Abstract
The impact of source and amount of lipid used to prepare a soy soft pretzel on the bioaccessibility and transport of isoflavones was investigated using the coupled in vitro digestion/Caco-2 human cell model. Pretzels were prepared without or with 2.9 or 6.0% exogenous lipid from either shortening, canola oil, ground almond, or ground hazelnut. The isoflavone backbone structure was stable during pretzel production, although there was partial conversion from malonyl and acetyl glucosides to simple glucosides and aglycones. Endogenous β-glucosidase activity in ground almond facilitated partial conversion of simple glucosides to aglycones during proofing, resulting in a slight decrease in bioaccessibility of isoflavones as compared with other sources of lipid. Amount and source of lipid did not affect bioaccessibility or uptake and metabolism of isoflavones by Caco-2 cells, although transport across the monolayer was greater with the lesser amount of shortening. These results suggest that the isoflavone structure, but not source or amount of lipid in a soy pretzel, may affect bioavailability of isoflavones.
Keywords: soy, isoflavones, fat, bioaccessibility, bioavailability, Caco-2 cells
INTRODUCTION
Soy foods have attracted much interest due to their proposed health benefits, such as alleviating hypercholesterolemia, reducing postmenopausal symptoms, and reducing the risk of some types of cancer.1 Although soy foods have been consumed as part of the traditional diet in East Asian countries for centuries, overall consumption of soy in the American diet remains low.2 Incorporation of soy into products that Americans regularly consume, such as carbohydrate-based snack foods, represents one strategy to increase soy consumption among this population.
Key components of soy that contribute to their health benefits include high-quality protein, fiber, and soy isoflavones. Health-promoting effects of soy isoflavones have been proposed to stem from their ability to bind to the estrogen receptor and affect various signaling pathways.1 To mediate such activities, soy isoflavones must be absorbed and transported to target tissues, that is, be bioavailable. Differences have been observed in the absorption and metabolism of soy isoflavones in response to the delivery matrix, genotype of the individual and their gut microflora, gender, and composition of one’s normal diet.3,4 The chemical species of isoflavones may be another factor affecting their bioavailability. Nonfermented soy foods are rich in isoflavone glucosides, but these compounds are not detected in circulation;5 the glucosides must be hydrolyzed by enzymes of host and microflora origin. Some,6,7 but not all,8–10 investigators have reported that the rate of absorption of isoflavones is greater for foods containing aglycones rather than glucosides.
We considered several factors for optimizing the isoflavone profile of a soy snack food to augment retention of isoflavones during preparation and maximize the proportion of isoflavone aglycones. First, a consumer-acceptable11 soy soft pretzel was selected as a model food. Processing conditions for the production of the pretzels (e.g., heat and changes in pH) could hydrolyze ester and glycosidic linkages of isoflavones to generate simple glucosides and/or aglycones12 while retaining the backbone structure.13 Also, preparation of soft pretzels involves submersion in a strong alkaline solution (lye) prior to the final proofing and baking, possibly inducing alkaline hydrolysis of isoflavone glucosides on the surface of the pretzel. Because raw almonds contain natural β-glucosidase activity that converts simple isoflavone glucosides to aglycones,14 we hypothesized that incorporation of ground almonds into a pretzel formulation would increase the aglycone content above that of other soy-based bakery products. This, in turn, might affect the stability of isoflavones during processing and digestion, as well as their bioaccessibility and absorption.
Because isoflavone aglycones require incorporation into mixed micelles for maximal intestinal uptake,13 the composition and size of mixed micelles may influence the rate and/or extent of uptake. Smaller micelles have been shown to promote more efficient uptake of compounds into enterocytes, although the size of the micelle is primarily affected by the ratio of bile acids to phospholipids and churning of the chyme, with limited impact of composition of the meal.15 The composition of the lipid components in mixed micelles (e.g., the degree of saturation and chain length of fatty acids) affects the intestinal cell uptake of hydrophobic dietary compounds including carotenoids16–18 and cholesterol.19 To our knowledge, the effects of the type, amount, and delivery matrix of lipid in a soy product formulation on the micellarization of soy isoflavones during digestion and their subsequent uptake into and transport across the mucosal epithelium have not been investigated.
The coupled in vitro digestion/Caco-2 human cell model provides a useful method for examining the relative stability, bioaccessibility, uptake, and transepithelial transport of dietary compounds from foods.20,21 Caco-2 cells have been shown to metabolize aglycones to their glucuronidated and sulfated metabolites, as in humans.22
Conversion of popular snack foods into functional foods with optimized nutritional properties represents a promising approach to improving the general quality of one’s diet. Herein, we evaluated the effects of source and amount of lipid in the soy soft pretzel on isoflavone retention during processing, bioaccessibility following digestion, and transport by Caco-2 human intestinal cells with the goal of maximizing isoflavone absorption. The specific aims of the experiments were (1) to assess the chemical stability of isoflavones during pretzel production and in vitro digestion with ground almond and other lipid sources and (2) to compare the effect of the source and amount of lipid in the pretzel formulation on the bioaccessibility, metabolism, and efflux of soy isoflavones from soy pretzels using the coupled in vitro digestion/Caco-2 cell model. We hypothesized that the presence of ground almonds as a source of β-glucosidase would increase the isoflavone aglycone content of the pretzel and that the source and amount of lipid would not significantly affect bioaccessibility. Additionally, we hypothesized that substitution of shortening with canola oil, a source of lipid with a relatively low degree of saturation that is commonly used in the food industry, may affect the extent of isoflavone uptake and transepithelial transport by highly differentiated cultures of Caco-2 human intestinal cells.
MATERIALS AND METHODS
Supplies
Reagents, solvents, and cell culture materials were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and Fisher Scientific (Fair Lawn, NJ, USA) unless noted otherwise. Isoflavone standards were purchased from LC Laboratories (Woburn, MA, USA). Fetal bovine serum (FBS) and antibiotics were purchased from Gibco Life Technologies (Grand Island, NY, USA).
