SUMMARY
Aims:
We report the safety and feasibility of a 3 days on/11 days off temozolomide regimen for the treatment of recurrent malignant gliomas.
Patients & methods:
Fifteen adult patients were treated; 14 were treated with 300 mg/m2 and one treated with 250 mg/m2.
Results:
We reviewed the toxicity, progression-free survival (PFS), overall survival and objective response rate. Two patients (13%) experienced grade 3 nausea/vomiting and six patients (40%) experienced grade 3 lymphopenia. Dose reduction and treatment delay occurred in eight (53%) cases. One patient discontinued treatment due to uncontrolled nausea/vomiting. Median PFS for glioblastoma patients was 4.1 months and 6-month PFS was 25%. Twelve patients exhibited stable disease (86%), one patient (7%) had progressive disease and one patient (7%) showed a partial response.
Conclusion:
The '3 on/11 off' temozolomide regimen for recurrent high-grade gliomas was tolerable and warrants further study in a larger, prospective study.
KEYWORDS: alternative regimens, chemotherapy, glioblastoma, glioma temodar, safety, temozolomide
Summary points.
Aims
The primary end point was to assess the safety and toxicity of an alternate dosing regimen (300 mg/m2 temozolomide [TMZ] 3 days on/11 days off) in recurrent malignant gliomas.
The secondary end points were objective response rate, progression-free survival (PFS) and overall survival.
Patients & methods
A total of 15 recurrent malignant gliomas, 13 glioblastoma, one anaplastic astrocytoma and one anaplastic pleomorphic xanthroastrocytoma were included.
All 13 glioblastomas had progressed on adjuvant TMZ.
Safety
Toxicity profile similar to reported toxicities of the adjuvant 5/23 TMZ regimen and other dose-intense TMZ regimens, with low frequency of grade 3/4 hematologic toxicities.
Response
At first imaging, 7% demonstrated an objective response (one patient); 86% were stable and 7% progressed.
For the glioblastoma patients, the PFS at 6 months was 25%, median PFS was 113.5 days (4.1 months) and overall survival was 350.5 days (13.8 months).
Conclusion
This 3/11 TMZ regimen was safe and comparable toxicity to other TMZ regimens.
We demonstrated a PFS of approximately 4 months in glioblastoma patients who had progressed on the standard 5/23 TMZ regimen.
High-grade gliomas (HGG) are the most common malignant primary brain tumors in adults, and it is generally recognized that these malignancies have a poor prognosis. While HGG are uncommon compared with other systemic malignancies, they cause a disproportionate amount of morbidity and mortality [1]. Treatment consists of surgical resection to the maximal feasible extent, followed by conformal brain radiotherapy, with concurrent and adjuvant temozolomide (TMZ) [2].
TMZ is a second-generation oral alkylating chemotherapeutic drug that has heralded a new era in the treatment of HGG. A large EORTC-NCIC randomized Phase III trial in patients with glioblastoma found that concomitant and adjuvant TMZ with radiotherapy prolonged the 2-year survival from 10.9 to 27.2% when compared with radiotherapy alone [1]. Follow-up analysis demonstrated a 5-year survival of 9.8% in the patients treated with TMZ and radiotherapy, compared with 1.9% in the patients that received radiotherapy only [3]. This was a significant improvement over previously used adjuvant nitrosourea-based chemotherapy, which only showed an absolute increase in 1-year survival of 6% [4]. TMZ is well tolerated and the systemic bioavailability is close to 100% after oral administration [1,5], and shows some CNS penetration of approximately 20% of plasma [6]. TMZ has become the standard of care for the treatment of patients with newly diagnosed HGG, and recent studies indicate that the drug also has efficacy in low-grade gliomas as well [7–9].
