Abstract
Elucidation of the regulatory controls on epithelial plasticity is pivotal not only to better understand the nature of metastasis, but also for the design of targeted therapies to prevent the earliest steps in migration and invasion from the primary tumor. This review will highlight the role of the novel TRIM protein DEAR1 (annotated as TRIM62) in the regulation of apical-basal polarity and acinar morphogenesis as well as its function as a chromosome 1p35 tumor suppressor and negative regulator of TGFβ-driven epithelial-mesenchymal transition (EMT). DEAR1 binds to and promotes the ubiquitination of SMAD3, the major effector of TGFβ-mediated EMT, as well as downregulates SMAD3 targets SNAIL1/2, master transcriptional regulators of EMT. Cumulative results suggest a novel paradigm for DEAR1 in the regulation of the breast tumor microenvironment, polarity and EMT. Because DEAR1 undergoes loss of function mutations, homozygous deletion as well as copy number losses in multiple epithelial cancers including breast cancer, DEAR1 has clinical utility as a predictive and prognostic biomarker as well as for stratifying breast cancers and potentially other epithelial tumor types for targeted therapies aimed at the pathways regulated by DEAR1.
Introduction
Aberrant signaling from the tumor microenvironment has been extensively documented both in vitro and in vivo to modulate epithelial plasticity and influence breast cancer proliferation and progression (1). Cumulative studies provide strong support for the importance of tumor-tumor microenvironment interactions in cancer initiation and progression; yet, the underlying genetic mechanisms differentially regulating communication from the microenvironment in normal and tumor cells are not clear. Loss of polarity and tissue architecture is one of the earliest hallmarks of breast cancer invasion and metastasis, suggesting a critical link between mechanisms governing polarity, signaling from the microenvironment, and the regulation of breast cancer progression.
In the mammary gland, an intricate tissue architecture exists characterized by a highly organized, branching ductal-lobular network culminating in terminal structural units called acini. The precise timing and developmental events characterizing acinar morphogenesis have been demonstrated both in in vivo model systems as well as in elegant three dimensional (3D) culture assays (2). Individual acini consist of a bi-layered epithelium surrounding a hollow lumen with an inner layer of polarized luminal secretory epithelial cells surrounded by myoepithelial cells which are separated from the extracellular milieu by an intact basement membrane. Contraction of the myoepithelial cells propels milk produced by the luminal epithelial cells into the lumen and subsequently the ductal tree. Epithelial cell polarity and tissue architecture are thus critical for proper functioning of the mammary gland. In fact, polarized epithelia are essential for the establishment of cellular orientation within tissues and for appropriate responses to temporal-spatial microenvironmental signaling. However, coincident with the onset of invasion and metastasis, the mammary gland loses proper organization and polarity and increases cell proliferation (3). Thus, loss of polarity in the mammary gland disrupts a delicate homeostasis balancing differentiation, proliferation and death, thereby potentially rendering the epithelial cell refractory to growth inhibition, differentiation or apoptotic signals and instead allowing for the inappropriate communication of growth factor signaling from the extracellular matrix (ECM) (4, 5). Loss of polarity is also associated with initial steps in epithelial-mesenchymal transition (EMT), a developmental program in which polarized epithelia are converted to motile, fibroblast-like cells, resulting in loss of polarity, remodeling of the extracellular matrix and actin cytoskeleton. Although polarity loss is a hallmark of early EMT, loss of a single polarity regulator thus far has proven insufficient to initiate EMT (6); however, loss of polarity has been shown to prime epithelial cells for cooperation with oncogenic signaling pathways to drive cancer invasion and metastasis. In that regard, loss of function of the polarity protein Scribble cooperates with c-myc to drive mammary tumorigenesis and activation of ErbB2 in cells lacking Scribble, Dig1 or Af-6 induced invasion (4, 5). Thus, loss of polarity is one of the earliest events in EMT and cooperates with signaling from the stroma to drive tumor progression.
