Abstract
CD3+CD56−, CD4 and CD8 double negative T (DNT) cells comprise 1–3% of peripheral blood mononuclear cells. Their role in tumor immunity remains largely unknown due to their limited numbers and lack of effective methods to expand them. Here we developed a novel protocol by which DNT cells can be expanded ex vivo to therapeutic levels in 2 weeks from 13 of 16 acute myeloid leukemia (AML) patients during chemotherapy-induced complete remission. The expanded DNT cells expressed similar or higher levels of IFN-γ and TNF-α, and Granzyme B as that seen in bulk activated CD8 T cells from the same patient but significantly higher levels of perforin. The expanded DNT cells could effectively kill both allogeneic and autologous primary CD34+ leukemic blasts isolated from peripheral blood of AML patients in a perforin-dependant manner. These results demonstrate, for the first time, that DNT cells from AML patients can be expanded ex vivo even after intensive chemotherapy, and are effective at killing both allogeneic and autologous primary leukemic blasts. These findings warrant studies further exploring the potential of DNT cells as a novel adjuvant immunotherapy to decrease the risk of relapse in patients with AML and, perhaps other cancers.
Keywords: Acute Myeloid Leukemia (AML), Double Negative T (DNT) cells, Adoptive Immunotherapy
Introduction
Combination chemotherapy is capable of eliminating clinically apparent disease and inducing complete remission (CR) in the majority of patients with acute myeloid leukemia (AML) (1). However, it is often ineffective at eliminating minimal residual disease (MRD) (2). Consequently, the majority of patients relapse within 2 years of treatment. Currently, treatment options to prevent relapse of leukemia include allogeneic or autologous hematopoietic cell transplantation (HCT). Allogeneic HCT is the treatment of choice for younger patients with high, and intermediate risk disease who are medically fit and have a suitable donor (3–5). Unfortunately, allogeneic HCT can cause debilitating graft-versus-host disease (GVHD). Compared with allogeneic HCT, autologous HCT has a lower transplant-related mortality but is associated with a higher relapse rate due to the lack of an immune-mediated graft-versus-leukemia effect. Infusion of unselected donor lymphocytes to treat relapse after HCT is effective in a minority of patients but rarely produces durable remission of disease (6–8).
Novel cellular therapies that can effectively eliminate MRD during chemotherapy-induced remission without causing severe damage to the host are needed to prevent disease relapse and improve long-term survival of AML patients. Adoptive cell therapy using ex vivo activated and expanded autologous T cells with potent anti-tumor activity could be an effective complementary approach to chemotherapy for eliminating residual tumor cells. The majority of T cells in human peripheral blood (PB) express either CD4 or CD8 molecules. Approximately 1–3% of them express CD3 but lack CD4, CD8 and CD56, and are termed double negative T (DNT) cells. The DNT cells may express either an αβ- or a γδ-T cell receptor (TCR). Unlike tumor infiltrating lymphocytes (TIL) or cytokine activated killer (CIK) cells whose anti-tumor effects have been investigated extensively (9–13), little is known about the role of DNT cells in tumor immunity. Previously, we have demonstrated in mouse models that a single infusion of pre-activated allogeneic αβ-DNT cells or clones could protect mice from a lethal dose of leukemia cells without causing GVHD (14, 15). In addition, a human αβ-DNT cell clone isolated from PB of a melanoma patient was shown to be able to kill gp-100 expressing melanoma cells (16). It is also known that human pan γδ-T cells have potent anti-tumor effects (17). However, due to the limited number of primary DNT cells that can be obtained in the blood and the lack of effective methods to expand this cellular population, the role of human DNT cells in anti-tumor responses remains elusive.
In order to evaluate the anti-tumor effect of human DNT cells, we developed a novel protocol to efficiently expand human DNT cells ex vivo. We found that DNT cells could be expanded to clinically useful numbers in a majority of AML patients during or just after the period of consolidation chemotherapy and that expanded DNT cells were effective in killing both allogeneic and autologous leukemic cells in vitro. The results obtained from these studies provide evidence that ex vivo expanded autologous DNT cells could be used as a novel immunotherapy to complement cytotoxic chemotherapy to prevent AML relapse by eliminating residual leukemic cells.
Materials and Methods
Patient population
The study was approved by the University Health Network-Research and Ethics Board (UHN-REB). Eligible patients included patients aged 18 years or older with AML in CR after standard chemotherapy. One month after conventional induction or consolidation chemotherapy, 20 ml of whole blood was drawn from AML patients.
Leukemic blasts and cell lines
Bone marrow (BM) or PB samples were obtained from newly diagnosed AML patients in the clinic under a UHN-REB approved protocol after obtaining appropriate informed consent. For in vitro studies, CD34+ cells were purified using CD34-FITC and anti-FITC-coated magnetic beads (Miltenyi Biotec) with >95% purity, and used as targets in standard 51Cr-release assays. U937 (histiocytic lymphoma) cell line was obtained from the American Type Culture Collection. Primary leukemic cell lines OCI/AML-2 (AML-2) and OCI/AML-3 (AML-3) were generated from AML patients as described previously (18, 19). All cell lines were cultured in RPMI-1640 supplemented with 10% FBS, and incubated at 37° C in 5% CO2.