Preparation of Pretzels
Soy soft pretzels were prepared as previously reported.11,23 Pretzels were produced with and without exogenous lipid. The endogenous lipid content of the pretzel was 1.9 ± 0.2% as determined gravimetrically using the Folch method.24 To prepare the pretzels, activated yeast, 43% of the total wheat flour, wheat gluten, dough conditioner, and 45% of the total water were mixed and proofed at 43 °C, 100% humidity (CM2000 Holding/Proofing Combination Module; InterMetro Industries Corp., Wilkes Barre, PA, USA). After 2 h, the remainder of the water and other ingredients were incorporated with or without one exogenous lipid being added last. Shortening was added at either 2.9% dry weight (traditional formulation) or at 6.0% dry weight. Similarly, ground almond was added to deliver 2.9% additional lipid (5.9% dry weight of ground almond, assuming almonds are 49% lipid25) or 6.0% additional lipid (12.2% dry weight). Nuts were ground fresh with a kitchen blender to a size <1.70 mm (no. 12 sieve, W. S. Tyler, Mentor, OH, USA) before addition to the formulation. As a control that provided lipid in a similar form as almond oil but lacking β-glucosidase activity, canola oil was added at either 2.9 or 6.0% dry weight. Hazelnut was used as a control matrix for almond because it contains similar amounts of total, saturated, and unsaturated fat as almond,25 but has very limited β-glucosidase activity. Hazelnuts were also ground to <1.70 mm and added to achieve 2.9 and 6.0% added lipid (4.7 and 9.8% ground hazelnut, by weight, respectively, because hazelnuts were assumed to contain 61% lipid25). The amount of wheat flour in the formulation was reduced to offset the addition of lipid and nonlipid components to maintain a consistent amount of soy components among all formulations. One batch of soy materials (Table 1) was used for quantitative analysis of retention and chemical stability of isoflavones during processing, simulated digestion, and partitioning into the aqueous fraction of the chyme, that is, bioaccessibility.
Table 1.
Isoflavone (IFN) Content of Individual Soy Ingredients and Baked Soy Pretzel without Almonds
| isoflavone | soy flour (nmol/g) | soy milk powder (nmol/g) | IFNs in soy pretzela
|
recoveries
|
||
|---|---|---|---|---|---|---|
| expected | measured | individual IFNs (%) | IFN families (%) | |||
| daidzin | 896.7 ± 32.5 | 1734.8 ± 85.6 | 292.0 ± 12.1 | 350.6 ± 15.8 | 120.1 | 89.7 |
| malonyldaidzin | 1977.4 ± 84.5 | 1795.3 ± 104.8 | 511.4 ± 23.7 | 299.8 ± 17.1 | 58.6 | |
| acetyldaidzin | 138.0 ± 18.3 | 115.7 ± 17.2 | 35.1 ± 4.8 | 29.4 ± 2.1 | 83.9 | |
| daidzein | 148.0 ± 24.2 | 168.7 ± 30.6 | 40.5 ± 6.8 | 108.4 ± 3.4 | 267.6 | |
| genistin | 764.4 ± 37.8 | 1761.1 ± 79.2 | 267.3 ± 12.7 | 357.8 ± 17.4 | 133.8 | 101.3 |
| malonylgenistin | 2059.7 ± 77.9 | 2004.9 ± 96.8 | 541.5 ± 21.8 | 349.7 ± 18.4 | 64.6 | |
| acetylgenistin | 209.9 ± 7.7 | 110.4 ± 10.9 | 49.0 ± 2.2 | 35.2 ± 2.7 | 71.7 | |
| genistein | 112.6 ± 12.8 | 263.3 ± 20.7 | 39.6 ± 3.9 | 166.7 ± 6.7 | 420.7 | |
| glycitin | 154.8 ± 5.7 | 67.6 ± 34.5 | 35.3 ± 3.4 | 32.7 ± 3.5 | 92.8 | 65.3 |
| malonylglycitin | 243.7 ± 25.0 | 89.2 ± 3.1 | 54.4 ± 5.2 | 28.7 ± 2.8 | 52.8 | |
| acetylglycitin | 80.2 ± 14.8 | 53.1 ± 11.5 | 19.5 ± 3.7 | ndb | nd | |
| glycitein | 198.8 ± 10.8 | 35.9 ± 12.5 | 42.0 ± 3.0 | 37.3 ± 3.9 | 88.8 | |
| total | 6984.1 | 8200.1 | 1927.5 | 1796.3 | 93.2 | |
Soy flour and soy milk comprise 18.5 and 6.2%, respectively, of pretzel dry weight.
Not detected.
Dough (71.0 g) was rolled into 60 cm ropes and twisted into a pretzel shape. The pretzel was submerged in a 1.0% sodium hydroxide solution at 60–65 °C for 60 s and then placed on a lightly greased baking sheet (Pam 100% canola cooking spray; ConAgra Foods, Omaha, NE, USA). The pretzels were proofed for an additional 25 min (43 °C, 100% humidity) and then baked at 149 °C for 16 min (JA14 Jet-Air oven; Doyon, Linière, QC, Canada). Pretzels were cooled and stored in individual pint size plastic bags overnight.
β-Glucosidase activity of almonds was determined before and after baking as detailed previously.26 One unit of enzyme activity (U) was defined as 1 μmol of p-nitrophenol (pNP) released per minute.
In Vitro Digestion
Pretzels were manually torn into pieces smaller than 1.0 g. Pieces totaling 25.0 g were combined with 166.7 g of “salt solution” (120 mM sodium chloride, 6 mM calcium chloride, 5 mM potassium chloride). After softening in the solution for 10–15 min, the mixture was homogenized at a rate of 6500 rpm for approximately 5 min until large pieces were no longer visible (Ika T25 Digital Ultra-Turrax Disperser with S 25N-18 G Dispersing Element, Ika Works, Inc., Wilmington, NC, USA). Homogenates were decanted into 50 mL polypropylene tubes and stored at −20 °C.
The in vitro digestion protocol was based on the method developed by Garrett et al.20 and modified by Thakkar et al.27 to include an oral phase in addition to gastric and small intestine phases. The oral phase was modified by decreasing α-amylase activity to 1.8 U per g pretzel and decreasing the pH of the small intestinal phase of digestion to 6.0 ± 0.1. Two individual pretzels from each batch were subjected to simulated digestion in triplicate for a total of six independent digestions per batch, and at least two different batches were analyzed per pretzel formulation to yield a total of n = 12 digestions per formulation. The resulting suspension after completion of the small intestinal phase of digestion is referred to as “chyme” throughout the remainder of the text.