Understanding the mechanism of action of TMZ provides insight into the development of chemoresistance, and suggests ways to overcome this problem. TMZ is hydrolyzed in aqueous solution to 5-(3-methyltriazen-1-y1)-imidazole-4-carboxamide, the active metabolite. 5-(3-methyltriazen-1-y1)-imidazole-4-carboxamide rapidly degrades to a reactive cation that causes methylation of guanines in DNA at the O6 position and subsequently leads to base pair mismatch. Despite the fact that guanine alkylation only constitutes 5% of the DNA adducts, this is primarily responsible for the cytotoxic effect of TMZ [10,11]. Intrinsic or acquired chemoresistance is the most important reason that TMZ is not effective or loses efficacy in HGG. O6-methylguanine-DNA methyltransferase (MGMT) is an enzyme that repairs the O6-alkylation adducts. Increased MGMT expression is thought to play a crucial role in chemoresistance to TMZ. Epigenetic silencing by promoter methylation of the MGMT gene has shown to significantly increase therapeutic benefit of TMZ [12].
MGMT is a suicide enzyme and is irreversibly inactivated after DNA repair. In theory, MGMT can be depleted if production of O6-alkylation adducts would exceed synthesis of MGMT. Prolonged exposure to TMZ would be able to overcome chemoresistance in this manner [13]. Standard adjuvant TMZ therapy consists of 150–200 mg/m2 given daily for 5 days every 28 days [1]. There have been a variety of alternative higher dosing schedules in patients with recurrent HGG [13]. Alternative dosing schedules are primarily focused on depleting MGMT [13]. Higher doses of TMZ demonstrate superiority depleting MGMT in preclinical studies [14]. Most research has focused on three alternative dose-dense regimens: a continuous low-dose daily schedule, a '7 days on/7 days off' and a '21 out of 28 days' schedule. These trials increased the TMZ dose given in one cycle by 2- to 2.8-fold compared with the standard adjuvant 5/23 regimen of 150–200 mg/m2 [13]. In this case series, we evaluated a dosing regimen of 300 mg/m2 TMZ daily for 3 days every 14 days for the treatment of recurrent HGG as previously studied in newly diagnosed patients [15]. The rationale for this regimen is that higher daily doses will allow more drug into the CNS such as has been shown for high-dose methotrexate and high-dose ara-C for CNS lymphoma. We hypothesize that increasing the total daily TMZ dose will improve CNS availability and further deplete MGMT in recurrent HGG and lead to a clinical response, including patients that have progressed on standard-dose TMZ regimens.
Patients & methods
• Patients & study design
This retrospective study was approved by the institutional review board of the UC San Diego Moores Cancer Center. Data from patients that were treated with a 3 days on/11 days off (3/11) TMZ regimen were identified in the neuro-oncology program database and reviewed. Patients included in this study were ≥18 years of age, with the presence of histologically proven HGG (WHO grade ≥3), Karnofsky performance status ≥60, and objective recurrence of disease after standard of care treatment. We collected data about patient characteristics, pathology, prior treatments, number of cycles of the 3/11 TMZ regimen, response and toxicities. Fifteen patients were identified for further evaluation. Thirteen patients developed first progression while on the 5/23 TMZ regimen and two patients after chemoradiation and were started on the 3/11 regimen at this first progression. Two patients were switched to the 3/11 regimen for clinical progression without clear imaging progression.
The primary objective of this study was to assess the toxicity profile of TMZ on the 3/11 schedule, measured by the occurrence of adverse events. Secondary objectives were progression-free survival (PFS), overall survival (OS) and objective response rate (ORR).
• Treatment regimen & safety
TMZ was administered orally once daily for 3 consecutive days at 300 mg/m2 daily, followed by 11 days without treatment. Patients were treated >28 days from prior TMZ regimen. This dose was established as the recommended dose for this schedule in a previous study [15]. Each treatment cycle was 28 days, with TMZ administration on days 1–3 and 15–17. Adverse events and laboratory values were assessed using Common Terminology Criteria for Adverse Events version 4.0. When hematological values were substandard at day 28, treatment was withheld for a minimum of 7 days until recovery. In addition, patients were dose reduced by 50 mg/m2 when they restarted treatment. Patients that exhibited signs of toxicity, which could not be reduced by delay of treatment or dose reduction, received an alternative salvage therapy. Otherwise, patients were kept on the 3/11 dosing schedule until they displayed clinical or radiographic progression of disease.
