Abstract
A number of epidemiological studies suggest that the consumption of green tea reduces the incidence of prostate cancer. Since the major catechins present in green tea are potent anti-oxidants, we hypothesized that genetic and cellular damage induced by oxygen free radicals could be significantly reduced by potent anti-oxidants in green tea, thus reducing the cumulative genetic and cellular damage with age, and slowing or preventing tumour formation.
Long-term administration of a decaffeinated green tea extract to Lobund-Wistar rats for periods up to 26 months almost halved the incidence of primary tumours in the genitourinary tract when compared to an aged-matched cohort receiving just water. We observed no inhibition of DNA adduct formation or lipid peroxidation in animals consuming green tea compared to animals consuming de-ionized water. The decrease in tumour formation was associated with an increase in 8-hydroxy-2’deoxyguanosine (8-OH-dG) and 4-hydroxynonenal (4-HNE) content (markers of DNA adduct formation and lipid peroxidation respectively) in the epithelium of the ventral prostate in aging animals. There was also an increase in 8-OH-dG expression, but no change in 4-HNE expression in the seminal vesicles of older animals.
There was an age associated increase in expression of the anti-oxidant enzymes MnSOD and catalase in the epithelium of the ventral prostate of aging animals. There was also an increase in MnSOD expression, but no change in catalase expression in the seminal vesicles of older animals.
These data demonstrate that consumption of green tea decreases the incidence of genitourinary tract tumours in the Lobund-Wistar rat, but has no effect on age associated DNA adduct formation and lipid peroxidation in the aging rat ventral prostate and seminal vesicles.
Keywords: Lobund-Wistar rat, green tea, ventral prostate, seminal vesicles, cancer, MnSOD, catalase, 8-hydroxy-2’deoxyguanosine (8-OH-dG), 4-hydroxynonenal (4-HNE)
INTRODUCTION
It is well established that genetic and hormonal factors are important in prostate cancer progression. However, there is considerable evidence to suggest that extrinsic factors, in particular diet, play a role in prostate cancer incidence (Dunn 1975). While epidemiological studies are not definitive, evidence suggests that consumption of green tea may reduce the incidence of prostate cancer and slow progression of the disease (Bettuzzi et al. 2006;Jian et al. 2004;Blot et al. 1996).
Green tea contains a number of polyphenols that have been tested alone and in combination in a variety of in vitro model systems of prostate cancer. The mechanism of action of its most active constituent epigallocatechin-3-gallate (EGCG) and other catechins found in green tea is still under active investigation.
There are a number of proposed mechanisms of action for green tea (Liao & Hiipakka 1995;Naasani et al. 1998;Ahmad et al. 2000;Nam et al. 2001), EGCG modulates cell cycle arrest and apoptosis in several prostate cancer cell lines including LNCaP, PC3 and DU-145 cells (Paschka et al. 1998;Agarwal 2000;Gupta et al. 2000). Several short term animal studies using the TRAMP mouse model of prostate cancer or subcutaneous xenografts of PC3 and LNCaP 104-R cells have demonstrated that the consumption of green tea inhibits tumour growth (Tam et al. 2006;Liao et al. 1995;Gupta et al. 2001).
While the studies outlined above suggest that green tea may affect tumour progression, no long-term studies have been performed in vivo to examine the chemopreventative effects of chronic green tea consumption on tumour incidence and progression.
An age-related increase in genetic and cellular damage by oxygen free radicals may be a significant factor in prostate cancer development (Aydin et al. 2006;Malins et al. 2001). There is considerable evidence that EGCG and other catechins found in green tea act as potent anti-oxidants (Guo et al. 1999;Toschi et al. 2000). The genetic and cellular damage by oxygen free radicals can be prevented by potent anti-oxidants like EGCG (Chung et al. 1993), reducing cumulative genetic damage to the cell, presumably decreasing oxidative damage that could lead to initiation and/or progression of primary tumour growth.
The current study examined the long term (greater then 2 years) effect of decaffeinated green tea consumption on autochthonous genitourinary tumour formation in Lobund-Wistar rats (Pollard 1973;Pollard 1998b). The incidence of primary tumours of the genitourinary tract (the ventral, dorsolateral prostate and seminal vesicles), the morphology of the glands, lipofuscin accumulation, DNA adduct formation, lipid peroxidation, MnSOD and catalase expression in the animals receiving the decaffeinated green tea extract were compared to an age matched cohort receiving water.
Chronic consumption of green tea, while not significant, delayed the initiation of tumour formation and reduced tumour incidence. This was associated with an increase in DNA adduct formation (assessed by 8-OH-dG staining) and MnSOD expression in the epithelium of both the prostate and seminal vesicles in older animals. There was also an increase in lipid peroxidation (assessed by 4-HNE staining) and catalase expression in the epithelium of the prostate with no apparent change in the seminal vesicles in older animals. This suggests that consumption of green tea while decreasing tumour incidence had no long term effect on DNA adduct formation or lipid peroxidation in these genitourinary tissues.
MATERIALS AND METHODS
Animal Husbandry
Male Lobund-Wistar rats from the original colony described by Pollard (Pollard 1973), were bred in the Friemann Life Sciences Center at the University of Notre Dame. The animals were maintained in a controlled environment (12 h light, 12 h dark) and were fed a full diet Teklad L-485 rodent diet (Harlan, Madison, WI) ad libitum. A total of 248 males were randomized into control or green tea arms at 4 months of age, at which point control animals were given de-ionized water ad libitum throughout the experiment while the experimental arm received de-ionized water until 4 months of age then they received a decaffeinated 0.6% green tea extract as their only source of fluids.
To ensure green tea consumption did not affect weight gain, food or liquid intake at 3 different times during the study (immediately, 6 months and one year after starting green tea administration) food and fluid intake was monitored for 2 week intervals and body weights were measured monthly. Animals were sacrificed using CO2 at specific time points throughout the study (i.e. 6; 10; 14; 18; 22 and 26 months of age). The number of animals sacrificed at each time point was 20 rats/group (with the exception of the 22 and 26 month control groups, which each had 24 animals).
