Summary
Primary effusion lymphoma (PEL) is a rare and aggressive B-cell non-Hodgkin's lymphoma that usually presents with malignant effusions without tumor masses. An extracavitary or solid variant of PEL has also been described. Human herpes virus 8/Kaposi sarcoma-associated herpes virus (HHV-8/KSHV) is universally associated with the pathogenesis of PEL. More than 70% of cases occur with concurrent Epstein-Barr virus infection, but its relation to the pathogenesis is unknown. Patients are found in the context of immunosuppressive states (HIV-1 infection, post-organ transplantation). PEL is usually treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like chemotherapy with antiretroviral therapy if HIV-1 is positive. However, it is generally resistant to chemotherapy with a short median survival of less than 6 months. The optimal treatment for PEL has not been established yet. More intensive chemotherapy, such as dose-adjusted EPOCH (DA-EPOCH; etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) and CDE (cyclophosphamide, doxorubicin, etoposide) are expected to show a favorable prognosis. Recently, the molecular steps in KSHV/HHV-8-driven oncogenesis have begun to be revealed, and molecular targeting therapies such as proteasome, NF-κB, cytokines and surface antigens would provide evidence for their clinical use.
Keywords: Primary effusion lymphoma (PEL), Human herpes virus-8/Kaposi sarcoma-associated herpes virus (HHV-8/KSHV), HIV-1/AIDS, combination antiretroviral therapy (cART), NF-κB, PEL xenograft mouse model
1. Introduction
Primary effusion lymphoma (PEL) is defined as “a large B-cell neoplasm usually presenting as serious effusions without detectable tumor masses, and is universally associated with human herpes virus 8/Kaposi sarcoma-associated herpes virus (HHV-8/KSHV)” by the WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (4th edition) (1). Rare HHV-8-positive lymphomas indistinguishable from PEL present as solid tumor masses, named extracavitary PEL (2).
PEL was first described in 1989 as an AIDS-related lymphoma of uncertain lineage that demonstrated B-cell derivation and included Epstein-Barr virus (EBV) (3). In 1995, Cesarman et al. identified KSHV DNA sequences within a distinct subtype of AIDS-related lymphoma presenting with lymphomatous effusions (4). In 1996, Nador et al. designated this lymphoma as “primary effusion lymphoma”, which is a distinct entity associated with HHV-8/KSHV (5). The majority of cases arise in young and middle-aged homosexual or bisexual men with HIV infection. The disease also occurs in elderly patients and post-transplantation patients (Table 1) (6–9). In the majority of PEL cases, co-infection with EBV has been detected. The latency of EBV is type I and the role of EBV in the PEL pathogenesis is still unclear. HIV-infected individuals have a 60–200-times higher relative risk of developing NHL than the HIV-negative population (10). Among HIV-associated lymphoma, PEL arises more frequently in the HIV-infected population. PEL accounts for approximately 4% of all HIV-associated NHL cases (11,12). PEL is described as a distinct entity and is also included in “lymphomas occurring more specifically in HIV-positive patients” among HIV-associated lymphoma in the WHO classification (13).
Table 1. Etiology of primary effusion lymphoma (PEL).
| Category | Characteristics |
|---|---|
| PEL in elderly persons | Especially in endemic areas of HHV-8/KSHV |
| Post-transplantation PEL | With immunosuppressive therapy |
| HIV-1-related PEL | Homosexuals have a high prevalence of HHV-8/KSHV |
In this review article, therapeutic evidence from case series and the potential use of drugs and novel therapeutic approaches from preclinical evaluation of this refractory lymphoma are discussed.
2. Clinical features
PEL is clinically characterized by lymphomatous effusions in body cavities (formerly called body cavity lymphoma) usually without extracavitary tumor masses, and the clinical symptoms depend on the cavities involved. The most common sites are the pleural, peritoneal and pericardical cavities, and joint space and meningeal space are rarely involved (14). Patients present with dyspnea from pleural or pericardial effusion, or abdominal distension from ascites, which are the results of mass effects of malignant effusions. Patients with PEL with more than one body cavity involved had a median overall survival (OS) of 4 months compared with 18 months in patients with only one cavity involved (15). PEL usually occurs in advanced AIDS patients with a decreased CD4 T-cell count at diagnosis. Approximately half of the patients have pre-existing or develop KS (16). HIV-negative patients with PEL are extremely rare but have been described in elderly men from the Mediterranean region (areas with high prevalence for HHV-8 infection) and immunocompromised patients after solid organ transplantation (17,18). Recently, rare cases of an extracavitary variant of PEL have been observed in the lymph nodes or extranodal sites, such as the gastrointestinal tract, skin, lung, and CNS without lymphomatous effusions (2,19). Since extracavitary PEL has immunoblastic-like and anaplastic features with CD30 expression, it is hard to diagnose without showing the existence of HHV-8/KSHV infection.
3. Laboratory features
Cytologic preparation (Cytospin) of the involved effusion fluid is used for pathological examination and diagnosis. PEL cells show nuclei that are large, round and irregular in shape, with prominent nuclei. The cytoplasm is deeply basophilic with occasional vacuolated cells.
PEL cells typically express a hematolymphoid marker, CD45, but they usually lack expressions of B-cell markers (CD19, CD20, CD79a, surface and cytoplasmic immunoglobulin) (20). PEL cells express plasma cell markers, including CD138, VS38c and MUM-1/IRF4. Moreover, the cells generally express various activation markers, such as CD30, CD38, CD71 and epithelial membrane antigen (EMA). They usually lack T-cell markers (CD2, CD3, CD4, CD5, CD7, CD8), although aberrant expression of T cell antigen may occur. Bcl-6 and c-myc are usually absent, and immunoglobulin gene rearrangement shows monoclonality of B-cell origin. Thus, PEL is a postgerminal center tumor at a pre-terminal stage prior to plasma cell differentiation (21). Transcript profiling confirmed this genesis (22).
