Abstract
Orthopaedic injuries are very common and a source of much misery and economic stress. Several relevant tissues, such as cartilage, meniscus and intra-articular ligaments, do not heal. And even bone, which normally regenerates spontaneously, can fail to mend. The regeneration of orthopaedic tissues requires four key components: cells, morphogenetic signals, scaffolds and an appropriate mechanical environment. Although differentiated cells from the tissue in question can be used, most cellular research focuses on the use mesenchymal stem cells (MSCs). These can be retrieved from many different tissues, and one unresolved question is the degree to which the origin of the cells matters. Embryonic and induced, pluripotential stem cells are also under investigation. Morphogenetic signals are most frequently supplied by individual, recombinant growth factors or native mixtures provided by, for instance, platelet-rich plasma; MSCs are also a rich source of trophic factors. Obstacles to the sustained delivery of individual growth factors can be addressed by gene transfer or smart scaffolds, but we still lack detailed, necessary information on which delivery profiles are needed. Scaffolds may be based upon natural products, synthetic materials, or devitalized extracellular matrix. Strategies to combine these components to regenerate tissue can follow traditional tissue engineering practices, but these are costly, cumbersome and not well suited to treating large numbers of individuals. More expeditious approaches make full use of intrinsic biological processes in vivo to avoid the need for ex vivo expansion of autologous cells and multiple procedures. Clinical translation remains a bottleneck.
“All the successes of orthopaedic surgeons are little more than a reflection of the body’s amazing ability to heal itself”
Henry Mankin, MD1
Introduction
With its wide range of competencies and frequent injury, the skeletal system provides a rich testing ground for the field of regenerative medicine. The long bones, for example, represent one of the few organs in the human body that normally regenerate spontaneously, without scarring, to restore full function. But large segmental defects in these same bones fail to heal 2, and the calvarial bones of the skull lack any inherent reparative ability in individuals older than 2 years of age 3. Likewise, intra-articular ligaments, articular cartilage and menisci have almost no ability to heal after injury. Extra-articular ligaments and tendons, on the other hand, mount a repair response, but produce a regenerate that is inferior to uninjured tissue.
It is not generally appreciated that orthopaedics has long been at the forefront of regenerative medicine. The osteoinductive properties of demineralized bone matrix (DBM), for example, were recognized nearly 50 years ago and it has been available for clinical use for decades 4. Two of its active ingredients, bone morphogenetic protein (BMP) -2 and -7, were cloned in the 1980s and have been approved for clinical use since the early 2000s. Cell therapy, in the form of autologous chondrocytes used to heal cartilagenous injuries, has been available since the 1990s 5. It should also be pointed out that the concept of a mesenchymal stem cell (MSC) grew out of the orthopaedic research community 6, as did the suggestion to use gene therapy for tissue regeneration 7. Despite these advantages, progress in developing approved regenerative products for clinical use has been slow.
This review summarizes recent developments and trends in this important area. Space does not permit comprehensive or detailed treatment of the subject matter, but recent reviews are cited for additional information. Emphasis is placed on translational aspects of the field.
Current Concepts
Strategies
Regeneration of the skeletal system, like many other organ systems, requires at a minimum cells, morphogenetic signals and matrices or scaffolds. Because a major function of the skeletal system is to bear load, mechanical stimuli are of additional importance, something that provides an interesting intersection between orthopaedic surgery and rehabilitation.
In the past, major effort was expended in developing traditional tissue engineering technologies for growing new tissue extracorporally prior to implantation (figure 1A). These usually involved harvesting autologous cells from the patient, expanding them in culture, seeding them onto a scaffold and incubating them in a bioreactor. Considerable successes have been logged by this approach 8, including the use of patients as their own bioreactors 9.
Figure 1. Strategies for regeneration.
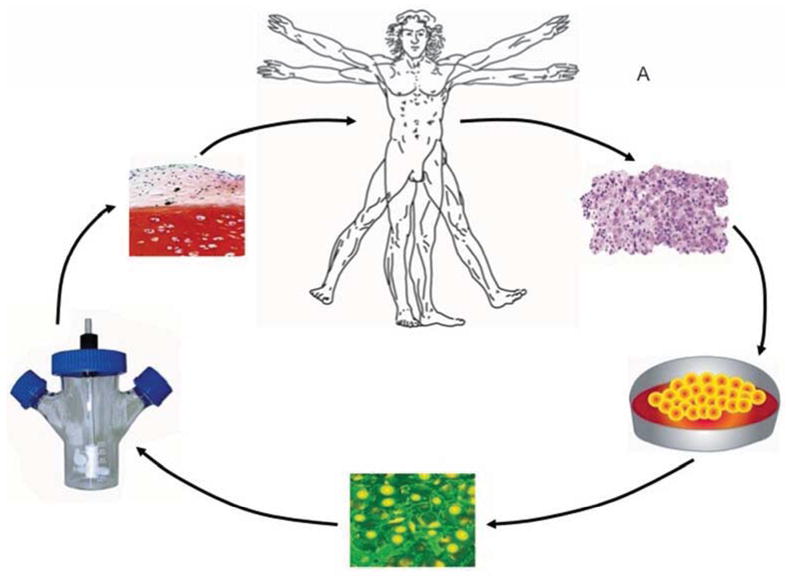
Panel A. In traditional tissue engineering approaches, a first procedure removes tissue biopsies. Autologous cells are isolated, seeded on a matrix and incubated in a bioreactor under conditions where new tissue is formed. The graft is implanted into the patient in a second procedure.
Panel B. Expedited approaches require a single percutaneous, minimally invasive, or intra-operative procedure.
Adapted from reference 10
Although technically feasible, the economics and complexities of such approaches mitigate against their widespread application 10. This point is arguable 11, but there is nevertheless increasing interest in using technologies that do not require ex vivo cultivation of autologous cells for each patient or more than one invasive procedure. This can be achieved with allograft cells, rapid isolation and manipulation techniques that can be used intra-operatively, or by provoking and facilitating endogenous repair processes (Figure 1B). For practical and economic reasons, there is a trend towards minimally invasive approaches.
Cells
Differentiated cells from the tissue to be repaired are already used clinically, as exemplified by autologous chondrocyte implantation (ACI) 5. However, attention is increasingly turning to the use of stem or progenitor cells as the basis for skeletal tissue regeneration.
MSCs derived from bone marrow were the first to be investigated, and they remain the cell of choice for many investigators 12. They can be obtained readily from bone marrow biopsies and easily expanded in culture. Because populations generated in this manner contain cells with the ability to differentiate along various lineages of importance to the skeletal system, they are of wide significance to regenerative orthopaedics. It is commonly believed that MSCs can be successfully allografted, which raises the possibility of facilitating clinical application with universal donor lines. Moreover, MSCs have anti-inflammatory properties of potential benefit when regeneration has to occur in a hostile inflammatory environment.
Initially identified in bone marrow aspirates, MSCs have since been harvested from almost every tissue and organ in the body, including periosteum, long recognized as a rich source of regenerative power in orthopaedics 13. Some newer sources, such as sub-dermal fat, are readily accessible and provide far more cells. Fresh lipoaspirates provide a “stromal vascular fraction” comprising, in addition to MSCs, endothelial precursor cells, smooth muscle cells, monocytes, macrophages and lymphocytes, among others. The equivalence between MSCs derived from different sources and their suitability for regenerating different tissues is still under discussion. However, there is an emerging consensus that not all MSC populations are equal, and regeneration is likely to be most successful when MSCs are recovered from the tissue to be regenerated. This is relevant to the suggestion that MSCs from the pulp of extracted human molars, or elsewhere, be banked for future use 14. Refinements to the use of MSCs include preimplantation sorting to enrich for progenitors of choice, and preconditioning to prime the cells to survive and differentiate, as required, after implantation 15. A rapidly growing number of companies are developing MSCs for clinical use in regenerative orthopaedics, but at the same time attracting increasing scrutiny by the FDA 16. Alternative sources of progenitor cells include blood 17, placenta and umbilical cord 18. There is increasing interest in the use of endothelial progenitor cells for orthopaedic purposes 19.
