Abstract
Objective
We investigated the incidence and clinical features of drug-induced lung injury during cetuximab therapy in Japanese patients with colorectal cancer in a prospective multicenter registry based on a central registration system.
Methods
We investigated and followed up patients with or suspected of having drug-induced lung injury among 2006 patients with cetuximab-treated colorectal cancer. A subcommittee of medical oncologists, pulmonologists and a radiologist evaluated and discussed each case of drug-induced lung injury that occurred during cetuximab therapy.
Results
Sixty-six patients were identified and further examinations of drug-induced lung injury were conducted during the registration period. We analyzed time to onset, patient characteristics and factors associated with mortality. Cetuximab-related drug-induced lung injury occurred in 24 (1.2%) patients, and was rated as Grade 3 or worse in 15 (0.7%) patients. Fourteen patients received steroid pulse therapy. Ten patients with drug-induced lung injury died, of whom eight received steroid pulse therapy. The incidence of drug-induced lung injury was significantly higher in elderly patients, and in patients with prior interstitial lung disease. There was no particular trend in the time to onset. Patients with early onset of drug-induced lung injury (within 90 days) after starting cetuximab therapy had higher mortality than patients with later onset (over 90 days).
Conclusions
The incidence of drug-induced lung injury in cetuximab-treated patients was 1.2%. Because drug-induced lung injury is potentially serious, it is important to promptly initiate appropriate treatments. Considering that early onset drug-induced lung injury during cetuximab therapy is associated with a poor prognosis, close monitoring is mandatory for these patients.
Keywords: biological therapy, cetuximab, chemotherapy, colorectal cancer, drug-induced lung injury
INTRODUCTION
Severe drug-induced lung injury (DLI) occurring during chemotherapy can result in respiratory failure, and may be fatal. Accurate diagnosis is complicated because it is difficult to distinguish DLI from cancer progression, infection or drug-induced heart failure (1). In addition, treatment with multiple drugs makes it difficult to identify the responsible drug.
The consequences of DLI are broadly classified into two categories: a direct cytotoxic effect in which the drug or an intermediate metabolite affects lung tissue directly, and an indirect cytotoxic effect in which the host's inflammatory response and immunological factors affect lung tissue (2,3). However, the etiology of DLI is unknown in many cases because its pathology varies between different drugs.
The incidence of DLI, including interstitial lung disease (ILD), during treatment with gefitinib, leflunomide and bleomycin, is higher in Japan than in the USA and Europe (4,5), suggesting there are racial differences in the risk of these lung disorders. Because ILD during gefitinib treatment occurred more frequently after its approval and marketing in Japan (6), physicians and the Pharmaceuticals Medical Devices Agency (PMDA) of Japan were interested in clarifying whether epidermal growth factor receptor (EGFR) inhibitors are associated with DLI in Japanese patients. The West Japan Thoracic Oncology Group retrospectively analyzed 1976 patients treated with gefitinib (7) and the incidence of gefitinib-induced ILD was 3.5%. Another prospective multicenter study reported that ILD occurred in 122 of 1872 (6.5%) of gefitinib-treated patients (6).
Cetuximab (Erbitux®) is a monoclonal antibody that specifically binds to EGFR and inhibits its downstream signaling. Clinical data for cetuximab as first-, second- and third-line therapy have been reported (8–12). In July 2008, cetuximab was approved in Japan for the treatment of EGFR-positive, curatively unresectable, advanced or recurrent colorectal cancer (CRC). This approval was granted on the condition that all patients scheduled for cetuximab treatment from September 2008 were to be included in a prospective registry study to evaluate the safety profile of this drug in clinical practice (13). Therefore, the safety data accumulated in the central registration system were collated and analyzed in a timely manner to determine the characteristics of DLI occurring during cetuximab therapy.
PATIENTS AND METHODS
Patients
Following the launch of cetuximab on 19 September 2008, all patients to be treated with cetuximab were enrolled in a central registration system in advance. Overall, 2126 patients across 637 institutions were registered between September 2008 and January 2009. Of these, 120 patients who did not receive cetuximab treatment were excluded from this study. Therefore, 2006 patients were included in the safety population. The median age of these patients was 64 years (range, 18–87 years), the male:female ratio was 1:0.6, and the median duration of treatment was 15.3 weeks (range, 1–73.9 weeks). CRC was EGFR positive in 98.5% of patients; 61.6% of patients had primary tumor sites in the colon and 38.6% in the rectum (including overlapping patients). In total, 93.2% of the patients received cetuximab as third-line or later therapy, and 99.7% had an Eastern Cooperative Oncology Group Performance Status (PS) score of 0 or 1. Furthermore, 405 patients (20.2%) had a history of other relevant diseases, including prior ILD in four patients (0.2%) and allergy in 306 patients (15.3%) (13). Except for four patients with prior ILD, the other patients had no evidence of interstitial changes before starting treatment, as determined from computed tomography (CT) images and the medical history recorded on case-report forms. Twenty-three cases of lung injury occurred during treatment with cetuximab and were reported by the primary physicians. Four-hundred and forty patients developed respiratory disorders, categorized by system organ class, and were further evaluated to identify possible cases of DLI.
