Abstract
By investigating host-pathogen interactions in zebrafish using intravital imaging, Davis and Ramakrishnan (2009) provide evidence that aggregates of immune cells known as granulomas, long thought to constrain mycobacterial infection, may instead facilitate its spread.
In criminal cases, the concept of cui bono (“who benefits”) is often invoked to characterize those who stood to gain from a crime. When the same principle of inquiry is applied to the complexities of host-pathogen interactions, the results can be surprising. Such is the case of Davis and Ramakrishnan (2009) who report in this issue the use of quantitative intravital imaging to study the initial steps of Mycobacterium marinum infection in a zebrafish model of tuberculosis. Their findings suggest that organized aggregates of immune cells called granulomas, which accumulate at sites of tuberculosis infection, are not necessarily beneficial to the host, at least in the early stages of infection. Instead, they show that granulomas may act as unwitting accomplices in the spread of M. marinum infection.
Mycobacterium tuberculosis, which causes tuberculosis in humans, is a highly successful bacterium that infects macrophages and dendritic cells and is hence well positioned to subvert and exploit host immune responses. M. tuberculosis poses a quandary for the immune system: although adaptive (CD4+ and CD8+ T cell) immunity is essential for control of bacterial growth, it cannot eradicate infection. The result is latent infection—a hallmark of tuberculosis—that persists for the lifetime of the host and reactivates with sufficient frequency to maintain a worldwide epidemic. Therefore, studies of host responses to virulent mycobacteria provide opportunities to identify the limitations of immunity. Understanding the shortcomings of the immune responses to M. tuberculosis and the virulence strategies it uses to evade immunity remain essential prerequisites for development of an effective tuberculosis vaccine.
The characteristic lesions of tuberculosis are granulomas, which have long been considered a host defense mechanism for containing persistent pathogens (Adams, 1976; Egen et al., 2008). Granulomas are classically defined as organized collections of macrophages and lymphocytes that form to eliminate foreign substances. Their unique cellular organization led to the belief that granulomas physically “wall off” persisting bacteria while preserving the function of neighboring tissue. An alternative model is that mature granulomas represent a stalemate or equilibrium between host and pathogen, characterized by adaptive immunity sufficient to arrest progressive infection but insufficient to kill the intracellular bacteria. Until recently, investigations of granulomas have been constrained by imaging techniques that required fixed tissue, limiting insights into cellular dynamics and the molecular mechanisms of formation and maintenance of their structure.
Fortunately, recent innovations in intravital imaging have enabled a more complete analysis. Real-time imaging of M. marinum infection in transparent zebrafish embryos has demonstrated that granuloma formation by infected macrophages occurs within 3 days of infection and does not require adaptive immunity (Davis et al., 2002). Further work in this model revealed that the cytokine tumor necrosis factor (TNF), which is essential for immunity to M. tuberculosis, is required for maintenance of granulomas but dispensable for their initial formation (Clay et al., 2008). Egen et al. (2008) produced the first real-time images of granulomas in mammals by examining liver granulomas in mice infected with Mycobacterium bovis BCG. This study also identified a role for TNF in granuloma maintenance and described the dynamic behavior of macrophages and T cells, whose interactions in the structure are thought to be critical for control of bacterial growth. Whereas granuloma T cells had rapid motility but were spatially constrained to the confines of the structure, macrophages in mature granulomas were largely immobile, forming a matrix for T cells to explore.
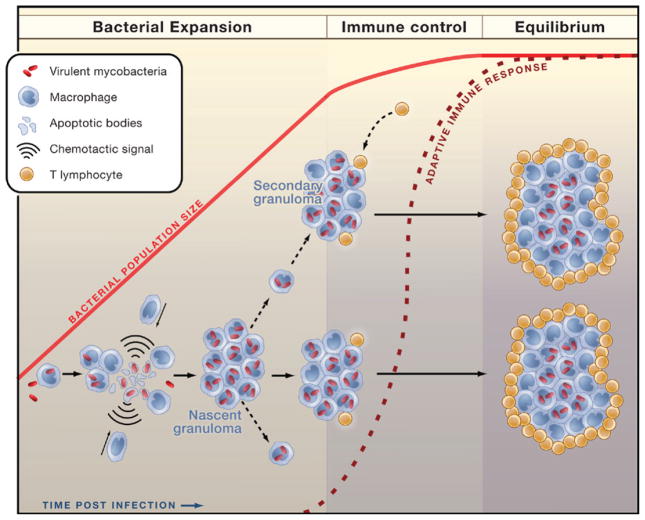
In contrast, Davis and Ramakrishnan now report that during early stages of granuloma formation, macrophages are highly motile and that the early granuloma actually benefits the pathogen by recruiting macrophages to the site of infection, thereby providing a steady supply of susceptible host cells to infect (Figure 1). The differences in macrophage motility observed by Egen et al. and by Davis and Ramakrishnan may be attributable to the stages of infection observed in each: early-stage granulomas exhibit high macrophage motility, whereas increasing stability of the structure is observed at later stages. Davis and Ramakrishnan also determined that efficient recruitment of uninfected macrophages to the site of M. marinum infection depends on the mycobacterial RD1 locus. The 9.5 kb RD1 locus is deleted in M. bovis BCG, the strain used by Egen et al., and contains genes that contribute to a secretion system termed the early secreted antigen 6 kDa (ESAT-6) secretion system 1 (ESX-1), which is essential for full virulence. There is considerable effort to understand the roles of ESX-1 proteins in mycobacterial virulence (Ernst et al., 2007), and the observations by Davis and Ramakrishnan provide important insight into the contributions of this virulence system to the success of M. marinum (and presumably, M. tuberculosis).