Preparation of the Green Tea
Freeze-dried decaffeinated green tea was kindly provided by Thomas J. Lipton Inc. (Englewood Cliffs NJ). (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG) and (−)-epigallocatechin-3-gallate (EGCG) accounted for 4.5%, 7.2%, 5.3%, and 10.6% of the dried weight respectively with a caffeine content of 0.2%. To ensure that there was no degradation of the catechins in solution with time (Chen et al. 2001), fresh 0.6% green tea extracts, were prepared every second day by dissolving the freeze dried extract in the appropriate volume of de-ionized water at 55°C (Shi et al. 1994). Assuming a cup of tea contains 250 mg of catechins, this concentration of green tea has been determined to be equivalent to approximately 16 cups of green tea per day for adult humans (Liao et al. 2004).
Tissue Processing and Staining
To standardize the assessment of the morphology of the urogenital complex, the entire complex including the seminal vesicles were excised intact and immediately frozen placed on a metal surface, oriented in a standardized manner, before freezing in liquid nitrogen to keep the morphology of the urogenital complex intact. The entire complex was sliced into five uniform segments and fixed in cassettes overnight in 10% neutral buffered formalin, incubated in 70% ethanol overnight and processed through a series of ethanol, toluene, and paraffin using an automated tissue processor (Shandon, Cheshire, England). Tissues were then sectioned to 8 μm, deparaffinized and stained with hematoxylin and eosin using the Optimax plus automated staining system (Biogenex, San Ramon, CA), dehydrated and mounted with permount. All histological slides were viewed using an Olympus SZX12 and Olympus AX70 microscope (Olympus Melville, NY) and photographed with a Spot RT digital camera (Diagnostic instruments Inc. Sterling Heights, MI).
Tissue Microarrays
0.6 mm cores of neutral buffered formalin fixed paraffin-embedded seminal vesicles and ventral prostate from 12 animals from each group (i.e. 6 month control; 6 month treated; 26 month control; 26 month treated) or tumoured tissue from treated or control animals consisting of 1 tissue microarray with 2 replicate cores per block were used for immunohistochemical analyses.
Immunohistochemistry
Immunohistochemistry was performed using the DAKO Chemmate Envision Kit (DAKO, K5007). Eight micron tissue microarray sections on charged slides (Biogenex) were deparaffinized and rehydrated. Antigen retrieval was performed for the anti-8-OH-dG and anti-4-HNE antibodies using antigen retrieval solution (15 mL of 1M sodium citrate and 15 mL of 1M citric acid in de-ionized water, pH 6.0), Sections were heated in a pressure cooker (Merarini Diagnostics, Berkshire, UK), incubated for a further 4 min, cooled and washed in PBS for 10 min. Non-specific binding was blocked using 10% casein in PBS for 25 min. Antibodies were diluted in DAKO diluent buffer. Sections were incubated with either 1:40 anti-8-OH-dG, or 1:40 anti-4-HNE (mouse monoclonal antibodies, Genox, Baltimore, MD), or 1:100 anti-catalase (StressGen), or 1:100 anti-MnSOD (StressGen). An IgG mouse control and elimination of primary antibody were used as negative controls. Sections were incubated with primary antibodies for 2 h at room temperature and washed three times in PBS + 0.05% Tween for 5 min at room temperature. Endogenous peroxidase activity was quenched using 3% hydrogen peroxide in PBS for 7 min. Sections were then washed three times in PBS + 0.05% Tween incubated with secondary antibody/HRP (DAKO, Glostrup, Denmark) for 30 min at room temperature, followed by 1:50 DAB in substrate buffer 10 min. Sections were washed with de-ionized water for 5 min, counterstained with 1:4 hematoxylin for 20 sec followed by a water wash for 5 min, dehydrated and mounted using Pertex mounting media and photographed using an Olympus DF-50 microscope equipped with a U-CAMP-2 camera.
Assessment of immunohistochemical stains
Immunostaining was assessed using the following four-point categorical compositional scale: 0=negative, 1=weak, 2=moderate, and 3=strong. The immunostain results were determined by consensus by two of the investigators (JOS and JS), and results were in excellent agreement.
Lipofuscin Autofluorescence
Eight micron sections of tissue prepared on charged slides (Biogenex) were deparaffinized and then rehydrated, washed 3 times in 10 mM PBS, mounted in Crystal/mount™ (Fisher) and photographed under indirect fluorescence (excitation 488 nm; emission: 568 nm) using an Olympus AX-70 microscope equipped with an RT-SPOT digital camera.
Statistical Analysis
Kaplan-Meier survival curve was constructed with related mortality as the end-point. Differences in survival between the control and EGCG animals were assessed using the logrank test. Multivariate survival analyses were performed with the Cox proportional hazards model using the Statistical Package for the Social Sciences (SPSS, Chicago, Illinois). Student t test was used to compare significant differences in antibody staining intensities between animal groups. All p-values are two-sided and p-values less than 0.05 were considered statistically significant in all analyses.
RESULTS
Diet and weight gain
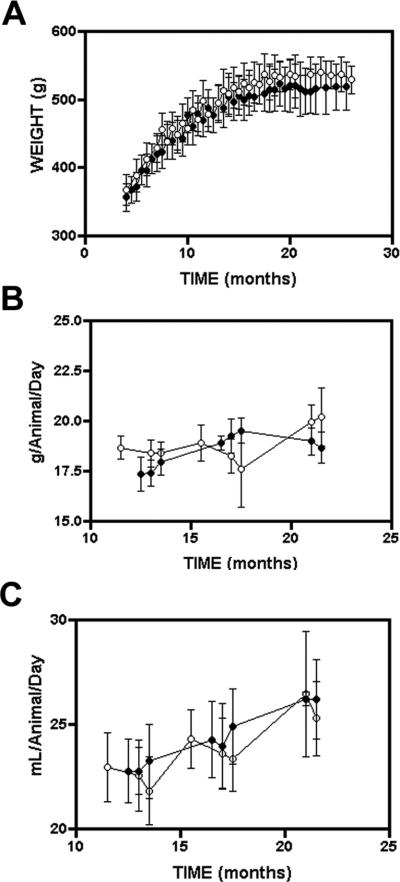
Food and liquid intake and weight gain were monitored at regular intervals (see material and methods) in green tea extract treated and control animals throughout the study (Figure 1. Panels A, B and C). Food intake by animals in both arms of the study remained relatively constant throughout study, between 17.5 and 20 grams of chow/day/rat. In contrast fluid consumption increased in both arms of the study with age. Animals in both arms of the study showed typical increase in body weight throughout the study, and although the green tea treated animals weighed slightly less than the control (untreated) animals from 14 months of age onwards, however, this difference in weight was not statistically significant. These data confirm that consumption of decaffeinated green tea did not significantly influence nutritional intake or animal growth rates during the study.