Evaluation of ILD
For patients who developed respiratory disorders during treatment, the physicians completed a case report form designed to evaluate the clinical characteristics of DLI. To exclude non-respiratory responses and respiratory events associated with infusion reactions as causative events, we assessed the following factors in these patients: causal relationship with cetuximab, patient characteristics and disease course. Based on these assessments, 43 of 440 patients were suspected of having DLI.
The 43 suspected and 23 reported cases were then reviewed by a DLI subcommittee. The DLI subcommittee, which consisted of two pulmonologists, one radiologist and two medical oncologists, convened seven times between April 2009 and September 2010 to review the patient data recorded in the registry and case report forms completed by the primary physicians. The committee members reviewed chest X-ray and CT images, occurrence of pulmonary metastasis, smoking history, results of consultation with a pulmonologist, measurement of interstitial pneumonitis markers (KL-6 and SP-D), blood gas measurements, tests for bacterial and fungal infection, bronchoalveolar lavage, lung biopsy and autopsy. DLI was diagnosed based on a combination of clinical symptoms (e.g. coughing, dry cough, shortness of breath/exertional dyspnea, and fever), clinical laboratory test results and chest X-ray and CT findings (revealing ground-glass opacities or infiltrates) during cetuximab therapy.
Statistical Analysis
Calculations and analyses of collected data were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA). Fisher's exact test or Wilcoxon's two-sample test was used to analyze the relationship between patient characteristics and the onset of DLI during cetuximab therapy. Factors associated with DLI mortality were also examined. Multivariate analysis using Cox's proportional hazard model was also performed in which the occurrence of DLI was used as the dependent variable and patient characteristics were used as independent variables. Values of P < 0.05 were considered statistically significant.
RESULTS
Patients
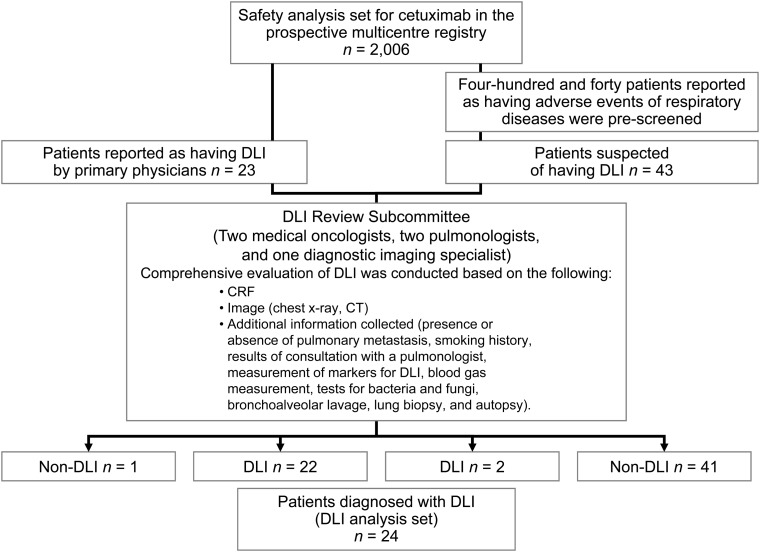
Figure 1 summarizes the disposition of patients and how they were diagnosed with DLI. Of 2006 patients included in the safety population, 23 were reported by their physician to have lung disease and were further assessed by the DLI subcommittee. Of these patients, one was thought to have pneumonia not related to DLI. Of the 43 patients suspected of having DLI, two patients were diagnosed with cetuximab-related DLI, although they were originally reported by their primary physicians to have lymphangitis carcinomatosa and radiation pneumonitis. Therefore, 24 patients were ultimately diagnosed with cetuximab-related DLI, and data for these patients were further analyzed (Fig. 1).
Figure 1.
Registry profile and identification of patients with drug-induced lung injury (DLI).
Incidence of Cetuximab-related DLI and Patient Characteristics
The incidence of DLI during treatment with cetuximab was 1.2% (n = 24/2006 patients). Grade 3 or worse DLI occurred in 0.7% of patients (n = 15). The characteristics of patients with DLI are shown in Table 1. DLI occurred in 18 males and six females, and the median age was 70 years (range, 45–80 years). PS score was 0 in 19 patients and 1 in five patients.