Figure 1. Stages of Granuloma Formation in Tuberculosis.
Findings by Davis and Ramakrishnan (2009) explain the initial stage of tuberculosis, which is characterized by expansion of the bacterial population in the absence of adaptive immunity. Bacterial multiplication and spread are facilitated by formation of the nascent granuloma. Infected macrophages undergo apoptosis and recruit uninfected macrophages to the site. These recruited macrophages phagocytose the remnants of infected cells and their bacterial contents. Some newly infected macrophages egress to seed secondary granuloma formation. When initiation of adaptive immunity eventually occurs, CD4+ and CD8+ effector T lymphocytes are recruited to infected tissue and curtail bacterial growth. Although essential for control of the infection, adaptive immunity cannot eradicate it. The mature granuloma represents an equilibrium between virulent mycobacteria and the host immune response.
The present paper establishes that during granuloma formation the macrophages that are initially infected die and actively recruit uninfected macrophages to the site of infection. The uninfected macrophages rapidly migrate toward and within the nascent granuloma. They then phagocytose infected cell remnants and their bacterial contents at a rate of 2.3 new macrophages for each old, infected cell. The recruited macrophages then phagocytose infected cell remnants and their bacterial contents. This multiplicative process ensures efficient spread and promotes expansion of the bacterial population. RD1-deficient M. marinum was defective in multiple steps of this process, including lower frequencies of death in infected cells and a lower frequency of phagocytosis by uninfected cells. Virulent M. marinum recruited 7-fold more uninfected macrophages to the site of infection than did RD1-deficient M. marinum.
The molecular basis for RD-1-dependent recruitment of uninfected cells remains to be determined. Whether this is a direct effect of an ESX-1 secretion product such as ESAT-6 or the induction of host chemokines or a response to the release of contents of dying cells (such as ATP or uric acid) is not yet clear. The possibility that virulent mycobacteria exploit a conserved process for recruiting macrophages to dispose of remnants of dead cells should be considered, as it may reveal another important connection between development and innate immunity. At the same time, pathogenic mycobacteria possess mechanisms to limit apoptosis. Two defined mutants of M. tuberculosis that induce apoptosis of infected cells have recently been identified. Although each of these proapoptotic mutants is attenuated in vivo, the mutant strain lacking a functional SecA2 secretion system (Hinchey et al., 2007) has decreased growth during the initial 3 weeks of infection, before the onset of adaptive immunity. In contrast, the mutant strain lacking NuoG, a subunit of type I NADH-dehydrogenase, (Velmurugan et al., 2007), has decreased growth during the chronic phase of infection. Together, these results suggest a complex role for apoptosis in tuberculosis, with benefits to either host or pathogen that depend on the stage of infection and the confluence of innate and adaptive immunity.
One feature of the zebrafish embryo model is the absence of adaptive immune cells; although this simplifies analysis of the early events in granuloma induction, it leaves many opportunities for studies in mice to define the interactions between infected phagocytes and T and B lymphocytes, such as those pioneered by Egen and colleagues. In particular, it will be essential to define the frequency of productive interactions of antigen-specific CD4+ and CD8+ T cells with mycobacterium-infected macrophages and dendritic cells in vivo. This will help determine whether some of the success of mycobacteria is due to their interfering with recognition of infected cells by antigen-specific lymphocytes. Another event noted by Davis and Ramakrishnan is the egress of infected macrophages from established granulomas. During the early stages of infection, this is a mechanism for establishing secondary granulomas; does the same mechanism account for egress of infected dendritic cells and their migration to lymph nodes to activate antigen-specific T cells later in infection (Wolf et al., 2008)? Understanding this process could help uncover the cause for the delay in initiation of adaptive immunity to M. tuberculosis and could provide valuable information for development of improved tuberculosis vaccines.
Advances in intravital imaging have already made major contributions to understanding cellular dynamics in mycobacterial granulomas, including observations that question the long-held tenet that granulomas only benefit the host. We can look forward to additional insights from imaging studies that will further refine our knowledge of the complex interactions between pathogen and host in tuberculosis and lead to new ways to perturb the equilibrium to our advantage.
References
- Adams DO. Am J Pathol. 1976;84:164–192. [PMC free article] [PubMed] [Google Scholar]
- Clay H, Volkman HE, Ramakrishnan L. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. Cell. 2009 doi: 10.1016/j.cell.2008.11.014. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JD, Trevejo-Nunez G, Banaiee N. J Clin Invest. 2007;117:1738–1745. doi: 10.1172/JCI31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, et al. J Clin Invest. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR, Jr, Porcelli SA, Briken V. PLoS Pat-hog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]