Figure 1. Body weight, food and liquid intake in Lobund-Wistar rats consuming de-ionized water or green tea.
Animals were provided with water or a green tea extract (GTE) as described in materials and methods. Animals were weighed once a month (Panel A). Food and liquid intake (Panel B and C) was measured over 3 two week periods, to ensure that the green tea extract did not have a significant affect on weight gain or dietary habits. Control (open circles) and green tea treated (filled circles). All results are expressed as mean ± SD.
Tumour Incidence
Previous pioneering studies by Pollard and colleagues (Pollard 1998a) have demonstrated that 27% of Lobund-Wistar rats develop autochthonous tumours of the urogenital complex by 24 months of age. This incidence rate was similar in our 26-month-old animal control arm. In this study, tumour incidence was monitored using gross morphology and histopathology.
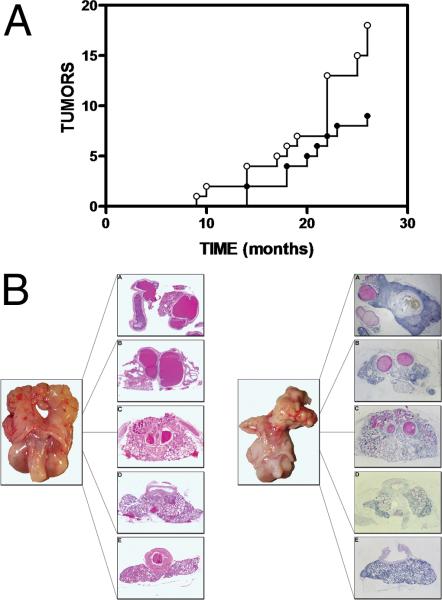
To examine tumour progression with age and to determine if green tea treatment delayed rather then prevented the onset of tumour formation, animals were sacrificed at specific stages in the study (Table 1). Tumour incidence increased in older animals and tumour incidence in green tea treated animals was nearly halved when compared to untreated controls (Figure 2A). While the tumour numbers in green tea treated animals were nearly halved when compared to untreated controls, this difference was not statistically significant (p=0.21). Some tumours were clearly visible upon gross morphological inspection others were detected upon histological inspection (Figure 2B).
Table 1.
Tumour incidence in Lobund-Wistar rats consuming de-ionized water or 0.6% green tea 128 rats were randomly assigned to the control group drinking deionized water (20 rats/group, with the exception of the control 22 and 26 month groups, which each had 24 animals). 120 rats were randomly assigned to the green tea group, consuming 0.6% green tea (20 animals per group). One animal from the 18 month control group was removed before the cause of death could be determined.
| Time of Sacrifice (months) | |||||||
|---|---|---|---|---|---|---|---|
| 6 | 10 | 14 | 18 | 22 | 26 | Total | |
| DE-IONIZED WATER | |||||||
| Seminal Vesicle | - | - | 2 | - | 1 | 6 | 9 |
| Ventral Prostate | - | - | - | - | 2 | 1 | 3 |
| Dorsolateral Prostate | - | - | - | - | - | 2 | 2 |
| Undefined | - | 2 | - | 2 | - | - | 4 |
| Total | 0 | 2 | 2 | 2 | 3 | 9 | 18 |
| GREEN TEA | |||||||
| Seminal Vesicle | - | - | 1 | - | 2 | 2 | 5 |
| Ventral Prostate | - | - | - | - | - | - | 0 |
| Dorso-lateral Prostate | - | - | 1 | 1 | - | - | 2 |
| Undefined | - | - | - | - | - | 2 | 2 |
| Total | 0 | 0 | 2 | 1 | 2 | 4 | 9 |
Figure 2. Tumour incidence in the urogenital complex of the aging Lobund-Wistar rat and accompanying morphology.
Panel A: Tumour incidence: At specified times of sacrifice, urogenital complexes were excised and tumour incidence assessed after staining with hematoxylin and eosin as described in materials and methods. Control (open circles) and green tea extract treated (filled circles). Panel B: Morphology: On the left is a urogenital complex from an unaffected male, showing ventral prostate, dorsolateral prostate, seminal vesicles and bladder. The urogenital complex was sectioned into 5 different regions, from the seminal vesicles (section A) to the ventral prostate (section E), paraffin embedded and stained with hematoxylin and eosin as described in Materials and Methods. On the right is a urogenital complex from an affected male showing evidence of tumour arising in the right lobe of the seminal vesicle (section A).
In control animals consuming de-ionized water (n=128), there were 18 tumours in total, 9 arising in the seminal vesicles, 3 within the ventral prostate, and 2 in the dorsolateral prostate, and 4 tumours that were too large and had disseminated throughout the urogenital complex, making it impossible to define their tissue of origin (Table 1). In animals consuming green tea (n=120), there were 9 tumours in total, 5 were found in the seminal vesicles, 2 in the dorsolateral prostate and 2 that were too advanced to identify the tissue of origin (Table 1).