In addition to loss of polarity, loss of cell-cell adhesion is also a critical initiating event in EMT with increased expression of a mesenchymal-like gene signature including upregulation of vimentin, N-cadherin, fibronectins, and integrins which couple the ECM to the actin cytoskeleton. Thus, a major function for cell adhesion molecules is to correctly “sense” the microenvironment and control cellular plasticity and movement by modulating responses between the cell and its environment. When EMT is inappropriately activated in cancer, loss of polarity and altered communication with the ECM results in migration, invasion, and subsequently, recurrence and metastasis (7). The genetic controls on cell polarity and EMT and their deregulation in cancer progression are critically important to elucidate and yet largely unknown. A novel class of tumor suppressors was hypothesized by Petersen et al to have the capacity “to sense” the microenvironment appropriately and to regulate the formation of three dimensional acinar structures (8). By this definition, this class of tumor suppressor would undergo loss of function mutations in cancer which would deregulate proper spatial restriction of signal transduction components and proper localization and function of polarity protein complexes and signaling from the microenvironment via disruption of appropriate communication through cell adhesion molecules, ultimately disrupting normal homeostasis. As such, this type of tumor suppressor could be pivotal regulators of EMT and master regulators of both polarity and cell adhesion, migration and metastasis through their regulation of epithelial plasticity and could silence communication from the microenvironment. For example p53, which is mutated in the germline in Li Fraumeni syndrome (9), also shows gain of function mutations that disrupt acinar morphogenesis, polarity and EMT (10, 11). LKB1 is a polarity regulator/tumor suppressor that undergoes loss of function germline mutations in Peutz-Jeghers syndrome, a syndrome that predisposes to cancer development and is also somatically mutated in a number of epithelial cancers (12). LKB1 loss of function also results in luminal filling of acini in 3D culture (13). As a master serine/threonine kinase, LKB1 phosphorylates multiple targets including AMPK to activate AMPK-mTOR signaling which are important for regulation of metabolism, growth control, cell polarity and epithelial architecture (14). Downregulation of some of the polarity regulators, including SCRIBBLE, and PAR3 have been shown to be intimately involved in the loss of polarity in breast cancer initiation and progression, and potentially function as context-dependent tumor suppressors (15). SCRIBBLE loss of function results in loss of basolateral polarity and filling of the luminal space reminiscent of early ductal carcinoma in situ (DCIS), an early precursor to invasive breast carcinoma. SCRIBBLE is also important for regulating the tight junction through EMT signaling by modulating MAPK-ERK activity in MCF10A cells (16). Loss of PAR3 produces mammary epithelial cells with multilayered ducts and is deleted or downregulated in esophageal tumors (15). In addition, genetic alterations resulting in loss of function of cell adhesion molecules that control EMT could also be included in the list of tumor suppressors inducing changes to polarity and epithelial plasticity. A classical example would be E-cadherin, in which germline mutations have been reported in gastric cancer as well as loss of function observed in the initial stages of EMT (17). Thus, characterization of tumor suppressors that undergo loss of function and lead to loss of polarity and EMT is crucial in uncovering therapeutic strategies to alter the course of metastatic disease.
A Novel Tumor Suppressor that Regulates Polarity and Tissue Architecture
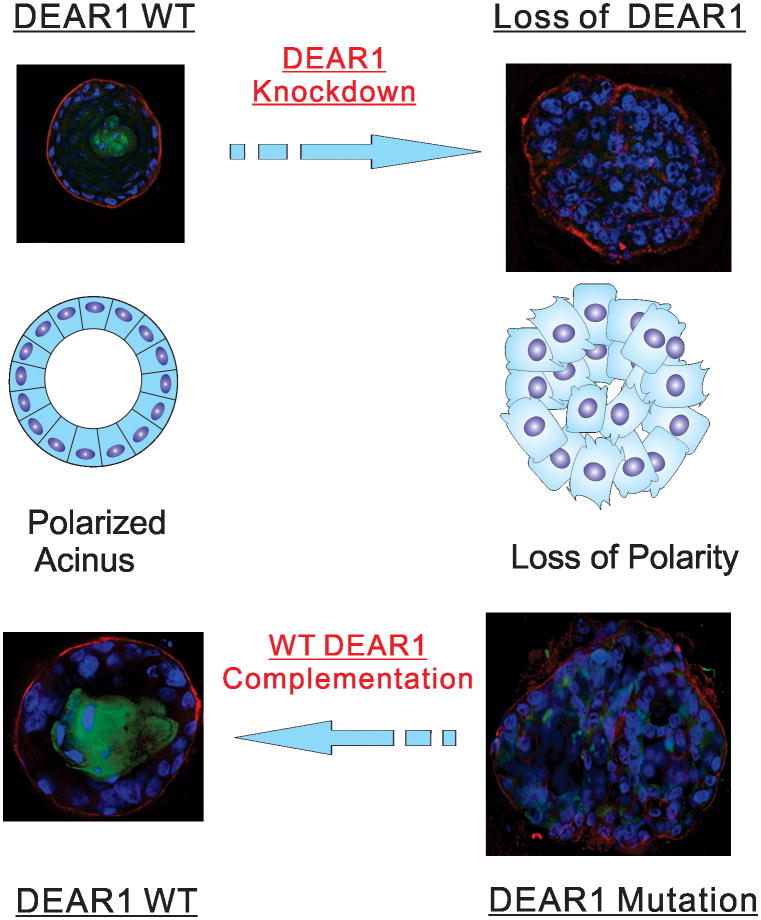