• Response assessment
Patients were examined at baseline and subsequently prior to every new cycle. Evaluation consisted of a detailed history and physical with neurological examination. In addition, laboratory tests (complete blood count with differential and a complete metabolic panel) were monitored weekly. Contrast-enhanced MRI scans were used to monitor disease progression. Scans were performed at baseline, every 8 weeks, and at clinical suspicion of disease progression. ORR was assessed by Response Assessment in Neuro-Oncology criteria [16]. PFS and OS were defined as the time from the first day of treatment with TMZ 3/11 until progression (PFS) or death (OS).
Results
• Patient characteristics
Fifteen eligible patients were prospectively included from January 2011 through November 2011. Patient characteristics are listed in Table 1; median age was 55 years (range: 33–73 years) and 12 (80%) patients were male. Thirteen patients (86.7%) had a diagnosis of glioblastoma. One patient (6.7%) had a diagnosis of anaplastic astrocytoma and one (6.7%) had a diagnosis of anaplastic pleomorphic xanthoastrocytoma. All 13 patients with glioblastoma had failed previous standard of care radiation and TMZ followed by adjuvant chemotherapy consisting of TMZ (standard 5/23 regimen).
Table 1. . Baseline characteristics of patients on temozolomide 3/11 schedule (n = 15).
| Patient | Sex | Age (years) | Diagnosis | Previous treatment | Past adjuvant CT | Cycles | Reason for enrollment | KPS at start of TMZ 3/11 | Diagnosis to start of TMZ 3/11 (months) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Radiotherapy | |||||||||
| 1 | F | 61 | GBM | STR | IMRT + TMZ | TMZ 5/23 | 2 | Stable disease, patients choice | 90 | 7 |
| 2 | F | 35 | GBM | B | IMRT + TMZ | TMZ 5/23 | 6 | MRI disease progression | 90 | 7 |
| 3 | M | 55 | GBM | B | IMRT + TMZ | TMZ 5/23 | 1 | Clinical progression | 80 | 7 |
| 4 | M | 55 | GBM | B | IMRT | TMZ 5/23 | 7 | MRI disease progression | 60 | 8 |
| 5 | M | 56 | GBM | GTR | IMRT + TMZ | TMZ 5/23 | 11 | MRI disease progression | 80 | 11 |
| 6 | M | 57 | GBM | B | IMRT + TMZ | None | – | Clinical progression | 70 | 4 |
| 7 | M | 36 | AA | B | IMRT + TMZ | TMZ 5/23 | 2 | MRI disease progression | 80 | 5 |
| 8 | M | 33 | APXA | GTR | IMRT + TMZ | TMZ 5/23 | 3 | MRI disease progression | 80 | 12 |
| 9 | M | 33 | GBM | B | IMRT | TMZ 5/23 | 12 | MRI disease progression | 80 | 16 |
| 10 | M | 49 | GBM | STR | IMRT + TMZ | TMZ 5/23 | 2 | MRI disease progression | 90 | 7 |
| 11 | M | 53 | GBM | B | IMRT + TMZ | TMZ 5/23 | 3 | MRI disease progression | 70 | 8 |
| 12 | M | 73 | GBM | GTR | IMRT + TMZ | TMZ 5/23 | 3 | MRI disease progression | 90 | 6 |
| 13 | M | 63 | GBM | GTR | IMRT + TMZ | TMZ 5/23 | 4 | MRI disease progression | 90 | 10 |
| 14 | M | 58 | GBM | STR | IMRT + TMZ | None | – | MRI disease progression | 80 | 3† |
| 15 | F | 56 | GBM | STR | IMRT + TMZ | TMZ 5/23 | 3 | MRI disease progression | 70 | 7 |
†Fluorodeoxyglucose-PET-positive recurrent disease.
AA: Anaplastic astrocytoma; APXA: Anaplastic pleomorphic xanthoastrocytoma; B: Biopsy; CT: Chemotherapy; F: Female; GBM: Glioblastoma; GTR: Gross total resection; IMRT: Intensity modulated radiation therapy; KPS: Karnofsky performance status; M: Male; STR: Subtotal resection; TMZ: Temozolomide.