Lipid Peroxidation in the Seminal Vesicles and Ventral Prostate
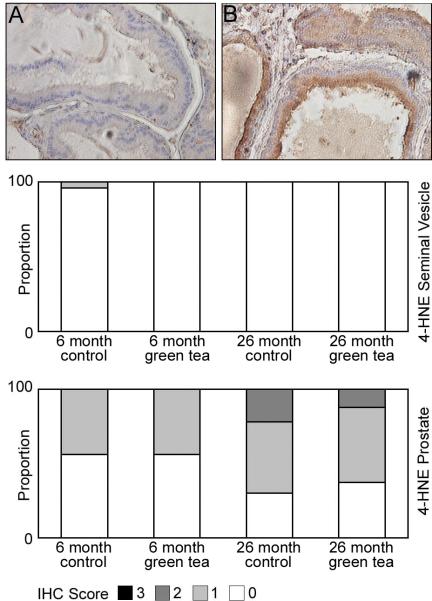
Lipid peroxidation, as monitored by 4-hydroxy-2-nonenal (4-HNE) immunohistochemistry was minimal in the seminal vesicles of 6 and 26-month-old animals. There was little to no staining observed in animals consuming de-ionized water or animals consuming green tea (Figure 3). Lipid peroxidation was evident in the ventral prostate of rats consuming de-ionized water at 6 months of age (Figure 3). Moderate staining was restricted to the cytoplasm of the epithelial cells, and was not present in stromal cells of the tissue. In animals consuming green tea for 2 months, from 4 months of age onward (Figure 3), there was no change in the staining intensity, suggesting that consumption of green tea did not protect the epithelium of the seminal vesicles from lipid peroxidation. By 26 months of age the level of lipid peroxidation in the seminal vesicles of control rats had increased (Figure 3), and consumption of green tea did not appear to provide any additional protective effect against lipid peroxidation (Figure 3). There was a significant increase in 4-HNE staining in the ventral prostate, with no significant difference in 4-HNE staining in the seminal vesicles of 6-month-old when compared to 26-month-old animals receiving de-ionized water.
Figure 3. Localization of lipid peroxidation in the seminal vesicles and ventral prostate of 6 and 26 month Lobund-Wistar rats consuming de-ionized water or green tea.
Eight micron paraffin sections of the seminal vesicles and ventral prostate were prepared from 6 and 26-month-old animals consuming de-ionized water or 0.6 % green tea extract. The sections were incubated with antibodies specific for 4-HNE as described in Materials and Methods. Panels A and B highlight intense and weak and moderate staining for 4-HNE in the epithelium of the ventral prostate in 6 and 26-month-old animals. Bar graphs underneath the panels display the intensity of staining for 4-HNE in the seminal vesicle and prostate, scoring two cores per animal (n=12). Legend: 3- strong staining, 2- moderate staining, 1- weak staining, 0- no staining.
Oxidative DNA Damage in Seminal Vesicles and Prostate
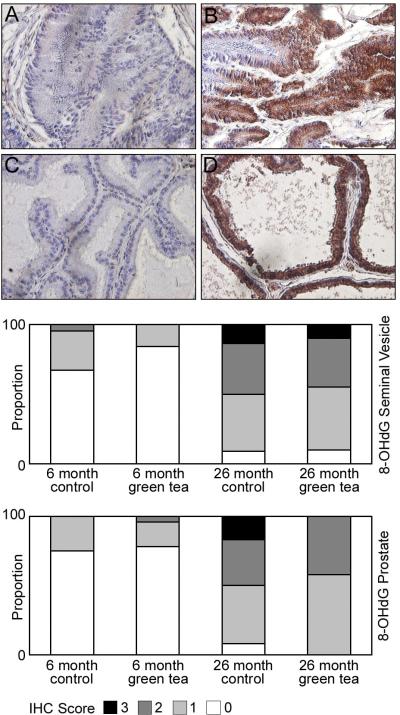
In the seminal vesicles of 6-month-old animals consuming de-ionized water, there was evidence of low levels of oxidative DNA damage, as measured by nuclear staining for 8 hydroxy-2'deoxyguanosine (8-OH-dG) (Figure 4). Green tea consumption for two months in 6-month-old animals had no effect on the level of DNA-adduct formation in the seminal vesicles (Figure 4). Unlike lipid peroxidation, the level of 8-OH-dG was elevated in the seminal vesicles of 26-month-old animals consuming de-ionized water and green tea (Figure 4). Green tea consumption did not reduce the level of DNA-adduct formation in the seminal vesicles (Figure 4).
Figure 4. Localization of 8-OH-dG in the seminal vesicles and ventral prostate of 6 and 26 month Lobund-Wistar rats consuming de-ionized water or green tea.
Eight micron paraffin sections of the seminal vesicles and ventral prostate were prepared from 6 and 26-month-old animals consuming de-ionized water or 0.6% green tea extract. The sections were incubated with antibodies specific for 8-OH-dG as described in Materials and Methods. Panels A and B highlight weak and intense staining for 8-OH-dG respectively in nuclear regions of the epithelium in the seminal vesicles in 6 and 26-month-old animals. Panels C and D highlight weak and intense staining for 8-OH-dG in nuclear regions of the epithelium in the ventral prostate in 6 and 26-month-old animals. Bar graphs underneath the panels display the intensity of staining for 8-OH-dG in the seminal vesicle and prostate, scoring two cores per animal (n=12) Legend: 3- strong staining, 2- moderate staining, 1- weak staining, 0- no staining.
The staining pattern for 8-OH-dG in the ventral prostate was similar to that of the seminal vesicles, with weak nuclear staining of 8-OH-dG (Figure 4) in the epithelium of the ventral prostate of 6-month-old animals consuming de-ionized water and green tea for 2 months (Figure 4). Oxidative DNA damage in the ventral prostates of 26-month-old rats consuming de-ionized water was clearly increased (Figure 4) compared to younger animals, and consumption of green tea or de-ionized water resulted in an increase in 8-OH-dG staining (Figure 4). While there was no statistical difference between the groups receiving green tea or de-ionized water, there were no animals with strong 8-OH-dG staining in the 26-month-old green tea group. There was a statistically significant difference in 8-OH-dG staining in the seminal vesicles and ventral prostate between 6 and 26-month-old animals receiving de-ionized water (p<0.001).