The detection of HHV-8 infection in neoplastic cells is needed for definitive diagnosis of PEL (1). Immunohistochemistry for latent nuclear antigen-1 (LANA-1) is currently the standard method to detect the presence of HHV-8/KSHV in lymphoma cells (14). Typically positive results are characterized by a nuclear dot-like pattern. Polymerase chain reaction (PCR) amplification using a DNA extract from lymphoma cells is also useful to detect HHV-8/KSHV and measure peripheral blood HHV-8/KSHV viral load (23) as HHV-8 can be detected in the plasma at the onset of PEL (24). Evidence of EBV infection is most reliably detected by in situ hybridization for EBV-encoded small RNA (EBER), while immunohistochemical staining for EBV latent membrane protein-1 (LMP-1) is negative (25).
High levels of interleukins (IL-6, IL-10) and soluble forms of antigens such as soluble CD30 might also help in the identification of a clinical marker for treatment (26,27). Onset of PEL is mostly related with immunosuppression (6) and is associated with HIV load and CD4 cell count in HIV-1 related PEL (28).
4. Molecular genetics
The HHV-8/KSHV genome has a 145 kb gene and PEL cells usually contain 40–80 copies of HHV-8/KSHV episomes per cell and express HHV-8/KSHV latent genes (Table 2, Figures 1 and 2). Five latent gene products, which are thought to play significant roles in PEL, are latency-associated nuclear antigen-1 (LANA-1), LANA-2/vIRF-3, viral cyclin (v-Cyclin), viral FLICE inhibitory protein (v-FLIP) and Kaposin (K12). LANA-1 binds to p53 and RB protein, inhibits their function, and impairs the apoptosis of HHV-8/KSHV-infected cells (29,30). v-Cyclin (viral homologue of cyclin D), binds to cyclin-dependent kinase 6 (CDK6) and inactivates RB protein (31). v-FLIP, a viral homologue of FLICE inhibitory protein (c-FLIP), inhibits apoptosis by blocking Fas-and TNF-mediated caspase activation and activates NF-κB thorough activation of IKKγ (32,33). Kaposin A has oncogenic potential through cytokesin-1 (34). Kaposin B stabilizes cytokine expressions, such as IL-6 and granulocyte-macrophage colony stimulating factor (GM-CSF), by stabilizing cytokine mRNA containing AU-rich elements, which plays a role in latent HHV-8/KSHV infection (35). vIL-6 is a homologue of cellular IL-6 (24.6% amino acid sequence identity), directly binds to gp130 without the cooperation of the IL-6 high affinity receptor, and triggers the JAK/STAT (Janus tyrosine kinases/signal transducers and activators of transcription) pathway (36). LANA-2/vIRF-3 has a potential role in developing drug resistance by binding to polymerized microtubules, reducing their stability (37). Furthermore, HHV-8/KSHV encodes homologous human interferon response factors (IRF), which inhibit interferon-mediated effects (38). These viral proteins are essential for the survival of PEL cells and could be a target of PEL treatment. The major latency-associated region of the HHV-8/KSHV genome also encodes 12 micro (mi) RNA genes. Of note, miR-K12-11 is a HHV-8/KSHV miRNA sharing full seed sequence homology with human miRNA, miR-155. Given that miR-155 promotes plasma cell differentiation, miR-K12-11 might contribute to HHV-8/KSHV lymphomagenesis (39,40).
Table 3. Classification and differential diagnosis of non-Hodgkin's lymphomas involving the serous body cavities and presenting as effusion lymphomas.
| Type of lymphoma | Primary effusion lymphoma | HHV-8/KSHV-unrelated PEL-like lymphoma | Extranodal large cell lymphoma | Extranodal Burkitt's lymphoma | Systemic lymphomas or body cavity-based mass-forming lymphomas |
|---|---|---|---|---|---|
| effusion | primary | primary | primary | primary | secondary |
| HHV-8/KSHV | + | − | − | − | − |
| EBV | + | +/− | +/− | +/− | various |
| CD20 | − | + (70–80%) | + | + | + |
| c-myc | − | − | − | + | − |
| Morphology | IBL/ALCL | IBL/DLBCL | BL | Various histoypes |
Attenuated from (13). IBL: Immunoblastic lymphoma, ALCL: Anaplastic large cell lymphoma, DLBCL: diffuse large B-cell lymphoma, BL: Burkitt's lymphoma.
Figure 1.
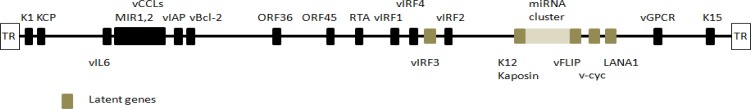
HHV-8/KSHV genome and viral gene expression in PEL. The latent HHV-8 genes LANA, v-cyclin, vFLIP, K12/Kaposine, and vIRF3 are shown as grey boxes.
Figure 2.
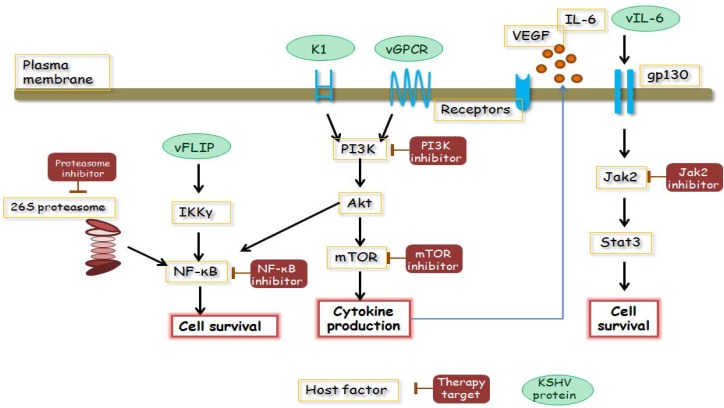
Potential candidate targeting molecules for the treatment of PEL. PEL constitutively activates NF-κB, JAK/STAT and PI3K/AKT/mTOR pathways, which are essential for the survival of PEL cells. These signaling pathways, cytokines and surface antigens are considered as targeting molecules for treatment.