Ex vivo expansion of human DNT cells
To enrich DNT cell population, CD4+ and CD8+ cells together with red blood cells were depleted using RosetteSep® depletion kit according to the manufacturer’s instruction (StemCell Technologies, Canada). CD4- and CD8-depleted PB mononuclear cells (PBMC) were either frozen viable for future use, or cultured fresh in 24-well plates with anti-CD3 mAb (OKT3, 5 μg/ml, eBioscience) in complete medium supplemented with recombinant human interleukin 2 (rhIL-2) (250U/ml; Chiron, Canada) and rhIL-4 (0.1 ng/ml, Biosource™, USA). The cells were washed and cultured with fresh media plus cytokines and soluble anti-CD3 antibody on day 7. On day 14, cells were harvested, counted and their viability, composition and purity were assessed by flow cytometry. The expanded DNT cells were either used directly or further purified by depleting CD4+ and CD8+ and CD56+ T cells prior to their use. In some experiments, DNT cells were further separated into αβ-DNT and γδ-DNT cells by FACS.
Antibodies and flow cytometry
The following anti-human antibodies for staining of cell surface markers and intracellular molecules were used: CD3-FITC, -PECy5 or -PECy7, CD4-FITC or -PE, CD8-FITC or -PE, CD34-FITC, CD56-PECy7, pan αβ-TCR-FITC, pan γδ-TCR-PECy5 or -FITC were purchased from Beckman Coulter, and FasL-PE from eBiosciences, Inc. (San Diego); perforin-PE, Granzyme B-PE, IFN-γ-APC, TNF-α-APC were from BD Biosciences (Mississauga). Isotype-matched mAbs were obtained from BD Biosciences and used as staining controls. For intracellular staining, cells were either stimulated with PMA/Ionomycin (Sigma-Aldrich, Canada) and GolgiSTOP (BD Biosciences) for 3 hours for IFN-γ and TNF-α staining or used without further stimulation for perforin and Granzyme B staining using Cytofix/Cytoperm Kit (BD Biosciences) according to the manufacturer’s instruction. Data acquisitions were performed using either FC500 (Beckman Coulter) or FACSCalibur or LSRII (BD Biosciences) Flow cytometers and data were analyzed using FlowJo software (Tree Star, Inc., Oregan, USA).
Cytotoxicity assay and blocking experiments
The cytotoxic activity of ex vivo expanded DNT cells was measured by standard 4-hr 51Cr release assay. Briefly, 106 target cells (either leukemic cell lines or freshly isolated leukemic blasts) were labeled with 100μCi of 51Cr for an hour, washed and coincubated with varying ratios of ex vivo expanded DNT cells. Percent specific target killing was calculated as described previously (20). For cytotoxicity blocking experiments, DNT cells were incubated with 100nM of Concanamycin A (CMA; Sigma-Aldrich) for 30 minutes and washed prior to co-incubation with targets.
Statistical analyses
Bar graphs were generated using Prism software (GraphPad, San Diego, CA). Data were expressed as means ± SD. The student t test was used for statistical data analysis. P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 16 AML patients in CR were enrolled into this study at the Princess Margaret Hospital, Toronto, Canada. The median age of the patients was 54 (range, 36–69), 50% were females. Characteristics of the study patients are shown in Table 1.
Table 1.
Characteristics of AML patients and cell expansion
| Patient Characteristics | Cell Expansion in Culture
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
total cell # (x106) | DNT# (x106) | |||||||||
| UPN | Age Gender |
Cytogenetics & molecular markers | WBC at diag | Did pt relapse | CR1 duration (month) | a | b1 | b2 | a | b1 | b2 |
| 1 | 54F | 46,XX,add(8)(q24.3),t(12;22)(p13;q?12)[19],46,XX[1] | 14.00 | yes | 8 | NE | NE | NE | NE | ||
| 2 | 69F | normal-intermediate risk | 24.00 | yes | 18 | 24 | 120 | 140 | 23 | 113 | 130 |
| 3 | 40F | 8;21 | 31.90 | yes | 13 | 6 | 26 | 5 | 14 | ||
| 4 | 66M | normal karyotype | 1.90 | yes | 10 | 6 | 25 | 3 | 3 | 14 | 2 |
| 5 | 56F | inversion 16 | 31.40 | no | 24 * | 14 | 20 | 35 | 13 | 12 | 15 |
| 6 | 51M | 46,XY,del(9)(q13q22)[20] | 32.20 | no | 24 * | 47 | 6 | 12 | 42 | 5 | 7 |
| 7 | 59F | 46,X,idic (x)(q13)[13], 46, XX[5] | 1.20 | no | 27 * | NE | NE | ||||
| 8 | 41M | inversion 16 | 40.30 | no | 12 * | 105 | NE | 45 | NE | ||
| 9 | 59F | normal karyotype | 18.70 | no | 12 * | 86 | 46 | ||||
| 10 | 42M | normal karyotype, ITD positive | 52.40 | yes | 3 | 90 | 66 | ||||
| 11 | 36F | normal karyotype, FLT3ITD positive | 45.80 | yes | 7 | 12 | 5 | ||||
| 12 | 53F | 46,XX,i(7)(p10)[20] | 0.90 | no | 12 * | 70 | 48 | 48 | 37 | ||
| 13 | 37M | 46,XY, t(11;17) [7] | 5.50 | no | 8 * | 218 | 41 | ||||
| 14 | 65M | 48,XY,+4,+8[20] | 2.80 | no | 8 * | 316 | 300 | ||||
| 15 | 48M | normal karyotype, NPM positive, ITD negative, TKD negative | 1.60 | no | 6 * | 60 | 11 | ||||
| 16 | 62M | normal karyotype, NPM positive, ITD negative, TKD positive | 103.00 | no | 7 * | 46 | 26 | ||||
|
| |||||||||||
| Median | 54 (36–69) | * ongoing remission | |||||||||
20 ml whole blood was drawn from 16 AML patients in complete remission at one month after induction chemotherapy (a), or one month after one (b1) or more (b2) consolidation chemotherapy. NE: No Expansion.