One of the most studied genomic intervals in human cancer lies within the short arm of human chromosome 1 in which loss of heterozygosity (LOH) within three separate intervals occurs at high frequency in a variety of epithelial cancers, including both sporadic breast cancers as well as in breast cancers with inherited predisposition (18-20). LOH within chromosome 1p has been shown to predict poor prognosis in node-negative breast cancers, and allelic deletions in the 1p36 and 1p32 region correlate with poor survival (20). Our laboratory discovered a novel gene DEAR1 (Ductal Epithelium Associated Ring Chromosome 1) by suppression subtractive hybridization cloning of genes differentially expressed between cells with and without functional complementation of the tumor suppressor locus NRC-1 within 3p12 (21-25). DEAR1 was identified as a differentially expressed cDNA obtained from the suppression library that mapped into chromosome 1p35.1, a genomic interval downstream of chromosome 3p loss in a cytogenetic pathway for the evolution of smoking related cancers including lung, kidney, and breast cancer (24). A 54kD protein, DEAR1 is a member of the TRIM (Tripartite Motif) family of proteins implicated in the organization and architecture of large protein complexes. To date, TRIM family members have been shown to be mutated in the germline in developmental disorders and play important roles in cancer, mostly in overexpression or oncogenic transformation or in the case of a few TRIM proteins, as tumor suppressors, such as the most well characterized TRIM protein PML that is translocated to RARalpha in the chromosome t(15;17) translocation associated with acute promyelocytic leukemia. TRIM proteins have been shown to play critical roles in the regulation of cell proliferation, apoptosis, DNA repair, and antiviral activity (26, 27). The first indication that DEAR1 might be a novel TRIM family tumor suppressor came from examination of its expression in normal tissue compared to tumors and cancer cell lines. DEAR1 is expressed in normal tissues but limited to the ductal and glandular epithelium, and undergoes loss or downregulation of expression in breast cancer cell lines as well as in DCIS (71%), one of the earliest histologic precursors of invasive breast cancer (24, 25) DEAR1 is also mutated (13%) and homozygously deleted in breast cancer, including early onset cases (24). Targeted disruption of Dear1 in the mouse determined that Dear1 was a bona fide tumor suppressor, the knockout of which resulted in late onset epithelial adenocarcinomas in multiple organs, including mammary gland, kidney, pancreas and lung as well as sarcomas and lymphomas (25). Interestingly, Dear1 heterozygous as well as homozygous null animals formed tumors with approximately equal frequency, suggesting that Dear1 might be a haploinsufficient tumor suppressor. Immunohistochemical staining of representative tumors from the mouse model indicated that in the majority of adenocarcinomas, expression was absent which would be expected for loss of function. However, in the majority of lymphomas, Dear1 was expressed in tumors (30%-80% of cells staining positively), indicating that Dear1 is a haploinsufficient tumor suppressor in certain tumor types (25). Unlike classical tumor suppressors that regulate cellular proliferation, however, DEAR1 loss to date has not been shown to affect growth rates of human mammary epithelial cells (HMECs) in both two dimensional culture (2D) (unpublished observations) and 3D culture (24). Rather, DEAR1 dramatically affects polarity and cellular architecture in 3D culture. Reintroduction of wild type DEAR1 to genetically complement a missense mutation in codon 187 restored acinar morphogenesis in 3D culture in the 21MT breast cancer cell line. Conversely, stable knockdown of DEAR1 resulted in irregular, apolar acini consistent with its role in the regulation of apical-basal polarity and indicative that DEAR1 is a dominant regulator of acinar morphogenesis in 3D culture (24). Thus, DEAR1 is a novel and critical tumor suppressor that regulates tissue architecture (24, 28) (Fig. 1). Moreover, DEAR1 not only regulates apical-basal polarity, but also connects polarity regulation with control of the most important pathway implicated in the regulation of epithelial plasticity, the transforming growth factor β (TGFβ) pathway, which is upregulated in the stroma and key to the onset of EMT, migration and invasion associated with breast cancer.
Figure 1. DEAR1 Regulates Cell polarity and Acinar Morphogenesis.