Three patients with glioblastoma received additional concomitant treatment. Two patients received bevacizumab 10 mg/kg intravenously every 2 weeks in addition to their TMZ treatment and one patient received intrathecal cytarabine liposome injection and methothrexate for leptomeningeal metastasis. In 12 cases, the reason for starting patients on the TMZ 3/11 schedule was radiographic disease progression on MRI, in two cases clinical progression and in one case the patient had a strong preference for this regimen. One patient had hematological toxicity with TMZ tolerability in the past and was started on 250 mg/m2 TMZ instead of 300 mg/m2.
• Safety
In total, 52 cycles of TMZ were given on the 3/11 schedule with a median of three cycles per patient (range: 0.5–9.5). The regimen was generally well tolerated and no grade >3 events were reported. All adverse events ≥grade 2 related to the TMZ 3/11 treatments are listed in Table 2. Grade 3 lymphopenia was seen in six patients (40%) and grade 3 nausea and vomiting were seen in two patients (13.3%), one of these patients chose to discontinue treatment when symptoms did not alleviate after a 50 mg/m2 dose reduction. The most common reason for dose reduction was a decreased thrombocyte count <100 × 109/l. This led to a dose reduction of 50 mg/m2 and holding treatment for at least 1 week in eight (53.3%) patients. After these measures, the platelet count normalized and seven out of eight patients continued treatment. One patient experienced disease progression before he could restart his TMZ treatment and was switched to an alternative salvage therapy. No patients experienced >grade 2 thrombocytopenia. Three patients experienced serious infectious adverse events, which required hospitalization. Two patients were admitted with pneumonia (non-Pneumocystis jirovecii) and one patient developed a jejunal perforation that was attributed to the steroid use by the treating physician (not on bevacizumab). TMZ treatment was withheld during hospitalization. One patient with pneumonia recovered, and was restarted on the TMZ 3/11 schedule after a 50 mg/m2 dose reduction. The second patient with pneumonia showed progression of disease and was switched to an alternative salvage therapy.