Despite their several advantages, cultures of MSCs display a Hayflick limit and, as they senesce, their differentiation properties alter. Embryonic stem cells (ESCs) and induced pluripotent stem (iPS) cells are of interest because they do not senesce on repeated sub-culture. Moreover, iPS cells obviate the ethical concerns surrounding ESCs. Conditions for their differentiation into cells of the skeletal system are being developed. Safety remains an issue, especially with the potential for teratoma formation.
Morphogenetic Stimuli
Most research has focused on particular growth factors that guide the relevant patterns of differentiation during embryogenesis. Thus BMPs have been widely explored in the context of osteogenesis and transforming growth factor (TGF) -β for chondrogenesis. The use of key transcription factors, such as scleraxis for tenogenesis, is also current. An alternative approach uses small inductive molecules. Patterns of differentiation can also be influenced by the chemical environment, such as hypoxia, which stimulates chondrogenesis.
Delivery of morphogenetic stimuli remains rate limiting for many applications. Although some promising agents, such as teriparatide (parathyroid hormone 1–34; Forteo), can be delivered systemically, for most purposes, it is necessary to deliver the agent locally to the site of regeneration for a period of several days or weeks. This has proved to be extremely difficult to accomplish. Much research thus focuses on the development of smart scaffolds that will provide controlled release of factors at suitable doses for an appropriate period of time. The literature on this topic is voluminous, yet no clinically approved product has appeared. Despite the popularity of this approach, it remains constrained by the reality that we still do not know exactly which factors we need to deliver, at what concentrations, and with what kinetics for individual applications.
Gene delivery offers an alternative strategy for delivering morphogens. In principle, the local delivery of genes (usually cDNAs) encoding the protein in question will result in focal synthesis of a nascent, endogenous product that has undergone authentic post-translational modification. Its synthesis will persist for as long as the cDNA is present and expressed, with the potential for regulated expression. Gene delivery also brings many advantages to the delivery of gene products that work intra-cellularly, such as transcription factors and inhibitory species of RNA; the latter are highly topical, and subject to increasing research. Gene transfer can be accomplished with viral or non-viral vectors, delivered by in vivo or ex vivo protocols; specific examples are given in later sections of this review.
Another option for the delivery of morphogenetic signals follows from the growing realization that MSCs are a rich source of trophic factors. One advantage of this approach is that it is not necessary a priori to know which morphogens are present or required, and the need for their isolation and purification is removed. The same is true of blood derived products such as autologous conditioned serum 20 and the latest orthopaedic panacea, platelet rich plasma (PRP) 21.
Scaffolds
Space filling scaffolds provide a substrate, preferably resorbable, upon which new tissue can form. Increasingly, the scaffold is not a passive conductive presence, but an agent that provides cells and signals to induce and inform the development of new tissue. Such signals may be biological, chemical or physical. Agents may be attached to scaffolds covalently or non-covalently by, for example, adsorption, encapsulation, or affinity binding 22. Two general delivery strategies exist. One provides agents at a uniform rate governed, for instance, by the rate of scaffold dissolution. Stimulus-responsive (“smart”) scaffolds deliver in response to changes in the local environment or in response to external stimuli, such as ultrasound or magnetic fields. Scaffolds can also be designed to resorb at rates that coordinate with tissue regeneration. This is achieved by including domains that are susceptible to lytic enzymes produced selectively by tissues during regeneration 23. Some scaffolds have intrinsic morphogenetic influence, such as those ceramics that, in addition to providing mechanical strength, are also osteoinductive, thus serving a dual mechanical and biological role 24.
Devitalized tissues often provide scaffolds that contain a rich cocktail of endogenous morphogens with inductive properties. A large number are commercially available and approved for clinical use 25; DMB 4 is an early example of this. Such materials are versatile, supplied as sheets, powders and gels, and may be shaped to need. Scaffolds may also be associated with gene delivery vectors to form gene-activated matrices (GAMs) that both provide support and deliver cDNA molecules 26.
A wide range of natural and synthetic materials has been evaluated as potential scaffolds (Table 1) and several are in clinical use. The choice of scaffold depends on a variety of factors, including porosity, biocompatibility, resorption rate and mechanical strength. One strategy selects mechanical properties to match those of the tissue being regenerated, so that, as the scaffold resorbs, load is progressively transferred to the new tissue. One disadvantage of this approach is the phenomenon of stress shielding whereby load bearing by the scaffold reduces mechanical stimulation (see next section) of the regenerate.
TABLE 1.
Materials used to form scaffolds for the regeneration of orthopaedic tissues.
| Class | Examples |
|---|---|
| Natural polymers | Collagen Gelatin Silk Fibrin Alginate Chitosan Hyaluronan Coral (hydroxyapatite) |
| Ceramics | Hydroxyapatite β-Tricalcium phosphate (TCP) Biphasic calcium phosphate (BCP) Calcium sulfate (plaster of Paris) Octacalcium phosphate Bioglass |
| Synthetic, biodegradable polymers | Polylactic acid Polyglycolic acid (vicryl) Poly (lactic-co-glycolic acid) Polycaprolactone Polyhydroxyalkanoate Polyurethane |
| Tissue extracellular matrix | Demineralized bone matrix (DBM) Small intestine sub-mucosa (SIS) Skin Dermis Fascia Pericardium |
| Other | Self-assembling peptides Hybrid scaffolds Collagen-glycosaminoglycan Collagen-hydroxyapatite Gelatin - hyaluronan Hyaluronan – polycaprolactone Polycaprolactone - polyurethane |
In recent years advanced fabrication technologies have been adapted for scaffold production. Electrospinning 27 allows the formation of nanofibers that can provide complex, anisotropic, biomimetic structures. Advanced imaging and fabrication techniques allow designer scaffolds, anatomically shaped to individual patients 8.
Although we usually think of scaffolds as solid implants, there is growing interest in using injectable liquids or pastes that can be applied in a minimally invasive fashion.
Mechanical Stimuli
Mechanical stimulation is essential to the development and function of skeletal tissues. Moreover, it has been appreciated since the 1960s that the mechanical environment can determine stem cell fate 28. Recent research that defines this interaction in greater mechanistic detail 29 promises to provide major advances. The question is how to incorporate this new information into improved regenerative strategies.
Many tissue engineering protocols use bioreactors to provide the appropriate forces 8. Control of the mechanical environment is more difficult when attempting in situ regeneration. In the case of bone healing, this has traditionally been addressed with fixtors of different designs. For accessible joints, such as the knee, it is possible to use passive motion machines 30. However, for many applications, control over the mechanical environment is rudimentary. This would seem to be a fruitful area for innovative rehabilitation protocols.
Progress
Bone
For an organ that often heals by itself, bone is proving surprisingly difficult to regenerate. Much present research is focused in four areas: fractures that fail to heal (non-unions), spine fusion, calvarial defects and critical size defects in long bones. The concept of a critical size is based upon the observation that segmental defects beyond a certain size will not heal spontaneously, even in healthy individuals who would otherwise heal a smaller defect 2. Much of the research uses young, healthy rodents and rabbits as model systems where osseous defects tend to heal readily. The challenge is to develop technologies robust enough to perform in demanding models using mature large animals, thereby justifying human trials 2.