Wild type DEAR1 Human mammary epithelial cells (HMECs) undergo acinar morphogenesis in 3D culture in which individual acini are formed containing a polarized layer of luminal epithelial cells surrounded by myoepithelial cells and an intact basement membrane as well as a hollow lumen formed by anoikis; with DEAR1 stable knockdown, HMECs lose apical basal polarity and proper lumen formation (figure adapted from Lott, et.al. Plos Medicine, (24)). In lower panel, introduction of wild type DEAR1 into the 21MT breast cancer cell line containing a missense mutation in DEAR1 rescued acinar morphogenesis.
DEAR1 as a master regulator of TGFβ Signaling and EMT
The tumor microenvironment has been shown to exert a profound influence on the progression and metastasis of primary tumors and yet the mechanisms regulating this effect are largely unclear. The TGFβ pathway is one of the most deregulated pathways in breast cancer and intimately associated with the induction of EMT. TGFβ is a critical cytokine, produced by the stroma, that functions as a potent tumor suppressor in epithelial cell types and has been shown to inhibit proliferation, and regulate differentiation and tissue architecture (29). However, accumulating evidence has indicated its important role in promoting EMT and metastasis in many cancers (29). TGFβ is regulated both in an autocrine and paracrine manner and induces cancer cell migration and invasion as well as deposition of extracellular matrix proteins (30). TGFβ signaling has been shown to be necessary for the survival of breast cancer cells and for the induction of EMT and cell migration, with clinical evidence indicative of a correlation between expression of TGFβ ligands and poor patient outcome (31).
The importance of DEAR1 in the regulation of TGFβ signaling from the ECM was discovered when immortal HMECs for which DEAR1 expression had been stably knocked down (DEAR1-KD), failed to undergo acinar morphogenesis when TGFβ was added to the 3D culture media as compared to wild type DEAR1 controls which formed proper acini (25). DEAR1-KD clones failed to upregulate Caspase 3, indicative that the cells were not undergoing apoptosis, but rather the cells upregulated Vimentin, an immediate early EMT marker, indicative that loss of DEAR1 resulted in a phenotype shift to a more mesenchymal phenotype in the presence of TGFβ and furthermore, in wild type (WT) DEAR1 expressing HMECs, DEAR1 restricted TGFβ signaling from the extracellular milieu. In addition, compared to HMEC clones that express DEAR1, TGFβ-treated DEAR1-KD clones exhibited pronounced migration across the wound in 2D culture as well as prolonged migration and invasion on top of and throughout the matrix in 3D culture (25). TGFβ-treated DEAR1-KD clones also displayed elevated levels of mesenchymal markers β-integrin and N-Cadherin, as well as acquired markedly enhanced anoikis resistance, another important marker of EMT, in which increased survival of epithelial tumor cells occurs in the presence of inadequate or inappropriate contact with the extracellular matrix (7, 32). Thus DEAR1 loss of function in the presence of TGFβ resulted in a failure of acinar morphogenesis, migration, invasion in 3D culture as well as anoikis resistance, indicative of alterations in the cell-matrix interaction, and key steps in TGFβ-induced EMT (25).
DEAR1 regulation of SMAD3 as a critical mechanism to control TGFβ-induced EMT
TGFβ mediates its effects on cell signaling pathways and cell behavior through both canonical and non-canonical pathways. The non-canonical pathway is related to the regulation of polarity (a SMAD-independent pathway), in which, TGFβ receptors can activate Ras/Erk MAP kinases, Par6/Rho GTPases and the PI3 kinase/Akt pathways and lead to EMT through their regulation of cytoskeleton organization and cell junctions (33). The TGFβ canonical pathway is mediated through SMAD signaling. After binding TGFβ, TGFβ receptors, TGFβRII and TGFβRI phosphorylate and activate receptor-activated (R)-SMADs (SMAD2/SMAD3), which form a complex with SMAD4 and then translocate from the cytoplasm to the nucleus in response to TGFβ signaling to transcriptionally activate distinct sets of genes that repress the proliferative response or activate EMT (34). Studies have found that SMAD2 and SMAD3 can play different roles in mediating TGFβ signaling. SMAD3, but not SMAD2, has recently been reported to be readily degraded by the proteasome at the steady state, thus, SMAD3 protein is less stable than SMAD2 (35). SMAD3, but not SMAD2 has the ability to directly bind DNA, thus has more versatile roles in transducing TGFβ signals (36, 37). Consistent with these studies, DEAR1 interacts strongly with SMAD3, but not SMAD2 or SMAD4, leading to the degradation of SMAD3, suggesting that DEAR1 specifically regulates SMAD3 (25). SMAD3 activation results in the transcription of EMT-markers, such as PAI-1, vimentin and β-integrin, as well as master EMT regulators, Snail1/2 and Zeb1/2 (36). In fact, treatment of DEAR1-KD clones with a SMAD3 inhibitor, SIS3 (38) or SMAD3 knockdown remarkably attenuated TGFβ-induced cell migration and EMT marker expression. These data suggest that regulation of SMAD3 protein levels could be a critical step in preventing EMT and importantly, that SMAD3 is a critical target of DEAR1, and underlies the mechanism by which DEAR1 inhibits TGFβ signaling and TGFβ-induced EMT (25).
