Abstract
Age related decline in balance performance is associated with deteriorating muscle strength, motor coordination and vestibular function. While a number of studies show changes in balance phenotype with age in rodents, very few isolate the vestibular contribution to balance under either normal conditions or during senescence. We use two standard behavioral tests to characterize the balance performance of mice at defined age points over the lifespan: the rotarod test and the inclined balance beam test. Importantly though, a custom built rotator is also used to stimulate the vestibular system of mice (without inducing overt signs of motion sickness). These two tests have been used to show that changes in vestibular mediated-balance performance are present over the murine lifespan. Preliminary results show that both the rotarod test and the modified balance beam test can be used to identify changes in balance performance during aging as an alternative to more difficult and invasive techniques such as vestibulo-ocular (VOR) measurements.
Keywords: Behavior, Issue 89, vestibular, behavior, balance, rotarod, balance beam, aging
Introduction
Our sense of balance is perhaps one of the most overlooked yet vital components of even the most basic motor activities including walking and turning. Balance is influenced by numerous factors including muscle strength, motor coordination and vestibular function, and it is only in the presence of vestibular neuropathies or during normal aging that the importance of a fully functioning balance system is appreciated. Disturbances to the vestibular system are often associated with experiences of vertigo or dizziness and disequilibrium resulting in an increased risk of falls and subsequent injuries1. This is particularly critical in older populations where falls are one of the leading causes of injury2.
Vestibular function tests are commonly based on the vestibular reflexes, in particular, the vestibulo-ocular (VOR) or the vestibulo-collic reflex (VCR). The VOR and VCR are essential for the stabilization of images on the retina and head position during movements of the head and body respectively. Commonly, VOR measurements require invasive implantation of search coils to measure eye movements or video tracking of eye movement3. This is challenging in mice due to the small nature of the mouse eye and the difficulty in detecting the pupil for video analysis3. As an alternative, the VCR has been used to measure stabilization of the head in response to body movements in mice without the need for invasive surgery4. Despite this, few studies focus specifically on how the vestibular system performs as a whole and more importantly how it changes during aging.
To assess overall balance performance simply and noninvasively we modified two commonly used behavioral tests. The rotarod and inclined balance beam tests assess different aspects of motor performance in rodents and in previous studies have been used in a test battery to acquire a complete profile of motor capability. This capability can be affected by disease or genetic modification, and is also sensitive to processes associated with normal development and aging5-7. Earlier work using the rotarod has shown that motor coordination in mice declines after 3 months of age8. In addition, rats show noticeable balance deficits with increasing age on the balance beam test9.
This paper describes the use of the rotarod and balance beam tests in conjunction with a vestibular stimulus in order to challenge the vestibular system and characterize the subsequent impact on balance performance in young and older mice. While the simple and noninvasive methods described are not designed as stand-alone measures of peripheral vestibular function, they do provide a useful and simple behavioral measure to compare cellular and subcellular changes at multiple stages of vestibular processing during normal aging in mice.
Protocol
1. Animals
Mice (C57/BL6) of ages 1, 9, and 13 months old were obtained from the Animal Resources Centre (Perth, Australia). These mice were housed in standard mouse cages in the Bosch Rodent Facility at the University of Sydney on a 12/12 hr light/dark cycle with access to food and water ad libitum. The procedures outlined below were approved by the University of Sydney Animal Ethics Committee.
Bring mouse cages into the testing room prior to each test for 10 min to allow mice to acclimatize to the testing environment.
2. Rotarod
- Set up the rotarod apparatus (Figure 1A):
- Install the dowels in each lane of the rotarod. Note: In this instance rat dowels (70 mm in diameter) are used instead of mouse dowels (32 mm in diameter) to discourage the mice from clinging to the dowel and performing “passive rotations”10.
- Position the magnetic landing platforms on the wire situated at the bottom of each lane of the rotarod making sure that they are not tilting to touch the floor of the rotarod and are placed as close as possible to the magnetic right wall of each lane without touching. Note: During rotarod testing mice are required to walk in a forward direction to stay on the rotating and accelerating dowels. When a mouse is no longer able to stay on the dowel, they fall and displace the landing platform that subsequently activates a magnetic sensor. The time taken to fall from the rotating dowel, the dowel rpm at time of falling and the distance traveled is automatically calculated for each mouse and recorded on the display screen at the front of the rotarod.