Table 2. . Grade ≥2 adverse events related to temozolomide 3/11 treatment regimen.
| Toxicity | Grade 2 | Grade 3 |
|---|---|---|
| Constipation | 2 | – |
| Diarrhea | 1 | – |
| Fatigue | 2 | – |
| Myalgia | 1 | – |
| Nausea | 1 | 2 |
| Thrombocytopenia | 1 | – |
| Vomiting | – | 2 |
| Leukopenia | 1 | – |
| Lymphopenia | 3 | 6 |
• Survival & response
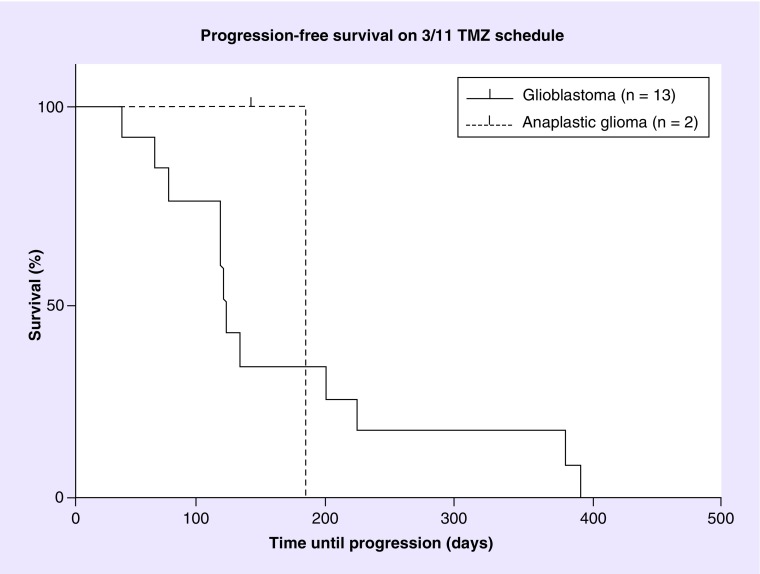
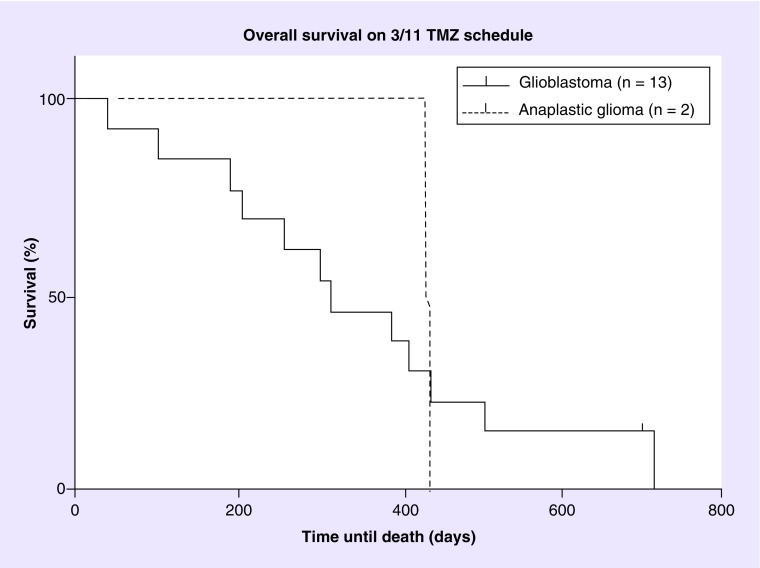
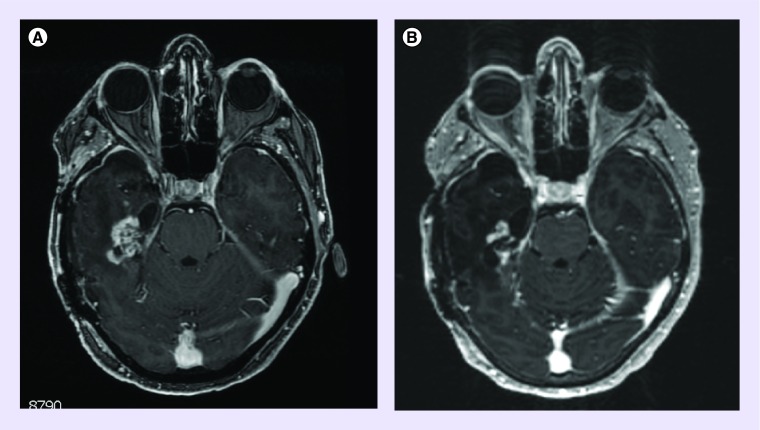
Dosage, length of therapy and treatment response for each patient is listed in Table 3. Out of 15 patients, one patient was alive at the time of analysis. Two patients (one glioblastoma, and one anaplastic pleiomorphic xanthoastrocytoma) were censored for progression after discontinuation due to toxicity or comorbidity, one for nausea and vomiting and one for jejunal perforation. The patient with nausea and vomiting discontinued treatment after 132 days (4.7 months), the patient with jejunal perforation only received half a cycle before he was admitted to the hospital with abdominal pain. The 6-month PFS (PFS6) was 25% and the median PFS was 113.5 (range: 35–388 days [4.1 months]) for the glioblastoma population. In the anaplastic glioma population, only one patient remained after censoring, and demonstrated a PFS of 177 days (6.3 months). The PFS for the glioblastoma group is shown in Figure 1. At the end of the study one patient (6.7%) was still alive. The primary cause of death in 13 of the 14 deceased patients was tumor progression. One patient died of peritonitis after a jejunal perforation induced by steroid use. The median OS for glioblastoma was 350.5 days (12.5 months) and the median OS for all patients was 388 (13.9 months) (Figure 2). Fourteen out of 15 patients were evaluable for objective response and demonstrated an ORR of 7%. Stable disease was seen in 12 (86%) patients, progressive disease in one (7%) patient and partial response in one (7%) patient (Figure 3). The partial response occurred in a patient with a glioblastoma who received no concomitant treatment. Unfortunately, molecular testing of MGMT and IDH1 was not routinely performed at the time of this review and were not available.