DNT cells can be expanded ex vivo from AML patients in CR during chemotherapy
DNT cells comprise 1–3% of PBMC from AML patients. In order to determine whether DNT cells can be used as a novel immunotherapy to improve AML patient survival by eliminating residual leukemic cells after CR, we first developed a method by which DNT cells could be expanded ex vivo. To prevent outgrowth of CD8+ and CD4+ T cells, CD4+ and CD8+ T cells were depleted together with red blood cells. The enriched DNT cells derived from 20 ml blood ranged from 2–5×105 cells, and were activated by cross-linking their TCR with anti-CD3 mAb for 3 days followed by addition of soluble anti-CD3 at day 7 for 3 days. The expanded cells were harvested on day 14. The total number of expanded cells and total number of DNT cells from each patient at different stages of chemotherapy are shown in Table 1. From a total of 28 cultures from 16 AML patients, we were able to expand DNT cells from 13 out of 16 patients (81%) including 24 of the 28 (86%) cultures. For those patients whose DNT cells expanded, we obtained a mean of 43×106 (range, 2–300 × 106) DNT cells from 20 ml blood, or an average of a 478±331-fold expansion in two weeks. The composition of expanded cells was examined by flow cytometric analysis, and the data from all cultures are shown in Supplementary Table 1. We found that 94±7% of expanded cells were CD3+ T cells, 66±23% were DNT cells, 30±20% were CD4+ or CD8+ T cells, and about 5% were CD56+CD3− natural killer (NK) cells. Representative data before and after expansion are shown in Fig. 1. These data show the feasibility of generating a large number of DNT cells from the blood of AML patients who had received chemotherapy following their initial diagnosis.
Figure 1. Composition of ex vivo expanded DNT cells.
Blood samples (20 ml) were collected from 16 AML patients in CR at one month after either induction or consolidation chemotherapy and DNT cells were enriched by depleting CD4+ and CD8+ T cells. Aliquots of the remaining cells were stained with anti-CD3-FITC, anti-CD4-PE, anti-CD8-PE and anti-CD56-PECy7. The remaining cells were expanded ex vivo as described in the Materials and Methods. On day 14, the expanded cells were harvested and stained as before. Data were analyzed by flow cytometry. Representative dot plots from the same patient before (day 0) and 14 days after ex vivo expansion are shown.
DNT cells can be expanded at different stages of chemotherapy from fresh or frozen/thawed samples
Since all newly diagnosed AML patients undergo several rounds of chemotherapy, the biology and the ability to collect and expand DNT cells might be affected by repeated exposure to chemotherapy. In order to determine the optimal time for DNT cell expansion and function during chemotherapy, blood samples were drawn from some patients either at one month after induction chemotherapy (sample a) or one month after repeated courses of consolidation therapies (sample b). There was no significant difference in either the total number of expanded cells (Fig. 2A) or total number of DNT cells (Fig. 2B) at the different time points examined, indicating that DNT cells can be expanded from the majority of AML patients one month after completing induction or consolidation chemotherapy.
Figure 2. DNT cells can be expanded from AML patients during chemotherapy and from fresh or frozen and thawed samples.
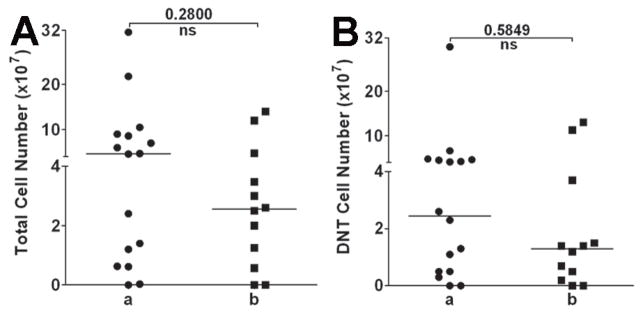
(A–B) 20 ml whole blood was collected one month after induction chemotherapy and before 1st consolidation therapy (sample a) and one month after 1–3 consolidation therapies (sample b). After 2 weeks of culture, cells were harvested and total number of cells in each culture was counted. DNT cell frequency was determined by staining the expanded cells with the antibodies as described in Fig. 1. Total number of expanded cells (A) as well as total number of DNT cells (B) obtained from blood samples that were collected at different treatment stages from each patient is shown. The medians for A and B are also shown. DNT cells were collected from AML patients. Half of the DNT cells were expanded without freezing, and the other half from the same patient were frozen in −80°C for 24 hours then thawed and expanded. The total numbers of DNT cells obtained from fresh versus frozen/thawed samples after 2 weeks of culture from 4 different patients (pt.) are shown in (C). Cytolysis against tumor targets U937 (D) AML-3 (E) and AML-2 (F) was compared between the DNT cells expanded from fresh versus frozen/thawed samples at 20:1 effector versus target ratio.