- Slide 2 clear plastic panels into the front of each rotarod lane with the shorter panels at the bottom and the longer panels above.
- Input the test parameters using the keypad located at the front of the rotarod. Follow steps 2.1.4.1 to 2.1.4.6 for the accelerating rotarod test parameters and steps 2.1.4.7 to 2.1.4.12 for the fixed speed rotarod test parameters.
- Set the maximum duration of the test to 60 sec.
- Set the number of lanes to be used (or the number of mice to be tested).
- Set the starting speed of the test to 5 rpm.
- Set the top speed of the test to 44 rpm.
- Set the ramp speed of the test to 60 sec.
- Set the size of the dowels chosen and the direction of rotation to rat dowels rotating in a forward direction.
- Set the maximum duration of the test to 240 sec.
- Set the number of lanes to be used to 1 as the mice are tested individually.
- Set the starting speed of the test to 15 rpm.
- Set the top speed of the test to 15 rpm.
- Set the ramp speed of the test to 0 sec.
- Set the size of the dowel chosen and the direction of rotation to a rat dowel rotating in a forward direction. Note: The settings above may be altered to suit the needs of different experiments.
- Place a camera in front of the rotarod for the fixed speed rotarod test, so that the behavior of the mouse during trials can be recorded and the videos used for later analysis to determine the duration of time the mice were able to stay on the rotarod.
- Follow steps 2.2.1 to 2.2.4 for the accelerating rotarod test:
- Place one mouse on each stationary dowel for 5 min to allow mice to acclimatize to the rotarod.
- Gently nudge the mice to face the back of the rotarod and start the rotarod test when all subjects are facing in this direction (see Figure 1B).
- Return all mice to their cages when they have fallen from the rotating dowels and leave them to rest for 10 min with access to food and water.
- Repeat steps 2.2.1 to 2.2.3 to complete a total of 8 trials making sure to clean the dowels, lanes, and landing platforms of the rotarod for urine and feces, and move the landing platforms back to its starting position betwen each trial. Note: The first 3-5 trials are used as training trials to allow the mice to familiarize themselves with the task. The time to fall, distance walked and end rpm of the dowel at the time of the fall for each subsequent trial is recorded for later analysis (Figure 2).
- Follow steps 2.3.1 to 2.3.8 for the fixed speed rotarod test:
- Place one mouse on a dowel for 5 min to allow it to acclimatize to the rotarod. Return the mouse back to its cage.
- Start video recording on the camera and press start on the rotarod. Then place the mouse on the rotating dowel ensuring it faces the back of the rotarod.
- Stop video recording on the camera when the mouse falls from the rotating dowels, and return the mouse to its cages for 10 min with access to food and water.
- Repeat steps 2.3.2 and 2.3.3 until a total of 8 trials is acquired making sure to clean the dowels, lanes, and landing platforms of the rotarod for feces and urine, and move the landing platforms back to its starting position between each trial.
- Switch on the custom built rotator at 3 Hz for 20 sec to allow the mice to become familiar with the sound. Stop the rotator after 20 sec by switching off the drill and place hands on either side of the running wheel to stop it from continuing to spin past the initial 20 sec. Note: The rotator itself consists of a rodent running wheel secured to a drill (Figure 3A). At the center of the running wheel is a small chamber with a mesh lid where the mouse is placed (Figure 3B). The rotator spins in a counter-clockwise direction about the vertical axis. The magnitude of the stimulus is in line with previous studies that show rotary stimulations ranging from 0.2 to 3 Hz are sufficient to generate VOR and VCR responses4,11,12.
- Place the mouse inside the chamber at the center of the rotator and replace the lid.
- Switch on the rotator at its lowest setting of 3 Hz for 20 sec. Start the rotarod and begin video recording on the camera during this time in preparation for the upcoming trial. Switch off the drill at the end of the 20 sec and place hands on either side of the running wheel to stop it from spinning. Retest the mouse on the rotarod immediately after by transferring it as quickly as possible to the rotating dowel.
- Stop video recording on the camera when the mouse falls from the dowel and return the mouse to its cage.
Clean the clear plastic panels with a mild detergent/water mix and the cylindrical dowels, lanes and metal landing platforms of the rotarod with 70% ethanol when all mice have been tested.