Duplicate aliquots (10 mL) of chyme were centrifuged at 5000g for 45 min at 4 °C (Beckman Coulter, Brea, CA, USA) to separate soluble from undigested materials. The supernatant, or aqueous fraction, was collected for analysis and use with Caco-2 cells.
Cell Culture
Differentiated monolayer cultures of Caco-2 human intestinal cells (HTB-37, American Type Culture Collection, Rockville, MD, USA) were used to investigate the uptake, metabolism, and transepithelial flux of soy isoflavones. Details for the growth, maintenance, and experimental use of these cultures are described elsewhere.20,28 For experiments, differentiated cultures of Caco-2 cells on inserts (21–25 days after reaching confluency) were used at passages 33 and 34.
Cellular Uptake and Transport of Isoflavones
The aqueous fraction of the chyme was filtered (0.22 μm pores; Millex GP, PES, Millipore Ireland Ltd., Tullagreen, Cork, Ireland) before diluting 1/4 with Dulbecco’s minimal essential medium (DMEM), pH 6.5, containing 500 μM phenol red to yield “test medium”. β-Glucosidase activity of gut microbes was mimicked by addition of 3 U/mL almond β-glucosidase for conversion of isoflavone glycosides to their respective aglycones.
Monolayers were washed once with basal DMEM before test medium was added to the apical compartment. The basolateral medium consisted of phenol red-free DMEM supplemented with 1.0% FBS and 1.0% nonessential amino acids. Cultures were incubated at 37 °C in a humidified atmosphere of 95% air/5% CO2. Test medium was also incubated in cell-free wells to assess the stability of the isoflavones in the cell culture environment. After 4 h, apical and basolateral media were collected into 15 mL polypropylene tubes and centrifuged at 800g (GR412 Jouan, Winchester, VA, USA). Supernatants were transferred to a new tube and stored at −20 °C until analysis. Monolayers were washed once with cold phosphate-buffered saline (PBS), pH 7.4, containing 2 g/L albumin and then twice with cold PBS, pH 7.4. Cells were collected in cold PBS and centrifuged (800g, 4 °C). The supernatant was discarded, and the cell pellet was stored at −20 °C until analysis.
Protein content of the cell pellet was determined using the bicinchoninic acid (BCA) protein assay kit (Pierce BCA Protein Assay, Thermo Scientific, Rockford, IL, USA). The barrier integrity of the monolayers grown on inserts was assessed by quantifying paracellular transfer of phenol red from apical to basolateral medium. The flux of phenol red from the apical to basolateral compartments did not exceed 0.012%/cm2/h for monolayers used in these experiments.
Assessment of Phase II Metabolites
Cell pellets were resuspended in 1.0 mL of PBS, pH 7.4, and lysed by sonication (2 × 10 s, amplitude setting 50; Vibra Cell, Sonics and Materials, Inc., Danbury, CT, USA). To quantify isoflavones that were metabolized to glucuronidated and sulfated derivatives, aliquots of spent media and cell lysates were treated with either glucuronidase (β-glucuronidase, type HP-2: from Helix pomatia) or sulfatase (type H-1, from H. pomatia). Both enzyme preparations exhibit glucuronidase, sulfatase, and glucosidase activities.5 Due to coelution of endogenous contaminants in the enzyme preparations, “glucuronidase” was used to estimate the phase II conjugation of daidzein and “sulfatase” was used to assess phase II conjugation of genistein. Cell homogenates or media were diluted with an equivalent volume of sodium acetate buffer, pH 5.5, with glucuronidase at a final concentration of 2.5 μL/mL, sulfatase at 5.4 mg/mL, or no added enzyme. Reaction tubes were incubated at 37 °C in a shaking water bath (85 rpm) for 2–3 h for the sulfatase and no enzyme treatments or for 16–20 h for treatment with glucuronidase. Total isoflavone content was calculated for the samples treated with either enzyme (isoflavones + phase II conjugates as aglycones) or buffer only (isoflavones that are not phase II conjugates). The difference between the two provided an estimate of phase II conjugates of isoflavones in the samples.
Isoflavone Extraction
Isoflavones were extracted from raw soy flour, soy milk powder, wheat flour, homogenized pretzel, chyme, aqueous fraction of the chyme, test media, spent media, and cells according to the method of Walsh et al.13 Extracts were dried under a stream of nitrogen gas and stored at −20 °C until analysis by high-performance liquid chromatography (HPLC). On the basis of the method of standard additions, recovery of isoflavones from samples was estimated to be >95%.
Isoflavone Quantification Using HPLC
The dried films containing isoflavones were resuspended in 80% methanol (400 μL), vortexed briefly (Vortex Genie 2, Fisher Scientific), and placed in a sonicating water bath for 60 s (B12 Ultrasonic Cleaner, Branson Cleaning Equipment Co., Shelton, CT, USA). Isoflavones were then quantified as in Walsh et al.13 with several modifications. A Symmetry C18 3.5 μm, 4.6 × 75 mm, column with an inline Symmetry C18 5 μm, 3.9 × 20 mm, guard column (Waters Corp., Milford, MA, USA) was used, held at 30 °C. For cell samples only, the injection volume was increased to 50 μL. The mobile phase initially consisted of a mixture of acetonitrile (solvent A) and 1% acetic acid in water (solvent B) at a flow rate of 1.0 mL/min. The solvent gradient began at 10% A/90% B with the gradient increasing linearly to 35% A from 1 to 23 min, to 75% A at 26 min, and returning to 10% A at 30 min to equilibrate for 5 min between samples. The chromatograms for isoflavone standards and samples were analyzed by their absorbance at 254 nm. Isoflavone standards for aglycones and simple glucosides were used for peak identification and quantification. Published retention times and spectra were used for identification of malonyl and acetyl isoflavone glucosides,13 and standard curves for the corresponding simple isoflavone glucosides were used to estimate the concentrations of these species. Percentages of total isoflavones are calculated on the basis of molar equivalents.