Next, to determine whether DNT cells can be expanded after freezing and thawing, blood samples were collected from 4 AML patients and after depleting CD4+ and CD8+ cells, half of the enriched DNT cells were expanded and half were frozen viably. The frozen DNT cells were thawed one day later and expanded. When the total cell number and function of expanded DNT cells from fresh or frozen/thawed samples was compared, no statistically significant difference in total cell number or cytolysis of leukemic cells was observed (Fig. 2 C–F). Collectively, these findings indicate that multiple blood samples may be collected for expanding DNT cells during chemotherapy and frozen to be used at later time points when treating patients.
Ex vivo expanded AML patient DNT cells express high levels of IFN-γ, TNF-α, perforin and Granzyme B, and are able to kill leukemic cell lines in vitro through perforin-dependant pathway
IFN-γ expression and in vitro tumor cell cytolysis have been shown to be correlated with the efficacy of adoptively transferred T cells (21). To evaluate the potential anti-tumor activity of ex vivo expanded DNT cells from AML patients, we first measured the expression level of IFN-γ and TNF-α, two well-known cytokines that can contribute to tumor immunity. We found that the majority of expanded DNT cells expressed high levels of intracellular IFN-γ and TNF-α, which were similar to or higher than that seen with CD8+ T cells that were isolated, activated and expanded from the same patient using the same protocol as DNT cells (Fig. 3A). Next, we assessed whether the ex vivo expanded DNT cells can kill allogeneic leukemic cell lines. Ex vivo expanded DNT cells from AML patients were used as effectors in standard 51Cr-release assays against the human monocytic leukemic cell line AML-3 or U937. DNT cell cultures containing <70% DNT cells were depleted of contaminating CD4+, CD8+ and CD56+ cells before cytotoxicity assays were performed. We consistently observed a dose-dependent killing of these leukemic cell lines (Fig. 3B). Furthermore, we found that DNT cell-mediated cytotoxicity of leukemic cells could be effectively blocked by pre-incubation of DNT cells with CMA, which blocks activation of the perforin molecule (22) (Fig. 3C), indicating that perforin plays an important role in DNT cell-mediated leukemia cytolysis in vitro. Although our expansion protocol resulted in very low frequency of CD3+CD56+ cells (on average 5%, range 2–9%), cultures sometimes contained significant numbers of CD4+ or CD8+ T cells. To evaluate the relative contribution of DNT cells versus CD4+ and CD8+ T cells to cytolysis of leukemic cells, we purified DNT, CD4+ and CD8+ T cells that were expanded from the same donor and used them as effectors. We found that whereas each expanded subpopulation displayed an anti-leukemic activity, DNT cells showed higher cytolysis of allogeneic leukemic cells than either CD4+ and/or CD8+ T cell subsets. However, when non-expanded DNT cells or CD4+ or CD8+ T cells from the same donor were used as effectors, no significant cytotoxicity was observed (Supplementary Fig. 1). Together, these data demonstrate that the ex vivo expanded DNT cells are cytolytic to allogeneic leukemic cell lines and perforin is critical for DNT cell-mediated cytotoxicity.
Figure 3. Perforin is important for DNT cell-mediated cytolysis against leukemia cells.
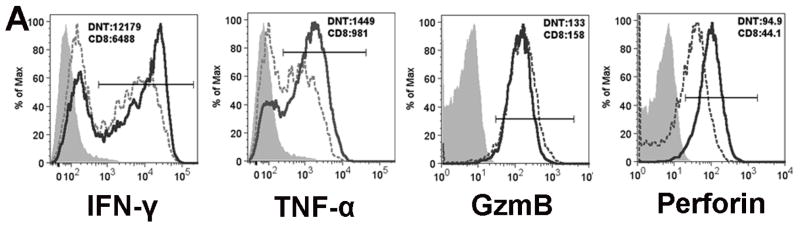

(A) Ex vivo expanded DNT cells express high levels of IFN-γ, TNF-α, Granzyme B and perforin. Ex vivo expanded cells were stained with CD3, CD4, CD8 and CD56 cell surface markers followed by intracellular staining of either Granzyme B or perforin. For cytokine detection, expanded cells were stimulated with PMA/Ionomycin for 3 hours then stained with CD3, CD4, CD8 and CD56 followed by intracellular staining of IFN-γ or TNF-α. Cells were gated on either CD3+CD4−CD8−CD56− (DNT) cells (solid lines) or CD8+ T cells (dotted lines). The expression of IFN-γ, TNF-α, Granzyme B and perforin was compared. Shaded areas are fluorescence minus one (FMO) as controls. The numbers shown are MFI (median florescence intensity) of gated DNT or CD8+ T cells. The data shown represent the results obtained from 3 independent experiments using expanded T cells from 3 different patients. (B) Cytotoxicity against human leukemic cell lines by ex vivo expanded DNT cells from AML patients. Ex vivo expanded DNT cells from 14 different AML patients in remission were used as effectors either directly after expansion if the DNT cells comprised more than 70% of total expanded population or further purified by depleting CD4+, CD8+ and CD56+ cells prior to their use. Human leukemia cell lines AML-3 and U937 were labeled with 51Cr and used as targets at the indicated ratios. Specific killing of tumor cells was determined by standard 51Cr-release assays. Mean specific killing of the tumor targets by DNT cells expanded from 14 different patients is shown. (C) Blocking of DNT cell-mediated killing of tumor cells by CMA. After depletion of CD4+, CD8+ and CD56+ cells, the ex vivo expanded DNT cells were either used directly as effector cells or preincubated with 100nM of Concanamycin A (CMA) for 30 minutes and washed prior to co-incubation with AML-3 or U937 tumor targets at 20:1 or 10:1. Specific killing of the tumor targets by CMA-treated and non-treated DNT cells is shown. This experiment was repeated 3 times using DNT cells expanded from different patients and similar results were obtained.