3. Balance Beam with Vestibular Challenge
- Set up the balance beam apparatus as seen in Figure 4A. Note: The balance beam apparatus was adapted from an apparatus described in Carter et al. (2001)13. For this test, mice walk from the lower end of the beam, which is 52.5 cm above the ground, to a darkened goal box (13 x 22 cm, with a 5 x 6 cm doorway) situated 60 cm above the ground (Figure 4A). Mice naturally seek out the darkness and protection of the goal box in favor of the exposed beam and are further encouraged to traverse the beam by the slight incline which exploits their natural escape mechanism to run in an upwards direction14. The beam itself is 1 m in length and has a circular cross section with a diameter of 14 mm. A tailored range of beam diameters can be used which allows the experimenter to adjust the sensitivity of the test or accommodate larger subjects. At the lower end of the balance beam a white line indicates the starting line. Another line has been drawn 60 cm from the start line at the higher end of the beam to indicate the finish line (Figure 4A).
- Position 2 cameras, one on either side of the balance beam, at the lower end of the balance beam (Figure 4B). Note: These cameras should be angled to capture the entire length of the balance beam and ensure that start and finish lines marked on the balance beam are clearly visible. These cameras will be used to video record the behavior of mice as they traverse the balance beam, with resulting videos being used for later analysis.
- Line the floor of the goal box with paper towel, to enable easy cleaning of urine and feces after testing each mouse, and place the housing dome from the subjects home cage inside the goal box.
- Place adequate foam or other cushioning material underneath the raised beam to protect any subjects that fall from the apparatus. Mice that fall will be picked up immediately by the experimenter and placed inside the goal box to rest.
Place one mouse in the goal box for 2 min so that it becomes familiar with this environment. Cover the opening to the goal box with a gloved hand for 5 sec if the mouse tries to walk onto the beam during this time to discourage this behavior.
Train the mouse by placing it on the beam just outside the opening to the goal box and allowing it to walk into the goal box. Continue to train the mouse by placing it on the beam progressively further away from the goal box until the mouse is able to walk from the start line to the goal box with no assistance and minimal hesitation. Leave the mouse to rest in the goal box for 1 min after each run.
- Begin testing the mouse when training is complete.
- Start video recording on cameras.
- Place the mouse at the start line of the beam and wait while it traverses the beam in the direction of the goal box.
- Stop video recording on cameras when the mouse reaches the box.
- Leave the mouse to rest in the goal box for 1 min. Remove any urine or feces that may have been deposited during the trial while waiting.
- Repeat steps 3.4.1 to 3.4.4 until a total of 5 trials have been completed.
Switch on the custom built rotator at 3 Hz for 20 sec (like in the fixed speed rotarod test) to allow the mice to become familiar with the sound. Stop the rotator after 20 sec by switching off the drill and place hands on either side of the running wheel to stop it from spinning.
Place the mouse inside the chamber at the center of the rotator and replace the lid.
Switch on the rotator at its lowest setting of 3 Hz for 20 sec. Start video recording on the cameras during this time, in preparation for the upcoming trial. Switch off the drill at the end of the 20 sec and place hands on either side of the running wheel to stop it from continuing to spin past the initial 20 sec. Transfer the mouse to the start of the balance beam as quickly as possible and wait while the mouse traverses the beam to the goal box.
Stop video recording on cameras when the mouse reaches the goal box and return the mouse to its cage.
Clean the balance beam apparatus with 70% ethanol and change the paper towel in the goal box after each mouse has been tested.
Representative Results
Rotarod
The motor performance of mice was described as the Time To Fall (TTF) recorded for each mouse over 8 trials. Using these measurements of TTF, training curves for each mouse can be plotted. Figure 2 shows examples of the motor performance of one 1 month-old mouse and one 9 month-old mouse over the course of 8 trials. These training curves show an increase in TTF during the first 3-5 trials followed by a subsequent plateau. Measurements of TTF recorded before the plateau were considered training (Figure 2), while measurements of TTF that form the plateau were recorded and used for data analysis (Figure 5).
Figure 5 shows that motor performance on the rotarod deteriorates with age. When compared with their 9 month-old counterparts (n = 8), 1 month-old mice (n = 6) were able to stay on the rotarod significantly longer (18.38 ± 4.66 vs. 14.13 ± 2.53 sec; p < 0.05, Student’s t-test). This shows that the rotarod and the test parameters used are sensitive to changes in motor performance with age.