Table 1.
Incidence of drug-induced lung injury (DLI) during cetuximab therapy according to patient characteristics
| Patient characteristic |
Safety population | Number of patients with DLI | Incidence of DLI | P value | |
|---|---|---|---|---|---|
| n | 2006 | 24 | 1.2% | ||
| Sex | Male | 1234 | 18 | 1.46% | 0.2083 |
| Female | 772 | 6 | 0.78% | ||
| Age | <65 years | 1032 | 6 | 0.58% | 0.0122 |
| ≥65 years | 971 | 18 | 1.85% | ||
| Unknown | 3 | 0 | 0.0% | ||
| PS | 0 | 1370 | 19 | 1.39% | 0.3762 |
| 1 | 630 | 5 | 0.79% | ||
| 2 | 2 | 0 | 0% | ||
| Other | 4 | 0 | 0% | ||
| Treatment line | Second line | 133 | 2 | 1.50% | 0.6711 |
| Third line or later | 1869 | 22 | 1.18% | ||
| Other | 4 | 0 | 0% | ||
| Prior interstitial lung disease (ILD) | – | 1955 | 21 | 1.07% | 0.0442 |
| + | 4 | 1 | 25% | ||
| Unknown | 47 | 2 | 4.26% | ||
| Complications | – | 1019 | 11 | 1.08% | 0.6833 |
| + | 974 | 13 | 1.33% | ||
| Unknown | 13 | 0 | 0% | ||
| Combination chemotherapy | – | 460 | 2 | 0.43% | 0.2083 |
| + | 1546 | 22 | 1.42% | ||
| CPT-11 alone | 1255 | 17 | 1.35% | ||
| FOLFIRI | 256 | 4 | 1.56% | ||
| Other | 35 | 1 | 2.86% | ||
Two patients received cetuximab as second-line therapy, while 22 received it as third-line therapy. One patient had prior ILD, and 13 had other medical histories. Twenty-two patients received cetuximab in combination with chemotherapy, including 17 who received cetuximab in combination with CPT-11 alone.
Of the 24 patients, 10 had a history of smoking and 10 were never smokers; smoking status was unknown in four patients. Image patterns of DLI were categorized according to the image findings evaluated by the DLI subcommittee. Images were classified as diffuse alveolar damage in eight patients and as ground-glass opacities in 14 patients; the images could not be determined in the other two patients.
Subgroup analyses based on patient characteristics revealed that the incidence of DLI was significantly higher in elderly patients (≥65 years) and in patients with prior ILD (Table 1). Therefore, we performed a multivariate analysis using Cox's proportional hazard model to investigate the relationship between DLI and patient characteristics, including sex, age (<65 vs ≥65 years old), treatment line (second-line vs third-line or later), PS (0 vs 1), prior ILD and combination chemotherapy (with vs without). The analysis showed that the incidence of DLI was significantly higher in patients with prior ILD (HR, 19.49; 95% CI, 1.22–311.73; P = 0.036).
Time to Onset
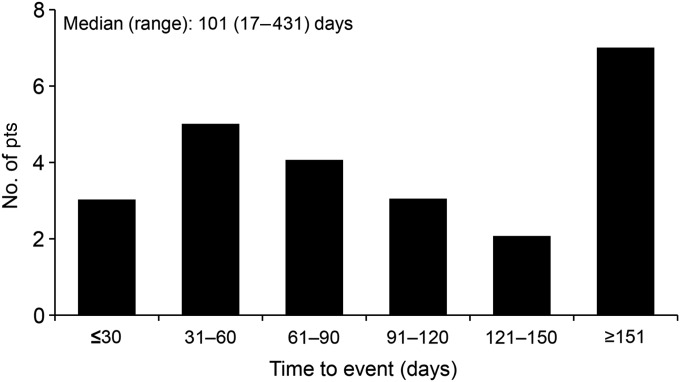
The median time to the onset of DLI from the start of cetuximab therapy was 101 days (range, 17–431 days; Fig. 2). DLI occurred within 30 days of starting cetuximab therapy in three patients, from 31 to 60 days in five patients, from 61 to 90 days in four patients, and on Day 91 or later in 12 patients.
Figure 2.
Time to the onset of DLI from the start of cetuximab administration.
Treatment of DLI
Steroid pulse therapy was administered to 14 of 24 patients. The time from the onset of DLI (initial symptoms) to the start of steroid pulse therapy was 3 days in six patients, 4–7 days in six patients and ≥8 days in two patients.
