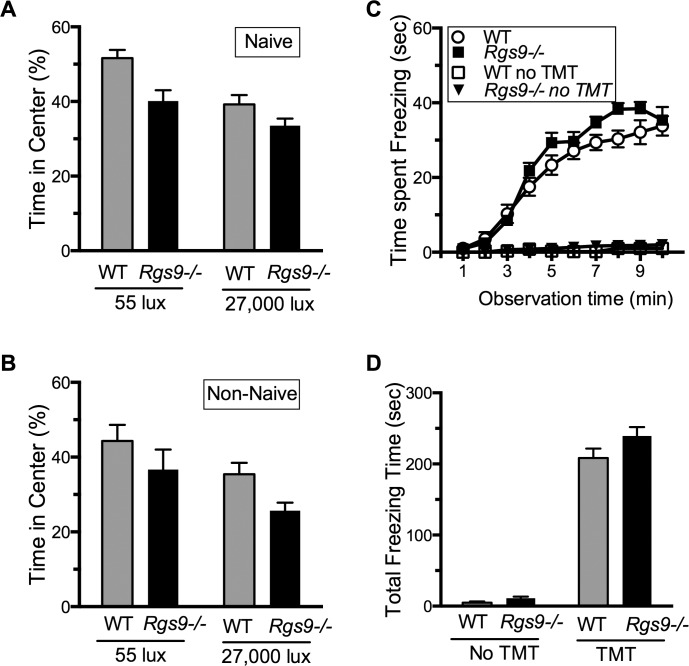
A potential complication of the light aversion and negative masking assays is that an alternatively spliced form of Rgs9 in the striatum has been implicated in reward behavior.26–28 Although Rgs9−/− mice were previously reported to have normal open field behavior,29 it was important to rule out an anxiogenic phenotype in our colony of Rgs9−/− mice, under both low and bright light intensities. To do this, we measured open field center avoidance and predator odor-evoked freezing behavior. Two naïve groups of mice were tested in the open field at 55 and 27,000 lux (Fig. 3A). For both groups, Rgs9−/− mice spent approximately the same time in the center as the wild-type mice did. To test whether repeated exposure to the light-dark box had an effect on the time spent in center, we tested two groups of Rgs9−/− and wild-type mice that had previously been tested in the light-dark assay, one at 55 lux and one at 27,000 lux. There were no differences in time spent in center at either light level (Fig. 3B). Likewise, there were no significant difference in predator odor-evoked freezing behavior between Rgs9−/− and wild-type mice (Figs. 3C, 3D). As a control, neither genotype showed any freezing behavior in the absence of odorant. Taken together, these results indicate that a general increase in anxiety is not likely to account for the increased sensitivity to light by Rgs9−/− mice.
Figure 3.
Assessment of anxiety in Rgs9−/− mice. (A) Naïve mice, not previously used for any other assay, were tested in an open field assay at either 55 lux or 27,000 lux. The mean time in the center (±SEM) is shown for wild-type (n = 12; gray bars) and Rgs9−/− (n = 12; black bars). (A, B) P > 0.05 between genotypes. (B) Mice previously tested in the light aversion assay were tested in an open field assay at 55 lux and 27,000 lux. The mean time (±SEM) in the center is shown for wild-type (n = 11; gray bars) and Rgs9−/− (n = 12; black bars). (C) Time spent freezing (seconds) during each minute following exposure of the mice to TMT is shown. The mice had previously been tested with the light aversion and open field assays. The mean (±SEM) is shown for wild-type (n = 11 with TMT; open circles), (n = 6 without TMT; open squares) and Rgs9−/− (n = 12 with TMT; closed squares; and n = 6 without TMT; closed triangles). Overall effect of genotype with TMT F(1,21) = 2.89, P = 0.1041; without TMT F(1,10) = 4.33, P = 0.06. (D) Total freezing behavior in 10 minutes. The mean (±SEM) is shown for wild-type (n = 11 with TMT, n = 6 without TMT; gray bars) and Rgs9−/− (n = 12 with TMT, n = 6 without TMT; black bars), P > 0.05.