Table 3. . Dosage and treatment response of patients on temozolomide 3/11 schedule (n = 15).
| Patient | TMZ 3/11 starting dose (mg/m2) | Cycles TMZ 3/11 | Dose reduced cycles of 250 mg/m2 | Concomitant treatment | PFS (months) | OS (months) | Best response |
|---|---|---|---|---|---|---|---|
| 1 | 300 | 6.5 | 5.5 | – | 7.8 | 25.4 | PR |
| 2 | 300 | 4 | 3 | IT CY + MTX | 4.1 | 14.6 | SD |
| 3 | 300 | 3 | 1.5 | – | 4 | 25 | SD |
| 4 | 300 | 1.5 | 0.5 | – | 2.1 | 7.3 | SD |
| 5 | 300 | 2 | – | – | 2.5 | 6.7 | SD |
| 6 | 300 | 4 | – | Bevacizumab | 4 | 9.1 | SD |
| 7 | 300 | 3 | 1 | – | 6.3 | 15.6 | SD |
| 8 | 300 | 4.5 | 0.5 | – | 4.7† | 15.4 | SD |
| 9 | 300 | 0.5 | – | – | 1.4† | 1.4 | PD |
| 10 | 300 | 4 | – | – | 4.5 | 11.2 | SD |
| 11 | 250 | 0.5 | 0.5 | – | 1.3 | 3.6 | PD |
| 12 | 300 | 9.5 | – | – | 13.5 | 15.6 | SD |
| 13 | 300 | 1 | – | – | 13.9 | 13.9 | SD |
| 14 | 300 | 6 | 3 | – | 6.9 | 18 | SD |
| 15 | 300 | 3 | – | Bevacizumab | 4 | 10.8 | SD |
†Censored.
IT CY+ MTX: Intrathecal cytarabine liposome and methothrexate; OS: Overall survival; PD: Progressive disease; PFS: Progression-free survival; PR: Partial response; SD: Stable disease; TMZ: Temozolomide.
Figure 1. . Progression-free survival for patients on temozolomide 3/11 schedule.
Kaplan–Meier progression-free survival for glioblastoma patients (n = 13; solid line) and anaplastic glioma (n = 2; dashed line) on the TMZ 3 days on/11 days off schedule.
TMZ: Temozolomide.
Figure 2. . Overall survival for patients on temozolomide 3/11 schedule.
Kaplan–Meier overall survival for glioblastoma patients (n = 13; solid line) and anaplastic glioma (n = 2; dashed line) on the TMZ 3 days on/11 days off schedule.
TMZ: Temozolomide.
Figure 3. . MRI of partial response on temozolomide 3/11 schedule.
(A) Shows pretreatment baseline axial T1, post-gadolinium MRI lesion and (B) shows 4 months after temozolomide 3/11 regimen showing a partial response on axial T1, post-gadolinium MRI.
Discussion
Alternative dosing schedules are primarily focused on depleting MGMT [13]. Higher doses of TMZ demonstrate superiority depleting MGMT in preclinical studies [14]. Most research has focused on three alternative dose-dense regimens: a continuous low-dose daily schedule, a '7 days on/7 days off' and a '21 out of 28 days' schedule. These trials increased the TMZ dose given in one cycle by 2- to 2.8-fold compared with the standard adjuvant 5/23 regimen of 150–200 mg/m2 [13]. By administering 300 mg/m2 TMZ daily in a 3/11 schedule the total dose per cycle is increased by approximately 1.8- to 2.4-fold.
The alternative dosing regimens described previously are promising, but prospective data regarding their efficacy is still lacking. Recently, the MRC BR12 trial failed to demonstrate any benefit of the '21 out of 28 days' regimen over the standard '5 out of 28' days regimen in patients with recurrent HGG who were chemotherapy-naive [17]. A recent large randomized Phase III study comparing a standard 5-day TMZ regimen to 21-day regimen in newly diagnosed glioblastoma failed to show improved efficacy with the dose-dense regimen [18]. At this time, no large trial has been able to confirm the effectiveness of dose-dense TMZ treatment [19].