Table 2. HHV-8/KSHV-encoded protein implicated in tumorigenesis.
| HHV-8/KSHV -encoded protein | Host cell homologue | Possible function |
|---|---|---|
| LANA-1 | Inhibition of p53, Rb and GSK3β | |
| Induce hTERT, Id-1 and IL-6 | ||
| LANA-2/vIRF-3 | Interferon regulatory factor | Inhibition of p53 |
| v-Cyclin | D-Type cyclin | Inactivation of pRB promotes G1 to S phase transition |
| v-FLIP | FLICE inhibitory protein (c-FLIP) | Activation of NF-κB pathway, |
| Inhibition of CD95L (FasL) and TNF induced apoptosis | ||
| Kaposin (K12) | Kaposin A: oncogenic potential | |
| Kaposin B: stabilize cytokine | ||
| K1 | ||
| Transformation | ||
| v-MIPs | CC chemokines | Chemoattraction, angiogenesis |
| v-IL-6 | IL-6 | Growth factor |
| v-Bcl2 | Bcl-2 family proteins | Inhibition of apoptosis |
| v-GPCR | IL-8 GPCR | Cellar growth signal |
| v-Ox-2 | N-CAM family proteins | Cellular adhesion molecule |
| ORF4 | CD21/CR2 complement binding protein | Escape form host immune response |
Approximately 50%–80% of PEL are co-infected with EBV (5). EBV gene expression in dually infected PEL cells is restricted to EBNA-1 and EBER (latency I). Although EBV-positive PEL exhibits a different pattern of gene expression from EBV-negative PEL, there is no evidence that EBV-positive PEL presents with the characteristic clinical manifestation, and the contribution of PEL features is unknown (13).
5. Differential diagnosis
The most common differential diagnoses in cases of PEL are other types of non-Hodgkin's lymphoma with lymphomatous effusion, such as diffuse large B cell lymphoma (DLBCL) and Burkitt's lymphoma (BL) with secondary effusion (Table 3). Recently classified HHV-8-negative PEL-like lymphoma shows similar clinical and laboratory features, except for being HHV-8/KSHV negative and CD20 positive (41), and the term “HHV-8/KSHV-negative effusion-based lymphoma” was proposed (42). This lymphoma also presents with lymphomatous effusion without detectable masses. HHV-8/KSHV is negative in all cases. Hepatitis C virus (HCV) and EBV are positive in nearly 30% of cases, respectively. Patients are generally elderly and have underlying medical conditions, such as cirrhosis or cardiovascular dysfunction. It is also considered to be associated with fluid overload states. Confirmation of the typical morphology and immunophenotype described previously and evidence of HHV-8/KSHV infection are required for the diagnosis of PEL (1).
Pyothorax-associated lymphoma (PAL) is a non-Hodgkin's B-cell lymphoma developing in the pleural cavity of patients after a long-term history of pyothorax resulting from an artificial pneumothorax for the treatment of pulmonary tuberculosis or tuberculous pleuritis (43). PAL is more common in Japan and usually occurs in elderly men with a history of pulmonary tuberculosis or tuberculous pleuritis. PAL usually shows the diffuse proliferation of large cells of B-cell type (diffuse large B-cell lymphoma; DLBL), and is strongly associated with EBV infection with the expression of EBV latent genes such as EBNA-2, LMP-1, together with EBNA-1 (latency III) and HHV-8/KSHV negative (44).
Plasmablastic lymphoma (PBL) is an aggressive non-Hodgkin's B-cell lymphoma that presents at both oral and extra-oral sites (especially the gastrointestinal tract) of chronically HIV-infected immunosuppressed young men. The morphology shows plasmablastic differentiation and plasma cell markers (CD20−, CD38+, CD138+) in all cases. EBV is detected in most cases, but HHV-8/KSHV is negative.
Because of their similar morphology and lack of a B-cell marker, T-cell anaplastic large cell lymphoma is sometimes confused with PEL (45). Immunohistochemistry for anaplastic lymphoma kinase (ALK) and the TCR gene rearrangement would be helpful in these cases.
If the morphological findings show large immunoblastic to plasmablastic with anaplastic morphology, virological analysis of HHV-8/KSHV and EBV is essential for diagnosis. HHV-8/KSHV can be demonstrated by PCR, in situ hybridization or by immuno-histochemistry against LANA-1, which is consistently expressed in HHV-8/KSHV infected cells.
6. Treatment
The prognosis of PEL is extremely poor with few long-term survivors. Owing to the rarity of the disease, there are very few longitudinal observational series of patients and prospective randomized clinical studies are not feasible; thus, treatment is mostly based on expert consensus opinion and small case series.
6.1. Chemotherapy
Traditional chemotherapy with cyclophosphamide, doxorubicin, vincristine and predonisolone (CHOP) is the most common chemotherapy regimen for treating non-Hodgkin's lymphoma (NHL), and has been attempted for the treatment of PEL; however, the prognosis of patients with PEL remains extremely poor. Boulanger et al. showed a median survival of 6.2 months and a 1-year overall survival rate of approximately 40% (46). Studies using CHOP-like regimens resulted in similar outcomes. Recently, an anti-CD20 monoclonal antibody (Rituximab) -containing regimen became the standard therapy for CD20-positive B cell NHL. Although most PEL cases do not express CD20, Rituximab can be considered for the treatment of rare cases of CD20-positive PEL (47,48).
Methotrexate-containing regimens, such as high-dose methotrexate and CHOP with methotrexate, have been studied. However, methotrexate accumulates in effusions, resulting in delayed clearance and an increased risk of systemic toxicity. Infusion therapy such as dose-adjusted EPOCH (DA-EPOCH; etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) and CDE (cyclophosphamide, doxorubicin, etoposide) has been shown to be well tolerated and effective in the treatment of AIDS-related aggressive B-cell lymphomas, and can be applied for the treatment of PEL (21).
6.2. Stem cell transplantation
The efficacy of high-dose chemotherapy with autologous stem cell transplantation (ASCT) for chemotherapy-sensitive relapsed disease in HIV-associated lymphoma has been reported (49,50). Only two cases have been reported in PEL (51,52): one failed to recover from PEL, while the other was successfully treated with high-dose chemotherapy with ASCT following complete remission 12 months post-transplantation. Successful treatment with reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation in second remission has been reported (53). This patient remained in complete remission 31 months post-transplantation only on cART with an undetectable HIV viral load.