AML patient DNT cells are cytotoxic to autologous CD34+ leukemic cells
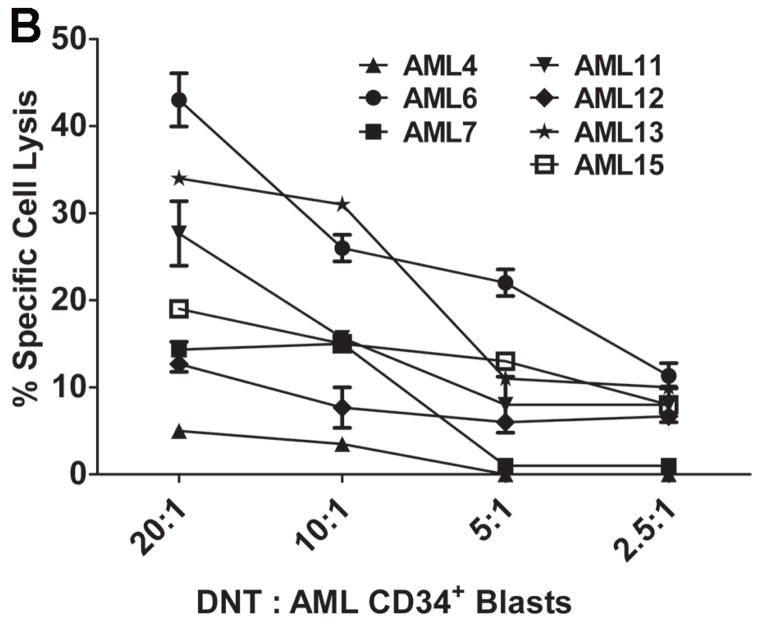
To further determine whether ex vivo expanded DNT cells from AML patients could kill autologous primary leukemic cells, leukemic blasts were collected from AML patients at diagnosis. Blood samples were collected after chemotherapy-induced CR and DNT cells were expanded ex vivo. For those patients whose DNT cells could be expanded, CD34+ cells from their leukemic blasts were purified (Fig. 4A) and used as targets. To exclude an effect of non-DNT populations on cytotoxicity, we first purified DNT cells to greater than 95% by depleting CD4+, CD8+ and CD56+ cells prior to their use as effectors. As shown in Fig. 4B, six out of 7 AML patients that were studied showed varying degrees of cytotoxicity against primary autologous CD34+ leukemic cells in vitro. These data demonstrated that ex vivo expanded DNT cells from AML patients can kill autologous CD34+ leukemic blasts.
Figure 4. Ex vivo expanded DNT cells from AML patients can kill autologous CD34+ leukemia blasts.

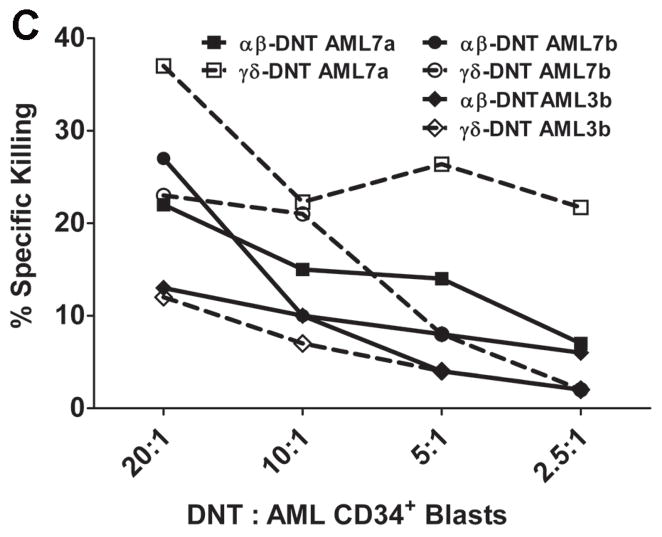
DNT cells were expanded ex vivo from 7 AML patients in remission, CD4+ and CD8+ and CD56+ cells were depleted and the remaining cells (>90% DNT) were used as effectors. Leukemic blasts were collected from PBMC of the same AML patients at the time of diagnosis. CD34+ leukemic blast cells were purified by staining the cells with CD34-FITC mAb followed by anti-FITC-coated magnetic beads, labeled with 51Cr and used as targets at the indicated effector versus target ratios. (A) Percentage of CD34+ cells in the leukemic blasts before and after purification. (B) Specific killing of autologous CD34+ leukemic cells was determined by standard 51Cr-release assay with 3 replicates for each patient. (C). DNT cells were expanded from 3 AML patients. After 14 days of culture, both αβ-TCR+CD4−CD8−CD56− DNT cells (αβ-DNT) and γδ-TCR+CD4−CD8− CD356− DNT cells (γδ-DNT) were purified by FACS and used separately as effectors. Freshly isolated CD34+ leukemic blasts from 3 different AML patients were labeled with 51Cr and used as targets. Specific killing of CD34+ cells was determined by standard cytotoxicity assay. Solid lines and dash lines show αβ-DNT and γδ-DNT cell mediated cytolysis of primary CD34+ targets, respectively.