Pupillary Constriction in Light Is More Sustained in Rgs9−/− Mice
We used pupillometry to more effectively record the time course of rod–cone-generated responses to light in an irradiance measurement pathway with rod/cone input to ipRGCs.30–33 At baseline, the dark-adapted pupil size was not significantly different between genotypes (mean ± SEM of the 10 seconds preceding the dim blue and bright red tests for wild-type = 1.75 mm ± 0.21; Rgs9+/− 1.73 mm ± 0.23; Rgs9−/− = 1.85 mm ± 0.33; 1 way ANOVA P = 0.37). Further, responses to a 20-minute stimulus showed that Rgs9−/− mice retained the ability to sustain pupil constriction for the duration of the light-aversion tests (Fig. 4A). Individual traces of pupil responses are presented in supplementary material (Supplementary Fig. S1). After an initial peak pupil constriction, pupils partially redilated over time, but the kinetics of redilation were significantly slower in Rgs9−/− mice (K = wild type [WT] 0.0087; Rgs9−/− 0.0025; P < 0.0001).
Figure 4.
Pupillary light reflex. (A) Mean pupil diameter is shown for wild-type and Rgs9−/− mice (n = 3 each) over a 20-minute bright blue stimulus. Mice were dark-adapted prior to stimulus-on at time zero. (B, C) Mean waveforms for pupil responses to 1-second stimuli are shown for wild-type, Rgs9+/−, and Rgs9−/− mice. Colored vertical lines behind panels indicate the color and timing of stimuli. (B) A dim blue stimulus (0.001 μW · cm−2 at 480 ± 3 nm, ~1 lux) selectively activates rods (wild type [WT] n = 8; Rgs9+/− = 7; Rgs9−/− = 10). (C) A bright red stimulus (10 μW · cm−2 at 622 ± 3nm, ~25,610 lux) predominantly activates rods and medium-wavelength sensitive cones (WT n = 7; Rgs9+/− = 8; Rgs9−/− = 9). Derived mean ± SEM pupil constriction is shown for the initial response (D) and the residual constriction at 30 seconds post stimulus (E). Pupil responses are shown as a percentage of constriction relative to pre-stimulus baseline. Significance is indicated above the bars: ns = not significant; ***P < 0.001.
Brief, 1-second stimuli were then used to further examine rod, cone, and melanopsin contribution to the pupil response, and results confirmed the presence of a rod or rod and cone generated abnormally sustained pupil constriction in Rgs9−/− mice (Figs. 4B–E). Wild-type mice showed an expected short latency of initial pupil constriction followed by a rapid post-stimulus redilation to both a dim blue stimulus and a bright red stimulus. There was no significant difference between wild-type and Rgs9+/− mice to either dim blue or bright red stimuli, which is consistent with our findings in the light aversion assay. The initial pupil constriction in Rgs9−/− mice was slightly but not significantly increased compared to that in controls. However, in contrast to wild-types, Rgs9−/− mice had a pronounced post-stimulus residual pupil constriction that was significantly greater than that of the controls for both dim blue and bright red stimuli. By using a dim blue stimulus that was too dim and brief to elicit an intrinsic melanopsin-generated response from the ipRGCs, we showed that the rod-generated input was sufficient to generate the abnormally sustained pupil constriction.30,32 The response to primarily rod/cone-activating bright red light supported this finding, although this stimulus does weakly activate melanopsin. Thus, pupillary light reflex responses in Rgs9−/− mice were consistent with prolonged rod/cone activation of ipRGC circuitry.
Discussion
Photophobia is a subjective experience that can be manifested to various degrees as ocular pain, exacerbation of headache pain, or a general level of discomfort in otherwise normal light. This variability in experience is not surprising given that different pathologies can underlie photophobia. With this in mind, an experimental advantage of bradyopsia-associated photophobia is that it can be caused by a monogenic RGS9 mutation. We have now shown that loss of the Rgs9 gene recapitulates photophobia-like behavior in a mouse model of bradyopsia and increases the response to light in multiple irradiance measurement pathways.