The ubiquitination of SMAD3 is a mechanism by which DEAR1 finely regulates the TGFβ pathway
Overactivation of the TGFβ pathway has been extensively shown to drive EMT and metastasis (29). However, abrogation of the TGFβ pathway or SMAD signaling in mouse models has also been reported to enhance metastasis (39). These studies indicate the context-dependent role of TGFβ and suggest that gain or loss of function of TGFβ pathway components may lead to metastasis. Therefore, fine regulation of the TGFβ pathway could be very important in preventing metastasis.
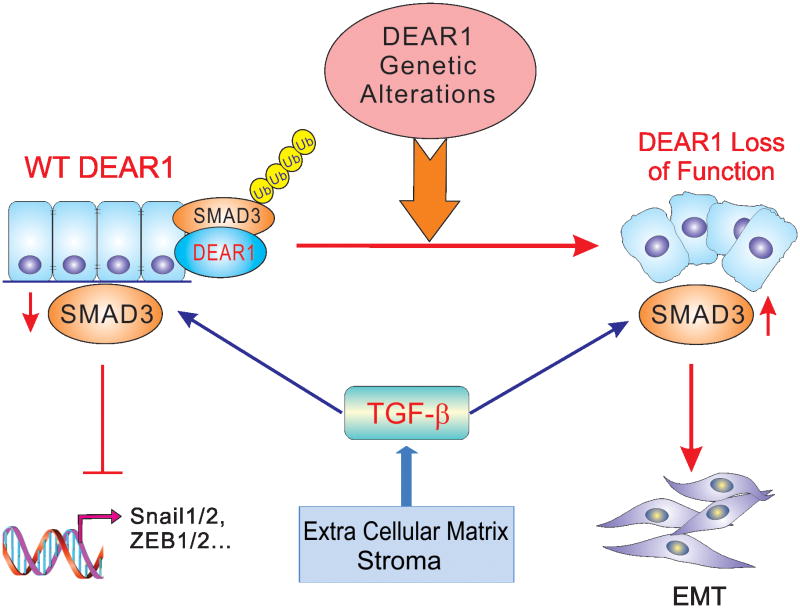
One of the main mechanisms of regulation of cellular responsiveness to TGFβ family ligands is through ubiquitination mediated degradation of SMAD proteins. Although a number of E3 ligases have been shown to affect SMAD3 levels in the activated ligand bound state, only a limited number of direct E3 ubiquitin ligases and other regulators have been reported to promote the ubiquitination of SMAD3 under nonactivated steady state levels to negatively regulate TGFβ signaling (40). Regulation of the levels of nonactivated SMAD3 are important as it determines the sensitivity of SMAD3 to TGFβ signaling. Both CHIP and Axin have been reported to promote the ubiquitination and degradation of nonactivated SMAD3 (35). Casein kinase 1 gamma 2 (CKIγ2), ROC1-SCF Fbw1a, WWP2-N and Nedd4L have been shown to promote the ubiquitination and proteasomal degradation of SMAD3 under an activated state (35, 40). Identification of additional ubiquitin ligases that regulate the ubiquitination of nonactivated SMAD3 will provide a better understanding of the physiological regulation of SMAD3. Most recently, we reported that DEAR1 can promote the polyubiquitination and proteasomal mediated degradation of nonactivated SMAD3 (25), highlighting that DEAR1 may play a pivotal role in the physiological regulation of SMAD3 signaling. DEAR1 promotes the polyubiquitination of nonactivated SMAD3, yet it remains to be determined whether DEAR1 functions as a direct E3 ubiquitin ligase for SMAD3 although DEAR1 has been shown to possess ligase activity (41). Since lysine-linkage specific ubiquitin chains ultimately decide the fate of ubiquitination, identifying the ubiquitin linkage of polyubiquitin chains supported by DEAR1 to promote the polyubiquitination of SMAD3 will provide novel insights into the mechanism of regulation of SMAD3 by DEAR1. Because DEAR1 undergoes mutations in multiple tumor types, including breast cancer, and since mutations in the RING domain of DEAR1 have also been reported in tumors (25), it will be important to determine whether tumor-derived mutations may differentially regulate SMAD3 ubiquitination and promote aberrant TGFβ signaling. Knockdown of DEAR1 in HMECs has been shown to dramatically elevate SMAD3 protein levels, however, HMECs expressing wild type DEAR1 still express a detectable amount of SMAD3, which is phosphorylated and enters the nucleus (25). Therefore, DEAR1 binding and ubiquitination may finely regulate SMAD3 protein levels. Once DEAR1 function is lost, more SMAD3 would be available for phosphorylation and nuclear translocation in the presence of TGFβ. Therefore, the rapid turnover of non-activated SMAD3 by DEAR1 ubiquitination may reflect the necessity for cells to more tightly control SMAD3 levels and thus control TGFβ signal transduction (Figure 2) (42).