Since expanded patient DNT cells contain both αβ- and γδ-TCR+ DNT cells, we next analyzed the cytolytic activity of αβ-TCR+ versus γδ-TCR+ DNT cells. Ex vivo expanded αβ-TCR+CD4−CD8−CD56− cells and γδ-TCR+CD4−CD8−CD56− cells were purified by FACS from 3 AML patients and used as effectors to kill CD34+ primary leukemia blasts purified from 3 different AML patients. As shown in Fig 4C, both αβ-TCR+ and γδ-TCR+ DNT cells showed a dose-dependent cytotoxicity against primary CD34+ leukemic cells. In 2 of 3 cases, cytotoxicity against AML cells by αβ-DNT cells compared to γδ-DNT cells was similar whereas in one patient γδ-TCR+ DNT cells had slightly higher lysis of AML blasts compared to αβ-DNT cells. When purified αβ-DNT and γδ-DNT cell-mediated cytolysis of leukemic blasts was compared to CD3+CD4−CD8−CD56− DNT cells, no synergistic effect between αβ-TCR+ and γδ-TCR+ DNT cells was observed (data not shown). These data demonstrate that ex vivo expanded DNT cells from AML patients can kill primary CD34+ leukemic blasts in vitro and that both DNT cell subsets contribute to this anti-tumor effect.
Discussion
In this study, we explored the use of ex vivo expanded DNT cells as a novel form of adoptive T cell therapy. We first developed a novel DNT cell expansion protocol which includes enrichment of DNT cells by depleting CD4+ and CD8+ T cells from the starting population, TCR-crosslinking of enriched DNT cells and timed addition of anti-CD3 mAb and cytokines. Using this protocol, we were able to obtain from 24 out of 28 cultures an average of 64×106 cells in two weeks from only 20 mls of blood collected from AML patients in CR at one month after chemotherapy, with the majority being DNT cells. Furthermore, we demonstrated that the majority of ex vivo expanded DNT cells from AML patients expressed high levels of IFN-γ and TNF-α, as well as perforin and Granzyme B (Fig. 3A), cytokines and molecules that are well-known to have direct and indirect anti-tumor activities. Other studies have shown a significant correlation between the ability of expanded T cells to lyse tumor cells in vitro and clinical outcome (23, 24). When ex vivo expanded AML patient DNT cells were used as effectors in cytotoxicity assays, we consistently observed a dose-dependent killing of leukemic cells in vitro. Moreover, blocking perforin activation in DNT cells abrogated 80–97% of their ability to kill leukemic cells, suggesting that perforin plays a major role in DNT cell-mediated cytolysis of tumor cells. Importantly, we used a fully autologous system where both DNT cells and leukemic blasts were derived from the same patient. We demonstrated that purified DNT cells from 6 out of 7 AML patients could kill autologous CD34+ leukemic blasts isolated from PB of the same patient (Fig. 4B). In contrast, when autologous PBMC or activated autologous CD8+ T cells were used as targets, we did not observe significant DNT cell-mediated cytotoxicity (data not shown). Collectively, these data demonstrate, for the first time, that DNT cells can be expanded from AML patients in CR and have potent anti-leukemia effects. These findings suggest the possibility of eliminating residual leukemic cells after chemotherapy by infusion of ex vivo expanded autologous DNT cells.
There are several potential advantages of ultilizing this unique adoptive T cell therapy: First, high numbers of DNT cells (up to 15 million/ml blood) could be generated ex vivo in a relatively short period of time. Most protocols used for ex vivo cell expansion for adoptive cellular therapy require between 3 to more than 6 weeks to obtain a sufficient number of cellular products (10, 25–28). The expansion technique utilized here has the capacity to produce therapeutic doses of DNT cells in only 2 weeks, which significantly reduces both the cost of cellular production and the chance of contamination, and limits the logistical inconvenience associated with delays that are incurred with autologous cellular production. In addition, the majority of our expanded cells are CD45RO+ and CD27+, which suggests a shorter expansion time favors the production of T cells that are at a central memory stage (29). This may be clinically relevant as central memory T-cells appear to be more effective in eliminating cancer cells after adoptive transfer than highly differentiated T cells (24). Second, we were able to expand DNT cells from AML patients in CR within one month of receiving dose intensive induction and/or consolidation chemotherapy. This potentially would permit protocols that adoptively transfer DNT cells during or soon after chemotherapy when the tumor cell burden is very low, thus increasing the chance of erradicating MRD. Third, it has been shown that preconditioning patients prior to adoptive transfer of T lymphocytes with lymphodepletion can enhance their efficacy by eliminating regulatory T cells and endogenous lymphocytes that compete with the transferred cells for homeostatic cytokines, thus facilitating homeostatic expansion of transferred T cells (21, 30–34). Recent studies suggest that the greater the depletion of the patient’s immune cells, the more effective the treatment of adoptive T-cell transfer against metastatic melanoma (26, 31). All AML patients receive chemotherapies after diagnosis of the disease. The ability to expand and administer DNT cells during or shortly after standard chemotherapy may facilitate in vivo expansion of DNT cells after adoptive transfer without the need for administering additional preconditioning therapy. Lastly, we showed that DNT cells could be expanded from both fresh or frozen/thawed samples with no significant difference in their expansion rate or anti-tumor ability. It is thus possible to collect multiple blood samples from AML patients while in CR to freeze DNT cells for subsequent expansion so that multiple doses of DNT cells could be administered to enhance the treatment effect. To be able to expand DNT cells from a frozen sample also provides the flexibility of transporting cells to centers and expanding them under GMP conditions which may not be available locally.