Urist’s observation that DBM has osteoinductive properties 31, led to its development as the first commercial product for bone regeneration. This product has stood the test of time and remains in wide clinical use for spine fusion, fracture healing, the repair of cysts, and periodontal reconstruction. 4. However, it has several shortcomings including batch-to-batch variability in osteoinductivity. Further research identified a number of morphogens associated with DBM that helped account for its osteoinductive properties. The recombinant forms of two of these, BMP-2 and BMP-7, are approved for specific human applications as the active ingredients of Infuse® (US) (InductOs in Europe) and OP-1® (Ossigraft), respectively. In 2005, the FDA approved the use of platelet-derived growth factor (PDGF) on a tricalcium phosphate (TCP) matrix (Gem21s) for periodontal regeneration. It is presently under review for approval in ankle fusions, although concern about the possible tumorigenicity of PDGF is delaying authorization. Additional factors, such as Nel 1 32 and wnt 33 are undergoing pre-clinical development. The latter is highly topical because antibodies against its major physiological antagonist, sclerostin, are coming into the clinic for treating osteoporosis. These antibodies are already in clinical trials for fracture healing (NCT00907296; NCT01081678). Parathyroid hormone 1–34 (teriparatide; Forteo) is clinically approved for treating osteoporosis and has potential use in bone regeneration 34; nine clinical trials are listed in this context on clinicaltrials.gov (visited 3/8/13). Despite current high enthusiasm for biological triggers of regeneration, interest remains in using small molecules 35. A recent novelty in this area involves the use of an organic salt of vanadium as an insulin mimetic to enhance fracture healing 36
Delivery of BMPs is problematic and the commercial products are simply implanted on collagen scaffolds. These provide only burst release, using very large amounts of the product, raising costs and provoking unwanted side-effects 37. Moreover, BMP-2 and BMP-7 have not proved as dramatically effective in human clinical practice as in certain pre-clinical animal models. Considerable research is directed towards the development of scaffolds that deliver growth factors in a controlled fashion 38,39. However, there is little information on exactly how much growth factor is needed, when it is needed and for how long. Indeed, it is possible to question the degree to which fine control is necessary. Reichert et al., for instance, recently reported very impressive healing of critical size tibial defects in skeletally mature sheep, a very demanding model, using a TCP-polycaprolactone (PCL) scaffold implanted in conjunction with a paste comprising BMP-7 mixed with type I collagen carrier 40. Certain ceramic scaffolds are able to induce bone formation without the need for exogenous cells or growth factors 24,41. Scaffolds for bone regeneration are reviewed in reference 42.
Cell-based approaches to regenerating bone also have a substantial history. The clinical use of unfractionated autologous bone marrow as a source of osteoprogenitors, goes back over 20 years 43. Hernigou and coworkers improved the efficiency of the procedure by enriching for MSCs with a cell sorter 44. Their data suggest that the injection of a minimum average of approximately 55,000 osteoprogenitors is required to achieve union. Harvest of osteoprogenitors may be aided by the development of improved recovery devices, such as the “reamer-irrigator-aspirator” 45. The cells recovered by this device have proved successful clinically as adjuncts to the healing of difficult nonunions and segmental defects, using dexamethasone to promote their osteogenic differentiation 46. In a similar fashion, Kim et al 47 used dexamethasone to pre-differentiate marrow-derived MSCs into osteoblasts prior to injection into fracture sites. Osteonecrosis has been also treated clinically with marrow 48, marrow-derived MSCs 49 or the stromal vascular fraction of fat 50. Of these publications, only that of Hernigou and Beaujean 48 reported a large study with long-term follow-up. They found that when marrow was administered before collapse of the femoral head, only 9 of 145 hips went on to require total joint replacement. In contrast, 25 of 44 hips required replacement when cells were administered after collapse. The MSC content of the marrow aspirates was measured, and better outcomes were associated with the administration of larger numbers of progenitor cells.
Considerable emphasis is being placed on the use of MSCs to repair large segmental defects. Following promising results in rat 51 and dog 52 models, marrow-derived MSCs were used to treat large segmental defects in 3 human subjects 53. All subjects responded well, and were still clinically successful after 7 years, although the slow resorption of the hydroxyapatite scaffold was of concern 54. In a related, dental application, autologous MSCs derived from adipose tissue were used successfully to repair calvarial defects in a 7-year old individual 55. Perhaps the most striking example of successful bone regeneration concerns the regeneration of the entire distal phalanx of the human thumb 56. This was achieved by implanting autologous, expanded osteoprogenitors from the patient’s periosteum loaded onto a coral (porous hydroxyapatite) scaffold (figure 2). No morphogens or other developmental cues were added.
Figure 2. Regeneration of the human phalanx.
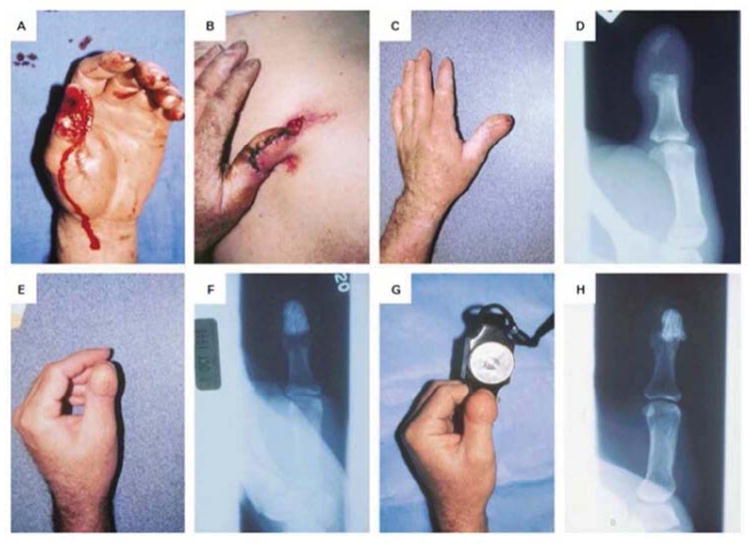
Panel A: Thumb at presentation
Panel B: Thumb at one week covered by a pedical of abdominal skin
Panel C: At six weeks, the thumb was well healed
Panel D: Radiograph of thumb prior to implantation of scaffold seeded with autologous periosteal cells.
Panel E: Six weeks after implantation, the thumb was of normal length
Panel F: Radiograph of implant, six weeks post-implantation
Panel G: At 28 months, patient had good pinch strength (2.3 kg).
Panel H: Radiograph at 28 months showing evidence of remodeling.
From reference 56
Based upon the productive interplay between osteoprogenitor cells and a suitable matrix, a system called “Cellect™” (DePuy AcroMed, Raynham, MA) was developed and made commercially available for the intra-operative harvest and implantation of marrow-derived MSCs. Marrow aspirates were filtered through a suitable scaffold, such as DMB or collagen-TCP, that preferentially retained the adherent MSCs. The entire structure was then implanted into the osseous lesion. Despite encouraging preliminary data for spine fusion 57 and osteonecrosis 58, this product was discontinued. Other innovative approaches under development include the use of matrix laid down by MSCs as they undergo osteogenesis in vitro. After devitalizing, the matrix is combined with MSCs that have been treated with an inducer of osteogenesis. Implantation of such a construct showed great efficacy in healing a murine calvarial defect 59. An additional type of biological, osteogenic membrane was discovered by accident. After surgeons remove large segments of bone, they will sometimes implant an inert spacer until the time of subsequent, reconstructive surgery. Masquelet and colleagues 60 noted that the spacers became surrounded by highly osteogenic membranes, which are now used to aid the regeneration of difficult, large osseous defects. Their high osteoinductivity may reflect an unique combination of osteogenic cells and potent osteogenic factors 61.
Gene transfer offers an elegant way to combine cell therapy with the delivery of growth factors. As pioneered by Lieberman’s group 62, marrow MSCs can be genetically modified with recombinant adenovirus vectors to express large amounts of BMP-2, thereby becoming potent osteogenic cells when implanted into a defect. To expedite this strategy, this team now uses lentivirus vectors in combination with buffy coat cells that can be isolated and transduced intra-operatively 63. Other gene therapy approaches, reviewed in references 64,65, include the direct injection of vectors carrying osteogenic genes 66 and the combination of vectors with scaffolds to create GAMs 26. An innovative application of the GAM concept coats allograft bone with recombinant adeno-associated virus vectors to achieve “allograft revitalization” 67.