Statistical Analysis
Statistical analyses were performed to fit the model Y = LipidType + LipidAmount + (LipidType × LipidAmount), where Y = the parameter of interest; LipidType = shortening, canola oil, almond, or hazelnut; LipidAmount = 0, 2.9, or 6.0%; and (LipidType × LipidAmount) is the interaction between the two variables. Analysis of variance and least-squares means for the (LipidType × LipidAmount) interaction were calculated using SAS 9.1 (SAS Institute, Cary, NC, USA). Statistical significance was deemed at p < 0.05. The average ± standard deviation is reported throughout.
RESULTS AND DISCUSSION
Isoflavone Retention during Production of Soy Soft Pretzels
To assess the impact of processing on the profile and retention of isoflavones in a soy soft pretzel, the total amount of isoflavones and the isoflavone profile were first determined in the raw soy ingredients (Table 1 and Figure 1). In the soy ingredients (defatted soy flour and soy milk powder at a 2:1 ratio23), 46.6 ± 1.3% of the isoflavones were members of the genistein family, 45.6 ± 1.4% were members of the daidzein family, and 7.8 ± 0.4% were members of the glycitein family. This is in accord with other studies showing that the quantity of isoflavones in the genistein family is similar to or exceeds that in the daidzein family and that glycitein represents a minor component of the isoflavones in soy foods.13,29,30 The forms of isoflavones in the soy mixture included 57.4% malonyl glucosides, 5.4% acetyl glucosides, 30.8% simple glucosides, and 6.4% aglycones. This profile is similar to previous reports that the soy flour has 1–3% isoflavone aglycones13,31 and soy milk powder has about 10% aglycones due to hydrolysis during processing.13,29 Both ingredients used in these studies had a greater total amount of isoflavones than we reported previously.13 This difference is likely explained by the variability between soybean crops and ingredient batch.32,33
Figure 1.
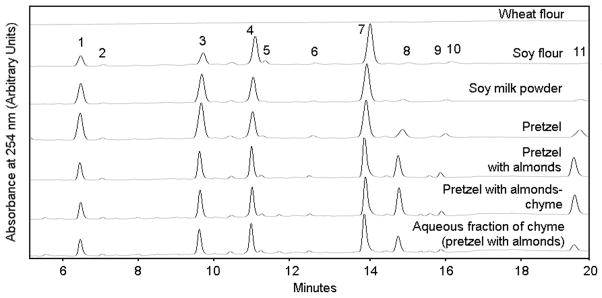
Representative HPLC chromatograms of isoflavone profiles in extracts from wheat flour, soy flour, soy milk powder, soy soft pretzel with 2.9% shortening, soy soft pretzel with 6.0% lipid from almond, chyme of the pretzel with 6.0% almond digested in vitro, and the aqueous fraction of the chyme. Isoflavones were identified using retention times and absorption spectra of standards from simple glucosides and aglycones and from published literature.13 Isoflavones eluted in the following order: (1) daidzin, (2) glycitin, (3) genistin, (4) malonyldaidzin, (5) malonylglycitin, (6) acetyldaidzin, (7) malonylgenistin, (8) daidzein, (9) glycitein, (10) acetylgenistin, (11) genistein. Acetylglycitin was not detected.
After dipping in lye, proofing, and baking the raw ingredients into the pretzel, 93.2% isoflavones were recovered, and their profile was altered in a manner that is consistent with the preparation of soy bread.13 More specifically, the percentage of malonyl- and acetyl-glucosides in a soy soft pretzel decreased to 38 and 4%, respectively, whereas the percentages of simple glucosides and aglycones increased to 41 and 17%, respectively. These changes in isoflavone profiles were consistent for both the genistein and daidzein families.
To determine how the alkaline dip itself affected conversion of malonyl and acetyl glucosides to simple glucosides or aglycones (compared with the proofing and baking steps), pretzels were proofed and baked without dipping, after dipping in water, or after dipping in lye (Table 2). Pretzels contained 80–100 μmol (42 ± 4 mg) isoflavones per serving (one serving = 59 g). On the basis of the observation that a portion of simple glucosides increased at the expense of the malonyl and acetyl-glucosides with minimal change in the amount of aglycones, it appears that hydrolysis of ester bonds of isoflavones, but not the glycosidic bonds, occurred. Similarly, Mathais et al.12 reported that alkaline conditions promoted hydrolysis of ester bonds of isoflavones to yield simple glucosides, and conversion was enhanced at elevated temperatures. The dip did not alter the total amount of isoflavones in each isoflavone family; that is, all pretzels, regardless of the type of dip, contained approximately 52, 44, and 4% of isoflavones in the genistein, daidzein, and glycitein families, respectively. Because the conversion was not complete, it is likely that the hydrolysis occurred mainly on and near the surface of the pretzel during dipping in lye. This finding suggests that an alkaline dip is a nondestructive, effective strategy to tailor the form of various nutrients.
Table 2.
Effects of Dipping on the Isoflavone Profile of a Soy Pretzela
| percentage of total | no dip (%) | water dip (%) | lye dip (%) |
|---|---|---|---|
| malonyl glucosides | 47.5 ± 2.9 a | 47.0 ± 2.0 a | 40.3 ± 1.7 b |
| acetyl glucosides | 8.2 ± 1.1 a | 10.5 ± 0.8 b | 7.1 ± 0.8 a |
| β-glucosides | 32.2 ± 2.7 a | 30.3 ± 2.0 a | 41.4 ± 1.8 b |
| aglycones | 12.0 ± 1.7 a | 12.3 ± 1.5 a | 11.2 ± 1.4 a |
Data are the mean ± standard deviation for n = 6. Means in a row with different letters are signicantly different (p < 0.05).
Effect of the Source and Amount of Lipid on the Isoflavone Profile in the Soy Soft Pretzel
To assess whether the type and/or amount of lipid affected the isoflavone profile, pretzels were prepared with either no exogenous lipid or 2.9 or 6.0% lipid added as shortening, canola oil, ground almond, or ground hazelnut. The endogenous lipid content of the pretzel was 1.9 ± 0.2%. Ground raw almonds were used because they possess β-glucosidase activity that preferentially cleaves simple isoflavone glucosides to aglycones14 and because almonds increased the relative amount of soy isoflavone aglycones in a soy bread matrix during proofing and baking.34 The other sources of lipid (shortening, canola oil, and hazelnut) were not expected to affect the isoflavone profile in the pretzel because they lack β-glucosidase activity.
