Abstract
Personality dimensions capturing individual differences in behavior, cognition, and affect have been described in several species, including humans, chimpanzees, and orangutans. However, comparisons between species are limited by the use of different questionnaires. We asked raters to assess free-ranging rhesus macaques at two time points on personality and subjective well-being questionnaires used earlier to rate chimpanzees and orangutans. Principal-components analysis yielded domains we labeled Confidence, Friendliness, Dominance, Anxiety, Openness, and Activity. The presence of Openness in rhesus macaques suggests it is an ancestral characteristic. The absence of Conscientiousness suggests it is a derived characteristic in African apes. Higher Confidence and Friendliness, and lower Anxiety were prospectively related to subjective well-being, indicating that the connection between personality and subjective well-being in humans, chimpanzees, and orangutans is ancestral in catarrhine primates. As demonstrated here, each additional species studied adds another fold to the rich, historical story of primate personality evolution.
Nonhuman primate personality traits capture impressions that are reliable in that they are consistent across raters and time (King & Figueredo, 1997; King, Weiss, & Sisco, 2008; Stevenson-Hinde, Stillwell-Barnes, & Zunz, 1980; Uher, Asendorpf, & Call, 2008; Weiss, King, & Perkins, 2006). Nonhuman primate personality traits are also valid in that they are related to other measures, including observed behavior (Capitanio, 1999; Konečná et al., 2008; Pederson, King, & Landau, 2005; Stevenson-Hinde, et al., 1980; Uher & Asendorpf, 2008).
Like the study of physical characteristics in different species, the comparative method can address questions concerning the evolution of stable personality traits (Gosling & Graybeal, 2007; Harvey & Pagel, 1991). King and Figueredo (1997) examined personality phylogeny by obtaining ratings of zoo chimpanzees on a questionnaire based on measures of the five major dimensions along which humans differ --- Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness (Digman, 1990). They found a broad chimpanzee-specific Dominance domain, and five additional domains that, while differing slightly on the trait level, were analogous to human personality domains (King & Figueredo, 1997). Thus, precursors of human personality were likely present in the common chimpanzee-human ancestor and early hominids, though dominance was no longer a key domain upon which members of the latter could be distinguished. A study of orangutan personality using an expanded version of the same questionnaire as that used to rate chimpanzees found variants of human and chimpanzee Neuroticism, Extraversion, and Agreeableness domains as well as a Dominance domain, suggesting that these domains may have existed in the common ancestors of great apes and humans (Weiss, et al., 2006). In addition, this same study found that, instead of making up separate domains, traits defining Openness and Conscientiousness defined a single domain in orangutans named Intellect, suggesting that selection may have favored separate Openness and Conscientiousness domains in species such as humans and chimpanzees, which have more complex social structures.
Although personality differences have been comprehensively described in great apes and humans, the historical patterns of personality evolution cannot be deduced from examining this taxon alone. Studying personality in Old World monkeys, which split off from hominoids between 25-30 mya (Andrews, 1986) could offer insights into the evolutionary origins of personality and selective factors which contribute to phylogenetic divergences in personality. Their distant relatedness to humans relative to hominoids makes Old World monkeys an important study species for understanding human evolution.
Rhesus macaques have been the most widely used nonhuman primate in behavioral research. Unlike hominoids, and what is often assumed to be the ancestral social structure for early hominids (Foley & Lee, 1989; Wrangham, 1987), rhesus macaque social structure is largely based on male dispersal (Colvin, 1986; Manson, 1995; Melnick & Pearl, 1987) with female philopatry (Gouzoules & Gouzoules, 1987). Like other macaque species, rhesus macaques live in multi-male, multi-female groups, which are characterized as matrilineal societies, with a matriline defined as all descendants of a founder female (Melnick & Pearl, 1987).
Early personality research on captive rhesus macaques using a questionnaire containing 33 behaviorally-defined adjectives identified three reliable and stable domains: Confident, which describes differences in boldness and submissiveness; Excitable, representing differences in curiosity, activity, and reactions to change; and Sociable, which captures differences in how much time individuals spends with others (Stevenson-Hinde, et al., 1980; Stevenson-Hinde & Zunz, 1978). Later studies using this same questionnaire identified an additional rhesus macaque personality domain labeled Equable (Bolig, Price, O'Neill, & Suomi, 1992; Capitanio, 1999), comprised of individual differences in reactions to conspecifics, the appropriateness of behaviors displayed towards conspecifics, and being slow, deliberate, and not hurried (Capitanio, 1999).