Another innovation takes advantage of the high osteogenic potential of skeletal muscle, as evidenced by the disease fibrodysplasia ossificans progressiva 68 and the prevalence of heterotopic ossification in muscle following blast injuries 69 and certain types of surgery 70. We have reported very efficient healing of femoral, segmental defects in rats using unprocessed muscle biopsies transduced with adenovirus carrying BMP-2 cDNA 71. A similar, although less reliable, effect was noted using genetically modified fat. This technology also supported healing of calvarial defects, but less dramatically 72. Ripamonti and Roden 73 have used morcellized muscle to improve the healing of calvarial defects in the baboon with TGF-β2. The osteogenic environment of muscle has also been exploited when using patients as their own bioreactors for growing new bone for jaw reconstruction 74,75.
Gene transfer technologies can also deliver inhibitory RNA molecules; knockdown of chordin 76 and noggin 77, two inhibitors of BMPs, enhances the osteogenic differentiation of MSCs.
Bone forms physiologically by two routes. Intramembranous, or direct, osteogenesis occurs when osteoprogenitors differentiate directly into osteoblasts. Endochondral ossification occurs via a cartilagenous intermediate that is replaced by bone. Strategies for the regeneration of bone have tended to focus on the former which has to confront the early need for a blood supply; this explains why vascular endothelial growth factor (VEGF) is a favored growth factor in many studies. However, it has proved very difficult to engineer an effective vascular supply for regenerating bone. Under these conditions, the endochondral route is attractive because it relieves the physician of the need to provide a blood supply 78,79. Chondrogenesis does not require angiogenesis and the biology of endochondral ossification supplies angiogenic signals spontaneously as the process evolves. Much recent attention is thus focused on the endochondral route to bone regeneration, especially as chondrogenesis is favored by the hypoxic, acidotic conditions of major lesions. It may be particularly useful in regenerating bone at sites, such as the diaphysis of long bones, where the endochondral process is the normal route of healing.
As noted, mechanical signals are important for osteogenesis and clinical research has confirmed that a certain level of micromotion promotes fracture healing. The concept of dynamization has also proved attractive, whereby bones are fixed rigidly to initiate healing and then allowed axial motion to promote maturation and remodeling. We have recently studied this experimentally in a rat, femoral, segmental defect model and found, unexpectedly, that healing is accelerated by first fixing loosely and then imposing high stiffness as bone begins to be deposited. We call this “reverse dynamization” 80. Sophisticated manipulation of the mechanical environment provides additional avenues for bone regeneration.
Cartilage
Cartilage is frequently damaged as a result of sporting injuries or other trauma, and is eroded in joints with arthritis. Damaged cartilage often leads to joint pain and can predispose to osteoarthritis (OA). Cartilage repair is indicated for symptomatic, but otherwise healthy, joints using one of a variety of approaches indicated in this section. Repair is not normally attempted in arthritic joints, because of the size and nature of the lesions, as well as recognition that the concomitant disease process could impair restoration of cartilage. Strategies for articular cartilage regeneration may differ, depending upon whether the lesion is restricted to the cartilage itself, or penetrates the underlying bone to form an osteochondral lesion.
Unlike bone, articular cartilage has almost no intrinsic ability to regenerate. The lack of a repair process is usually ascribed to the low cellularity of cartilage and the absence of blood, lymph or innervation. One way to obviate this is to allow communication between the cartilagenous defect and the underlying marrow by piercing the sub-chondral bone. A clot forms where MSCs from the marrow differentiate and synthesize cartilagenous repair tissue. A simple, arthroscopic procedure, such as microfracture, is often clinically effective, especially for lesions <2cm2, even though the repair tissue is a fibrocartilagenous scar rather than true hyaline cartilage. The major reasons for failure are the inferior mechanical properties of the repair tissue and bone invasion.
ACI is an alternative clinical procedure in which healthy cartilage is harvested from a non-weight bearing part of the joint as a source of autologous chondrocytes. These are expanded in monolayer culture and, in its original configuration, reimplanted as a suspension under a flap of periosteum. Despite the phenotypic modulation of the chondrocytes during serial, monolayer passage and the absence of a scaffold, this procedure is surprisingly effective, and produces a clinical response arguably equivalent to microfracture 83, but suitable for larger defects. Improvements to the technology include using a collagen flap to replace the need for periosteum, and seeding the chondrocytes onto a collagen scaffold prior to implantation in a procedure known as MACI (matrix associated chondrocyte implantation). Selection of the most chondrogenic cells prior to implantation may also improve outcomes 84.
The major disadvantages of ACI are the need for two invasive procedures and the extensive expansion of cells for each patient. Moreover, there is evidence that harvest of donor cartilage initiates degenerative changes elsewhere in the joint 85. Because cartilage is thought to be an immunologically privileged site, there is interest in using allografted chondrocytes, especially from juvenile donors that may be less antigenic 86. One such product, DeNovo® NT (Zimmer, Warsaw, IN), is already on the market.
MSCs provide an attractive alternative. Marrow-derived MSCs undergo chondrogenesis in vitro in response to TGF-β, but there is a concern that they will undergo further differentiation to osteoblasts, leading to the formation of bone where there should be cartilage. This property may account for the observation of bone invasion after microfracture. Nevertheless, marrow-derived MSCs have been used successfully in the clinic to repair cartilage lesions 87,88. O’Driscoll has pioneered the use of periosteum to regenerate cartilage 89 and has used this tissue to treat lesions clinically 90. The stromal vascular fraction of adipose tissue has also been evaluated in two patients 50. A protocol using progenitors from umbilical cord is in clinical trials (NCT01041001).
Archer’s group have identified a population of chondroprogenitor cells that exists in the superficial zone of articular cartilage 91. These cells undergo chondrogenesis without forming bone and are undergoing pre-clinical evaluation as allografts in large animal models of cartilage repair. Protocols for the chondrogenic differentiation of ESCs 92 and iPS cells 93 are being developed.
Because cartilage is a highly hydrated tissue, many of the scaffolds being developed for cartilage repair are hydrogels. Materials include fibrin, hyaluronic acid, collagen, chitosan, silk, alginate and synthetic polymers such as poly lactic acid (PLA) and poly glycolic acid (PGA). Newer hydrogels are based on poly ethylene glycol (PEG), self-assembling peptides and electrospun nanofibers. Devitalized cartilage provides another scaffold of interest 94, whose investigation is encouraged by the successful regeneration of a trachea, an organ which contains articular cartilage, from devitalized donor tissue 95. Another approach dispenses with matrices altogether and instead allows chondrocytes in culture to develop their own extracellular matrices, creating implantable grafts 96 of the type exemplified by the DeNovo® NT product mentioned above.
Biological and mechanical integration of engineered cartilage grafts in articular cartilage is problematic 97, exacerbated by the fact that, unlike bone, articular cartilage does not remodel rapidly or extensively. One strategy for improving biological integration involves local digestion of the surrounding cartilage using enzymes or catabolic cytokines 98. Mechanical integration may be helped by recent research suggesting that exposure of neo-cartilage to a combination of fibroblast growth factor-2 and TGF-β promotes rapid maturation with enhanced mechanical properties 99. Another approach subjects chondrocytes to hydrostatic pressure in a bioreactor to accelerate maturation prior to implantation 100. This product is being evaluated in a Phase III human protocol (NCT01066702).
Rather than implant rigid, pre-formed tissue grafts, there is interest in applying soluble materials that can take on the shape of the defect and then solidify in situ. This leads to greater filling of the defect and enhanced integration with surrounding cartilage. One approach uses a hyaluronan-based polymer that is liquid at room temperature and solidifies at body temperature 101. A recent publication describes a polymer based on PEG that solidifies in response to light. It has been used successfully in a pilot clinical study (figure 3) to improve the outcome of microfracture by providing a reliable matrix for colonization and chondrogenesis by MSCs entering from the marrow 102. In rabbits, entry of MSCs into defects can be increased by incorporation of the chemotactic protein, stromal cell-derived factor 1 103.
Figure 3. Clinical procedure for adhesive implantation into a cartilage defect.