6.3. Combination antiretroviral therapy (cART)
Prior to the administration of cART, the therapeutic results with chemotherapy were unsatisfactory in HIV-1 associated lymphomas. The prognostic impact of cART in combination with chemotherapy has been reported in PEL (46), although the impact of cART is lower than for other HIV-1 associated lymphomas such as DLBCL and BL (54,55). In addition, complete remission of PEL patients with cART but without chemotherapeutic drugs has been reported (56–58). Thus, implementation of cART is recommended when treating PEL patients with HIV-1 infection.
It is important to avoid major drug-drug interactions during chemotherapy (Table 4). Among antiretroviral agents, protease inhibitors modify the metabolism of cytotoxic drugs and potentiate myelotoxicity by inhibiting the CYP3A4 enzyme to various extents (59). Thus, anticancer drugs, which rely on Cytochrome P450, should be used carefully with protease inhibitorbased regimens to avoid inadvertent toxicity. Currently, integrase strand transfer inhibitors (INSTI), raltegravir and dolutegravir, are recommended by many experts to anchor cART regimens in patients receiving chemotherapy (Table 5). Another INSTI, elvitegravir is only available as a component of four-drug combination product, which contains potent CYP3A inhibitor.
Table 4. Adverse effects of anti-HIV-1 reagents during chemotherapy.
| Agents | Adverse effects |
|---|---|
| AZT | Bone marrow suppression, contraindication |
| d4T/ddI | Peripheral nerve disorder/ileus (avoid with VCR) Liver dysfunction (toxic for mitochondria) |
| Protease inhibitor (PI) RTV > IDV = APV > NFV> = SQV | High blood level of anti-cancer agents (inhibition of CYP450-3A4) |
| NNRTI Efavirenz (EFV) | Reduced function of anti-cancer agents (activate CYP450) |
| Nevirapine (NVP) | |
| Abacavir (ABC) | Hypersensitivity |
| Tenofovir (TDF) | Renal dysfunction |
Table 5. Anti-HIV-1 treatment during cancer chemotherapy.
| Recommended therapy | EFV + TDF/FTC |
| RAL + TDF/FTC | |
| Alternative therapy | EFV + ABC/3TC |
| RAL + ABC/3TC |
EFV, efavirenz; TDF, tenofovir; FTC, emtricitabine; RAL, raltegravir; ABC, abacavir; 3TC, lamivudine. Summarized from lines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents (http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf).
6.4. Treatment of opportunistic infections
Supportive treatment of opportunistic infections is important in HIV-infected patients and post-organ transplantation patients with PEL. Granulocyte-colony stimulating factor (G-CSF) helps reduce chemotherapy-induced neutropenic complications. All patients need to receive prophylaxis for Pneumocystis carinii pneumonia with trimethoprim-sulfamethoxazole, regardless of the CD4 cell count. For patients who have severe neutropenia with chemotherapy, alternation of trimethoprim-sulfamethoxazole for Pneumocystis carinii prophylaxis can be considered, including dapsone or aerosolized pentamidine. Infectious complications may be minimized by using prophylactic fluroquinolone antibiotics and azoles during periods of protracted neutropenia. Prophylaxis for Mycobacterium avium complex (MAC), Toxoplasmosis and other opportunistic infection should be also considered as PEL usually arises in an immunodeficient state and chemotherapy induces myelosuppression. Prophylaxis against infection during chemotherapy may include drugs that interact with cART and anticancer agents. Careful attention must be paid for the adverse effects and drug-drug interaction among these agents (60).
6.5. Clinical trial
On the basis of recent preclinical data and translational studies, several new targeted therapies are being explored, and several clinical trials have been performed based on expert consensus opinions and evidence in preclinical studies.
A proteasome inhibitor, bortezomib, is expected to show clinical effects against PEL. Despite the promising results of in vitro experiments and a mouse model (61,62), bortezomib treatment either alone or in combination with chemotherapy showed no clinical improvement (20,63). The optimization of treatment protocol and combination therapy with bortezomib may be needed to show the preferable effects of bortezomib.
Lenalidomide is an immunomodulatory drug that is commonly used to treat newly diagnosed and relapsed multiple myeloma as well as a variety of hematological malignancies. It exerts its antitumor action through various mechanisms, such as activation of the immune system, inhibition of angiogenesis and direct antineoplastic effects. Treatment with lenalidomide has never been reported in PEL patients with favorable results (64). As lenalidomide was also successfully used to treat three patients with advanced refractory Kaposi sarcoma, this novel agent is expected to be used in prospective studies.
Antiviral treatment can be induced to effect the lytic phase of HHV-8/KSHV viral replication. Complete remission has been reported after the administration of an antiviral nucleotide analogue, cidofovir (57,65,66), an antiviral agent with broad activity against multiple DNA viruses, inducing lytic replication of HHV-8/KSHV.
PEL cells are quite sensitive to irradiation in culture and in a xenograft mouse model (67). It was reported that chemotherapy-refractory PEL patients achieved remission and survived for more than 12 months with radiation therapy (68). Irradiation therapy should be considered as part of the treatment recommendation for patients with chemotherapy-refractory PEL-associated solid masses or localized effusions.
6.6. Mental support
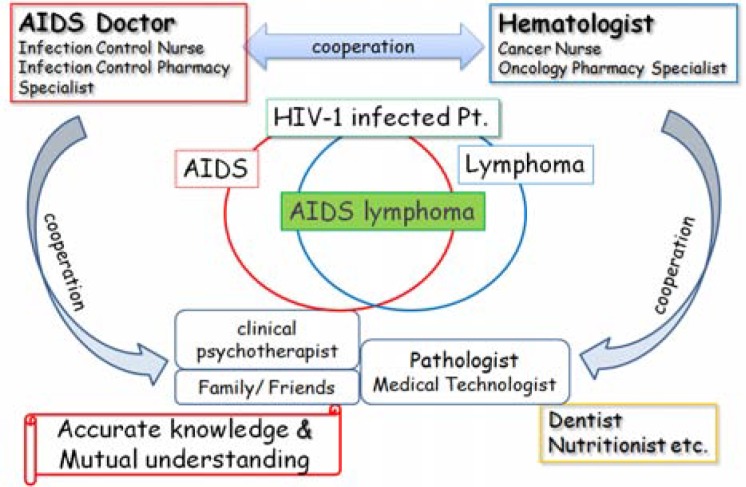
There are considerable difficulties in the treatment of AIDS-related lymphoma, including the mental care of patients. The close cooperation of AIDS doctors and hematologists, intensive care by nurse specialists, support from pharmacy specialists, and other co-medical staff is essential. Mental care from a psychiatrist, clinical psychotherapist, and the patient's family and friends are quite supportive for patients. It is especially important to ensure an organic link with the specialist as well as family and friends for treatment (Figure 3).
