Abstract
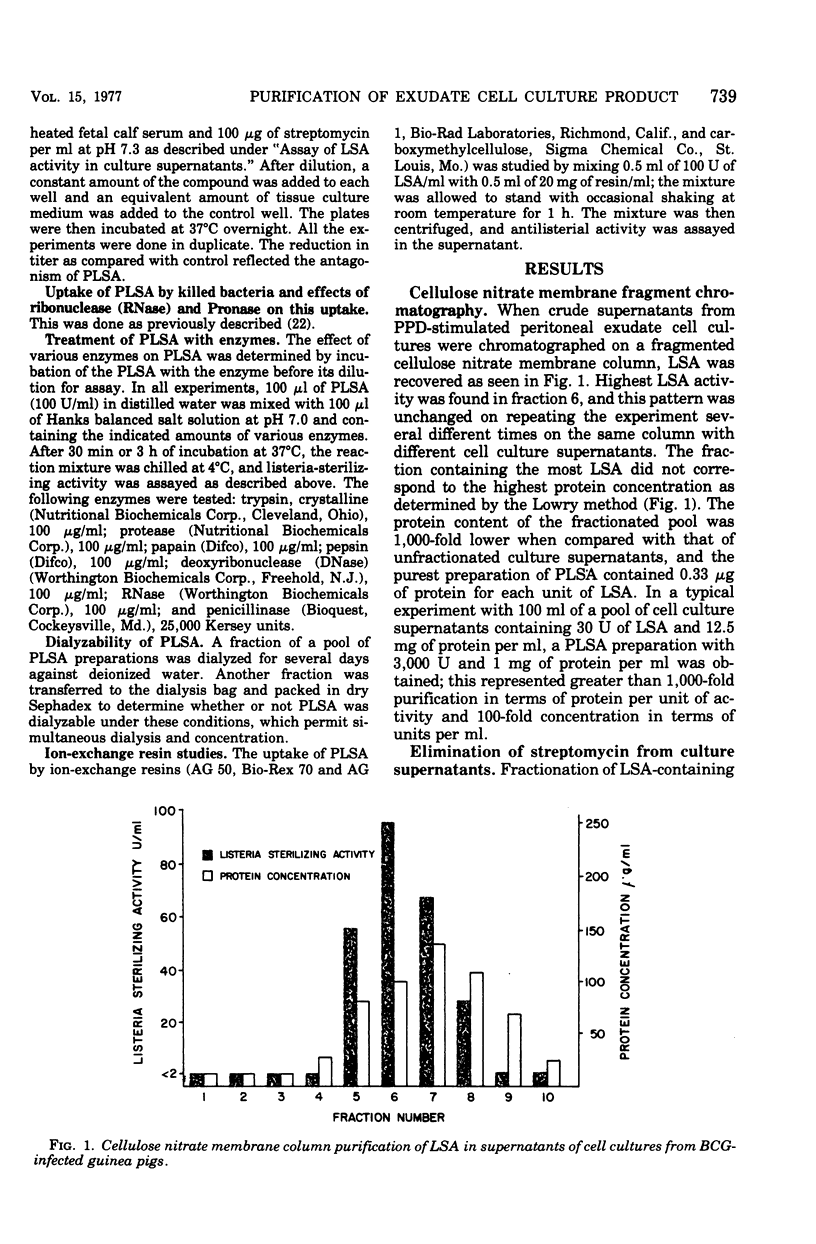
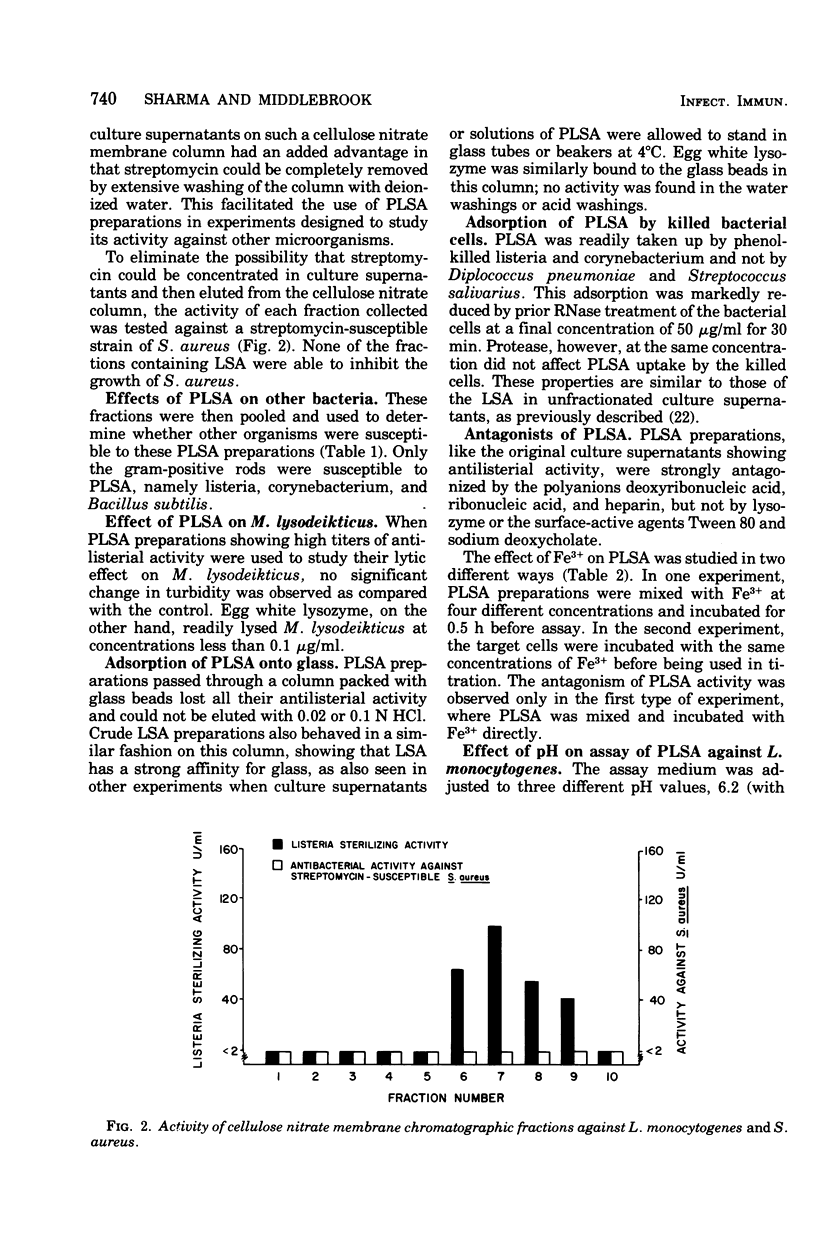
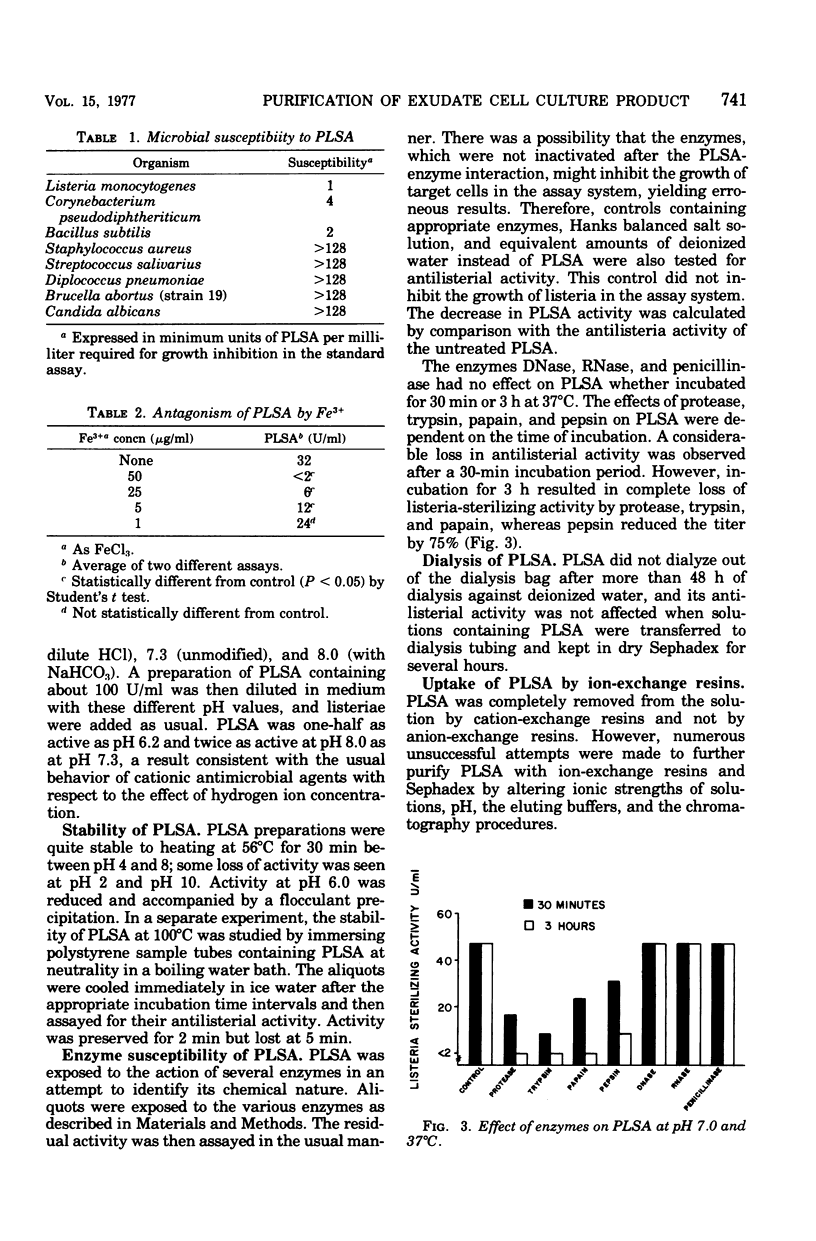
Peritoneal exudates elicited in BCG-infected guinea pigs with caseinate yield cell cultures that have been shown to produce soluble material capable of sterilizing certain bacteria if the cultures are incubated with the specific antigen purified protein derivative or the lectin phytohemagglutinin. This material is now shown to have the following properties: (i) strongly adsorbable to glass; (ii) strongly adsorbable to cation- and not to anion-exchange resins but not elutable with mineral acid or solutions of high ionic strength; (iii) strongly adsorbable to cellulose nitrate membrane filter materials and quantitatively elutable with dilute HCl, providing a convenient method for partial purification; (iv) relatively stable over a wide range of pH and temperature; (v) antagonized by polyanions and by iron ions; (vi) active against the three gram-positive bacilli tested and not against the other organisms tested: (vii) more active in alkaline than in acidic media; and (viii) inactivated by proteolytic enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast R. C., Jr, Cleveland R. P., Littman B. H., Zbar B., Rapp H. J. Acquired cellular immunity: extracellular killing of Listeria monocytogenes by a product of immunologically activated macrophages. Cell Immunol. 1974 Feb;10(2):248–259. doi: 10.1016/0008-8749(74)90116-6. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon J., Kiely J. M., Lefko J. L., Unanue E. R. The modulation of lymphocyte functions by molecules secreted by macrophages. I. Description and partial biochemical analysis. J Exp Med. 1975 Jul 1;142(1):151–164. doi: 10.1084/jem.142.