Outcomes
In terms of the outcomes of DLI, 10 patients (41.7%) died, two patients showed full recovery, six patients had partial recovery, five patients showed no recovery, and the outcome was unknown in one patient. Eight of the patients who died had received steroid pulse therapy.
Factors Associated with Mortality
Univariate analyses were performed to investigate potential associations between mortality and patient characteristics, including sex, treatment line, PS, combination chemotherapy, pulmonary metastasis, time to onset, steroid pulse therapy including the timing and history of smoking. As a result, patients with early onset of DLI (within 90 days of starting cetuximab) had significantly higher mortality than those with later onset (over 90 days).
There were no significant associations between mortality and other characteristics, including smoking history and the time to the start of steroid pulse therapy. However, it is worth noting that two of the six patients who started steroid pulse therapy within 3 days died, compared with six of the eight patients who started steroid pulse therapy after 4 days (Table 2).
Table 2.
Univariate analysis of mortality among 24 patients with DLI during cetuximab therapy
| Number of patients (of 24) | Number of deaths (of 10) | P value | ||
|---|---|---|---|---|
| Sex | Male | 18 | 7 | 0.6653 |
| Female | 6 | 3 | ||
| Treatment line | Second line | 2 | 1 | 1.000 |
| Third line or later | 22 | 9 | ||
| PS | 0 | 19 | 8 | 1.000 |
| 1 | 5 | 2 | ||
| Combination chemotherapy | Cetuximab alone | 2 | 0 | 0.4928 |
| In combination with CPT-11 or FOLFIRI | 22 | 10 | ||
| Lung metastasis | − | 10 | 5 | 0.6785 |
| + | 14 | 5 | ||
| Classification of images | Diffuse alveolar damage | 8 | 5 | n/a |
| Ground-glass opacity | 14 | 5 | ||
| Unable to be classified | 2 | 0 | ||
| Time to onset of DLI from the start of cetuximab treatment | <90 days | 12 | 8 | 0.0361 |
| ≥91 days | 12 | 2 | ||
| Steroid pulse therapy | − | 10 | 2 | 0.1041 |
| + | 14 | 8 | ||
| Time to the start of steroid pulse therapy from the onset of DLI | Within 3 days | 6 | 2 | 0.2744 |
| 4 days or later | 8 | 6 | ||
| Smoking history | − | 10 | 5 | 1.000 |
| + | 10 | 4 | ||
| Unknown | 4 | 1 |
Case Report
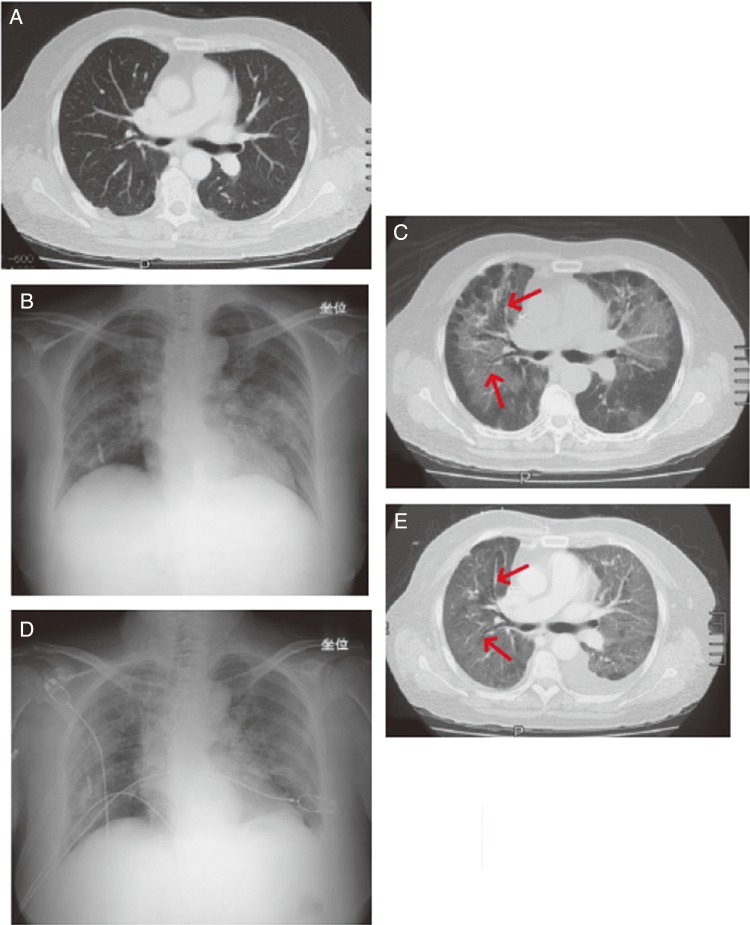
A female patient in her 60s had primary colon cancer with liver and lymph node metastases (Fig. 3A) that was previously treated with FOLFOX (folinic acid, fluorouracil and oxaliplatin) and FOLFIRI (folinic acid, fluorouracil and irinotecan). Cetuximab (initial dose of 400 mg/m2 with subsequent weekly doses of 250 mg/m2) in combination with CPT-11 (140 mg/m2, biweekly) was then started (Day 1). The patient experienced breathing difficulties 3 days after the sixth dose of cetuximab (∼Day 57). Three days later (Day 60), chest X-ray and CT images showed ground-glass opacity (Fig. 3B and C), and she was diagnosed with DLI. The patient was hospitalized and treated with supplemental oxygen, antibacterial drugs and antifungal drugs (micafungin sodium, sulfamethoxazole-trimethoprim). On Day 61, treatment with methylprednisolone (1 g) was started. Piperacillin was added at a dose of 4 g/day on Day 62, and cyclophosphamide was added at a dose of 250 mg/day on Day 67. Despite these treatments, her symptoms did not improve (Fig. 3D), and signs of diffuse alveolar damage were found on Day 68 (Fig. 3E). The patient died 22 days after the onset of symptoms (Day 79).