Figure 2. DEAR1 Regulates Epithelial Plasticity by Inhibiting the TGFβ/SMAD3 pathway.
Normal DEAR1 expression promotes the fine regulation of SMAD3 and inhibits TGFβ-induced EMT-associated gene expression in HMECs, while loss of function of DEAR1 by genetic alterations, such as mutation, copy number alteration or loss of expression, results in loss of polarity, a dramatic increase of SMAD3 levels, and in the presence of TGFβ, the initiation of EMT.
The importance of genomic alteration of DEAR1 in cancer development and progression
In the 2011 revised version of Hanahan and Weinberg's hallmarks of cancer, genomic instability and the accumulation of mutations were noted as enabling characteristics instrumental in the expansion of tumor development, further promoting the acquisition of the integral hallmarks of cancer (43). Chromosome 1 which undergoes LOH at a relatively high frequency in multiple epithelial tumor types (29-72%), including breast, colorectal, lung, stomach, and kidney, with chromosomal regions 1p31, 1p34-35 and 1p36 experiencing the highest frequency of copy number loss (19, 44). LOH of chromosome 1p has even been found to occur preferentially in ER+ breast tumors and is associated in a multivariate analysis in breast cancer with a 2.7 fold increase in relative risk of death (44, 45). One of the consequences of chromosome 1p loss would be coordinate loss of important tumor suppressors mapping into this genomic interval, including p73 and CHD5, within 1p36, CDKN2C (p18INK4C) mapping to 1p32, DEAR1 localized to 1p35.1, and the base excision repair gene, MUTYH, localized to 1p34. Loss of a single copy of chromosome 1p would be especially important if tumor suppressors in this genomic region were also haploinsufficient. Targeted disruption of Dear1 in the mouse indicated both that Dear1 was a “bona fide” tumor suppressor and in some cases functioned as a haploinsufficient tumor suppressor, suggesting that loss of a single copy of Dear1 might be sufficient as a “driver” event in certain human cancers. Thus, heterozygous loss of DEAR1 in combination with loss of additional chromosome 1p tumor suppressors could result in loss of growth control as well as increased migratory, invasive behavior due to loss of DEAR1 (24, 28). In that regard, Muthuswamy and coworkers have made a strong case for the role of loss of polarity regulation in combination with overexpression of oncogenes driving malignancy and metastasis (46). We propose that in the case of DEAR1, loss of function would involve not only disrupting polarity but also causing loss of its ability to function in ubiquitination of at least SMAD3, and potentially other targets yet to be discovered. Furthermore, loss of function of DEAR1 by mutation, copy number loss or loss of expression in conjunction with overexpression of key cytokines in the microenvironment, such as TGFβ, activation of oncogenes driving growth, or loss of other chromosome 1p tumor suppressors, would result in enhanced migration, accelerated invasion, and metastasis (Figure 2).
DEAR1 has also been found to be mutated, at rare frequencies, in multiple cancer types (24). The rare mutation rate observed in DEAR1 may be due to the fact that currently most sequencing methods have involved low coverage techniques and the sequencing of the primary tumor. Ultra-deep sequencing might be useful to detect rare variants in primary tumors that are also represented in metastatic lesions and therefore might represent “driver” events for early metastasis. To date, most of the mutations in DEAR1 are exonic and predicted by algorithms to be potentially deleterious (24, 25). Mutations known to be in domains important to binding to other cell signaling regulators could potentially effect downstream signaling with large phenotypical changes. Moreover, ultra-rare loss of function nonsense and frameshift mutations of DEAR1 have been identified from the cBIO database that add further support to the tumor spectrum in the Dear1 mouse model and suggest that DEAR1 could be involved in multiple epithelial cancers.