MnSOD expression in the Seminal Vesicles and Ventral Prostate
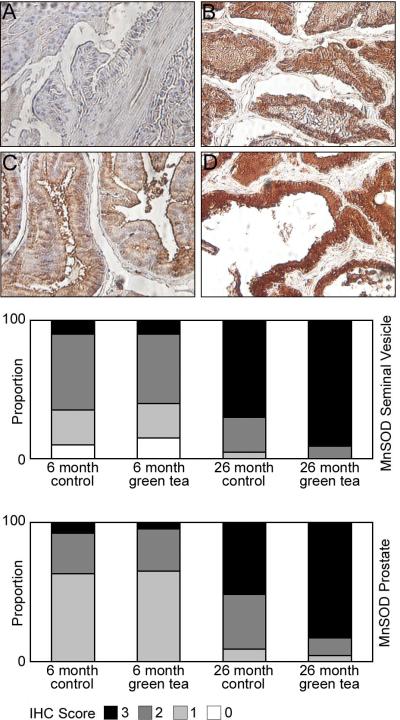
MnSOD was expressed in the mitochondrial fraction of the cell. MnSOD staining intensity was similar in the seminal vesicles of 6-month-old animals consuming de-ionized water and 6-month-old animals consuming green tea (Figure 5). Increased intense MnSOD expression was present in the seminal vesicles of 26-month-old animals consuming de-ionized water and animals consuming green tea (Figure 5). MnSOD staining in the ventral prostate was similar to the seminal vesicles, with moderate staining in 6-month-old animals, with increased intense staining in 26-month-old animals (Figure 5). There was a statistically significant difference in MnSOD staining in the seminal vesicles and ventral prostate between 6 and 26-month-old animals receiving green tea or de-ionized water (p<0.001). There was also an increase in MnSOD staining in the seminal vesicles of 26-month-old animals consuming de-ionized water and 26-month-old animals consuming green tea green tea, but it was not significant (p=0.06). However, there was a significant increase in MnSOD staining in the ventral prostate of 26-month-old animals consuming green tea compared to 26-month-old animals consuming de-ionized water (p<0.04).
Figure 5. Localization of MnSOD in the seminal vesicles and ventral prostate of 6 and 26 month Lobund-Wistar rats consuming de-ionized water or green tea.
Eight micron paraffin sections of the seminal vesicles and ventral prostate were prepared from 6 and 26-month-old animals consuming de-ionized water or 0.6% green tea extract. The sections were incubated with antibodies specific for MnSOD as described in Materials and Methods. Panels A and B highlight moderate and intense staining for MnSOD respectively in cytoplasmic regions of the epithelium in the seminal vesicles in 6 and 26-month-old animals. Panels C and D highlight weak and intense staining for MnSOD in cytoplasmic regions of the epithelium in the ventral prostate in 6 and 26-month-old animals. Bar graphs underneath the panels display the intensity of staining for MnSOD in the seminal vesicle and prostate, scoring two cores per animal (n=12) Legend: 3- strong staining, 2- moderate staining, 1- weak staining, 0- no staining.
Catalase expression in the Seminal Vesicles and Ventral Prostate
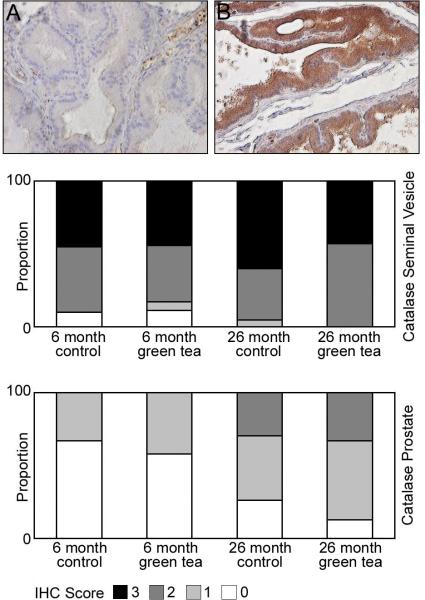
Catalase appeared to be highly expressed in the cytosol of the seminal vesicles. Similar staining for catalase was observed in the seminal vesicles of 6 and 26-month-old animals consuming de-ionized water or animals green tea (Figure 6). We observed less catalase expression in the ventral prostate with weak staining in 6-month-old animals, and increased moderate staining in 26-month-old animals (Figure 6). There was a significant increase in catalase staining in the ventral prostate of 26-month-old animals compared to 6-month-old animals receiving green tea or de-ionized water (p<0.001).
Figure 6. Localization of catalase in the seminal vesicles and ventral prostate of 6 and 26 month Lobund-Wistar rats consuming de-ionized water or green tea.
Eight micron paraffin sections of the seminal vesicles and ventral prostate were prepared from 6 and 26-month-old animals consuming de-ionized water or 0.6% green tea extract. The sections were incubated with antibodies specific for catalase as described in Materials and Methods. Panels A and B highlight weak and moderate staining for catalase in cytoplasmic regions of the epithelium in the ventral prostate in 6 and 26-month-old animals. Bar graphs underneath the panels display the intensity of staining for catalase in the seminal vesicle and prostate, scoring two cores per animal (n=12) Legend: 3- strong staining, 2- moderate staining, 1- weak staining, 0- no staining.
Assessment of 8-OH-dG, 4-HNE, MnSOD and catalase expression in primary tumours
Immunostaining was consistent in the primary tumours of 22 and 26-month-old animals, with no differences observed between animals consuming green tea extract and animals receiving de-ionized water. MnSOD and catalase displayed weak to moderate expression in all tumours. 8-OH-dG displayed moderate to intense staining with 4-HNE displaying little to no staining.