Balance Beam
Figure 6 shows the times to traverse (TTT) the balance beam and cross the finish line before and after the vestibular stimulus for 1 month-old, 9 month-old and 13 month-old mice. In 1 month-old mice (n = 9), the vestibular stimulus had a minimal effect on the time taken for them to traverse the balance with similar TTT before (3.49 ± 0.62 sec) and after (3.81 ± 0.66 sec) the stimulus. In contrast, 9 month-old mice (n = 6) required more time to traverse the balance beam after the vestibular stimulus (4.85 ± 1.67 vs. 8.45 ± 2.59 sec; p <0.05, Student’s t-test). In 13 month-old mice (n = 5) TTT increased following the vestibular stimulus (6.48 ± 2.19 vs. 9.24 ± 4.11 sec) but this was not statistically significant.
To further examine the interaction between age and vestibular stimulus-related changes in TTT we used repeated-measures ANOVA with a Tukey post hoc test. Figure 6 shows that the impact of vestibular stimulation on balance beam performance is significantly greater in 9 month-old (p < 0.01) and 13 month-old (p < 0.001) mice when compared with 1 month-old mice. Together these results indicate that the simple balance beam apparatus can be used in conjunction with the custom built rotator to measure vestibular-related changes in balance performance throughout the murine lifespan.
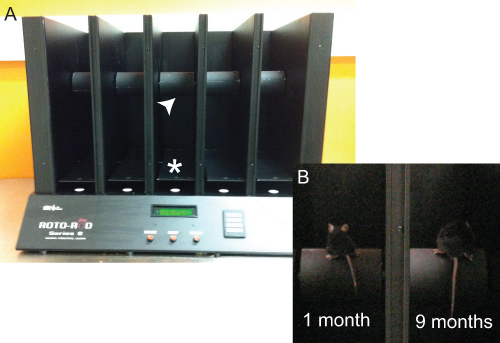 Figure 1. The rotarod apparatus. (A) The rotarod apparatus has 5 lanes and is able to test a maximum of 5 rodents at any one time. The cylindrical dowels (arrowhead) upon which mice are placed are situated above the metal landing platforms (*) that trigger pressure sensors for data acquisition. (B) A photo of one 1 month-old mouse and one 9 month old mouse sitting on the dowels facing the back of the rotarod in preparation of a test.
Figure 1. The rotarod apparatus. (A) The rotarod apparatus has 5 lanes and is able to test a maximum of 5 rodents at any one time. The cylindrical dowels (arrowhead) upon which mice are placed are situated above the metal landing platforms (*) that trigger pressure sensors for data acquisition. (B) A photo of one 1 month-old mouse and one 9 month old mouse sitting on the dowels facing the back of the rotarod in preparation of a test.
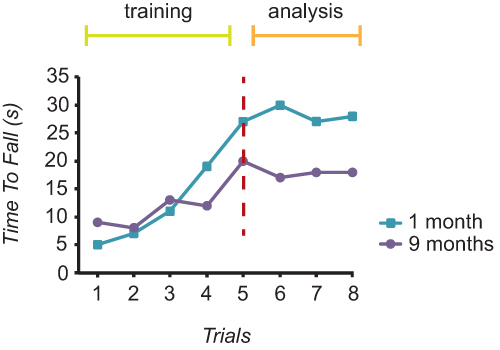 Figure 2. Rotarod training curves. An example of one 1 month-old and one 9 month-old mouse and their measurements of time to fall on the rotarod. The measurements of time to fall for both mice increased steadily during trials 1 to 5 and therefore were considered as training trials. Once trials stabilized (performance increases plateaued; dashed line) measurements of time to fall were recorded for data analysis.
Figure 2. Rotarod training curves. An example of one 1 month-old and one 9 month-old mouse and their measurements of time to fall on the rotarod. The measurements of time to fall for both mice increased steadily during trials 1 to 5 and therefore were considered as training trials. Once trials stabilized (performance increases plateaued; dashed line) measurements of time to fall were recorded for data analysis.
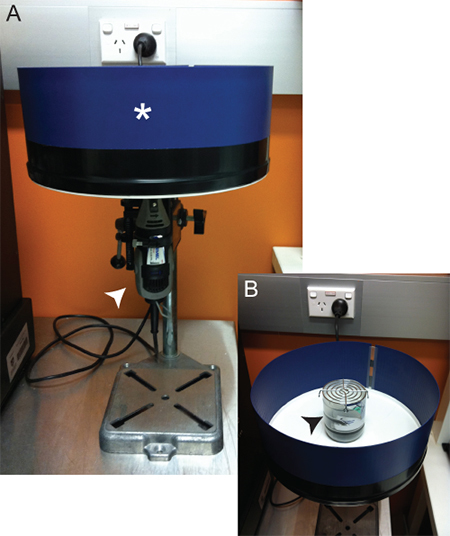 Figure 3. The custom built rotator.(A) The custom-built rotator is used to stimulate the vestibular system of mice. This rotator consists of a Dremel (arrowhead) and a rodent running wheel (*). (B) A superior view of the rotator. Mice are placed inside the chamber (arrowhead) at the center of the running wheel.