Figure 3.
Treatment and support of AIDS-related malignant lymphoma -Team Medical Care-
6.7. Molecular-targeted preclinical studies
Since PEL cells display constitutive activity of many signaling pathways and survival, including NF-κB, JAK/STAT and PI3K/AKT pathways, these molecules and HHV-8/KSHV latent proteins are considered ideal for targeted therapy (Figure 2). In particular, vFLIP has the ability to activate the NF-κB pathway by binding to the IκB kinase (IKK) complex (32,33), and NF-κB activation is known to be the key player in PEL oncogenesis, so various NF-κB and proteosome inhibitors have been investigated in a preclinical trial. Xenograft PEL mouse models and in vitro culture of PEL cell lines were used in preclinical studies, and promising preclinical results were reported with multiple NF-κB inhibitors, such as cepharanthine (69), diethyldithiocarbamate (70), berberine (71), and heat-shock protein 90 (72,73). Xenograft mouse models using severe immunodeficient mice are a powerful tool to confirm the effects and adverse effects of candidate reagents in a preclinical study.
The PI3K/AKT pathway, JAK2/STAT3 pathway and mTOR are also activated in PEL cell lines and could be promising targets (74–76). Several inhibitors are currently undergoing clinical trials in patients with hematological malignancies and can be used for the treatment of PEL in the near future (77).
Interferon-α and AZT induced TRAIL-mediated apoptosis of PEL (78,79). IFN-α upregulates TRAIL in PEL cells while AZT sensitizes them to TRAIL, resulting in the activation of a suicide program. The efficacy of this approach needs to be validated in clinical trials.
6.8. Immunotherapy
Although rituximab, a chimeric anti-CD20 antibody, has provided a significant survival advantage for B-cell NHL in combination with standard chemotherapy, rituximab does not play a significant therapeutic role in PEL because CD20 is not usually expressed on the surface of PEL cells. Rare cases expressing CD20 have been reported to respond to rituximab (47).
CD30 is expressed significantly in case of PEL. Brentuximab vedotin (SGN-35) is an antibody-drug conjugate in which a chimeric anti-CD30 antibody is combined with the synthetic microtubule-disrupting agent monomethylauristatin E (MMAE) (80). Since treatment with brentuximab vedotin also prolonged the survival of a PEL xenograft mouse model (81), brentuximab is expected to be a candidate for the treatment of PEL.
PEL cells secrete vascular endothelial growth factor (VEGF)-A (82), and treatment with mouse anti-human VEGF-A monoclonal antibody inhibited the development of ascites in a xenograft mouse model. Because bevacizumab, a humanized VEGF-A monoclonal antibody, is clinically used for the treatment of a variety of human cancers, including colorectal, non-small-cell lung, ovarian and metastatic renal cell carcinoma (83), it is also expected to be a novel target of treatment.
7. Conclusion
PEL is a rare but aggressive form of NHL, mostly arising in immunodeficient patients. PEL is commonly resistant to conventional chemotherapy and has a poor prognosis. Currently, more intensive chemotherapy with cART is recommended. The management of opportunistic infection is also needed since PEL arises in immunodeficient states. Drug interaction between anti-cancer reagents and cART, especially protease inhibitors, should be carefully monitored in HIV-1-positive individuals. Close communication among the oncologist, the patient's primary HIV-treating physician, and co-medical staff is needed for the intensive treatment of AIDS-related PEL patients. It is also important to avoid drug interactions in chemotherapy. Several molecular-targeted therapies are in clinical trial and preclinical stages, and their clinical use is anticipated. Since PEL is mostly associated with immunodeficient states, early diagnosis and treatment of HIV-1 may prevent the onset of PEL.