The clinical utility of DEAR1 mutation/copy number alteration or loss of expression in cancer
Genetic alterations and loss of expression of DEAR1 could have important clinical implications. Mutations in DEAR1 were found in early onset cases of breast cancer and loss of expression of DEAR1 was a strong predictive biomarker of local recurrence in 123 cases from young women (24). In addition, 70% of mutations identified for which clinical information was available, although only a small population (n=10), were associated with lymph node involvement or metastasis (25). Moreover, heterozygous loss of the DEAR1 locus along with SNAIL2 alteration at the mRNA or chromosomal level significantly correlated with overall poor survival in 889 cases of breast cancer independent of subtype (25). Probably of most importance, though, would be the utility of mutations, copy number losses or expression losses, for stratification of breast cancers and potentially other epithelial cancers for risk assessment and for targeted therapies. Importantly, clinical application of TGFβ inhibitors has proven difficult because of the broad role of TGFβ in cancer and thus, selective targeting could provide a novel avenue for therapy (47, 48). Because DEAR1 inhibits TGFβ-induced EMT through targeting SMAD3, more effective treatments with specific targeted inhibitors of this pathway might be possible in the near future.
References
- 1.Ronnov-Jessen L, Bissell MJ. Breast cancer by proxy: can the microenvironment be both the cause and consequence? Trends Mol Med. 2009;15:5–13. doi: 10.1016/j.molmed.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 3.Reichmann E. Oncogenes and epithelial cell transformation. Semin Cancer Biol. 1994;5:157–65. [PubMed] [Google Scholar]
- 4.Osanai M, Murata M, Nishikiori N, Chiba H, Kojima T, Sawada N. Occludin-mediated premature senescence is a fail-safe mechanism against tumorigenesis in breast carcinoma cells. Cancer Sci. 2007;98:1027–34. doi: 10.1111/j.1349-7006.2007.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichert M, Müller T, Hunziker W. The PDZ Domains of Zonula Occludens-1 Induce an Epithelial to Mesenchymal Transition of Madin-Darby Canine Kidney I Cells. Journal of Biological Chemistry. 2000;275:9492–500. doi: 10.1074/jbc.275.13.9492. [DOI] [PubMed] [Google Scholar]
- 6.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGF[beta] signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–8. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Yan W, Chen X. Mutant p53 Disrupts MCF-10A Cell Polarity in Three-dimensional Culture via Epithelial-to-mesenchymal Transitions. Journal of Biological Chemistry. 2011;286:16218–28. doi: 10.1074/jbc.M110.214585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Z, Jones R, Liu JC, Deng T, Robinson T, Chung PE, et al. RB1 and p53 at the crossroad of EMT and triple-negative breast cancer. Cell Cycle. 2011;10:1563–70. doi: 10.4161/cc.10.10.15703. [DOI] [PubMed] [Google Scholar]
- 12.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 13.Partanen JI, Tervonen TA, Myllynen M, Lind E, Imai M, Katajisto P, et al. Tumor suppressor function of Liver kinase B1 (Lkb1) is linked to regulation of epithelial integrity. Proc Natl Acad Sci U S A. 2012;109:E388–E397. doi: 10.1073/pnas.1120421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halaoui R, McCaffrey L. Rewiring cell polarity signaling in cancer. Oncogene. 2014 doi: 10.1038/onc.2014.59. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- 16.Elsum IA, Martin C, Humbert PO. Scribble regulates an EMT polarity pathway through modulation of MAPK-ERK signaling to mediate junction formation. J Cell Sci. 2013;126:3990–9. doi: 10.1242/jcs.129387. [DOI] [PubMed] [Google Scholar]
- 17.Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer. 2002;34:255–68. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 18.Millikan RC, Ingles SA, Diep AT, Xue S, Zhou N, Florentine BD, et al. Linkage analysis and loss of heterozygosity for chromosome arm 1p in familial breast cancer. Genes Chromosomes Cancer. 1999;25:354–61. [PubMed] [Google Scholar]
- 19.Borg A, Zhang QX, Olsson H, Wenngren E. Chromosome 1 alterations in breast cancer: allelic loss on 1p and 1q is related to lymphogenic metastases and poor prognosis. Genes Chromosomes Cancer. 1992;5:311–20. doi: 10.1002/gcc.2870050406. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto K, Ito N, Yoshimoto M, Kasumi F, Akiyama F, Sakamoto G, et al. Allelic loss on chromosome 1p is Associated with progression and lymph node metastasis of primary breast carcinoma. Cancer. 1998;82:317–22. [PubMed] [Google Scholar]