These four rhesus macaque personality domains roughly approximate personality domains of other species. Confident is described by items similar to those which describe chimpanzee Dominance (Dutton, 2008; King & Figueredo, 1997) and orangutan Neuroticism (Weiss, et al., 2006). Traits similar to those making up the Excitable, Sociable, and Equable domains are found in the Neuroticism, Extraversion, and Agreeableness domains, respectively, of humans (Costa & McCrae, 1992), chimpanzees (Dutton, 2008; King & Figueredo, 1997), and orangutans (Weiss, et al., 2006). In addition to predicting behavior (Capitanio, 1999), these domains predicted immunocompetence (Capitanio, Mendoza, & Baroncelli, 1999; Maninger, Capitanio, Mendoza, & Mason, 2003) and corticosteroid response (Capitanio, Mendoza, & Bentson, 2004).
Subjective well-being or “happiness” is a construct closely related to personality and describes individual differences in positive affect (Diener, 2000). The study of subjective well-being was, in part, a reaction to the near exclusive focus of much psychological research on the study of psychopathology (Seligman & Csikszentmihalyi, 2000). Like human personality domains (McCrae & Costa, 2003), subjective well-being is mostly stable throughout life (Diener, 2000). Moreover, humans who are higher in subjective well-being tend to be lower in Neuroticism and higher in Extraversion (Steel, Schmidt, & Shultz, 2008). This personality-well-being nexus is partially genetic, as the genetic variance in subjective well-being can be accounted for by genes also influencing personality (Weiss, Bates, & Luciano 2008).
Research on rater assessed chimpanzee subjective well-being also found high interrater reliability, stability over time, and correlations with personality like those described in human research (King & Landau, 2003; Weiss, et al., 2009). A study of well-being in orangutans using the same questionnaire also found correlations like those described in humans and chimpanzees (Weiss, et al., 2006). Finally, as in humans, chimpanzee subjective well-being is genetically correlated with personality (Weiss, King, & Enns 2002).
The above findings regarding human, chimpanzee, orangutan, and rhesus macaque personality suggest conservation of some basic personality dimensions over evolutionary time. We therefore expect to find rhesus macaque variants of traits similar to the Sociable, Excitable, Equable, and Confident domains identified in prior studies (Bolig et al., 1992; Capitanio, 1999; Stevenson-Hinde, et al., 1980; Stevenson-Hinde & Zunz, 1978). However, these previous studies of rhesus macaque personality did not identify distinct Conscientiousness or Openness domains. There are three possible explanations for the failure to find such domains in rhesus macaques. The first possibility is that the earlier assessment instrument was developed prior to the adoption of what has come to be known as the human Five-Factor Model (Digman, 1990) and may therefore not have been comprehensive enough to capture all rhesus macaque personality domains (Uher, 2008). The second possibility is that prior studies were based on captive samples which may not have been able to fully express their behavioral repertoires (Uher, 2008). The third possibility is that rhesus macaques cannot be differentiated on these domains and that the distinct clustering of individual differences in exploratory behavior and predictability are a more recent adaptation.
As such, our primary goal was to investigate which personality domains are present in rhesus macaques, and, in particular, whether distinct Conscientiousness or Openness domains are present. To rule out the possibility that limitations of previous rhesus macaque personality studies led to the absence of separable Conscientiousness or Openness domains we used a broad instrument which identified these domains in chimpanzees. To exclude the possibility that the limited behavioral repertoires of small samples of captive rhesus macaques did not permit the detection of Conscientiousness and Openness, we conducted this study with free-ranging individuals. These results could therefore address the third possibility, that these personality differences are the result of adaptation, and thus yield valuable insights into the evolutionary origins of personality. For example, if we find distinct Conscientiousness or Openness domains, this would suggest that they were present in the common ancestor of Old World monkeys and hominoids and that the orangutan Intellect domain is derived. On the other hand, if we fail to find distinct Conscientiousness or Openness domains this would suggest that these domains arose in the common ancestor of chimpanzees and humans before they speciated some 5-6 mya (Chimpanzee Sequencing and Analysis Consortium, 2005).
The subjective well-being findings in humans and great apes strongly suggest that the relationship between personality traits such as Extraversion and Neuroticism and higher and lower subjective well-being, respectively is ancestral. This possibility is strongly supported by the fact that these relationships are genetically mediated in humans (Weiss et al., 2008) and chimpanzees (Weiss et al., 2002). Thus, we asked several raters to assess the same set of rhesus macaques on the subjective well-being measure used in previous studies of chimpanzees (King & Landau, 2003; Weiss, et al., 2009) and orangutans (Weiss, et al., 2006). If personality and subjective well-being are related in the same way as they are in humans, chimpanzees, and orangutans, this would suggest that these relationships existed in the common ancestor of cercopithecoids and hominoids and were conserved.