Acknowledgements
This study was supported in part by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour, and Welfare of Japan (H25-AIDS-I-002), and Grants-in-Aid for Science Research (No. 25114711) from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- 1. Said J, Cesarman E. Primary effusion lymphoma. In: Steven H, Swerdlow E, Campo M.D., Lee Nancy, Harris M.D., Elaine S., Jaffe M.D., editor. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. Lyon: International Agency for Research on Cancer, 2008:260-261 [Google Scholar]
- 2. Pan ZG, Zhang QY, Lu ZB, Quinto T, Rozenvald IB, Liu LT, Wilson D, Reddy V, Huang Q, Wang HY, Ren YS. Extracavitary KSHV-associated large B-Cell lymphoma: A distinct entity or a subtype of primary effusion lymphoma? Study of 9 cases and review of an additional 43 cases. Am J Surg Pathol. 2012; 36:1129-1140 [DOI] [PubMed] [Google Scholar]
- 3. Knowles DM, Inghirami G, Ubriaco A, Dalla-Favera R. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989; 73:792-799 [PubMed] [Google Scholar]
- 4. Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995; 332:1186-1191 [DOI] [PubMed] [Google Scholar]
- 5. Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996; 88:645-656 [PubMed] [Google Scholar]
- 6. Riva G, Luppi M, Barozzi P, Forghieri F, Potenza L. How I treat HHV8/KSHV-related diseases in posttransplant patients. Blood. 2012; 120:4150-4159 [DOI] [PubMed] [Google Scholar]
- 7. Song JY, Jaffe ES. HHV-8-positive but EBV-negative primary effusion lymphoma. Blood. 2013; 122:3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Y, Hou Y, Hu Q, Su J, Zeng H, Tan Y. A rare case of HHV-8-positive/HIV-negative/EBV-negative primary effusion lymphoma in a renal transplant recipient. Cytopathology. 2012; 23:137-139 [DOI] [PubMed] [Google Scholar]
- 9. Mettler TN, Cioc AM, Singleton TP, McKenna RW, Pambuccian SE. Pleural primary effusion lymphoma in an elderly patient. Diagn Cytopathol. 2012; 40:903-905 [DOI] [PubMed] [Google Scholar]
- 10. Beral V, Peterman T, Berkelman R, Jaffe H. AIDSassociated non-Hodgkin lymphoma. Lancet. 1991; 337:805-809 [DOI] [PubMed] [Google Scholar]
- 11. Simonelli C, Spina M, Cinelli R, Talamini R, Tedeschi R, Gloghini A, Vaccher E, Carbone A, Tirelli U. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: A single-institution study. J Clin Oncol. 2003; 21:3948-3954 [DOI] [PubMed] [Google Scholar]
- 12. Ota Y, Hishima T, Mochizuki M, et al. Classification of AIDS-related lymphoma cases between 1987 and 2012 in Japan based on the WHO classification of lymphomas, fourth edition. Cancer Med. 2014; 3:143-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009; 113:1213-1224 [DOI] [PubMed] [Google Scholar]
- 14. Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007; 12:569-576 [DOI] [PubMed] [Google Scholar]
- 15. Castillo JJ, Shum H, Lahijani M, Winer ES, Butera JN. Prognosis in primary effusion lymphoma is associated with the number of body cavities involved. Leuk Lymphoma. 2012; 53:2378-2382 [DOI] [PubMed] [Google Scholar]
- 16. Ansari MQ, Dawson DB, Nador R, Rutherford C, Schneider NR, Latimer MJ, Picker L, Knowles DM, McKenna RW. Primary body cavity-based AIDS-related lymphomas. Am J Clin Pathol. 1996; 105:221-229 [DOI] [PubMed] [Google Scholar]
- 17. Jones D, Ballestas ME, Kaye KM, Gulizia JM, Winters GL, Fletcher J, Scadden DT, Aster JC. Primary-effusion lymphoma and Kaposi's sarcoma in a cardiac-transplant recipient. N Engl J Med. 1998; 339:444-449 [DOI] [PubMed] [Google Scholar]
- 18. Klepfish A, Sarid R, Shtalrid M, Shvidel L, Berrebi A, Schattner A. Primary effusion lymphoma (PEL) in HIV-negative patients - a distinct clinical entity. Leuk Lymphoma. 2001; 41:439-443 [DOI] [PubMed] [Google Scholar]
- 19. Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol. 2004; 28:1401-1416 [DOI] [PubMed] [Google Scholar]
- 20. Boulanger E, Meignin V, Oksenhendler E. Bortezomib (PS-341) in patients with human herpesvirus 8-associated primary effusion lymphoma. Br J Haematol. 2008; 141:559-561 [DOI] [PubMed] [Google Scholar]
- 21. Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012; 119:3245-3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenner RG, Maillard K, Cattini N, Weiss RA, Boshoff C, Wooster R, Kellam P. Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphoma has a plasma cell gene expression profile. Proc Natl Acad Sci USA. 2003; 100:10399-10404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan L, Milligan L, Michaeli J, Cesarman E, Knowles DM. Polymerase chain reaction detection of Kaposi's sarcoma-associated herpesvirus-optimized protocols and their application to myeloma. J Mol Diagn. 2001; 3:32-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simonelli C, Tedeschi R, Gloghini A, Bortolin MT, Spina M, Bidoli E, Cinelli R, De Paoli P, Carbone A, Tirelli U. Characterization of immunologic and virological parameters in HIV-infected patients with primary effusion lymphoma during antiblastic therapy and highly active antiretroviral therapy. Clin Infect Dis. 2005; 40:1022-1027 [DOI] [PubMed] [Google Scholar]
- 25. Cesarman E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 2011; 305:163-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drexler HG, Meyer C, Gaidano G, Carbone A. Constitutive cytokine production by primary effusion (body cavity-based) lymphoma-derived cell lines. Leukemia. 1999; 13:634-640 [DOI] [PubMed] [Google Scholar]
- 27. Michai M, Goto H, Hattori S, Vaeteewoottacharn K, Wongkham C, Wongkham S, Okada S. Soluble CD30: A possible serum tumor marker for primary effusion lymphoma. Asian Pac J Cancer Prev. 2012; 13:4939- 4941 [DOI] [PubMed] [Google Scholar]
- 28. Tedeschi R, Enbom M, Bidoli E, Linde A, De Paoli P, Dillner J. Viral load of human herpesvirus 8 in peripheral blood of human immunodeficiency virus-infected patients with Kaposi's sarcoma. J Clin Microbiol. 2001; 39:4269-4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friborg J, Jr., Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999; 402:889-894 [DOI] [PubMed] [Google Scholar]