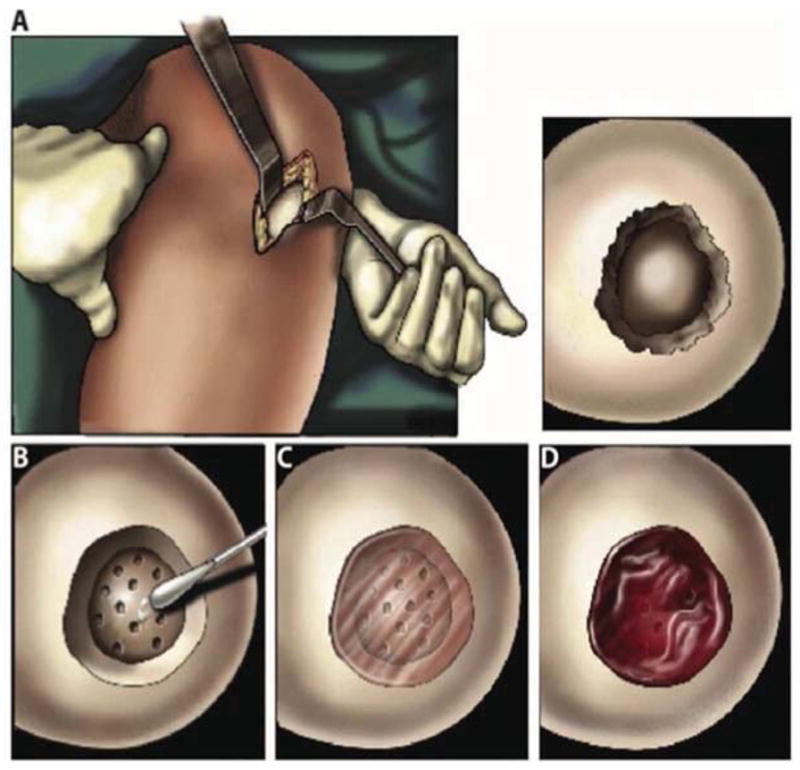
Panel A: A mini-incision exposed the cartilage defect.
Panel B: The adhesive was applied to the base and walls of the defect followed by surgical microfracture.
Panel C: The hydrogel solution was injected into the defect and photopolymerized in situ with light.
Panel D: Bleeding from the microfracture holes was trapped in and around the hydrogel.
From reference 102
Our group has combined this type of approach with gene transfer. Marrow was harvested from rabbits, mixed with recombinant adenovirus vectors, and allowed to clot thus forming a “gene plug”. The soft clot could be press-fit into full thickness defects, with the adenovirus vector transferring genes to MSCs as they entered from the marrow 104. The high efficiency of gene transfer using adenovirus vectors associated with hydrogels, including fibrin, helps explain the success of this technology 105. Promising data were subsequently obtained using this method to deliver TGF-β in a sheep, chondral defect model 106.
The ex vivo transfer of genetically modified chondrocytes 107,108 could be piggy-backed on standard ACI procedures. Indeed, a clinical trial is about to start in Korea in which retrovirally transduced chondrocytes that over-express TGF-β will be implanted as allografts into lesions in cartilage (Bumsup Lee, personal communication).
Gene therapy could also be used in conjunction with MSCs from various sources 109,110. Preliminary data suggest that genetically modified fat and muscle grafts might also be effective 71. Madry’s group has pioneered the direct application of recombinant adeno-associated virus to sites of articular cartilage defect, with encouraging results in animal models 111.
Gene therapy approaches are reviewed by Cucchiarini and Madry 112.
The discussion so far refers to the healing of small to medium sized lesions in cartilage. As the field of cartilage regeneration develops, it confronts the need to restore very large lesions or even resurface entire joints. Mao’s group succeeded in resurfacing the rabbit proximal humerus using an anatomically correct, implantable scaffold of PCL and hydroxyapatite in conjunction with a collagen hydrogel containing TGF-β3 113. The data suggest that TGF-β3 both recruited chondroprogenitor cells, possibly from the synovium, and promoted their differentiation into chondrocytes.
Repair of cartilage defects in OA is more challenging because there is a concomitant disease process creating an unfavorable repair environment. For example, our group has shown that inflammatory cytokines strongly inhibit the chondrogenic differentiation of MSCs 114. Moreover, there are data to suggest that marrow-derived MSCs from individuals with OA are intrinsically less chondrogenic 115. One approach to regenerating cartilage in joints with OA, already in clinical trials (NCT01671072), involves the intraarticular injection of genetically modified, allogeneic chondrocytes expressing elevated amounts of TGF-β1 116.
The influences of mechanical forces on chondrogenesis are widely appreciated, but incompletely understood 117. Recent data suggest that shear forces are important in this regard 118, promoting the endogenous production of TGF-β These are the sorts of forces that could be produced by a modified continual passive motion machine of the type already used in rehabilitation after knee surgery 30.
Other organs
Intervertebral disc
The IVD is a major load bearing structure of the spine, and its degeneration is associated with back pain. This is currently treated surgically by removing the degenerate disc and fusing the adjacent vertebrae, often using BMP-2 to enhance the process. Prosthetic discs have been developed, but are not a clinical success. Attempts to regenerate the disc have to accomodate the anatomy of the disc, where a highly hydrated, gelatinous nucleus pulposus exists within a fibrous, collagenous annulus fibrosis. These two structures differ considerably in biology and mechanical properties 121.
Attempts to regenerate the nucleus pulposus by the intra-discal injection of growth factors has met with some success in animal models, and the FDA has given permission for clinical trials using BMP-7 and growth and differentiation factor -5 (GDF-5; also BMP-14) in this fashion 122. Recognizing the probably transient effect of injected growth factors, there is interest in using gene transfer to provide sustained delivery 123. As the disc is so physiologically isolated and cell turnover is low, it is possible to obtain remarkably long periods of transgene expression, even using highly antigenic vectors such as adenovirus.
Cell therapy is attractive because IVD degeneration is associated with cell death; both disc cells and MSCs have been evaluated in this regard. Because the nucleus pulposus of the disc is highly acidotic and hypoxic there are concerns about the survival of transplanted cells. Preconditioning has been suggested to prepare cells for this environment and help engraftment. In a small clinical trial, suspensions of autologous, expanded, nucleus pulposus cells were injected into discs following surgery for disc prolapse. Preliminary data are encouraging 124. Yoshokawa et al. reported the intra-discal injection of autologous MSCs in two patients 125. The discovery of progenitor cells within the disc 126,127 adds new possibilities for this type of therapy. One impediment to research into cellular therapies for IVD regeneration is the lack of good markers for the relevant disc cells.
Survival and function of cells could be aided by a suitable scaffold, and a variety of hydrogels based upon chitosan, hyaluronan, alginate, cellulose, and composites of collagen/hyaluronan, and chondroitin sulfate have been investigated. The annulus fibrous has much greater tensile strength and materials explored in annulus regeneration include PLA, poly octanedial malate, gelatin, silk, and poly caprolactone triol malate. Electrospun PLA has been examined as a way of forming the alternating, lamellar structure of the annulus.
As noted earlier in the context of OA, degeneration of the IVD is often part of a disease process that needs to be controlled to allow successful regeneration. The research of LeMaitre and colleagues 128 makes a strong case for the involvement of interleukin-1 in IVD degeneration. Moreover, IVD degeneration is associated with calcification of end plates, which limits diffusion. Another limitation to progress in this area is the lack of good animal models 129.
Meniscus
The menisci are responsible for load transmission through the knee and are frequently injured. At one time it was common to remove the offending menisci; realization that this pre-disposed to OA spurred efforts to repair or regenerate menisci. The outer third of the meniscus (the red zone) is attached to the synovium and has a blood supply, providing it with some potential for repair. The inner two thirds (the white zone) lack a blood supply and cannot regenerate.