Lipofuscin Deposition
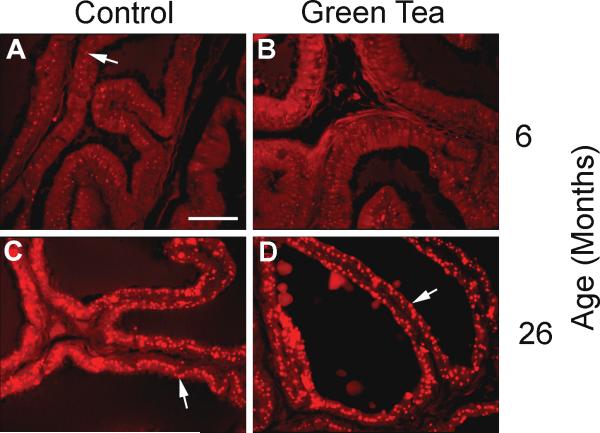
Lipofuscin accumulation in the ventral prostate may be caused by the accumulation of advanced glycation end products and is an indicator of generalized oxidative stress in the aging prostate (Morrissey et al. 2002). Autoflourescent lipofuscin accumulation occurs in the prostate of the Lobund-Wistar rat with increasing age. In 6-month-old rats consuming de-ionized water or green tea, small deposits of lipofuscin were present in the prostate epithelium (Figure 7, Panel A and B). Lipofuscin deposits increased in size and intensity in 26-month-old animals, whether the animals were consuming de-ionized water or green tea (Figure 7, Panel C and D).
Figure 7. Lipofuscin deposition in the ventral prostate of control and green tea treated rats.
Eight micron paraffin sections of the ventral prostate were prepared from 6 and 26-month-old animals consuming de-ionized water or 0.6% green tea and were photographed in the red fluorescence wavelength. Arrows indicate autofluorescent lipofuscin bodies. Bar = 50 μm.
DISCUSSION
There has been considerable debate over the origin of the urogenital tumours in the Lobund-Wistar rat. This has been further complicated by the use of MNU to initiate rapid tumour development in many studies, which may alter the natural history of these tumours. Early pioneering studies by Pollard reported that 27% of Lobund-Wistar rats develop tumours in the urogenital complex by 24 months of age. Using animals from this original colony, this figure is similar to the rates in our study (26-month-old cohort (6/24)).
In this animal model, tumour initiation appears to be stochastic, and tumours develop throughout the life span of the rats, however once initiated, these tumours grow rapidly and infiltrate the entire urogenital complex, making it difficult to determine the origin of the tumours. Previous studies have identified tumour-bearing animals based on palpation of the tumour, at which point most of the tumours have disseminated through the urogenital complex. By sacrificing rats at specified times throughout the life span of the animals, this study has provided the first opportunity to identify small, non-palpable tumours and hence establish their origin. Histological analysis provided unequivocal confirmation that 14 of the 28 tumours identified in this study originated in the seminal vesicles, 4 in the dorsolateral prostate and 3 in the ventral prostate. A further 6 tumours were too advanced to unequivocally determine the tissue of origin, although at least two of these appeared to originate in the seminal vesicles. These data suggest that the majority of the tumours arise in the seminal vesicles, as several other investigators have suggested (Cohen et al. 1994;Tamano et al. 1996).
Thus, for most purposes, the Lobund-Wistar rat does not adequately recapitulate human prostate cancer, and is not appropriate for studies of prostate tumour progression and metastasis. However, this does not preclude its use as a model to study dietary manipulation on tumour formation. Indeed since the Lobund-Wistar rat has a genetic predisposition to cancer that is clearly sensitive to oxidative stress and other dietary manipulations; it is probably still a preferred model for dietary studies when compared to the TRAMP mouse, which develops autochthonous tumours as a result of the expression of a powerful transgene in 100% of the animals.
This study was designed to test the efficacy of a green tea extract on an autochthonous model of prostate cancer (Pollard 1973;Pollard 1998b). We demonstrate that consumption of green tea delays the appearance of autochthonous genitourinary tumours in the Lobund-Wistar rat and nearly halving the overall incidence of tumours. However, this decrease in tumour incidence did not reach statistical significance. This level of green tea consumption for periods as short as 8 days has been shown to result in the substantial accumulation of EC, EGC and EGCG in the prostate of Sprague-Dawley rats, reaching steady state levels of 234.5 ∀ 59.2 ng/g, 250.6 ∀ 66.1 ng/g and 57.7 ∀ 20.9 ng/g respectively (Kim et al. 2000).
The concentration of green tea administered to the animals was above normal dietary levels. Assuming a cup of tea contains 250 mg of catechins, this concentration of green tea has been determined to be equivalent to approximately 16 cups of green tea per day for adult humans. (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG) and (−)-epigallocatechin-3-gallate (EGCG) accounted for 6.5 mg, 10.4 mg, 7.6 mg, and 15.3 mg of the dried weight/Animal/Day respectively with a caffeine content of 0.3 mg/Animal/Day.
The ventral prostate and seminal vesicles play a major role in the synthesis and storage of citrate, a major component of the seminal fluid. This places both tissues under very high levels of oxidative stress during the reproductive life of the animal, which has been proposed to contribute to tumour progression in prostate cancer (Costello & Franklin 1997).
We hypothesized that the anti-oxidant activity of green tea would decrease the formation of urogenital tumours in the Lobund-Wistar rat. We assessed lipid peroxidation and DNA-adduct formation (as measured by staining for 4-HNE and 8-OH-dG respectively) in the ventral prostate and seminal vesicles in aging Lobund-Wistar rats. While there was a reduction in tumour incidence, it was not statistically significant and we observed no significant changes in lipid peroxidation or DNA-adduct formation following the consumption of the green tea extract. These data suggest that the anti-oxidant activity did not affect these markers of oxidative damage in the Lobund-Wistar rat. While green tea consumption did not significantly affect lipid peroxidation or DNA-adduct formation. There was a decrease in the intensity of staining for 8-OHdG in the ventral prostates of 26-month-old animals administered green tea when compared to 26-month-old animals receiving de-ionized water.