There are two major new findings in this study. First, we quantified sensitivity to light in two operant behaviors. In particular, the light aversive response is an indicator of photophobia in mice. The increased light-sensitive behavior in Rgs9−/− mice establishes their use as a model for studying mechanisms and treatments of photophobia. Second, we have shown that Rgs9−/− mice have a prolonged pupillary response to light. Not only does this mean we can discount an increase in light entering the eye due to an enlarged pupil as the cause of increased behavioral responses to light, but this observation demonstrates the increase in sensitivity to light in multiple ipRGC-dependent irradiance response axes mediated by distinct central nuclei. An ipRGC irradiance measurement circuit has been proposed to be the primary retinal input to photophobic behaviors in mice and is the likely input to photophobia pathways in humans.14,16–18,34 The prolonged activation is consistent with previous single cell recordings of the photoreceptor cells.12 Furthermore, because ipRGCs receive rod/cone input and RGS9 does not act on melanopsin phototransduction, our observed behavioral and pupil phenotypes are consistent with an effect of prolonged rod/cone responses to light on the ipRGCs.35
We hypothesize that ocular photophobia can be caused by an amplified response in irradiance measurement pathways. Given the previous demonstration of prolonged activation of rods and cones in Rgs9−/− mice, the most obvious explanation for an increased response to light is an amplified response originating in the rods and cones, then mediated by the ipRGCs. This explanation is consistent with growing evidence that the ipRGCs mediate the retinal input that generates photophobia: ipRGCs have been identified with effects of light on headache in humans and demonstrated to be the major retinal input to light-aversion pathways in mice.14–16,34,36 In the case of migraine-associated photophobia, Burstein et al.3 revealed convergence of signals from ipRGCs and dura-sensitive nociceptive neurons in the posterior thalamus. In the absence of headache, animal studies have shown that bright light can activate the trigeminal nerve presumably by intraocular mechanisms. These intraocular mechanisms may involve either a parasympathetic reflex37 or ocular associational ganglion cells.38 There is also potential for nontrigeminal pathways to be invoked by abnormal activation of ipRGCs. For example, ipRGC neurons also innervate the amygdala and other limbic structures39 that could potentially contribute to light-induced discomfort in the absence of pain.40 Indeed, Ahn et al.36 have shown that aversive responses to light by newborn mice involved neuronal activation in the amygdala and posterior thalamus but not the trigeminal nucleus.
Our finding that a photophobia phenotype can be recapitulated in Rgs9−/− mice provides a clearly defined genetic model for studying pathways involved in eye-based photophobia. Rgs9−/− mice could also be used for preclinical drug screening and genetic crosses to identify modifier alleles that may compensate for the loss of Rgs9 function. Because the degree of photophobia can be quantified, the effects of interventions or treatments can be objectively tested in Rgs9−/− mice, something that is not routinely possible in patients in whom photophobia is an obvious but subjective and self-reported symptom of disease. Furthermore, to our knowledge, this is the first example of a retinal disease causing a pathologically sustained constriction of the pupil following a short light stimulus under conditions that are not activating an intrinsic melanopsin-mediated response. This phenotypic marker will be useful in studying the mechanisms of abnormal irradiance measurement in Rgs9−/− mice. The sustained pupillary response should prove to be an effective clinical test for diagnosing this phenotype in humans and as a means for assessing new treatments of photophobia.
Acknowledgments
The authors thank C. Johnson for performing light measurements, E. Kaiser for assistance with data analysis and statistics, C. K. Chen for kindly providing Rgs9−/− mice, and A. Raddant, M. Anderson and A. Recober for helpful discussions.
Supported by National Institutes of Health Grants NS075599 (AFR) and EY12682 (NOA), Department of Defense Grant W81XWH-11-1-0561 (RHK, AFR), Iowa City Veterans Affairs Center for the Prevention and Treatment of Visual Loss (C6810-C) (RHK, AFR), and the Stephen A. Wynn Institute for Vision Research (ST). The authors report no conflict of interest.