Figure 3.
Case report of a female patient with recurrent colorectal cancer in her 60s. (A) Image before the onset of DLI taken at 32 days after starting cetuximab therapy. Metastatic nodes were observed in the inferior lobe of both lungs. An infiltrate and ground-glass opacity were not observed. (B) X-ray image taken 60 days after starting cetuximab therapy, 3 days after the onset of symptoms. Ground-glass opacity was predominantly observed in the bilateral upper lung field. (C) Computed tomography (CT) scan on Day 60. Ground-glass opacity was observed from the hilus to the middle section of both lungs. The lesion in the periphery of the lung field was lightly spared. Hypertransradiancy of bronchi (arrow) was observed, but there was no obvious traction bronchiectasis, and it could not be diagnosed as diffuse alveolar damage from the images. (D) X-ray image taken on Day 67. The abnormal shadows in both lung fields had spread and were exacerbated. (E) CT image taken on Day 68. The shadows in both lungs were exacerbated and extended to the periphery of each lung. Hypertransradiancy of bronchi with bellows-like dilation (arrow) was observed in the shadows, and was considered to be traction bronchiectasis. The organizing stage of diffuse alveolar damage was observed, which was considered to be a sign of lesion progression. Left pleural effusion was also observed.
DISCUSSION
This was the first registry to analyze and investigate the incidence and risk of DLI during cetuximab-based therapy in patients with CRC. In recent years, lung disease associated with EGFR inhibitors (gefitinib and erlotinib) used to treat non-small cell lung cancer (NSCLC) has become a social concern in Japan (4,6). As a condition for the approval of cetuximab in Japan, the PMDA requested to continuously monitor and analyze cases of DLI occurring during cetuximab therapy. Therefore, we examined all Japanese patients who had received cetuximab-based therapies between September 2008 and January 2009.
DLI occurred in 24 of 2006 patients (1.2%) included in the present registry, while 10 of these 24 patients died. In contrast, the incidence of cetuximab-related ILD ranged from 0.2 to 0.6% in clinical studies performed in other countries (Table 3). Based on these clinical trials, it seems that the incidence of DLI, including ILD, is greater in Japan than in other countries, suggesting that there is a racial difference in this disease. A good example of this difference is that the incidence of ILD during gefitinib treatment in the US was ∼0.3% among 23 000 patients (FDA Approval Letter) while the incidence of ILD during gefitinib treatment was as high as 3.5–5.8% in Japan (7,14,15). As another example, the incidence of ILD in patients with leflunomide, a treatment for rheumatoid arthritis, was 0.02% (of 400 000 patients) and 1.2% (of 5054 patients) worldwide and in Japan, respectively (16,17). These results also highlight the differences in the incidence of ILD in Japan and other countries (5).
Table 3.