Pretzels contained approximately 25 mg of isoflavone aglycone equivalents per serving (59 g), regardless of formulation. Pretzels produced without added lipid or with shortening, canola oil, or hazelnut contained 15–18% of total isoflavones as aglycones. With the addition of almonds (2.9% lipid) to the pretzel, the relative amount of aglycones increased to 28.5 ± 1.1% (p < 0.001 compared to all formulations without almonds) and was further increased to 32.3 ± 2.9% in pretzels containing 6.0% lipid from almond (p < 0.001 when compared to all formulations without almond). These increases in aglycone content resulted from proportional decreases in simple glucosides without changes in malonyl and acetyl glucosides. This suggests that simple isoflavone glucosides are the primary substrate for almond β-glucosidase.14 This observation also demonstrated that almond β-glucosidase was active during proofing and perhaps baking.34 Activity in raw almonds was 1.0 U/mg almond. However, there was no detectable β-glucosidase activity in baked pretzels, likely due to thermal denaturation during baking. This suggests that further enzymatic cleavage of isoflavone glucosides is minimal during product storage and/or digestion.
Digestive Stability and Bioaccessibility
At least 95% of the isoflavones in the pretzels were recovered after simulated oral, gastric, and small intestinal digestions (Figure 1). Such stability of isoflavones has also been observed in soy bread digested in vitro13 and tofu digested by rats.35 The mean relative amount of isoflavone aglycones in the chyme generated during digestion of pretzels without almond increased from 16.8 ± 1.8 to 21.6 ± 1.8%. Similarly, for pretzels made with ground almond, the relative amount of aglycones increased from 28.5 ± 1.1 to 32.8 ± 1.4% during digestion of formulations containing 2.9% lipid from almond (p < 0.05) and from 32.3 ± 2.9 to 36.4 ± 0.8% with 6.0% lipid from almond (p < 0.05). The increase in aglycone content in the chyme is likely due to acid hydrolysis ester and glycosidic bonds during the simulated gastric phase of digestion.12 The similar degree of increase in aglycone content with pretzels with and without added almond during digestion (about 4–5%) further suggests that β-glucosidase activity from almond was limited during digestion.
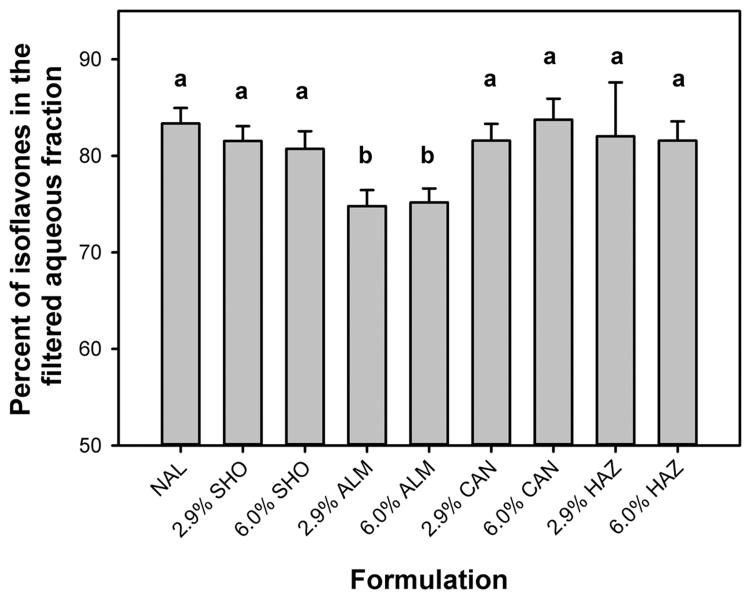
Bioaccessibility, as defined by the partitioning of isoflavones into the aqueous fraction, was 80–85% for all digested pretzels other than those containing almond (Figure 2). By comparison, the bioaccessibility of isoflavones from pretzels prepared with almond was about 75% (Figure 2, p < 0.05). Regardless of formulation, the relative amount of aglycones partitioning into the aqueous fraction was only 40–60%, whereas partitioning of isoflavone malonyl glucosides and simple glucosides exceeded 87% for all formulations (data not shown). Because isoflavone aglycones are more lipophilic than glucosides, maximum bioaccessibility may require a greater amount of bile extract during the small intestinal phase to facilitate further incorporation into mixed micelles. Indeed, Walsh et al.13 showed that increased bile increased the bioaccessibility of isoflavones from soy bread. When the bioaccessibility of isoflavone families was assessed, the efficiency of partitioning in the aqueous fraction was greater for the daidzein family (82.9 ± 0.4%) than for the genistein family (75.0 ± 0.1%, p < 0.001). In accord with these findings, Walsh et al.13 did not observe genistein in the aqueous fraction of chyme when bile extract was omitted from the digestion procedure, although 26% of daidzein was soluble in the chyme. This difference is likely due to the greater hydrophilicity of daidzein compared to genistein.36,37 These findings suggest that although almonds increased the percentage of isoflavone aglycones in the soft pretzel, the bioaccessibility of total isoflavones decreased slightly.
Figure 2.
Addition of almond to pretzel formulation reduced the relative bioaccessibility of isoflavones. Data are the mean ± standard deviation for at least 12 independent digestions of pretzels with indicated source and amount of lipid. NAL, no added lipid; SHO, shortening; ALM, lipid from ground almond; CAN, lipid from canola oil; HAZ, lipid from ground hazelnut. The amount of lipid from these sources added to formulations was either 2.9 or 6.0% lipid, dry weight. Endogenous lipid content from soy components was 1.9 ± 0.2%. Different letters over error bars indicate that differences in means are statistically significant (p < 0.05).
Prior studies have shown that the amount and type of lipid consumed in the food or meal affects the composition of mixed micelles and therefore affects the bioaccessibility of some compounds including carotenoids, cholesterol, and cocoa polyphenols.16,18,19,38,39 For example, the chain length of fatty acids16 and the degree of saturation40 have been reported to influence micellarization of some compounds during digestion. Goltz et al.18 observed increased absorption of carotenoids from a vegetable salad with higher levels of lipids and that unsaturated fat promoted carotenoid absorption to a greater extent than saturated fat. The amount of lipid added to pretzel formulations did not affect apparent bioaccessibility of isoflavones in digested soy pretzels. Huo et al.16 reported that only 0.5–1.0% of total triglyceride as either triolein or canola oil was required for optimal micellarization of the majority of carotenoids from a vegetable salad during simulated digestion. Because the octanol–water partitioning coefficient is much higher for carotenoids (e.g., 17.6 for β-carotene) than for isoflavones (3.0 for genistein and 2.5 for daidzein36), it is not surprising that the presence of additional lipid in the pretzel formulations or substitution of shortening with other lipids minimally affected partitioning of isoflavones in the aqueous fraction.