Figure 3. The custom built rotator.(A) The custom-built rotator is used to stimulate the vestibular system of mice. This rotator consists of a Dremel (arrowhead) and a rodent running wheel (*). (B) A superior view of the rotator. Mice are placed inside the chamber (arrowhead) at the center of the running wheel.
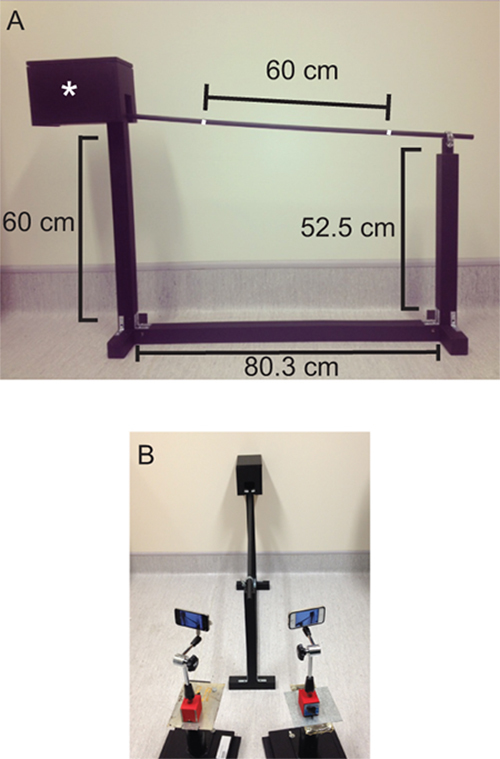 Figure 4. Balance beam apparatus. (A) The inclined balance beam apparatus has an 80.3 cm long base, with the start of the beam situated 52.5 cm above the ground and the goal box (*) raised 60 cm above the ground. (B) The behavior of mice on the balance beam is recorded by two cameras placed at the lower end of the beam. The videos recorded provide left and right views of the mice as they traverse the beam and are used for later analysis.
Figure 4. Balance beam apparatus. (A) The inclined balance beam apparatus has an 80.3 cm long base, with the start of the beam situated 52.5 cm above the ground and the goal box (*) raised 60 cm above the ground. (B) The behavior of mice on the balance beam is recorded by two cameras placed at the lower end of the beam. The videos recorded provide left and right views of the mice as they traverse the beam and are used for later analysis.
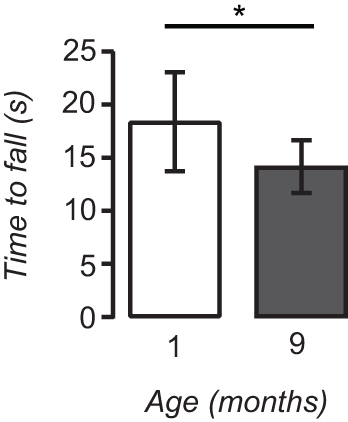 Figure 5. Rotarod performance decreases with age. 1 month-old mice (n = 6) were able to stay on the rotating dowels significantly longer than 9 month-old (n = 8) mice (*; p < 0.05). Data are represented as mean ± SD.
Figure 5. Rotarod performance decreases with age. 1 month-old mice (n = 6) were able to stay on the rotating dowels significantly longer than 9 month-old (n = 8) mice (*; p < 0.05). Data are represented as mean ± SD.