Incident rate of DLI, including ILD, in cetuximab studies
| Trial |
Regimen | n | All grades | ≥Grade 3 | |
|---|---|---|---|---|---|
| Large prospective registry in Japanese patientsa | Cetuximab alone | 2006 | 24 (1.20%) | 15 (0.7%) | |
| Cetuximab + CPT-11 | |||||
| Cetuximab + FOLFIRI | |||||
| Phase II in Japanese patients | Cetuximab + CPT-11 | 39 | 1 (2.60%) | − | |
| Phase II | BOND | Cetuximab + CPT-11 | 327 | − | |
| Cetuximab alone | |||||
| MABEL | Cetuximab + CPT-11 | 1147 | 3b (0.30%) | 1 (0.10%) | |
| OPUS | Cetuximab + FOLFOX4 | 170 | 1c (0.60%) | − | |
| Phase III | EPIC | Cetuximab + CPT-11 | 638 | − | |
| CO.17 | Cetuximab alone | 288 | − | ||
| CRYSTAL | Cetuximab + FOLFIRI | 600 | 1c (0.20%) | − | |
Evaluated by NCI-CTC Ver. 2.0.
aEvaluated by NCI-CTC Ver. 3.0.
bNot related to cetuximab.
cCounted adverse events related to both cetuximab and chemotherapy.
(Data source: Merck Serono internal documents).
It is also important to compare the incidence in Japan and other Asian countries. In Taiwan, for example, the incidence of ILD was reported to be 5.8% among patients with NSCLC treated with gefitinib (18,19). Meanwhile, in a Chinese Phase II study of gefitinib in patients with NSCLC, the incidence of ILD was 7.5% (20). These reports suggest that Asian patients are more susceptible to DLI, including ILD, than Caucasian patients. In fact, a survey conducted in the USA examining the presence of idiopathic pulmonary fibrosis (IPF) at death between 1989 and 2007, showed that White and Black populations were less likely to die of/with IPF than Hispanic and other ethnic/racial populations (21). Although the reason for this racial difference has not been clarified, some studies have suggested that genetic differences, including MUC5B promoter polymorphism and telomere shortening, may play some roles in the onset of pulmonary fibrosis (22,23).
There have been several studies of DLI, including ILD, in patients with CRC. In a retrospective analysis of 734 Japanese patients with CRC given standard chemotherapy consisting of FOLFOX or FOLFIRI, ILD occurred in 11 patients (1.5%), four of whom died (24). In a large prospective post-marketing registry of bevacizumab, ILD occurred in six of 3727 patients (0.16%), and one of these patients died (24). In a post-marketing surveillance study of oxaliplatin, ILD occurred in 13 of 5008 patients (0.26%) (24). In our registry, DLI occurred in two patients (0.43%) treated with cetuximab alone, in 17 patients (1.35%) receiving cetuximab in combination with CPT-11, and in four patients (1.56%) receiving cetuximab in combination with FOLFIRI.
The variations in the occurrence rates of ILD in these earlier studies can be explained by differences in study settings. First, the retrospective analysis of FOLFOX- or FOLFIRI-related ILD was based on data from a single central hospital. Second, patients included in the post-marketing surveillance study of bevacizumab received bevacizumab as first- or second-line therapy, and the patients included in our registry received cetuximab as third-line or later therapy. Finally, the oxaliplatin-related ILD study suggests that the duration of oxaliplatin treatment may have effect on onset of ILD (24). For these reasons, the incidence rates of DLI, including ILD, should not be directly compared among studies.
Other EGFR inhibitors, such as erlotinib and gefitinib, are often used to treat NSCLC. Among gefitinib-treated patients, risk factors for gefitinib-related ILD included older age (≥55 years), smoking habit, poor PS (≥2), and prior ILD (6). In our registry, older age and prior ILD were the primary factors associated with the onset of DLI. Although the sample size was limited, the results of multivariate analysis showed that prior ILD was the main risk factor for DLI during cetuximab therapy. It is noteworthy that the incidence of DLI in patients with CRC tends to be lower than that in patients with lung cancer, but mortality was similar. The incidence and risk factors for DLI in other cancers remain to be investigated.
In our registry, the median time to the onset of DLI was 101 days (range, 17–431 days) and there was no trend in the time to onset. In contrast, it was reported that ILD associated with gefitinib and erlotinib tends to occur within 4 weeks. Even though all of these drugs ultimately inhibit EGFR activity, the time to the onset of DLI, including ILD, varies by the type of cancer and the specific target site of each drug.
In our registry, patients with onset of DLI within 90 days had a worse prognosis than patients with later onset. For example, in a case presented in this report, the patient in her 60s developed DLI within 90 days after cetuximab administration. Although steroid pulse therapy was initiated on the third day of her symptom, she did not recover and later died. The prognosis of gefitinib-treated patients with early onset of ILD was also poor (25). Therefore, it is necessary to closely monitor patients for the first 3 months of treatment for signs of DLI. It is also notable that, in our registry, two of six patients who started steroid pulse therapy early died compared with six of eight patients who started the treatment later after the onset of DLI, although this was not significantly different. The response of patients with DLI to steroid therapy varies depending on the etiology and pathogenesis of DLI. However, it is strongly recommended that in cases of suspected or confirmed DLI, the cetuximab-based chemotherapy should be discontinued immediately, and adequate approaches including consultation with a pulmonologist and steroid therapy (steroid pulse therapy with methylprednisolone 500–1000 mg/day for 3 days), should be implemented as soon as possible.