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDSON D. M., ELLSWORTH B., MATHESON A. SEPARATION AND PURIFICATION OF BETA-LYSIN FROM NORMAL SERUM. J Immunol. 1964 Jun;92:896–901. [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. Effect of iron on the bactericidal proteins from rabbit polymorphonuclear leukocytes. Nature. 1970 Aug 22;227(5260):849–851. doi: 10.1038/227849a0. [DOI] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. O. Electrophoretic behavior and crystal violet adsorption capacity of ribonuclease treated bacterial cells. J Bacteriol. 1953 May;65(5):518–521. doi: 10.1128/jb.65.5.518-521.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Antimicrobial factors in tissues and phagocytic cells. Bacteriol Rev. 1960 Mar;24(1):133–140. doi: 10.1128/br.24.1.133-140.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGO R., JACOX R. F. Cellular source and charcter of a heatstable bactericidal property associated with rabbit and rat platelets. J Exp Med. 1961 Apr 1;113:701–711. doi: 10.1084/jem.113.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. B., Donaldson D. M. Purification of staphylocidal beta-lysin from rabbit serum. J Bacteriol. 1968 Sep;96(3):589–595. doi: 10.1128/jb.96.3.589-595.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Pellis N. R., Pfohl D. G. Effects of normal and activated cell fractions on the growth of tubercle bacilli. Infect Immun. 1972 Aug;6(2):142–148. doi: 10.1128/iai.6.2.142-148.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Middlebrook G., Salmon B. J., Kreisberg J. I. Sterilization of Listeria monocytogenes by guinea pig peritoneal exudate cell cultures. Cell Immunol. 