Using the culture conditions developed in this study, we could expand DNT cells from the majority (13/16, 81%) of the patients. However, as was seen with ex vivo expanded CIK cells from leukemia and lymphoma patients (10, 35), there was a large range in the DNT cell expansion rate among AML patients. Due to the heterogeneity of AML and relatively small number of patients that were studied, we were unable to elicit the factors that determine the expansion rate of DNT cells, although patient age and gender do not appear to impact DNT cell expansion. Interestingly, the highest expansion (300 million DNT cells from 20 ml blood) was obtained from a 65 year-old patient (Table 1). The oldest patient of this cohort study was a 69 year-old and the 3 samples collected at different time points after induction or consolidation chemotherapy from this patient yielded an average of 88.7×106 DNT cells from 20 ml blood (Table 1). These data suggest the possibility of applying adoptive DNT cell therapy to elderly AML patients during or after chemotherapy to help eliminate MRD. This finding is significant because elderly AML patients have the highest relapse rate and less than 5% survive more than 5 years after diagnosis with conventional chemotherapy and only a minority are candidates for HCT (36, 37). No correlation was observed between white blood cell counts at the time of diagnosis and DNT cell expansion rate. We also compared the total number of expanded DNT cells between patients with second CR with those in first CR. Although the average expanded cell numbers were higher in non-relapsed patients, the difference was not statistically significant (Table 1). Future studies in a larger cohort of AML patients could allow us to define factors such as disease type, cytogenetics and prior therapies that predict the ability to successfully expand DNT cells ex vivo.
Many studies have shown that ex vivo expanded autologous CD8+ T cells, CD4+ T cells, CD3+CD56+ cells, γδ-T cells and NK cells can all contribute to anti-tumor activity either alone or in concert (10, 17, 21, 38–40). As seen in most reports, our expanded cells are a heterogeneous population with the majority being DNT cells (Supplementary Table 1). It is possible that other expanded cell subsets could also contribute to the cytotoxicity against leukemic cells. Nevertheless, the following findings indicate that expanded DNT cells have potent anti-leukemia effect: When purified DNT cells were compared with CD4+ or CD8+ T cells expanded from the same donor in the same assay, DNT cells showed higher cytotoxicity towards allogeneic leukemic cells than CD4+ and CD8+ T cells (Supplementary Fig. 1). More importantly, when CD4+, CD8+ and CD56+ cells were depleted from expanded cells so that the effector cells comprised on average 95% DNT cells (Supplementary Fig. 2), we observed dose-dependent killing of primary CD34+ leukemic blasts (Fig. 4B and 4C). Together, these data clearly demonstrate that the ex vivo expanded DNT cells from AML patients possess potent anti-leukemia effects in vitro. Future studies should examine the fate and function of each subset of expanded cells in vivo to ensure that they do not exert significant toxicity to cancer patients before using them as autologous cell therapy for cancer.
In summary, we have developed a novel protocol by which DNT cells from AML patients in CR shortly following dose intensive chemotherapy can be purified from PB and expanded ex vivo to doses that would be potentially useful clinically within 2 weeks. We demonstrate, for the first time, that ex vivo expanded DNT cells from patients with AML can effectively kill leukemia cell lines and primary autologous CD34+ leukemic blasts. Our data highlight the potential of using DNT cells as a novel adjuvant immunotherapy to improve the survival of AML patients by eliminating MRD. Our findings also open a new window for adoptive T cell therapy of other human cancers.
Supplementary Material
Acknowledgments
This study was funded by the Ontario Institute for Cancer Research through funding provided by the Government of Ontario (LZ), and the Canadian Cancer Society (grant #020516). LZ is a Maria H. Bacardi Chair in Transplantation. The authors would like to thank Dr. Andre Schuh for his insightful comments.
Abbreviations
- AML
acute myeloid leukemia
- BM
bone marrow
- CIK
cytokine activated killer
- CR
complete remission
- DNT cells
double negative T cells
- GVHD
graft-versus-host-disease
- HCT
hematopoietic cell transplantation
- mAb
monoclonal antibody
- MRD
minimal residual disease
- NK
natural killer
- PB
peripheral blood
- TCR
T cell receptor
- TIL
tumor infiltrating lymphocytes
Footnotes
Conflict-of-interest
The authors declare no competing financial interests.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu).