The 3/11 TMZ dosing schedule has been previously described as a treatment for recurrent primary brain tumors by Vera et al. [15]. At the time of that study, the TMZ 5/23 regimen was not yet standard of care. Patients had only received prior nitrosourea-based chemotherapy before they were treated with TMZ on the 3/11 schedule. We therefore describe the first study in which patients who experienced recurrence on the standard 5/23 TMZ regimen were treated on the TMZ 3/11 schedule. In the previous study by Vera et al., the recommended dose for the schedule was found to be 300 mg/m2 because of its favorable toxicity profile. Hematological adverse events described at this dosing level were grade 3 neutropenia (8%) and thrombocytopenia (13%), with nonhematologic toxicities of nausea and vomiting. No grade 4 toxicities were reported. The schedule was well tolerated in the majority of patients and nonhematological toxicity could be managed effectively by prophylactic antiemetics [15]. This toxicity profile is similar to the toxicity profile in the adjuvant phase of the upfront 5/23 regimen by Stupp et al., which reported 12% grade 3–4 thrombocytopenia, 7% leukopenia, and 7% grade 3–4 neutropenia (lymphopenia not reported, although reported as 55% in recurrent anaplastic studies in prescribing information) [1]. In our study, we did have one gastrointestinal perforation that was attributed to high-dose steroids. Colonic diverticular perforation has been strongly associated with corticosteroid use [20,21]. The risk of perforation has been attributed to chronic corticosteroid use resulting in a reduction of collagen turnover, which compromises mucosal barrier function and colonic wall integrity. This is particularly in those who are over 50 years of age and have received a cumulative dose of at least 2240 mg of methylprednisolone given over at least 6 days [22].
Vera et al. report an ORR of 14% and a median PFS of 5.9 months for glioblastoma patients treated with TMZ 200–350 mg/m2 on the 3/11 schedule [15]. In our glioblastoma patient population, we saw one partial response and stable disease was seen in the majority of cases (11/13; 85%). We found a PFS6 of 25% in this population, which compares favorably to a 15% PFS found in historic controls [23]. A study of daily TMZ in recurrent glioblastoma at 50 mg/m2 reported a PFS6 of 23.9%, but 15.8% developed grade 3 lymphopenia [24]. Another recent study of 100–150 mg/m2 given weekly on alternate weeks demonstrated a PFS6 of 29% and an ORR of 8%, but reported 56% with grade 3/4 lymphopenia [25]. Although activity was not a primary end point in either study, when compared with bevacizuma, the PFS6 is lower [26].
In this study, we could not ascertain if the dose-dense TMZ 3/11 regimen can overcome MGMT-mediated resistance. MGMT promoter methylation analysis could only be performed in one patient. Therefore, we could not assess if there is a difference in activity of the TMZ 3/11 regimen between patients with methylated and unmethylated MGMT promoter tumors. Future studies should stratify all patients by MGMT methylation status to confirm the ability of this schedule to overcome MGMT-mediated resistance. In addition, PFS suggests activity, but OS is difficult to assess since patients received various salvage therapies at progression.
Conclusion & future perspective
In this limited, small retrospective study, we confirm that patients can be safely restarted on a high-dose schedule of TMZ in a 3/11 schedule after progression on the standard 5 days on/23 days off regimen. With the alternate 3/11 regimen, there was a median PFS of approximately 4 months in the glioblastoma patients who previously progressed on the adjuvant 5/23 TMZ schedule. Toxicity was similar to the standard 5/23 regimen and other dose-intense TMZ regimens but needs to be further confirmed in a prospective study. We conclude that the 300 mg/m2 TMZ 3/11 schedule as treatment for recurrent HGG is tolerable; however, activity is not as good as bevacizumab. One implication of this study is that early failure may represent low CNS dose of TMZ at 200 mg/m2 rather than DNA repair-mediated resistance. It is unlikely that dose-intense schedules of TMZ can adequately suppress MGMT expression to overcome resistance. Tolcher et al. [14] examined MGMT in peripheral blood lymphocytes, and TMZ concentrations in serum have been reported to be three- to five-fold higher than in the CNS. There is an increasing appreciation that clinical resistance to TMZ is not solely mediated by MGMT. Future studies using the 3/11 regimen and measuring plasma and tumor tissue levels of TMZ and correlating to response will be of interest.