The implantation of a cell-free, collagen scaffold is already used clinically with highly promising results 131. Scaffolds based upon polyurethane are in clinical trials 132 and a product based on silk is expected to launch this year. Pre-clinical research is evaluating alternative scaffolds, such as poly (lactic-co-glycolic acid) (PLGA), hyaluronan-PCL, and devitalized meniscus, augmented with VEGF or PRP or cells. Types of cells include MSCs, and autologous or allogeneic meniscus cells. A provocative paper by Murphy et al. 133 reporting the regeneration of the meniscus in goats following the injection of marrow-derived, autologous MSCs suspended in hyaluronan, has prompted two clinical trials (NCT00225095; NCT00702741).
A small number of studies (e.g. 134–136) have explored the use of gene therapy
Approaches to meniscus regeneration have been catalogued by Pereira et al. 137.
Ligament and Tendon
Although ligaments and tendons are different tissues, it is convenient to combine their discussion in a short review. Most pre-clinical research in this area uses injuries to the anterior cruciate ligament, Achilles tendon or rotator cuff as experimental models.
Two types of regenerative requirements exist, depending upon whether the need is to regenerate tissue in the center of the structure, or the insertion site to the bone or tendon-muscle junction. The former is simplified by the relative homogeneity of the surrounding tissue, whereas the insertion and junction sites are complex, multi-tissue, graded entities 138.
Because ligaments and tendons are collagenous, there is much interest in using growth factors that promote collagen synthesis, including TGF-β, PDGF, and insulin-like growth factors, as well as autologous conditioned serum 20 and the ubiquitous PRP. BMPs -12, -13, and -14 (GDFs -7, -6, -5) 139, and the transcription factor scleraxis 140, are of particular interest because they promote the differentiation of progenitor cells into tenocytes and ligament cells. Because scleraxis is an intracellular protein, gene transfer is a useful modality for its delivery that has been used successfully to promote tenogenesis in vitro 141,142. Other gene therapy strategies include various viral and non-viral vectors used in vivo and ex vivo (summarized within reference 143).
Most cell-based approaches to regeneration have adopted ex vivo strategies using tenocytes, ligament cells and skin fibroblasts 144. However, both tendons and ligaments have been found to possess local populations of stem cells 145,146, raising the possibility of stimulating endogenous repair processes. Collagen, synthetic polymers such as PLGA, and devitalized extracellular matrix preparations are frequently the scaffold of choice (reviewed in reference 147).
Mechanical forces are important in the formation and maturation of ligament and tendon; tensile stress, for instance, upregulates the expression of scleraxis by MSCs 148. There is interest in harnessing mechanobiological processes to regenerate the region where tendon or ligament inserts into bone. This is challenging, because this includes a fibrocartilagenous intermediary zone. An alternative approach uses multiphasic scaffolds containing multiple cells types. In one example, scaffold was seeded with ligament cells at one end, osteoblasts at the other, and chondrocytes, in between 149. A more practical approach may be to use a single progenitor cell type and induce zone-specific differentiation by spatial differences in the matrix 150.
Ma et al 151 recently engineered a scaffold-free, bone-ligament-bone anterior cruciate ligament graft in vitro using marrow-derived MSCs from sheep. Six months after implantation into ovine knee joints, the engineered structure had attained much of the biology and mechanical properties of the native, sheep ACL.
Unlike the case with the other tissues described in this review, there have been no major clinical trials adopting the strategies of regenerative orthopaedics for healing ligaments and tendons. However, a variety of scaffolds derived from devitalized extracellular matrix are used clinically to augment repair.152
Perspectives
As reflected in the opening quotation, biology provides many of the tools needed to restore orthopaedic tissues, and our research can be viewed as attempts to harness these tools to achieve regeneration in clinical settings. This review indicates strategies for achieving this goal. Despite many pre-clinical successes, there are few clinical trials and even fewer new products for the regeneration of orthopaedic tissues are entering clinical use. The reasons for this are a matter of discussion, ranging from regulatory burdens, funding restrictions, gaps in scientific knowledge, inadequate animal models and psychosocial issues 153. Health care economics play an increasingly important role, which is one reason, among others, that this author favors approaches that facilitate endogenous repair 10. The key scientific components of success appear to be in place and it would seem only a matter of time before a variety of new products are available to address the growing need for effective and affordable regeneration of skeletal structures.
Acknowledgments
Financial support
The author’s work in this area has been funded by NIH grant R01AR50243 from NIAMS, grant W81XWH-10-1-0888 from the Department of Defense, and by the AO Foundation (GENDEF).
I thank Dr. Henry Mankin for permission to use his quote.
Abbreviations
- ACI
Autolgous chondrocyte implantation
- BMP
Bone morphogenetic protein
- DBM
Demineralized bone matrix
- ESC
Embryonic stem cell
- GAM
Gene activated matrix
- GDF
Growth and differentiation factor
- iPS
Induced, pleuripotent stem
- IVD
Intervertebral disc
- MSC
Mesenchymal stem cell
- OA
Osteoarthritis
- PCL
Poly caprolactone
- PDGF
Platelet-derived growth factor
- PEG
Poly (ethylene glycol)
- PGA
Poly glycolic acid
- PLA
Poly lactic acid
- PLGA
Poly (lactate-co-glycolate)
- PRP
Platelet rich plasma
- TCP
Tricalcium phosphate
- TGF
Transforming growth factor
- VEGF
Vascular endothelial growth factor
Footnotes
Disclosure
The author is on the Board of Directors of Orthogen AG, a member of the Scientific Advisory Board of TissueGene Inc. and a consultant for Synthes Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mankin H. Personal Communication.
- 2.Muschler GF, Raut VP, Patterson TE, Wenke JC, Hollinger JO. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 16:123–145. doi: 10.1089/ten.TEB.2009.0658. [DOI] [PubMed] [Google Scholar]
- 3.Szpalski C, Barr J, Wetterau M, Saadeh PB, Warren SM. Cranial bone defects: current and future strategies. Neurosurg Focus. 29:E8. doi: 10.3171/2010.9.FOCUS10201. [DOI] [PubMed] [Google Scholar]
- 4.Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 64:1063–1077. doi: 10.1016/j.addr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 7.Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995;77:1103–1114. doi: 10.2106/00004623-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Grayson WL, Frohlich M, Yeager K, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 107:3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 10.Evans CH, Palmer GD, Pascher A, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 11.Salter E, Goh B, Hung B, Hutton D, Ghone N, Grayson WL. Bone tissue engineering bioreactors: a role in the clinic? Tissue Eng Part B Rev. 18:62–75. doi: 10.1089/ten.TEB.2011.0209. [DOI] [PubMed] [Google Scholar]
- 12.Steinert AF, Rackwitz L, Gilbert F, Noth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 1:237–247. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 30:1869–1878. doi: 10.1002/jor.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry BC, Zhou D, Wu X, et al. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14:149–156. doi: 10.1089/ten.tec.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisiday JD, Lee CM, McIlwraith CW, Frisbie DD. Induction of bone marrow mesenchymal stem cell chondrogenesis following short-term suspension culture. J Orthop Res. 29:26–32. doi: 10.1002/jor.21200. [DOI] [PubMed] [Google Scholar]
- 16.DeFrancesco L. Adult stem cell therapies walk the line. Nat Biotechnol. 30:739–741. doi: 10.1038/nbt.2321. [DOI] [PubMed] [Google Scholar]
- 17.Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 26:1685–1693. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- 18.Longo UG, Loppini M, Berton A, La Verde L, Khan WS, Denaro V. Stem cells from umbilical cord and placenta for musculoskeletal tissue engineering. Curr Stem Cell Res Ther. 7:272–281. doi: 10.2174/157488812800793054. [DOI] [PubMed] [Google Scholar]
- 19.Keramaris NC, Kaptanis S, Moss HL, Loppini M, Pneumaticos S, Maffulli N. Endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) in bone healing. Curr Stem Cell Res Ther. 7:293–301. doi: 10.2174/157488812800793081. [DOI] [PubMed] [Google Scholar]
- 20.Majewski M, Ochsner PE, Liu F, Fluckiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37:2117–2125. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- 21.Mehta V. Platelet-rich plasma: a review of the science and possible clinical applications. Orthopedics. 33:111. doi: 10.3928/01477447-20100104-22. [DOI] [PubMed] [Google Scholar]