Cellular Uptake, Metabolism, and Efflux of Isoflavones in the Caco-2 Cell Model
Isoflavone glycosides are transformed into aglycones by human lactase phlorizin hydrolase (LPH) that resides on the brush border membrane of enterocytes41 and by gut microbiota residing in the large intestine as well as the small intestine.42 Caco-2 cells express very limited brush border β-glucosidase activity,37,43,44 and intestinal bacteria and archaea were not present in the cell cultures. Therefore, β-glucosidase was added to the aqueous fraction at 3 U/mL before addition to cell cultures. This effectively converted simple isoflavone glucosides, but not acetyl or malonyl glucosides, to aglycones (data not shown). In test media, aglycone content increased from 1.31 ± 0.24 to 6.74 ± 0.56 nmol/mL, representing 13.5 and 60.1% of total isoflavones in the apical compartment, respectively.
To compare the effect of source and amount of lipid in the soy pretzel formulation on the uptake, metabolism, and transepithelial flux of soy isoflavones, pretzels were prepared with either shortening at 2.9 or 6.0% or canola oil at 2.9 or 6.0%. Pretzels were subjected to in vitro digestion, and the aqueous fraction of chyme was used to prepare test media that contained exogenous β-glucosidase (3 U/mL). Due to differences in soy flour batches used in these experiments, the amounts of aglycones in the test media prepared with aqueous fraction of digested pretzels with shortening and canola oil were 15–18 and 28–31 nmol/mL, respectively. After incubation with cells for 4 h, >98% of isoflavones added to the medium were recovered in the wells, demonstrating isoflavone stability in the cell culture environment. Slightly less than 50% of the isoflavones present in the apical compartment were conjugated with glucuronide, sulfate, or both, indicating that these isoflavones entered the cell, underwent phase II metabolism, and again traversed the apical membrane. As expected,37 <1% of the isoflavones in the well was present in the monolayer of cells (50–150 pmol/mg protein). The isoflavones that were transported into the basolateral compartment were considered to be “bioavailable”. After 4 h, 17– 30% of total isoflavones in the wells were present in the basolateral compartment (1–3 nmol/mL). Of these, ≤30% were present as phase II conjugates. These data are consistent with the results of Walsh and Failla,28 who reported that 26% of the equol added to the apical compartment was transported by Caco-2 cells to the basolateral compartment after a 4 h incubation. Overall, these data are also similar to those of Murota et al.37 and Steensma et al.,44 who reported that approximately 30–40% of isoflavone aglycones were transported into the basolateral compartment by Caco-2 cells, respectively. It is interesting that Chen et al.22 reported a preferential efflux of sulfated isoflavones into the apical compartment and glucuronidated isoflavones into the basolateral compartment. However, our choice of enzymes precluded such distinction in the present study.
The lower limit of isoflavones taken up from the apical compartment during the course of the experiment was estimated by summing isoflavones in the basolateral and cellular compartments, as well as conjugated isoflavones in the apical compartments. About 50–65% of total isoflavones appear to have been taken up, which is similar to the total amount of aglycones present in the system and is somewhat similar to previous results when genistein, daidzein, and equol were added to the apical compartment.28,37 Passive diffusion has been suggested to be the main route of isoflavones into cells,5,8 which implies that substrate concentration may be rate-limiting for the uptake of isoflavones.
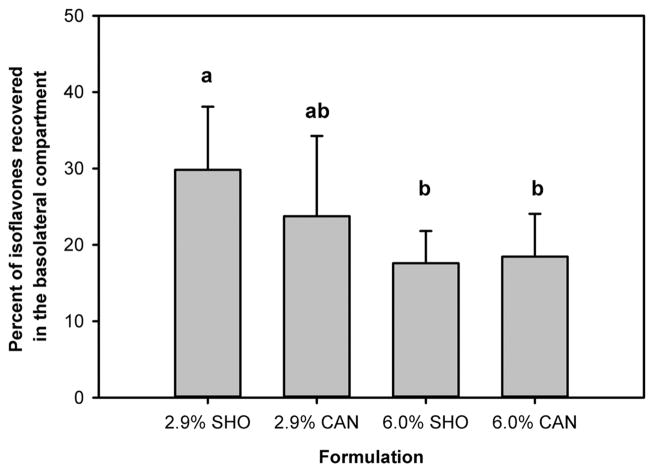
It did not appear that either source (shortening or canola oil) or amount of lipid (added at 2.9 or 6.0% to yield 4.8 and 7.9% total lipid in the pretzel formulation) strongly affected transepithelial flux of soy isoflavones (Figure 3). A statistically significant difference in transepithelial flux of isoflavones was observed between pretzels made with 2.9% shortening and both of the pretzel formulations with 6.0% added lipid. It is unknown if such an effect would be observed in vivo. Differences in food matrix, gender, age, efficiency of enterohepatic recycling, and gut microflora4,7,45 have also been shown to affect bioavailability.
Figure 3.
Flux of isoflavones across the differentiated monolayer of Caco-2 cells. Apical media were prepared from simulated digestions of soy soft pretzels made with 2.9% shortening (SHO), 2.9% canola oil (CAN), and 6.0% shortening or 6.0% canola oil. Different letters above bars indicate means are significantly different (p < 0.05).
Isoflavones most likely enter the enterocyte by passive diffusion8 and are transported across the apical membranes into the intestinal lumen by multidrug-resistant proteins.46 However, it is currently unknown if aglycones and/or phase II metabolites traverse the basolateral membrane via transport proteins or within chylomicrons. Whereas aglycones are not detected in circulation,5 first-pass conjugation of isoflavone aglycones in the liver is likely.3 The type and amount of lipid digestion products available to enterocytes affect chylomicron assembly.47 Shortening and canola oil are both composed of mostly C16 and C18 fatty acids (Table 3) and differ primarily in their degree of saturation.25 Studies by van Greevenbroek et al.47 have shown that the chylomicrons are smaller and denser when Caco-2 cells are incubated with palmitic acid compared to oleic acid. Effects on lipoprotein packaging and transport of isoflavones into circulation are unknown. Further studies investigating the effects of fatty acid chain length and degree of saturation are needed for a more thorough evaluation of the influence of type of dietary lipids on isoflavone bioavailability.