The increase in DNA-adduct formation and lipid peroxidation may still relate to the increased tumour incidence with age in these animals. It has been suggested that the decreased activity of the oxidant repair enzymes contributes to a progressive accumulation of oxidant damage with age (Pacifici & Davies 1991), and anti-oxidant enzymes such as catalase, superoxide dismutase, glutathione peroxidase, and glutathione reductase are known to display lobe specific age-related decreases in the rat prostate (Suzuki et al. 1997;Ghatak & Ho 1996). Pechenino and Brown (2006) (Pechenino & Brown 2006), observed no changes in MnSOD in the aging ventral prostate of the Brown Norway rat, however, in the Lobund-Wistar model, the level of MnSOD expression increases in the seminal vesicles and the ventral prostate, with a subtle increase in MnSOD expression in animals consuming green tea. This increase in MnSOD expression correlates with an increase in catalase expression, DNA-adduct formation, lipid peroxidation, and lipofuscin with age in the ventral prostate of the Lobund-Wistar rats. While there may be a decrease in the rate of cellular metabolism in older animals (Lau et al. 2003), the accumulated oxidative damage is still present in the older animals. We hypothesize that in the Lobund-Wistar rat the rate of cellular metabolism does not decrease considerably, contributing to the increased MnSOD expression, accumulated DNA-adduct formation and tumour incidence in these animals.
The ability to mount an effective response to oxidative stress may decline with age, with the initiation of tumour formation is dependent on the rate of cellular metabolism and the activity of repair enzymes, including the base excision repair system that recognizes and repairs 8-OH-dG adducts. Furthermore, apoptosis of cells that accumulate excessive oxidative damage, and the resultant turnover of damaged cells may also directly impact the rate of tumour initiation (Tenniswood 1997).
Furthermore, the consumption of green tea has no apparent effect on lipofuscin accumulation in the prostate, a marker of chronic, generalized oxidative stress (Lopez-Torres et al. 1993;Morrissey et al. 2002), this is not surprising as the green tea extract appears to have no effect on lipid peroxidation in the seminal vesicles and prostate.
The administration of green tea had no significant effect on 8-OH-dG adduct formation, lipid peroxidation, MnSOD or catalase expression, in primary tumours from 22 and 26 month-old animals. The increase in 8-OH-dG staining suggests the tumour cells are accumulating oxidative damage. The lack of 4-HNE may be related to the loss of the differentiated epithelial phenotype by the tumour cells, resulting in a decrease in secretory metabolism and secretory products.
These data suggest that long-term consumption of green tea can inhibit or delay the incidence of genitourinary tract tumours in the Lobund-Wistar rat. However, this effect is not sufficient to have a significant effect on tumour growth and development or on the accumulation oxidative damage in the glandular epithelium of the seminal vesicles and ventral prostate in the Lobund-Wistar rat.
Nonetheless there was a significant increase in MnSOD expression and a loss of strong 8-OHdG staining in the ventral prostates of 26-month-old green tea administered animals and those receiving de-ionized water. This may be significant as anti-oxidants have been previously shown to significantly upregulate phase II detoxifying enzymes such as glutathione-S-transferase, catalase and superoxide dismutase (Das et al. 2004). It is possible, that at low concentrations of EGCG and its metabolites, activation of MAPK may lead to ARE-mediated gene expression including phase II detoxifying enzymes. Whereas at higher concentrations of EGCG, sustained activation of MAPKs such as JNK leads to apoptosis (Chen et al. 2000;Morrissey et al. 2007). The ARE-mediated gene expression of phase II detoxifying enzymes may increase the rate of removal of noxious compounds. Low concentrations of EGCG may alter cell cycle kinetics in prostate cancer cells (Gupta et al. 2000;Morrissey et al. 2007).
In conclusion, while the long term consumption of the green tea extract did not appear to influence the accumulation of DNA-adducts in the seminal vesicles or ventral prostate of the Lobund-Wistar rat the activity of the green tea extract may have contributed in some measure to inhibiting the formation and delaying the onset of disease in this model system.
ACKNOWLEDGEMENTS
We would like to thank Kenneth Jones and John Scolaro for their technical assistance; and the staff of the Freimann Life Science Center for their help with animal husbandry. We would like to acknowledge Drs. Glendon Zinser and JoEllen Welsh for their advice and assistance with this study. The financial support of the Coleman Foundation is gratefully acknowledged. CM was supported in part by a Career Development Award from the Pacific Northwest Prostate Cancer SPORE P50 CA097186.
Footnotes
This work was published in part in the proceedings of the VII International Congress of Andrology.