- 22.Martino MM, Tortelli F, Mochizuki M, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 3:100ra189. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 23.Bahney CS, Hsu CW, Yoo JU, West JL, Johnstone B. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB J. 25:1486–1496. doi: 10.1096/fj.10-165514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barradas AM, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 21:407–429. doi: 10.22203/ecm.v021a31. discussion 429. [DOI] [PubMed] [Google Scholar]
- 25.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 27.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 28.Pauwels F. A new theory on the influence of mechanical stimuli on the differentiation of supporting tissue. The tenth contribution to the functional anatomy and causal morphology of the supporting structure. Z Anat Entwicklungsgesch. 1960;121:478–515. [PubMed] [Google Scholar]
- 29.Huebsch N, Arany PR, Mao AS, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salter RB. Continuous passive motion: from origination to research to clinical applications. J Rheumatol. 2004;31:2104–2105. [PubMed] [Google Scholar]
- 31.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 32.Zhu S, Zhang B, Man C, Ma Y, Hu J. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthritis Cartilage. 19:743–750. doi: 10.1016/j.joca.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Rosen V. Harnessing the parathyroid hormone, Wnt, and bone morphogenetic protein signaling cascades for successful bone tissue engineering. Tissue Eng Part B Rev. 17:475–479. doi: 10.1089/ten.TEB.2011.0265. [DOI] [PubMed] [Google Scholar]
- 34.Dhillon RS, Schwarz EM. Teriparatide Therapy as an Adjuvant for Tissue Engineering and Integration of Biomaterials. J Mater Res. 4:1117–1131. doi: 10.3390/ma4061117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aravamudhan A, Ramos DM, Nip J, et al. Osteoinductive Small Molecules: Growth Factor Alternatives for Bone Tissue Engineering. Curr Pharm Des. doi: 10.2174/1381612811319190008. [DOI] [PubMed] [Google Scholar]
- 36.Paglia DN, Wey A, Park AG, et al. The effects of local vanadium treatment on angiogenesis and chondrogenesis during fracture healing. J Orthop Res. 30:1971–1978. doi: 10.1002/jor.22159. [DOI] [PubMed] [Google Scholar]
- 37.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 64:1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichert JC, Cipitria A, Epari DR, et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 4:141ra193. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- 41.Yuan H, Fernandes H, Habibovic P, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A. 107:13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 30:546–554. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection for delayed unions of the tibia: a preliminary report. J Orthop Trauma. 1989;3:276–282. doi: 10.1097/00005131-198912000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 45.Porter RM, Liu F, Pilapil C, et al. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J Orthop Res. 2009;27:42–49. doi: 10.1002/jor.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 35:599–605. doi: 10.1007/s00264-010-1013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SJ, Shin YW, Yang KH, et al. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(Ossron) injection to treat fractures. BMC Musculoskelet Disord. 2009;10:20. doi: 10.1186/1471-2474-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Kawate K, Yajima H, Ohgushi H, et al. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs. 2006;30:960–962. doi: 10.1111/j.1525-1594.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 50.Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 5:296. doi: 10.1186/1752-1947-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 52.Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 54.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 55.Lendeckel S, Jodicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–1514. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 57.Muschler GF, Matsukura Y, Nitto H, et al. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005:242–251. doi: 10.1097/01.blo.0000149812.32857.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee K, Goodman SB. Cell therapy for secondary osteonecrosis of the femoral condyles using the Cellect DBM System: a preliminary report. J Arthroplasty. 2009;24:43–48. doi: 10.1016/j.arth.2008.01.133. [DOI] [PubMed] [Google Scholar]
- 59.Zeitouni S, Krause U, Clough BH, et al. Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med. 4:132ra155. doi: 10.1126/scitranslmed.3003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 41:27–37. doi: 10.1016/j.ocl.2009.07.011. table of contents. [DOI] [PubMed] [Google Scholar]
- 61.Gruber HE, Riley FE, Hoelscher GL, et al. Osteogenic and chondrogenic potential of biomembrane cells from the PMMA-segmental defect rat model. J Orthop Res. 30:1198–1212. doi: 10.1002/jor.22047. [DOI] [PubMed] [Google Scholar]
- 62.Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Virk MS, Sugiyama O, Park SH, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 19:960–968. doi: 10.1038/mt.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans C. Gene therapy for the regeneration of bone. Injury. 42:599–604. doi: 10.1016/j.injury.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans CH. Gene delivery to bone. Adv Drug Deliv Rev. 64:1331–1340. doi: 10.1016/j.addr.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Betz OB, Betz VM, Nazarian A, et al. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am. 2006;88:355–365. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 67.Ito H, Koefoed M, Tiyapatanaputi P, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forsberg JA, Pepek JM, Wagner S, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 70.Zeckey C, Hildebrand F, Frink M, Krettek C. Heterotopic ossifications following implant surgery--epidemiology, therapeutical approaches and current concepts. Semin Immunopathol. 33:273–286. doi: 10.1007/s00281-011-0240-5. [DOI] [PubMed] [Google Scholar]
- 71.Evans CH, Liu FJ, Glatt V, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu F, Porter RM, Wells J, Glatt V, Pilapil C, Evans CH. Evaluation of BMP-2 gene-activated muscle grafts for cranial defect repair. J Orthop Res. 30:1095–1102. doi: 10.1002/jor.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ripamonti U, Roden LC. Induction of bone formation by transforming growth factor-beta2 in the non-human primate Papio ursinus and its modulation by skeletal muscle responding stem cells. Cell Prolif. 43:207–218. doi: 10.1111/j.1365-2184.2010.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Moghadam HG, Urist MR, Sandor GK, Clokie CM. Successful mandibular reconstruction using a BMP bioimplant. J Craniofac Surg. 2001;12:119–127. doi: 10.1097/00001665-200103000-00005. discussion 128. [DOI] [PubMed] [Google Scholar]
- 76.Kwong FN, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res Ther. 2008;10:R65. doi: 10.1186/ar2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takayama K, Suzuki A, Manaka T, et al. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J Bone Miner Metab. 2009;27:402–411. doi: 10.1007/s00774-009-0054-x. [DOI] [PubMed] [Google Scholar]
- 78.Farrell E, Both SK, Odorfer KI, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glatt V, Miller M, Ivkovic A, et al. Improved healing of large segmental defects in the rat femur by reverse dynamization in the presence of bone morphogenetic protein-2. J Bone Joint Surg Am. 94:2063–2073. doi: 10.2106/JBJS.K.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bueno EM, Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol. 2009;5:685–697. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 82.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 85.Lee CR, Grodzinsky AJ, Hsu HP, Martin SD, Spector M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res. 2000;18:790–799. doi: 10.1002/jor.1100180517. [DOI] [PubMed] [Google Scholar]
- 86.Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem Cell Res. 4:57–68. doi: 10.1016/j.scr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 88.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 89.O’Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop Relat Res. 2001:S190–207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 90.O’Driscoll SW. Technical considerations in periosteal grafting for osteochondral injuries. Clin Sports Med. 2001;20:379–402. vii. doi: 10.1016/s0278-5919(05)70312-4. [DOI] [PubMed] [Google Scholar]
- 91.Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 92.Oldershaw RA, Baxter MA, Lowe ET, et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 28:1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 93.Diekman BO, Christoforou N, Willard VP, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A. 109:19172–19177. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwarz S, Koerber L, Elsaesser AF, et al. Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng Part A. 18:2195–2209. doi: 10.1089/ten.TEA.2011.0705. [DOI] [PubMed] [Google Scholar]