Table 3.
Fatty Acid Composition of Lipid Sources Used in the Formulation of a Soy Soft Pretzel as Percent Total Lipid25
| fatty acid | saturated fats
|
monounsaturated fat
|
polyunsaturated fats
|
total fat | |||||
|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C18:0 | total | C18:1 | C18:2 | C18:3 | total | ||
| canola oil | 5.1 | 2.0 | 8.0 | 58.5 | 23.3 | 5.8 | 29.1 | 95.6 | |
| shortening | 0.1 | 11.6 | 10.7 | 23.2 | 64.4 | 6.9 | 7.0 | 94.6 | |
| hazelnut | 5.1 | 2.1 | 7.4 | 75.3 | 12.9 | 0.2 | 13.0 | 95.7 | |
| almond | 6.2 | 1.3 | 7.4 | 62.3 | 24.5 | 0.0 | 24.5 | 94.3 | |
| soybean oil | 0.5 | 10.6 | 3.5 | 14.6 | 22.1 | 49.7 | 6.5 | 56.8 | 93.5 |
We observed that daidzein was transported across the Caco-2 monolayer more efficiently than genistein. Although daidzein and genistein each accounted for approximately 50% of the total isoflavone equivalents and aglycones in the test media, 60–80% of the isoflavones in the basolateral compartment were daidzein. In contrast, Chen et al.22 observed a greater flux of genistein than daidzein in a Caco-2 cell model. Murota et al.37 observed preferential formation of sulfate/glucuronide conjugates for daidzein compared to genistein after a 2 h incubation of isoflavones with Caco-2 cells. Reported relative rates of absorption and excretion of daidzein and genistein in vivo are also inconsistent. In accord with our results, Xu et al.48 observed that daidzein was more bioavailable than genistein from soy milk. Similarly, both King and Bursill49 and Shelnutt et al.50 observed a greater rate of urinary excretion of daidzein compared to genistein when a soybean flour-based meal was administered to healthy men. However, others7,22,49,50 observed similar pharmacokinetics for the postprandial plasma concentrations for both compounds. Individual differences in expression of brush border enzymes (e.g., LPH), differences in gut microflora, and effects of food matrix may also contribute to observed differences in isoflavone uptake by enterocytes and transfer into circulation. More research is required to better discern the reported differences.
In conclusion, the soft pretzel provides a distinct soy delivery system with a relatively high percentage of isoflavone aglycones. The addition of almond to the soft pretzel promoted partial conversion of isoflavones to aglycones during proofing, but this in turn slightly decreased the bioaccessibility of total isoflavones. The transport and metabolism of isoflavones from digested soy soft pretzels in an in vitro Caco-2 model did not appear to be markedly affected by the source or amount of lipid in the snack food.
Acknowledgments
Funding
This work was funded by the Ohio Agricultural Research and Development Center (OARDC), Project OHOA1440.
ABBREVIATIONS USED
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- HPLC
high-performance liquid chromatography
- IFN
isoflavones
- LPH
lactase phlorizin hydrolase
- nd
not detected
- PBS
phosphate-buffered saline
- PDA
photodiode array
- pNP
p-nitrophenol
Footnotes
The authors declare no competing financial interest.
References
- 1.Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: a review. Mol Cell Endocrinol. 2009;304:30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr. 2008;138:1244S–1249S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 3.Larkin T, Price WE, Astheimer L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit Rev Food Sci Nutr. 2008;48:538–552. doi: 10.1080/10408390701542716. [DOI] [PubMed] [Google Scholar]
- 4.Ahn-Jarvis J, Clinton SK, Riedl KM, Vodovotz Y, Schwartz SJ. Impact of food matrix on isoflavone metabolism and cardiovascular biomarkers in adults with hypercholesterolemia. Food Funct. 2012;3:1051–1058. doi: 10.1039/c2fo10284f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setchell KDR, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 6.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Human nutrition and metabolism soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 7.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KDR. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;3:45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 9.Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside. Am J Clin Nutr. 2003;77:1459–1465. doi: 10.1093/ajcn/77.6.1459. [DOI] [PubMed] [Google Scholar]
- 10.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by β-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002;132:2587–2592. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 11.Simmons AL, Miller CK, Clinton SK, Vodovotz Y. A comparison of satiety, glycemic index, and insulinemic index of wheat-derived soft pretzels with or without soy. Food Funct. 2011;2:678–683. doi: 10.1039/c1fo10125k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathias K, Ismail B, Corvalan CM, Hayes KD. Heat and pH effects on the conjugated forms of genistin and daidzin isoflavones. J Agric Food Chem. 2006;54:7495–7502. doi: 10.1021/jf061322a. [DOI] [PubMed] [Google Scholar]
- 13.Walsh KR, Zhang YC, Vodovotz Y, Schwartz SJ, Failla M. Stability and bioaccessibility of isoflavones from soy bread during in vitro digestion. J Agric Food Chem. 2003;51:4603–4609. doi: 10.1021/jf0342627. [DOI] [PubMed] [Google Scholar]
- 14.Ismail B, Hayes K. β-Glycosidase activity toward different glycosidic forms of isoflavones. J Agric Food Chem. 2005;53:4918–4924. doi: 10.1021/jf0404694. [DOI] [PubMed] [Google Scholar]
- 15.Wilson FA, Dietschy JM. Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J Clin Invest. 1972;51:3015–3025. doi: 10.1172/JCI107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huo T, Ferruzzi MG, Schwartz S, Failla M. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J Agric Food Chem. 2007;55:8950–8957. doi: 10.1021/jf071687a. [DOI] [PubMed] [Google Scholar]
- 17.Yonekura L, Tsuzuki W, Nagao A. Acyl moieties modulate the effects of phospholipids on β-carotene uptake by Caco-2 cells. Lipids. 2006;41:629–636. doi: 10.1007/s11745-006-5013-x. [DOI] [PubMed] [Google Scholar]