1974 Nov;14(2):270–283. doi: 10.1016/0008-8749(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Jackson R. C., Lemke H., Huget R., Flad H. D. Biochemical characterization of a factor released by macrophages. Cell Immunol. 1975 Jul;18(1):70–75. doi: 10.1016/0008-8749(75)90037-4. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalone R. M., Page R. C. Lymphokine-induced production and release of lysosomal enzymes by macrophages. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2091–2094. doi: 10.1073/pnas.72.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. P., Lucas Z. J. Cytotoxic activity of lymphocytes. V. Role of soluble toxin in macrophage-inhibited cultures of tumor cells. J Immunol. 1975 Aug;115(2):395–404. [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957 Dec;21(4):273–294. doi: 10.1128/br.21.4.273-294.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Teschner M., Brandis H. In vitro antilisterial activity of soluble product(s) released from Listeria-immune murine peritoneal macrophages. Infect Immun. 1974 Oct;10(4):960–962. doi: 10.1128/iai.10.4.960-962.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. D., Middlebrook G. Antibacterial activity of stimulated guinea pig peritoneal exudate cell culture supernates. Cell Immunol. 1976 Jun 1;24(1):123–131. doi: 10.1016/0008-8749(76)90138-6. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Wilder M. S. Cytotoxicity of rabbit blood for Listeria monocytogenes. Infect Immun. 1971 Dec;4(6):703–708. doi: 10.1128/iai.4.6.703-708.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L. D., Wilder M. S. Fate of listeria monocytogenes in normal rabbit serum. Infect Immun. 1973 Feb;7(2):289–297. doi: 10.1128/iai.7.2.289-297.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