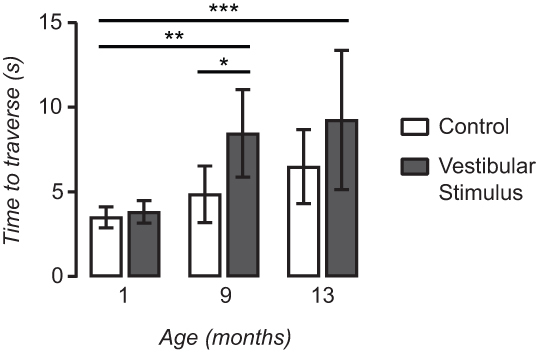 Figure 6. The impact of vestibular stimulation on balance beam performance is greater with age. Vestibular stimulation increased the time to traverse in 9 month-old (n = 6) and 13 month-old (n = 5) mice but not in 1 month-old (n = 9) mice. When the interaction between age and vestibular stimulus-related changes in TTT is assessed the impact of vestibular stimulation on balance beam performance is significantly greater in 9 month-old and 13 month-old mice when compared with 1 month-old mice. Data are represented as mean ± SD. *; p < 0.05 **; p < 0.01 ***; p < 0.001.
Figure 6. The impact of vestibular stimulation on balance beam performance is greater with age. Vestibular stimulation increased the time to traverse in 9 month-old (n = 6) and 13 month-old (n = 5) mice but not in 1 month-old (n = 9) mice. When the interaction between age and vestibular stimulus-related changes in TTT is assessed the impact of vestibular stimulation on balance beam performance is significantly greater in 9 month-old and 13 month-old mice when compared with 1 month-old mice. Data are represented as mean ± SD. *; p < 0.05 **; p < 0.01 ***; p < 0.001.
Discussion
Critical Steps within the Protocol
Previous work has shown that it is easy to overtrain mice on both the rotarod and balance beam apparatus and as a consequence, the acquisition of accurate measurements can be challenging15. For example, overtraining on the rotarod can lead to mice intentionally jumping off the dowels during both the acclimatization and trial periods, while overtraining on the balance beam can lead to more frequent stopping (exploratory behavior) and travelling in the opposite direction (i.e. towards the start line)15. Ultimately, overtraining can lead to underestimates of actual motor capability. It is therefore critical that training curves be assessed prior to analysis.
Another critical step within the rotarod protocol is ensuring that the mice face the correct direction (opposite direction to rotation) prior to starting each trial. Mice facing the wrong direction when the dowels start to rotate have difficulty maintaining balance on the dowel and consequently fall early, potentially overestimating the impact of the test. Further, in the balance beam test, it is important to transfer the mice as quickly as possible from the rotator to the balance beam as recovery from the vestibular challenge begins immediately. This can mean that disequilibrium caused by the rotator and subsequent reduction in performance can be underestimated.
Modifications and Troubleshooting
Modifications can be made to both the rotarod and the balance beam test in order to alter the sensitivity of the tests. The rotarod test can easily be modified to change the level of difficulty of the motor task necessary to detect balance and motor deficits. This is can be achieved by manipulating the speed at which the dowels rotate during the test, and also whether or not these rotations accelerate over the duration of the test. For the balance beam test different beam widths can be used to adjust the sensitivity of the test, with smaller beam widths engendering a higher level of difficulty. Beams with rectangular cross-sections can also be used, although in a previous study using this approach, it was shown that mice were able to grip onto the sides of the beam which lead to aberrant measurements of time to traverse15. In both rotarod and balance beam tests, mice can be challenged with the vestibular stimulus and retested on the apparatus up to 3 times. However, it should be noted that mice are often reluctant to complete the task after undergoing the first trial with the vestibular stimulus.
Limitations
Measurements of balance and motor ability can be affected by the size and weight of individual mice being tested14. This means that there is a possibility that the impact of age on motor performance can be augmented by the effects of gravity and center of mass. Indeed, mice with relatively higher body mass have been shown to perform worse on the rotarod test16. The application of the vestibular rotator however minimizes the extent to which balance performance is confounded by weight, and facilitates the attribution of balance performance to the impact of aging on the vestibular system.
Significance of the Technique with respect to Existing Methods and Alternative Methods
There have been few studies that directly investigate aging in the vestibular system of any species. Commonly these studies have used the VOR to assess vestibular function and have shown that VOR function is preserved up until 60 weeks of age with only small changes after adulthood is reached17,18. In addition VOR tests typically require a degree of invasiveness to attach the recording coils to the animal’s cornea, and often require a recovery period3. Due to the small size of the mouse eye the most commonly used alternative, video eye tracking, is also difficult to achieve. Together these difficulties have limited the number of VOR studies in the murine model.