Disclosure: A. Kuburas, None; S. Thompson, None; N.O. Artemyev, None; R.H. Kardon, None; A.F. Russo, None
References
- 1. Russell MB, Rasmussen BK, Fenger K, Olesen J. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996; 16: 239–45 [DOI] [PubMed] [Google Scholar]
- 2. Rasmussen BK, Jensen R, Olesen J. A population-based analysis of the diagnostic criteria of the International Headache Society. Cephalalgia. 1991; 11: 129–34 [DOI] [PubMed] [Google Scholar]
- 3. Noseda R, Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol. 2011; 24: 197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishiguchi KM, Sandberg MA, Kooijman AC, et al. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature. 2004; 427: 75–78 [DOI] [PubMed] [Google Scholar]
- 5. Cheng JY, Luu CD, Yong VH, Mathur R, Aung T, Vithana EN. Bradyopsia in an Asian man. Arch Ophthalmol. 2007; 125: 1138–1140 [DOI] [PubMed] [Google Scholar]
- 6. Hartong DT, Pott JW, Kooijman AC. Six patients with bradyopsia (slow vision): clinical features and course of the disease. Ophthalmology 2007; 114: 2323–2331 [DOI] [PubMed] [Google Scholar]
- 7. Michaelides M, Li Z, Rana NA, et al. Novel mutations and electrophysiologic findings in RGS9- and R9AP-associated retinal dysfunction (Bradyopsia). Ophthalmology 2010; 1171): 120–127 [DOI] [PubMed] [Google Scholar]
- 8. Druey KM, Blumer KJ, Kang VH, Kehrl JH. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature. 1996; 379: 742–746 [DOI] [PubMed] [Google Scholar]
- 9. Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002; 54: 527–559 [DOI] [PubMed] [Google Scholar]
- 10. Anderson GR, Lujan R, Semenov A, et al. Expression and localization of RGS9-2/G 5/R7BP complex in vivo is set by dynamic control of its constitutive degradation by cellular cysteine proteases. J Neurosci. 2007; 27: 14117–14127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009; 54: 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000; 403: 557–560 [DOI] [PubMed] [Google Scholar]
- 13. Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology 2010; 58: 156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Semo M, Gias C, Ahmado A, et al. Dissecting a role for melanopsin in behavioural light aversion reveals a response independent of conventional photoreception. PLoS One 2010; 5:e15009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matynia A, Parikh S, Chen B, et al. Intrinsically photosensitive retinal ganglion cells are the primary but not exclusive circuit for light aversion. Exp Eye Res. 2012; 105: 60–6 9 [DOI] [PubMed] [Google Scholar]
- 16. Thompson S, Recober A, Vogel TW, et al. Light aversion in mice depends on nonimage-forming irradiance detection. Behav Neurosci. 2010; 124: 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson S, Foster RG, Stone EM, Sheffield VC, Mrosovsky N. Classical and melanopsin photoreception in irradiance detection: negative masking of locomotor activity by light. Eur J Neurosci. 2008; 27: 1973–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003; 20: 989–999 [DOI] [PubMed] [Google Scholar]
- 19. Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997; 17: 8024–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci. 2012; 32: 15439–15449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson S, Philp AR, Stone EM. Visual function testing: a quantifiable visually guided behavior in mice. Vision Res. 2008; 48: 346–352 [DOI] [PubMed] [Google Scholar]
- 22. Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009; 29: 8798–8804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 5348–5354 [DOI] [PubMed] [Google Scholar]
- 24. Dvorkin A, Szechtman H, Golani I. Knots: attractive places with high path tortuosity in mouse open field exploration. PLoS Comput Biol. 2010; 6:e1000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thiels E, Hoffman EK, Gorin MB. A reliable behavioral assay for the assessment of sustained photophobia in mice. Curr Eye Res. 2008; 33: 483–491 [DOI] [PubMed] [Google Scholar]
- 26. Rahman Z, Schwarz J, Gold SJ, et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron 2003; 38: 941–952 [DOI] [PubMed] [Google Scholar]
- 27. Zachariou V, Georgescu D, Sanchez N, et al. Essential role for RGS9 in opiate action. Proc Natl Acad Sci U S A 2003; 100: 13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kovoor A, Seyffarth P, Ebert J, et al. D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005: 25: 2157–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blundell J, Hoang CV, Potts B, Gold SJ, Powell CM. Motor coordination deficits in mice lacking RGS9. Brain Res. 2008; 1190: 78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008; 453: 102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007; 47: 946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001; 4: 621–626 [DOI] [PubMed] [Google Scholar]
- 33. Gooley JJ, Ho Mien I, St Hilaire MA, et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012; 32: 14242–14253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010; 13: 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martemyanov KA, Arshavsky VY. Biology and functions of the RGS9 isoforms. Prog Mol Biol Transl Sci. 2009; 86: 205–2 27 [DOI] [PubMed] [Google Scholar]
- 36. Delwig A, Logan AM, Copenhagen DR, Ahn AH. Light evokes melanopsin-dependent vocalization and neural activation associated with aversive experience in neonatal mice. PLoS One 2012; 7:e43787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain 2010; 149: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 2011; 52: 7852–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006; 497: 326–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russo AF, Recober A. Unanswered questions in headache: so what is photophobia, anyway? Headache. 2013; 53: 1677–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]