- 30. Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med. 2000; 6:1121-1127 [DOI] [PubMed] [Google Scholar]
- 31. Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997; 390:184-187 [DOI] [PubMed] [Google Scholar]
- 32. Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, Scaffidi C, Krammer PH, Peter ME, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997; 386:517-521 [DOI] [PubMed] [Google Scholar]
- 33. Matta H, Chaudhary PM. Activation of alternative NF-κB pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc Natl Acad Sci USA. 2004; 101:9399-9404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kliche S, Nagel W, Kremmer E, Atzler C, Ege A, Knorr T, Koszinowski U, Kolanus W, Haas J. Signaling by human herpesvirus 8 kaposin A through direct membrane recruitment of cytohesin-1. Mol Cell. 2001; 7:833-843 [DOI] [PubMed] [Google Scholar]
- 35. McCormick C, Ganem D. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science. 2005; 307:739-741 [DOI] [PubMed] [Google Scholar]
- 36. Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997; 272:19625-19631 [DOI] [PubMed] [Google Scholar]
- 37. Munoz-Fontela C, Marcos-Villar L, Hernandez F, Gallego P, Rodriguez E, Arroyo J, Gao SJ, Avila J, Rivas C. Induction of paclitaxel resistance by the Kaposi's sarcoma-associated herpesvirus latent protein LANA2. J Virol. 2008; 82:1518-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao SJ, Boshoff C, Jayachandra S, Weiss RA, Chang Y, Moore PS. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene. 1997; 15:1979-1985 [DOI] [PubMed] [Google Scholar]
- 39. Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, Braich R, Manoharan M, Soutschek J, Ohler U, Cullen BR. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007; 450:1096- 1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol. 2007; 81:12836-12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu W, Youm W, Rezk SA, Zhao X. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma: Report of a rare case and review of 54 cases in the literature. Am J Clin Pathol. 2013; 140:258-273 [DOI] [PubMed] [Google Scholar]
- 42. Alexanian S, Said J, Lones M, Pullarkat ST. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol. 2013; 37:241-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakatsuka S, Yao M, Hoshida Y, Yamamoto S, Iuchi K, Aozasa K.Pyothorax-associated lymphoma: A review of 106 cases. J Clin Oncol. 2002; 20:4255-4260 [DOI] [PubMed] [Google Scholar]
- 44. Aozasa K, Takakuwa T, Nakatsuka S. Pyothorax-associated lymphoma: A lymphoma developing in chronic inflammation. Adv Anat Pathol. 2005; 12:324-331 [DOI] [PubMed] [Google Scholar]
- 45. Chan AC, Chan JK, Yan KW, Kwong YL. Anaplastic large cell lymphoma presenting as a pleural effusion and mimicking primary effusion lymphoma. A report of 2 cases. Acta Cytol. 2003; 47:809-816 [DOI] [PubMed] [Google Scholar]
- 46. Boulanger E, Gerard L, Gabarre J, Molina JM, Rapp C, Abino JF, Cadranel J, Chevret S, Oksenhendler E. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005; 23:4372-4380 [DOI] [PubMed] [Google Scholar]
- 47. Lim ST, Rubin N, Said J, Levine AM. Primary effusion lymphoma: Successful treatment with highly active antiretroviral therapy and rituximab. Ann Hematol 2005; 84:551-552 [DOI] [PubMed] [Google Scholar]
- 48. Carbone A, Gloghini A. KSHV/HHV8-associated lymphomas. Br J Haematol. 2008; 140:13-24 [DOI] [PubMed] [Google Scholar]
- 49. Re A, Cattaneo C, Michieli M, et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J Clin Oncol. 2003; 21:4423-4427 [DOI] [PubMed] [Google Scholar]
- 50. Bayraktar UD, Ramos JC, Petrich A, Gupta N, Lensing S, Moore PC, Reid EG, Aboulafia DM, Ratner L, Mitsuyasu R, Cooley T, Henry DH, Barr P, Noy A. Outcome of patients with relapsed/refractory acquired immune deficiency syndrome-related lymphoma diagnosed 1999-2008 and treated with curative intent in the AIDS Malignancy Consortium. Leuk Lymphoma. 2012; 53:2383-2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waddington TW, Aboulafia DM. Failure to eradicate AIDS-associated primary effusion lymphoma with highdose chemotherapy and autologous stem cell reinfusion: Case report and literature review. AIDS Patient Care STDS. 2004; 18:67-73 [DOI] [PubMed] [Google Scholar]
- 52. Won JH, Han SH, Bae SB, Kim CK, Lee NS, Lee KT, Park SK, Hong DS, Lee DW, Park HS. Successful eradication of relapsed primary effusion lymphoma with high-dose chemotherapy and autologous stem cell transplantation in a patient seronegative for human immunodeficiency virus. Int J Hematol. 2006; 83:328- 330 [DOI] [PubMed] [Google Scholar]
- 53. Bryant A, Milliken S. Successful reduced-intensity conditioning allogeneic HSCT for HIV-related primary effusion lymphoma. Biol Blood Marrow Transplant. 2008; 14:601-602 [DOI] [PubMed] [Google Scholar]
- 54. Weiss R, Mitrou P, Arasteh K, Schuermann D, Hentrich M, Duehrsen U, Sudeck H, Schmidt-Wolf IG, Anagnostopoulos I, Huhn D. Acquired immunodeficiency syndrome-related lymphoma: Simultaneous treatment with combined cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy and highly active antiretroviral therapy is safe and improves survival - results of the German Multicenter Trial. Cancer. 2006; 106:1560-1568 [DOI] [PubMed] [Google Scholar]
- 55. Lim ST, Karim R, Nathwani BN, Tulpule A, Espina B, Levine AM. AIDS-related Burkitt's lymphoma versus diffuse large-cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: Significant differences in survival with standard chemotherapy. J Clin Oncol. 2005; 23:4430-4438 [DOI] [PubMed] [Google Scholar]
- 56. Oksenhendler E, Clauvel JP, Jouveshomme S, Davi F, Mansour G. Complete remission of a primary effusion lymphoma with antiretroviral therapy. Am J Hematol. 1998; 57:266. [DOI] [PubMed] [Google Scholar]
- 57. Hocqueloux L, Agbalika F, Oksenhendler E, Molina JM. Long-term remission of an AIDS-related primary effusion lymphoma with antiviral therapy. AIDS. 2001; 15:280-282 [DOI] [PubMed] [Google Scholar]