- 21.Sanchez Y, El-Naggar A, Pathak S, Killary AM. A tumor suppressor locus within 3p14-p12 mediates rapid cell death of renal cell carcinoma in vivo. Proc Natl Acad Sci U S A. 1994;91:3383–7. doi: 10.1073/pnas.91.8.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lott ST, Chandler DS, Curley SA, Foster CJ, El-Naggar A, Frazier M, et al. High frequency loss of heterozygosity in von Hippel-Lindau (VHL)-associated and sporadic pancreatic islet cell tumors: evidence for a stepwise mechanism for malignant conversion in VHL tumorigenesis. Cancer Res. 2002;62:1952–5. [PubMed] [Google Scholar]
- 23.Zhang K, Lott ST, Jin L, Killary AM. Fine mapping of the NRC-1 tumor suppressor locus within chromosome 3p12. Biochem Biophys Res Commun. 2007;360:531–8. doi: 10.1016/j.bbrc.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 24.Lott ST, Chen N, Chandler DS, Yang Q, Wang L, Rodriguez M, et al. DEAR1 is a dominant regulator of acinar morphogenesis and an independent predictor of local recurrence-free survival in early-onset breast cancer. PLoS Med. 2009;6:e1000068. doi: 10.1371/journal.pmed.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N, Balasenthil S, Reuther J, Frayna A, Wang Y, Chandler DS, et al. DEAR1 Is a chromosome 1p35 tumor suppressor and master regulator of TGF-β driven epithelial-mesenchymal transition. Cancer Discovery. 2013;3:1172–89. doi: 10.1158/2159-8290.CD-12-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140–51. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 28.Muthuswamy SK. A new tumor duppressor that regulates tissue architecture. PLoS Med. 2009;6:e1000073. doi: 10.1371/journal.pmed.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meulmeester E, ten Dijke P. The dynamic roles of TGF-β in cancer. J Pathol. 2011;223:206–19. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 30.Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–52. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- 31.Moses H, Barcellos-Hoff MH. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 33.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–76. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuzaki K, Seki T, Okazaki K. TGF-beta during human colorectal carcinogenesis: the shift from epithelial to mesenchymal signaling. Inflammopharmacology. 2006;14:198–203. doi: 10.1007/s10787-006-1536-2. [DOI] [PubMed] [Google Scholar]
- 35.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3-ß control Smad3 protein stability and modulate TGF-ß signaling. Genes Dev. 2008;22:106–20. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millet C, Zhang YE. Roles of Smad3 in TGF-beta signaling during carcinogenesis. Crit Rev Eukaryot Gene Expr. 2007;17:281–93. doi: 10.1615/critreveukargeneexpr.v17.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, et al. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and Its effect on transforming growth factor-β1-Induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 39.Pickup M, Novitskiy S, Moses HL. The roles of TGF[beta] in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–99. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Boeck M, ten Dijke P. Key role for ubiquitin protein modification in TGFβ signal transduction. Ups J Med Sci. 2012;117:153–65. doi: 10.3109/03009734.2012.654858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang F, Xiao H, Sun BL, Yang RG. Characterization of TRIM62 as a RING finger E3 ubiquitin ligase and its subcellular localization. Biochemical and Biophysical Research Communications. 2013;432:208–13. doi: 10.1016/j.bbrc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Lonn P, Moren A, Raja E, Dahl M, Moustakas A. Regulating the stability of TGFbeta receptors and Smads. Cell Res. 2009;19:21–35. doi: 10.1038/cr.2008.308. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Ragnarsson G, Eiriksdottir G, Johannsdottir JT, Jonasson JG, Egilsson V, Ingvarsson S. Loss of heterozygosity at chromosome 1p in different solid human tumours: association with survival. Br J Cancer. 1999;79:1468–74. doi: 10.1038/sj.bjc.6690234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZC, Lin M, Wei LJ, Li C, Miron A, Lodeiro G, et al. Loss of heterozygosity and its correlation with expression profiles in subclasses of invasive breast cancers. Cancer Res. 2004;64:64–71. doi: 10.1158/0008-5472.can-03-2570. [DOI] [PubMed] [Google Scholar]
- 46.Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–78. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3:1011–22. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 48.Nagaraj NS, Datta PK. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin Investig Drugs. 2010;19:77–91. doi: 10.1517/13543780903382609. [DOI] [PMC free article] [PubMed] [Google Scholar]