- 95.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 96.Jubel A, Andermahr J, Schiffer G, et al. Transplantation of de novo scaffold-free cartilage implants into sheep knee chondral defects. Am J Sports Med. 2008;36:1555–1564. doi: 10.1177/0363546508321474. [DOI] [PubMed] [Google Scholar]
- 97.Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Noth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan IM, Gonzalez LG, Francis L, et al. Interleukin-1beta enhances cartilage-to-cartilage integration. Eur Cell Mater. 22:190–201. doi: 10.22203/ecm.v022a15. [DOI] [PubMed] [Google Scholar]
- 99.Khan IM, Francis L, Theobald PS, et al. In vitro growth factor-induced bio engineering of mature articular cartilage. Biomaterials. 34:1478–1487. doi: 10.1016/j.biomaterials.2012.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crawford DC, Heveran CM, Cannon WD, Jr, Foo LF, Potter HG. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med. 2009;37:1334–1343. doi: 10.1177/0363546509333011. [DOI] [PubMed] [Google Scholar]
- 101.Mortisen D, Peroglio M, Alini M, Eglin D. Tailoring thermoreversible hyaluronan hydrogels by “click” chemistry and RAFT polymerization for cell and drug therapy. Biomacromolecules. 11:1261–1272. doi: 10.1021/bm100046n. [DOI] [PubMed] [Google Scholar]
- 102.Sharma B, Fermanian S, Gibson M, et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 5:167ra166. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sukegawa A, Iwasaki N, Kasahara Y, Onodera T, Igarashi T, Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A. 18:934–945. doi: 10.1089/ten.TEA.2011.0380. [DOI] [PubMed] [Google Scholar]
- 104.Pascher A, Palmer GD, Steinert A, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11:133–141. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 105.Neumann AJ, Schroeder J, Alini M, Archer CW, Stoddart MJ. Enhanced adenovirus transduction of hMSCs using 3D hydrogel cell carriers. Mol Biotechnol. 53:207–216. doi: 10.1007/s12033-012-9522-y. [DOI] [PubMed] [Google Scholar]
- 106.Ivkovic A, Pascher A, Hudetz D, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 17:779–789. doi: 10.1038/gt.2010.16. [DOI] [PubMed] [Google Scholar]
- 107.Kang R, Marui T, Ghivizzani SC, et al. Ex vivo gene transfer to chondrocytes in full-thickness articular cartilage defects: a feasibility study. Osteoarthritis Cartilage. 1997;5:139–143. doi: 10.1016/s1063-4584(97)80007-6. [DOI] [PubMed] [Google Scholar]
- 108.Madry H, Kaul G, Cucchiarini M, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 109.Matsumoto T, Cooper GM, Gharaibeh B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cucchiarini M, Venkatesan JK, Ekici M, Schmitt G, Madry H. Human mesenchymal stem cells overexpressing therapeutic genes: from basic science to clinical applications for articular cartilage repair. Biomed Mater Eng. 22:197–208. doi: 10.3233/BME-2012-0709. [DOI] [PubMed] [Google Scholar]
- 111.Cucchiarini M, Madry H, Ma C, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005;12:229–238. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 112.Cucchiarini M, Madry H. Gene therapy for cartilage defects. J Gene Med. 2005;7:1495–1509. doi: 10.1002/jgm.824. [DOI] [PubMed] [Google Scholar]
- 113.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 376:440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wehling N, Palmer GD, Pilapil C, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 116.Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 14:247–256. doi: 10.3109/14653249.2011.629645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grad S, Eglin D, Alini M, Stoddart MJ. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 469:2764–2772. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schatti O, Grad S, Goldhahn J, et al. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 22:214–225. doi: 10.22203/ecm.v022a17. [DOI] [PubMed] [Google Scholar]
- 119.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnstone B, Alini M, Cucchiarini M, et al. Tissue engineering for articular cartilage repair – the state of the art. Eur Cell Mater. 2013 doi: 10.22203/ecm.v025a18. In Press. [DOI] [PubMed] [Google Scholar]
- 121.Chan SC, Gantenbein-Ritter B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy--feasible or fiction? Swiss Med Wkly. 142:w13598. doi: 10.4414/smw.2012.13598. [DOI] [PubMed] [Google Scholar]
- 122.An HS, Masuda K, Cs-Szabo G, et al. Biologic repair and regeneration of the intervertebral disk. J Am Acad Orthop Surg. 19:450–452. [PubMed] [Google Scholar]
- 123.Woods BI, Vo N, Sowa G, Kang JD. Gene therapy for intervertebral disk degeneration. Orthop Clin North Am. 42:563–574. ix. doi: 10.1016/j.ocl.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008;17 (Suppl 4):492–503. doi: 10.1007/s00586-008-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976) 35:E475–480. doi: 10.1097/BRS.0b013e3181cd2cf4. [DOI] [PubMed] [Google Scholar]
- 126.Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 128.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kepler CK, Anderson DG, Tannoury C, Ponnappan RK. Intervertebral disk degeneration and emerging biologic treatments. J Am Acad Orthop Surg. 19:543–553. doi: 10.5435/00124635-201109000-00005. [DOI] [PubMed] [Google Scholar]
- 131.Harston A, Nyland J, Brand E, McGinnis M, Caborn DN. Collagen meniscus implantation: a systematic review including rehabilitation and return to sports activity. Knee Surg Sports Traumatol Arthrosc. 20:135–146. doi: 10.1007/s00167-011-1579-9. [DOI] [PubMed] [Google Scholar]
- 132.Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs EL. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 39:774–782. doi: 10.1177/0363546511398040. [DOI] [PubMed] [Google Scholar]
- 133.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 134.Goto H, Shuler FD, Lamsam C, et al. Transfer of lacZ marker gene to the meniscus. J Bone Joint Surg Am. 1999;81:918–925. doi: 10.2106/00004623-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 135.Steinert AF, Palmer GD, Capito R, et al. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-beta 1 complementary deoxyribonucleic acid. Tissue Eng. 2007;13:2227–2237. doi: 10.1089/ten.2006.0270. [DOI] [PubMed] [Google Scholar]
- 136.Cucchiarini M, Schetting S, Terwilliger EF, Kohn D, Madry H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Ther. 2009;16:1363–1372. doi: 10.1038/gt.2009.91. [DOI] [PubMed] [Google Scholar]
- 137.Pereira H, Frias AM, Oliveira JM, Espregueira-Mendes J, Reis RL. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthroscopy. 27:1706–1719. doi: 10.1016/j.arthro.2011.08.283. [DOI] [PubMed] [Google Scholar]
- 138.Smith L, Xia Y, Galatz LM, Genin GM, Thomopoulos S. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect Tissue Res. 53:95–105. doi: 10.3109/03008207.2011.650804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 141.Alberton P, Popov C, Pragert M, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 21:846–858. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 39:1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 143.Longo UG, Lamberti A, Maffulli N, Denaro V. Tissue engineered biological augmentation for tendon healing: a systematic review. Br Med Bull. 98:31–59. doi: 10.1093/bmb/ldq030. [DOI] [PubMed] [Google Scholar]
- 144.Tremblay P, Cloutier R, Lamontagne J, et al. Potential of skin fibroblasts for application to anterior cruciate ligament tissue engineering. Cell Transplant. 20:535–542. doi: 10.3727/096368910X536482. [DOI] [PubMed] [Google Scholar]
- 145.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 146.Steinert AF, Kunz M, Prager P, et al. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A. 17:1375–1388. doi: 10.1089/ten.tea.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ge Z, Yang F, Goh JC, Ramakrishna S, Lee EH. Biomaterials and scaffolds for ligament tissue engineering. J Biomed Mater Res A. 2006;77:639–652. doi: 10.1002/jbm.a.30578. [DOI] [PubMed] [Google Scholar]
- 148.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 149.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A. 2008;86:1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 150.Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering graded tissue interfaces. Proc Natl Acad Sci U S A. 2008;105:12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ma J, Smietana MJ, Kostrominova TY, Wojtys EM, Larkin LM, Arruda EM. Three-dimensional engineered bone-ligament-bone constructs for anterior cruciate ligament replacement. Tissue Eng Part A. 18:103–116. doi: 10.1089/ten.tea.2011.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 153.Evans CH. Barriers to the clinical translation of orthopedic tissue engineering. Tissue Eng Part B Rev. 17:437–441. doi: 10.1089/ten.teb.2011.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]