REFERENCES
- Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem.Pharmacol. 2000;60:1051–1059. doi: 10.1016/s0006-2952(00)00385-3. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch.Biochem.Biophys. 2000;376:338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, Ozgok Y, Dimovski A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin.Biochem. 2006;39:176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Blot WJ, Chow WH, McLaughlin JK. Tea and cancer: a review of the epidemiological evidence. Eur.J.Cancer Prev. 1996;5:425–438. [PubMed] [Google Scholar]
- Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch.Pharm.Res. 2000;23:605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J.Agric.Food.Chem. 2001;49:477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- Chung FL, Morse MA, Eklind KI, Xu Y. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis by compounds derived from cruciferous vegetables and green tea. Ann.N.Y.Acad.Sci. 1993;686:186–201. doi: 10.1111/j.1749-6632.1993.tb39174.x. [DOI] [PubMed] [Google Scholar]
- Cohen MB, Heidger PM, Lubaroff DM. Gross and microscopic pathology of induced prostatic complex tumors arising in Lobund-Wistar rats. Cancer Res. 1994;54:626–628. [PubMed] [Google Scholar]
- Costello LC, Franklin RB. Citrate metabolism of normal and malignant prostate epithelial cells. Urology. 1997;50:3–12. doi: 10.1016/S0090-4295(97)00124-6. [DOI] [PubMed] [Google Scholar]
- Das RK, Ghosh S, Sengupta A, Das S, Bhattacharya S. Inhibition of DMBA/croton oil-induced two-stage mouse skin carcinogenesis by diphenylmethyl selenocyanate. Eur.J.Cancer Prev. 2004;13:411–417. doi: 10.1097/00008469-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Dunn JE. Cancer epidemiology in populations of the United States--with emphasis on Hawaii and California--and Japan. Cancer Res. 1975;35:3240–3245. [PubMed] [Google Scholar]
- Ghatak S, Ho SM. Age-related changes in the activities of antioxidant enzymes and lipid peroxidation status in ventral and dorsolateral prostate lobes of noble rats. Biochem.Biophys.Res.Commun. 1996;222:362–367. doi: 10.1006/bbrc.1996.0749. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim.Biophys.Acta. 1999;1427:13–23. doi: 10.1016/s0304-4165(98)00168-8. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol.Appl.Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc.Natl.Acad.Sci.U.S.A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int.J.Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee MJ, Hong J, Li C, Smith TJ, Yang GY, Seril DN, Yang CS. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr.Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- Lau KM, Tam NN, Thompson C, Cheng RY, Leung YK, Ho SM. Age-associated changes in histology and gene-expression profile in the rat ventral prostate. Lab.Invest. 2003;83:743–757. doi: 10.1097/01.lab.0000069519.06988.24. [DOI] [PubMed] [Google Scholar]
- Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, Seril DN, Yang CS. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr.Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- Liao S, Hiipakka RA. Selective inhibition of steroid 5 alpha-reductase isozymes by tea epicatechin-3-gallate and epigallocatechin-3-gallate. Biochem.Biophys.Res.Commun. 1995;214:833–838. doi: 10.1006/bbrc.1995.2362. [DOI] [PubMed] [Google Scholar]
- Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96:239–243. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M, Perez-Campo R, Fernandez A, Barba C, Barja d.Q. Brain glutathione reductase induction increases early survival and decreases lipofuscin accumulation in aging frogs. J.Neurosci.Res. 1993;34:233–242. doi: 10.1002/jnr.490340211. [DOI] [PubMed] [Google Scholar]
- Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61:6025–6028. [PubMed] [Google Scholar]
- Morrissey C, Brown M, O'Sullivan J, Weathered N, Watson RW, Tenniswood M. Epigallocatechin-3-gallate and bicalutamide cause growth arrest and apoptosis in NRP-152 and NRP-154 prostate epithelial cells. Int.J.Urol. 2007;14:545–551. doi: 10.1111/j.1442-2042.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Morrissey C, Buser A, Scolaro J, O'Sullivan J, Moquin A, Tenniswood M. Changes in hormone sensitivity in the ventral prostate of aging Sprague-Dawley rats. J.Androl. 2002;23:341–351. [PubMed] [Google Scholar]
- Naasani I, Seimiya H, Tsuruo T. Telomerase inhibition, telomere shortening, and senescence of cancer cells by tea catechins. Biochem.Biophys.Res.Commun. 1998;249:391–396. doi: 10.1006/bbrc.1998.9075. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J.Biol.Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Pacifici RE, Davies KJ. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 1991;37:166–180. doi: 10.1159/000213257. [DOI] [PubMed] [Google Scholar]
- Paschka AG, Butler R, Young CY. Induction of apoptosis in prostate cancer cell lines by the green tea component, (-)-epigallocatechin-3-gallate. Cancer Lett. 1998;130:1–7. doi: 10.1016/s0304-3835(98)00084-6. [DOI] [PubMed] [Google Scholar]
- Pechenino AS, Brown TR. Superoxide dismutase in the prostate lobes of aging Brown Norway rats. Prostate. 2006;66:522–535. doi: 10.1002/pros.20364. [DOI] [PubMed] [Google Scholar]
- Pollard M. Spontaneous prostate adenocarcinomas in aged germfree Wistar rats. J.Natl.Cancer Inst. 1973;51:1235–1241. doi: 10.1093/jnci/51.4.1235. [DOI] [PubMed] [Google Scholar]
- Pollard M. Dihydrotestosterone prevents spontaneous adenocarcinomas in the prostate-seminal vesicle in aging L-W rats. Prostate. 1998a;36:168–171. doi: 10.1002/(sici)1097-0045(19980801)36:3<168::aid-pros4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Pollard M. Lobund-Wistar rat model of prostate cancer in man. Prostate. 1998b;37:1–4. doi: 10.1002/(sici)1097-0045(19980915)37:1<1::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Shi ST, Wang ZY, Smith TJ, Hong JY, Chen WF, Ho CT, Yang CS. Effects of green tea and black tea on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bioactivation, DNA methylation, and lung tumorigenesis in A/J mice. Cancer Res. 1994;54:4641–4647. [PubMed] [Google Scholar]
- Suzuki K, Oberley TD, Pugh TD, Sempf JM, Weindruch R. Caloric restriction diminishes the age-associated loss of immunoreactive catalase in rat prostate. Prostate. 1997;33:256–263. doi: 10.1002/(sici)1097-0045(19971201)33:4<256::aid-pros6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Tam NN, Nyska A, Maronpot RR, Kissling G, Lomnitski L, Suttie A, Bakshi S, Bergman M, Grossman S, Ho SM. Differential attenuation of oxidative/nitrosative injuries in early prostatic neoplastic lesions in TRAMP mice by dietary antioxidants. Prostate. 2006;66:57–69. doi: 10.1002/pros.20313. [DOI] [PubMed] [Google Scholar]
- Tamano S, Rehm S, Waalkes MP, Ward JM. High incidence and histogenesis of seminal vesicle adenocarcinoma and lower incidence of prostate carcinomas in the Lobund-Wistar prostate cancer rat model using N-nitrosomethylurea and testosterone. Vet.Pathol. 1996;33:557–567. doi: 10.1177/030098589603300511. [DOI] [PubMed] [Google Scholar]
- Tenniswood M. Apoptosis, tumour invasion and prostate cancer. Br.J.Urol. 1997;79(Suppl 2):27–34. doi: 10.1111/j.1464-410x.1997.tb16918.x. [DOI] [PubMed] [Google Scholar]
- Toschi TG, Bordoni A, Hrelia S, Bendini A, Lercker G, Biagi PL. The protective role of different green tea extracts after oxidative damage is related to their catechin composition. J.Agric.Food.Chem. 2000;48:3973–3978. doi: 10.1021/jf000499g. [DOI] [PubMed] [Google Scholar]