Our registry has several limitations. First, although this registry was planned before approval of cetuximab, chest X-rays or CT scans were not mandatory during treatment, except for patients with respiratory symptoms. In addition, although history of smoking increases the risk of DLI, smoking status was not recorded for all patients. Second, because this registry was conducted in 637 hospitals under the insurance reimbursement program, the characteristics of patients varied considerably compared with those in clinical trials. Nevertheless, we think that the incidence, mortality and risk factors identified in this registry will provide useful information to guide future treatment strategies.
Conclusion
Although DLI is rare, it can result in respiratory failure and may be fatal. Accurate diagnosis is complicated, and identifying the drug responsible for DLI is difficult. This registry examined 2006 patients treated with cetuximab following its approval in Japan. We reviewed all patients reported as having lung disease by their primary physicians, as well as patients suspected of having DLI during cetuximab therapy. The incidence of ILD in this registry was 1.2%. Older age and prior ILD were risk factors for the onset of DLI. Although no particular trend was noted in the time to onset, DLI occurring within 90 days of starting cetuximab had a poor prognosis. Therefore, we strongly recommend patients should be closely monitored, especially for the first 90 days of treatment with cetuximab.
Acknowledgements
We are grateful to all the physicians and patients for their cooperation in this study, as well as Merck Serono Co. Ltd, Japan and Bristol-Myers K.K., Japan.
Funding
This work was supported by Merck Serono Co. Ltd, Japan and Bristol-Myers K.K., Japan. The sponsors were involved in protocol development and data analysis through the contributions of S.I. and M.I., who are employees of the sponsors. Funding to pay the Open Access publication charges for this article was provided by Merck Serono Co., Ltd., Japan and Bristol-Myers K.K., Japan.
Conflict of interest statement
Taroh Satoh has received consulting fees and honoraria from Merck Serono Co. Ltd and Bristol-Myers K.K., and departmental research grants from Chugai Pharmaceutical Co. Ltd and Yakult Honsha Co. Ltd.
Akihiko Gemma has received lecture fees from Merck Serono Co. Ltd.
Shoji Kudoh has no potential conflicts of interest to declare.
Fumikazu Sakai has provided consultancy services to the Japanese Ministry of Health, Labour and Welfare, the Japanese Agency of the Environment and Restoration. Japanese Organization of Research for Thoracic Radiology, Takeda Pharmaceuticals Co. Ltd, Chugai Pharmaceutical Co. Ltd, MSD Co. Ltd, AstraZeneca Co. Ltd, Pfizer Co. Ltd, Bayer Co. Ltd, Jansen Pharmaceuticals Co. Ltd, Kyowa Kirin Co. Ltd, Bristol-Meyers Co. Ltd and Shionogi Pharmaceuticals Co. Ltd. Fumikazu Sakai has also received lecture fees from Daiichi-Sankyo Co. Ltd, Kyorin Pharmaceuticals Cl. Ltd and Dainihon-Sumitomo Pharmaceuticals Co. Ltd; research grants from the Japanese Ministry of Health, Labour and Welfare, the Japanese Agency of the Environment and Restoration, the Japanese Ministry of Environment, Eizai Co. Ltd, Bayer Co. Ltd, AstraZeneca Co. Ltd and LTT Bio Co. Ltd. Fumikazu Sakai has also provided expert testimony on behalf of Maruyama Memorial Hospital and Osaka District Labour Agency of the Japanese Ministry of Health, Labour and Welfare.
Kensei Yamaguchi has received lecture fees from Merck Serono Co. Ltd, Bristol-Myers K.K. and Chugai Pharmaceutical Co. Ltd.
Toshiaki Watanabe has received consultancy fees, honoraria and research funding from Merck Serono Co. Ltd and Bristol-Myers K.K., and lecture fees and research funding from Taiho Pharmaceutical Co. Ltd, Chugai Pharmaceutical Co. Ltd, Yakult Honsha Co. Ltd and Takeda Pharmaceutical Company.
Megumi Ishiguro has received lecture fees from Merck Serono Co. Ltd, Bristol-Myers K.K., Taiho Pharmaceutical Co. Ltd, Chugai Pharmaceutical Co. Ltd and Yakult Honsha Co. Ltd.
Shogo Inoshiri is an employee of Merck Serono Co. Ltd.
Makiko Izawa is an employee of Bristol-Myers K.K.