- 18.Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res. 2012;56:866–877. doi: 10.1002/mnfr.201100687. [DOI] [PubMed] [Google Scholar]
- 19.Schneider CL, Cowles RL, Stuefer-Powell CL, Carr TP. Dietary stearic acid reduces cholesterol absorption and increases endogenous cholesterol excretion in hamsters fed cereal-based diets. J Nutr. 2000;130:1232–1238. doi: 10.1093/jn/130.5.1232. [DOI] [PubMed] [Google Scholar]
- 20.Garrett D, Failla M, Sarama R. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J Agric Food Chem. 1999;47:4301–4309. doi: 10.1021/jf9903298. [DOI] [PubMed] [Google Scholar]
- 21.Failla M, Chitchumroonchokchai C. In vitro models as tools for screening the relative bioavailabilities of provitamin A carotenoids in foods. Harvest Plus Tech Rep. 2005;3 www.harvestplus.org/pubs.html. [Google Scholar]
- 22.Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55:159–169. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 23.Vodovotz Y, Ballard C. Compositions and processes for making high soy protein-containing bakery products. 7,592,028 B2. US Patent. 2009
- 24.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.U.S. Department of Agriculture. USDA National Nutrient Database for Standard Reference. 2011 http://ndb.nal.usda.gov/
- 26.Riedl KM, Zhang YC, Schwartz SJ, Vodovotz Y. Optimizing dough proofing conditions to enhance isoflavone aglycones in soy bread. J Agric Food Chem. 2005;53:8253–8258. doi: 10.1021/jf0508549. [DOI] [PubMed] [Google Scholar]
- 27.Thakkar SK, Maziya-Dixon B, Dixon AGO, Failla ML. β-Carotene micellarization during in vitro digestion and uptake by Caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava. J Nutr. 2007;137:2229–2233. doi: 10.1093/jn/137.10.2229. [DOI] [PubMed] [Google Scholar]
- 28.Walsh KR, Failla M. Transport and metabolism of equol by Caco-2 human intestinal cells. J Agric Food Chem. 2009;57:8297–8302. doi: 10.1021/jf9011906. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Murphy PA. Isoflavone content in commercial soybean foods. J Agric Food Chem. 1994;42:1666–1673. [Google Scholar]
- 30.Franke AA, Custer LJ, Hundahl SA. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutr Cancer. 2004;50:141–154. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 31.da Silva LH, Celeghini RMS, Chang YK. Effect of the fermentation of whole soybean flour on the conversion of isoflavones from glycosides to aglycones. Food Chem. 2011;128:640–644. [Google Scholar]
- 32.Barion G, Hewidy M, Mosca G, Vamerali T. Intraspecific variability for soybean cotyledon isoflavones in different cropping and soil conditions. Eur J Agron. 2010;33:63–73. [Google Scholar]
- 33.Riedl KM, Lee JH, Renita M, St Martin SK, Schwartz SJ, Vodovotz Y. Isoflavone profiles, phenol content, and antioxidant activity of soybean seeds as influenced by cultivar and growing location in Ohio. J Sci Food Agric. 2007;1206:1197–1206. [Google Scholar]
- 34.Zhang YC. PhD Thesis. The Ohio State University; Columbus, OH: 2004. Physiochemical Properties and Isoflavone Content of Bread Made with Soy. [Google Scholar]
- 35.Andlauer W, Kolb J, Fürst P. Isoflavones from tofu are absorbed and metabolized in the isolated rat small intestine. J Nutr. 2000;130:3021–3027. doi: 10.1093/jn/130.12.3021. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell JA, Day AJ, Morgan MRA. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J Agric Food Chem. 2005;53:4355–4360. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- 37.Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr. 2002;132:1956–1961. doi: 10.1093/jn/132.7.1956. [DOI] [PubMed] [Google Scholar]
- 38.Ortega N, Reguant J, Romero MP, Macià A, Motilva MJ. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J Agric Food Chem. 2009;57:5743–5749. doi: 10.1021/jf900591q. [DOI] [PubMed] [Google Scholar]
- 39.Yonekura L, Tsuzuki W, Nagao A. Acyl moieties modulate the effects of phospholipids on β-carotene uptake by Caco-2 cells. Lipids. 2006;41:629–636. doi: 10.1007/s11745-006-5013-x. [DOI] [PubMed] [Google Scholar]
- 40.Raju M, Lakshminarayana R, Krishnakantha TP, Baskaran V. Micellar oleic and eicosapentaenoic acid but not linoleic acid influences the β-carotene uptake and its cleavage into retinol in rats. Mol Cell Biochem. 2006;288:7–15. doi: 10.1007/s11010-005-9091-5. [DOI] [PubMed] [Google Scholar]
- 41.Day AJ, Cañada FJ, Díaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 42.Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora – implications for health. Mol Nutr Food Res. 2007;51:765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 43.Henry C, Vitrac X, Decendit A, Ennamany R, Krisa S, Mérillon JM. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J Agric Food Chem. 2005;53:798–803. doi: 10.1021/jf048909e. [DOI] [PubMed] [Google Scholar]
- 44.Steensma A, Noteborn HP, Jagt RC, Polman TH, Mengelers MJ, Kuiper HA. Bioavailability of genistein, daidzein, and their glycosides in intestinal epithelial Caco-2 cells. Environ Toxicol Pharmacol. 1999;7:209–212. doi: 10.1016/s1382-6689(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 45.Mortensen A, Kulling SE, Schwartz H, Rowland I, Ruefer CE, Rimbach G, Cassidy A, Magee P, Millar J, Hall WL, Kramer Birkved F, Sorensen IK, Sontag G. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res. 2009;53:S266–S309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Hu M. Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30:370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 47.van Greevenbroek MMJ, Robertus-Teunissen MG, Erkelens DW, de Bruin TWA. Participation of the microsomal triglyceride transfer protein in lipoprotein assembly in Caco-2 cells: interaction with saturated and unsaturated dietary fatty acids. J Lipid Res. 1998;39:173–185. [PubMed] [Google Scholar]
- 48.Xu X, Wang HJ, Murphy PA, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994;124:825–832. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]
- 49.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 50.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]