References
- 1.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010 Jan 21;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 2.Litzow MR, Othus M, Cripe LD, Gore SD, Lazarus HM, Lee SJ, et al. Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: a report from the Eastern Cooperative Oncology Group. British journal of haematology. 2010 Jan;148(2):217–225. doi: 10.1111/j.1365-2141.2009.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litzow MR, Tarima S, Perez WS, Bolwell BJ, Cairo MS, Camitta BM, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010 Mar 4;115(9):1850–1857. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demirer T, Barkholt L, Blaise D, Pedrazzoli P, Aglietta M, Carella AM, et al. Transplantation of allogeneic hematopoietic stem cells: an emerging treatment modality for solid tumors. Nature clinical practice. 2008 May;5(5):256–267. doi: 10.1038/ncponc1104. [DOI] [PubMed] [Google Scholar]
- 5.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009 Jun 10;301(22):2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995 Sep 1;86(5):2041–2050. [PubMed] [Google Scholar]
- 7.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997 Feb;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 8.Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002 Jan 15;20(2):405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. The Journal of experimental medicine. 1991 Jul 1;174(1):139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linn YC, Lau LC, Hui KM. Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. British journal of haematology. 2002 Jan;116(1):78–86. doi: 10.1046/j.1365-2141.2002.03247.x. [DOI] [PubMed] [Google Scholar]
- 11.Alvarnas JC, Linn YC, Hope EG, Negrin RS. Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2001;7(4):216–222. doi: 10.1053/bbmt.2001.v7.pm11349808. [DOI] [PubMed] [Google Scholar]
- 12.Linn YC, Lau SK, Liu BH, Ng LH, Yong HX, Hui KM. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology. 2009 Mar;126(3):423–435. doi: 10.1111/j.1365-2567.2008.02910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998 Nov 1;92(9):3318–3327. [PubMed] [Google Scholar]
- 14.Young KJ, Kay LS, Phillips MJ, Zhang L. Antitumor activity mediated by double-negative T cells. Cancer research. 2003 Nov 15;63(22):8014–8021. [PubMed] [Google Scholar]
- 15.Young KJ, DuTemple B, Zhang Z, Levy G, Zhang L. CD4(−)CD8(−) regulatory T cells implicated in preventing graft-versus-host and promoting graft-versus-leukemia responses. Transplantation proceedings. 2001 Feb-Mar;33(1–2):1762–1763. doi: 10.1016/s0041-1345(00)02670-1. [DOI] [PubMed] [Google Scholar]
- 16.Voelkl S, Moore TV, Rehli M, Nishimura MI, Mackensen A, Fischer K. Characterization of MHC class-I restricted TCRalphabeta+ CD4− CD8− double negative T cells recognizing the gp100 antigen from a melanoma patient after gp100 vaccination. Cancer Immunol Immunother. 2009 May;58(5):709–718. doi: 10.1007/s00262-008-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer research. 2007 Jan 1;67(1):5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 18.Trus MR, Yang L, Suarez Saiz F, Bordeleau L, Jurisica I, Minden MD. The histone deacetylase inhibitor valproic acid alters sensitivity towards all trans retinoic acid in acute myeloblastic leukemia cells. Leukemia. 2005 Jul;19(7):1161–1168. doi: 10.1038/sj.leu.2403773. [DOI] [PubMed] [Google Scholar]
- 19.Rayappa C, McCulloch EA. A cell culture model for the treatment of acute myeloblastic leukemia with fludarabine and cytosine arabinoside. Leukemia. 1993 Jul;7(7):992–999. [PubMed] [Google Scholar]
- 20.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nature medicine. 2000 Jul;6(7):782–789. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature reviews. 2008 Apr;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996 May 15;156(10):3678–3686. [PubMed] [Google Scholar]
- 23.Aebersold P, Hyatt C, Johnson S, Hines K, Korcak L, Sanders M, et al. Lysis of autologous melanoma cells by tumor-infiltrating lymphocytes: association with clinical response. Journal of the National Cancer Institute. 1991 Jul 3;83(13):932–937. doi: 10.1093/jnci/83.13.932. [DOI] [PubMed] [Google Scholar]
- 24.Schwartzentruber DJ, Hom SS, Dadmarz R, White DE, Yannelli JR, Steinberg SM, et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994 Jul;12(7):1475–1483. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 25.June CH. Adoptive T cell therapy for cancer in the clinic. The Journal of clinical investigation. 2007 Jun;117(6):1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008 Nov 10;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg M, Lundqvist A, McCoy P, Jr, Samsel L, Fan Y, Tawab A, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11(3):341–355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. The New England journal of medicine. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 29.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. The Journal of clinical investigation. 2005 Jun;115(6):1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nature clinical practice. 2006 Dec;3(12):668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Current opinion in immunology. 2005 Apr;17(2):195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005 Mar 1;174(5):2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. The Journal of experimental medicine. 2005 Oct 3;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. The Journal of clinical investigation. 2002 Jul;110(2):185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005 Mar;11(3):181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007 Feb 15;109(4):1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 37.Gupta V, Chun K, Yi QL, Minden M, Schuh A, Wells R, et al. Disease biology rather than age is the most important determinant of survival of patients > or = 60 years with acute myeloid leukemia treated with uniform intensive therapy. Cancer. 2005 May 15;103(10):2082–2090. doi: 10.1002/cncr.21006. [DOI] [PubMed] [Google Scholar]
- 38.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. The New England journal of medicine. 2008 Jun 19;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009 Jun 11;113(24):6120–6127. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamb LS, Jr, Lopez RD. gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant. 2005 Mar;11(3):161–168. doi: 10.1016/j.bbmt.2004.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.