The methods described in this paper employ apparatus commonly used to assess motor coordination and balance. In addition, these methods have been used to investigate the changes that occur during development and aging and those due to genetic modifications5,7,19,20. As motor coordination and balance has been shown to decline after 3 months of age, the additional use of a simple vestibular stimulus in this paper facilitates the investigation of the vestibular system in an aging murine model without the use of more difficult and invasive techniques outlined above8. This information can then be used to correlate behavior with underlying cellular and subcellular changes that occur in the vestibular system with age.
Future Application or Directions after Mastering this Technique
Although the methods described here do not quantify the level of disequilibrium experienced by the animals post-rotation, further application of a vestibular stimulus can be modified to include a scoring system based on the presence of symptoms including urination, defecation and tremors21. Other ways of quantifying the amount of disequilibrium experienced by mice include measuring saccharin and Kaolin uptake as shown previously11,21. Ultimately, the ability to score the vestibular-related effect of aging in an individual mouse allows for investigation on correlations between balance performance and cellular/subcellular processes using subsequent electrophysiological, molecular and two-photon microscopy techniques22.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgments
The authors would like to acknowledge The Garnett Passe and Rodney Williams Memorial Foundation and the Bosch Institute Animal Behavioural Facility.
References
- Agrawal Y, et al. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch. Intern. Med. 2009;169:938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- Schwab CW, Kauder DR. Trauma in the geriatric patient. Arch. Surg. 1992;127:701–706. doi: 10.1001/archsurg.1992.01420060077011. [DOI] [PubMed] [Google Scholar]
- Stahl JS, et al. A comparison of video and magnetic search coil recordings of mouse eye movements. J. Neurosci. Methods. 2000;99:101–110. doi: 10.1016/s0165-0270(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Takemura K, King WM. Vestibulo-collic reflex (VCR) in mice. Exp. Brain Res. 2005;167:103–107. doi: 10.1007/s00221-005-0030-1. [DOI] [PubMed] [Google Scholar]
- Carter RJ, et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J. Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JE, et al. Motor and reflexive behavior in the aging rat. J. Gerontol. 1980;35:364–370. doi: 10.1093/geronj/35.3.364. [DOI] [PubMed] [Google Scholar]
- Ingram DK, et al. Differential effects of age on motor performance in two mouse strains. Neurobiol. Aging. 1981;2:221–227. doi: 10.1016/0197-4580(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Serradj N, Jamon M. Age-related changes in the motricity of the inbred mice strains 129/sv and C57BL/6j. Behav. Brain Res. 2007;177:80–89. doi: 10.1016/j.bbr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gage FH, et al. Spatial learning and motor deficits in aged rats. Neurobiol. Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Rustay NR, et al. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav. Brain Res. 2003;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Xiaocheng W, et al. Expression of calcitonin gene-related peptide in efferent vestibular system and vestibular nucleus in rats with motion sickness. PloS One. 2012;7 doi: 10.1371/journal.pone.0047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraneck M, et al. Ontogeny of mouse vestibulo-ocular reflex following genetic or environmental alteration of gravity sensing. PloS One. 2012;7 doi: 10.1371/journal.pone.0040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, et al. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001. [DOI] [PubMed]
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user's guide. Nat. Rev. Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- Luong TN, et al. Assessment of motor balance and coordination in mice using the balance beam. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]
- McFadyen MP, et al. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–219. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Shiga A, et al. Aging effects on vestibulo-ocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiol. Neurootol. 2005;10:97–104. doi: 10.1159/000083365. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Eye movements of the murine P/Q calcium channel mutant rocker, and the impact of aging. J. Neurophysiol. 2004;91:2066–2078. doi: 10.1152/jn.01068.2003. [DOI] [PubMed] [Google Scholar]
- Fahlstrom A, et al. Behavioral changes in aging female C57BL/6 mice. Neurobiol. Aging. 2011;32:1868–1880. doi: 10.1016/j.neurobiolaging.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Bâ A, Seri BV. Psychomotor functions in developing rats: ontogenetic approach to structure-function relationships. Neurosci. Biobehav. Rev. 1995;19:413–425. doi: 10.1016/0149-7634(94)00042-y. [DOI] [PubMed] [Google Scholar]
- Yu X, et al. A novel animal model for motion sickness and its first application in rodents. Physiol. Behav. 2007;92:702–707. doi: 10.1016/j.physbeh.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Tung VW, et al. An isolated semi-intact preparation of the mouse vestibular sensory epithelium for electrophysiology and high-resolution two-photon microscopy. J. Vis. Exp. 2013. [DOI] [PMC free article] [PubMed]