Kenichi Sugihara has received lecture fees and research funding from Taiho Pharmaceutical Co. Ltd, Chugai Pharmaceutical Co. Ltd, Merck Serono Co. Ltd, Takeda Pharmaceutical Co. Ltd, Bristol-Myers K.K., Daiichi-Sankyo Co. Ltd and Kyowa Kirin Co. Ltd.
Yuh Sakata has received financial support to travel to meetings from Merck Serono Co. Ltd and Bristol-Myers K.K., and has received lecture fees from Taiho Pharmaceutical Co. Ltd, Yakult Honsha Co. Ltd and International Inc Synergy.
References
- 1.Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361:137–9. doi: 10.1016/S0140-6736(03)12190-3. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: cytotoxic drugs. Am Rev Respir Dis. 1986;133:321–40. doi: 10.1164/arrd.1986.133.2.321. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 2: noncytotoxic drugs. Am Rev Respir Dis. 1986;133:488–505. doi: 10.1164/arrd.1986.133.3.488. [DOI] [PubMed] [Google Scholar]
- 4.Tsuboi M, Le Chevailier T. Interstitial lung disease in patients with non-small-cell lung cancer treated with epidermal growth factor receptor inhibitors. Med Oncol. 2006;23:161–70. doi: 10.1385/MO:23:2:161. [DOI] [PubMed] [Google Scholar]
- 5.Azuma A, Kudoh S. High prevalence of drug-induced pneumonia in Japan. Jap Med Assoc J. 2007;50:405–11. [Google Scholar]
- 6.Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case–control study. Am J Respir Crit Care Med. 2008;177:1348–57. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 7.Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–56. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 9.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 10.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–9. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 12.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro M, Watanabe T, Yamaguchi K, et al. A Japanese post-marketing surveillance of cetuximab (Erbitux®) in patients with metastatic colorectal cancer. Jpn J Clin Oncol. 2012;42:287–94. doi: 10.1093/jjco/hys005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takano T, Ohe Y, Kusumoto M, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004;45:93–104. doi: 10.1016/j.lungcan.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Hotta K, Kiura K, Tabata M, et al. Interstitial lung disease in patients with non-small cell lung cancer receiving gefitinib: an analysis of risk factors and treatment outcomes in Okayama Lung Cancer Study Group. Cancer J. 2005;11:417–24. doi: 10.1097/00130404-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Sawada T, Inokuma S, Sato T, et al. Leflunomide-induced interstitial lung disease: prevalence and risk factors in Japanese patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1069–72. doi: 10.1093/rheumatology/kep052. [DOI] [PubMed] [Google Scholar]
- 17.McCurry J. Japan deaths spark concerns over arthritis drug. Lancet. 2004;363:461. doi: 10.1016/S0140-6736(04)15527-X. [DOI] [PubMed] [Google Scholar]
- 18.Chou CL, Ko HW, Wang CW, Yu CT, Kuo HP, Huang CD. Erlotinib-associated near-fatal interstitial pneumonitis in a patient with relapsed lung adenocarcinoma. Chang Gung Med J. 2010;33:100–5. [PubMed] [Google Scholar]
- 19.Shih YN, Chiu CH, Tsai CM, Perng RP. Interstitial pneumonia during gefitinib treatment of non-small-cell lung cancer. J Chin Med Assoc. 2005;68:183–6. doi: 10.1016/S1726-4901(09)70246-1. [DOI] [PubMed] [Google Scholar]
- 20.Lin WC, Chiu CH, Liou JL, Chen YM, Perng RP, Tsai CM. Gefitinib as front-line treatment in Chinese patients with advanced non-small-cell lung cancer. Lung Cancer. 2006;54:193–9. doi: 10.1016/j.lungcan.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Swigris JJ, Olson AL, Huie TJ, et al. Ethnic and racial differences in the presence of idiopathic pulmonary fibrosis at death. Respir Med. 2012;106:588–93. doi: 10.1016/j.rmed.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–12. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–37. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimura T, Fuse N, Yoshino T, et al. Clinical features of interstitial lung disease induced by standard chemotherapy (FOLFOX or FOLFIRI) for colorectal cancer. Ann Oncol. 2010;21:2005–10. doi: 10.1093/annonc/mdq061. [DOI] [PubMed] [Google Scholar]
- 25.Hotta K, Kimura K, Tabata M, et al. Interstitial lung disease in Japanese patients with non-small cell lung cancer receiving gefitinib: an analysis of risk factors and treatment outcomes in Okayama Lung Cancer Study Group. Cancer J. 2005;11:417–24. doi: 10.1097/00130404-200509000-00010. [DOI] [PubMed] [Google Scholar]