Footnotes
Financial & competing interests disclosure
This research was partly funded by the Dutch Cancer Society to V van Vugt and grant from NIH (NIH 3P30CA023100–25S8) to S Kesari and from Brain Cancer Research funds. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Monje ML, Ramakrishna NR, Young G, et al. Durable response of a radiation-induced, high-grade cerebellar glioma to temozolomide. J. Neurooncol. 2007;84(2):179–183. doi: 10.1007/s11060-007-9354-y. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 5.Diez BD, Statkevich P, Zhu Y, et al. Evaluation of the exposure equivalence of oral versus intravenous temozolomide. Cancer Chemother. Pharmacol. 2010;65(4):727–734. doi: 10.1007/s00280-009-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin. Cancer Res. 2009;15(22):7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kesari S, Schiff D, Drappatz J, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin. Cancer Res. 2009;15(1):330–337. doi: 10.1158/1078-0432.CCR-08-0888. [DOI] [PubMed] [Google Scholar]
- 8.Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68(21):1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 9.Pouratian N, Gasco J, Sherman JH, Shaffrey ME, Schiff D. Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J. Neurooncol. 2007;82(3):281–288. doi: 10.1007/s11060-006-9280-4. [DOI] [PubMed] [Google Scholar]
- 10.Tisdale MJ. Antitumor imidazotetrazines--XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem. Pharmacol. 1987;36(4):457–462. doi: 10.1016/0006-2952(87)90351-0. [DOI] [PubMed] [Google Scholar]
- 11.Baer JC, Freeman AA, Newlands ES, Watson AJ, Rafferty JA, Margison GP. Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br. J. Cancer. 1993;67(6):1299–1302. doi: 10.1038/bjc.1993.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 13.Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro. Oncol. 2009;11(1):69–79. doi: 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br. J. Cancer. 2003;88(7):1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vera K, Djafari L, Faivre S, et al. Dose-dense regimen of temozolomide given every other week in patients with primary central nervous system tumors. Ann. Oncol. 2004;15(1):161–171. doi: 10.1093/annonc/mdh003. [DOI] [PubMed] [Google Scholar]
- 16.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-oncology Working Group. J. Clin. Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 17.Brada M, Gabe R, Stenning S, Al E. 33rd Meeting of the European Society for Medical Oncology. Stockholm, Sweden: 12–16 September 2008. A randomised trial of temozolomide vs PCV for recurrent maligant glioma (MRC BR12) Presented at. [Google Scholar]
- 18.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized Phase III clinical trial. J. Clin. Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen PY. Therapy for recurrent high-grade gliomas: does continuous dose-intense temozolomide have a role? J. Clin. Oncol. 2010;28(12):1977–1979. doi: 10.1200/JCO.2009.27.6014. [DOI] [PubMed] [Google Scholar]
- 20.Piekarek K, Israelsson LA. Perforated colonic diverticular disease: the importance of NSAIDs, opioids, corticosteroids, and calcium channel blockers. Int. J. Colorectal Dis. 2008;23(12):1193–1197. doi: 10.1007/s00384-008-0555-4. [DOI] [PubMed] [Google Scholar]
- 21.Humes DJ, Fleming KM, Spiller RC, West J. Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut. 2011;60(2):219–224. doi: 10.1136/gut.2010.217281. [DOI] [PubMed] [Google Scholar]
- 22.Weiner HL, Rezai AR, Cooper PR. Sigmoid diverticular perforation in neurosurgical patients receiving high-dose corticosteroids. Neurosurgery. 1993;33(1):40–43. doi: 10.1227/00006123-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto Phase II clinical trials. J. Clin. Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 24.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J. Clin. Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 25.Taal W, Segers-Van Rijn JM, Kros JM, et al. Dose dense 1 week on/1 week off temozolomide in recurrent glioma: a retrospective study. J. Neurooncol. 2012;108(1):195–200. doi: 10.1007/s11060-012-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccioni DE, Selfridge J, Mody RR, et al. Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro. Oncol. 2014;16(6):815–822. doi: 10.1093/neuonc/nou028. [DOI] [PMC free article] [PubMed] [Google Scholar]