- 58. Ripamonti D, Marini B, Rambaldi A, Suter F. Treatment of primary effusion lymphoma with highly active antiviral therapy in the setting of HIV infection. AIDS. 2008; 22:1236-1237 [DOI] [PubMed] [Google Scholar]
- 59. Bower M, McCall-Peat N, Ryan N, Davies L, Young AM, Gupta S, Nelson M, Gazzard B, Stebbing J. Protease inhibitors potentiate chemotherapy-induced neutropenia. Blood. 2004; 104:2943-2946 [DOI] [PubMed] [Google Scholar]
- 60. Torres HA, Mulanovich V. Management of HIV Infection in Patients With Cancer Receiving Chemotherapy. Clin Infect Dis. 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. An J, Sun Y, Fisher M, Rettig MB. Antitumor effects of bortezomib (PS-341) on primary effusion lymphomas. Leukemia. 2004; 18:1699-1704 [DOI] [PubMed] [Google Scholar]
- 62. Sarosiek KA, Cavallin LE, Bhatt S, Toomey NL, Natkunam Y, Blasini W, Gentles AJ, Ramos JC, Mesri EA, Lossos IS. Efficacy of bortezomib in a direct xenograft model of primary effusion lymphoma. Proc Natl Acad Sci USA. 2010; 107:13069-13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siddiqi T, Joyce RM. A case of HIV-negative primary effusion lymphoma treated with bortezomib, pegylated liposomal doxorubicin, and rituximab. Clin Lymphoma Myeloma. 2008; 8:300-304 [DOI] [PubMed] [Google Scholar]
- 64. Antar A, El Hajj H, Jabbour M, Khalifeh I, El-Merhi F, Mahfouz R, Bazarbachi A. Primary effusion lymphoma in an elderly patient effectively treated by lenalidomide: Case report and review of literature. Blood Cancer J. 2014; 4:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luppi M, Trovato R, Barozzi P, Vallisa D, Rossi G, Re A, Ravazzini L, Potenza L, Riva G, Morselli M, Longo G, Cavanna L, Roncaglia R, Torelli G. Treatment of herpesvirus associated primary effusion lymphoma with intracavity cidofovir. Leukemia. 2005; 19:473-476 [DOI] [PubMed] [Google Scholar]
- 66. Halfdanarson TR, Markovic SN, Kalokhe U, Luppi M. A non-chemotherapy treatment of a primary effusion lymphoma: Durable remission after intracavitary cidofovir in HIV negative PEL refractory to chemotherapy. Ann Oncol. 2006; 17:1849-1850 [DOI] [PubMed] [Google Scholar]
- 67. Shiraishi Y, Gotoh K, Towata T, Shimasaki T, Suzu S, Kojima A, Okada S. Therapeutic effects of gamma-irradiation in a primary effusion lymphoma mouse model. Exp Ther Med. 2010; 1:79-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cassoni A, Ali U, Cave J, Edwards SG, Ramsay A, Miller RF, Lee SM. Remission after radiotherapy for a patient with chemotherapy-refractory HIV-associated primary effusion lymphoma. J Clin Oncol. 2008; 26:5297-5299 [DOI] [PubMed] [Google Scholar]
- 69. Takahashi-Makise N, Suzu S, Hiyoshi M, Ohsugi T, Katano H, Umezawa K, Okada S. Biscoclaurine alkaloid cepharanthine inhibits the growth of primary effusion lymphoma in vitro and in vivo and induces apoptosis via suppression of the NF-kappaB pathway. Int J Cancer. 2009; 125:1464-1472 [DOI] [PubMed] [Google Scholar]
- 70. Matsuno T, Kariya R, Yano S, Morino-Koga S, Taura M, Suico MA, Shimauchi Y, Matsuyama S, Okamoto Y, Shuto T, Kai H, Okada S. Diethyldithiocarbamate induces apoptosis in HHV-8-infected primary effusion lymphoma cells via inhibition of the NF-κB pathway. Int J Oncol. 2012; 40:1071-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goto H, Kariya R, Shimamoto M, Kudo E, Taura M, Katano H, Okada S. Antitumor effect of berberine against primary effusion lymphoma via inhibition of NF-κB pathway. Cancer Sci. 2012; 103:775-781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gopalakrishnan R, Matta H, Chaudhary PM. A purine scaffold HSP90 inhibitor BIIB021 has selective activity against KSHV-associated Primary Effusion Lymphoma and blocks vFLIP K13-induced NF-kappaB. Clin Cancer Res. 2013; 19:5016-5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nayar U, Lu P, Goldstein RL, Vider J, Ballon G, Rodina A, Taldone T, Erdjument-Bromage H, Chomet M, Blasberg R, Melnick A, Cerchietti L, Chiosis G, Wang YL, Cesarman E. Targeting the Hsp90-associated viral oncoproteome in gammaherpesvirus-associated malignancies. Blood. 2013; 122:2837-2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003; 101:1535-1542 [DOI] [PubMed] [Google Scholar]
- 75. Uddin S, Hussain AR, Al-Hussein KA, Manogaran PS, Wickrema A, Gutierrez MI, Bhatia KG. Inhibition of phosphatidylinositol 3′-kinase/AKT signaling promotes apoptosis of primary effusion lymphoma cells. Clin Cancer Res. 2005; 11:3102-3108 [DOI] [PubMed] [Google Scholar]
- 76. Sin SH, Roy D, Wang L, Staudt MR, Fakhari FD, Patel DD, Henry D, Harrington WJ, Jr, Damania BA, Dittmer DP. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007; 109:2165-2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goto H, Okada S. New approaches to treating primary effusion lymphoma. Expert Opinion on Orphan Drugs. 2013; 1:1019-1029 [Google Scholar]
- 78. Toomey NL, Deyev VV, Wood C, Boise LH, Scott D, Liu LH, Cabral L, Podack ER, Barber GN, Harrington WJ, Jr. Induction of a TRAIL-mediated suicide program by interferon alpha in primary effusion lymphoma. Oncogene. 2001; 20:7029-7040 [DOI] [PubMed] [Google Scholar]
- 79. Wu W, Rochford R, Toomey L, Harrington W, Jr, Feuer G. Inhibition of HHV-8/KSHV infected primary effusion lymphomas in NOD/SCID mice by azidothymidine and interferon-alpha. Leuk Res. 2005; 29:545-555 [DOI] [PubMed] [Google Scholar]
- 80. Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, DeBlanc R, Toki BE, Law CL, Doronina SO, Siegall CB, Senter PD, Wahl AF. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003; 102:1458-1465 [DOI] [PubMed] [Google Scholar]
- 81. Bhatt S, Ashlock BM, Natkunam Y, Sujoy V, Chapman JR, Ramos JC, Mesri EA, Lossos IS. CD30 targeting with brentuximab vedotin: A novel therapeutic approach to primary effusion lymphoma. Blood. 2013; 122:1233-1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Aoki Y, Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood. 1999; 94:4247-4254 [PubMed] [Google Scholar]
- 83. Shih T, Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006; 28:1779-1802 [DOI] [PubMed] [Google Scholar]