One defining characteristic of human personality traits (Roberts & DelVecchio, 2000) and subjective well-being are that they are mostly stable over time (Eid & Diener, 2004). Thus, for the present study, personality and subjective well-being ratings were collected in at two waves separated in time by over one year. These data will therefore enable us to determine how stable these constructs are in rhesus macaques and whether the relationship between these constructs is also stable. Thus, they will offer further insight into how rhesus macaque personality and subjective well-being compare to like constructs in chimpanzees, orangutans, and humans.
Methods
Subjects and Raters
Subjects were 125 rhesus macaques (Macaca mulatta) from the free-ranging population on Cayo Santiago (for more details see Rawlins & Kessler, 1986) who were rated in two waves. The Wave 1 sample consisted of 124 subjects (51 males and 73 females) rated between February 2006 and July 2007. The mean age of Wave 1 subjects was 7.71 years (SD = 6.21). Wave 1 males had a mean age of 7.17 years (SD = 6.48) and Wave 1 females had a mean age of 8.08 years (SD = 6.03). The Wave 2 sample consisted of 71 subjects (26 males and 45 females) rated 13.9 to 18.0 months later between August 2007 and May 2008. Of the Wave 2 subjects, all but one male had been rated in Wave 1. The mean age of Wave 2 subjects was 6.26 years (SD = 5.53). Wave 2 males had a mean age of 3.09 years (SD = 0.09) and Wave 2 females had a mean age of 8.09 years (SD = 6.27).
Subjects were rated by multiple raters who, for 3 to 24 months prior to this study, had been conducting unrelated research, and could reliably identify individual subjects. There were 11 raters in Wave 1. Ratings in Wave 2 were made by three raters from Wave 1 and three new raters.
Instruments
We modified questionnaires used to rate the personality and subjective well-being of captive chimpanzees (King & Figueredo, 1997; King & Landau, 2003; Weiss, et al., 2009) and orangutans (Weiss, et al., 2006) for use in rating free-ranging monkeys. Modification involved changing the words “enclosure” and “chimpanzee” to “environment” and “monkey,” respectively. Raters were instructed to base ratings on overall impressions of the subjects and to not discuss their ratings. Ratings were made on a 7-point Likert scale.
Personality measure
The Hominoid Personality Questionnaire consists of 54 adjectives, each followed by one to three sentences defining the adjective within the context of nonhuman primate behavior. For example, the item fearful is “FEARFUL: Subject reacts excessively to real or imagined threats by displaying behaviors such as screaming, grimacing, running away or other signs of anxiety or distress.” The original version included 43 items and was used to rate chimpanzees (King & Figueredo, 1997). Of these items, 41 were sampled from the factors of Goldberg's (1990) taxonomy of the Big Five and 2 (autistic and clumsy) were created by the King and Figueredo. This questionnaire was later increased by five items for a study of orangutan personality (Weiss et al., 2006). Of these new items, anxious and vulnerable were based on facets of the Revised NEO Personality Inventory Neuroticism domain (Costa & McCrae, 1992), curious and conventional were based on markers of Openness from an adjectival questionnaire (McCrae & Costa, 1985), and cool was created by the researchers to capture low Neuroticism. In a later revision (Weiss et al., 2009) the 48 item version of the questionnaire was expanded by adding 3 Conscientiousness and 3 Openness items. Two Conscientiousness items (thoughtless and quitting) were from an adjectival questionnaire McCrae and Costa (1985) and one (distractible) was devised by the researchers. All three Openness markers (individualistic, innovative, and unperceptive) were created by these researchers.
Subjective well-being measure
This questionnaire includes four items derived from measures of human subjective well-being, which assessed the balance of positive and negative moods, pleasure derived from social interactions, the ability to achieve personal goals, and how “happy” raters think they would be if they were the target individual. Because of a clerical error, the third item of this questionnaire referred to “enclosure”; it was thus left out of further analyses.
At Wave 1 one subject was missing one item (curious) while 13 subjects were missing 18 to 39 items. Data were not missing at random but came from raters who did not provide ratings for any subject on a subset of items. Twenty-three subjects were not assessed by one of their raters on a single subjective well-being item, although every subject had a rating from at least one rater on each item.
Statistical Analyses
Interrater reliability analyses of items
Interrater reliabilities of personality and subjective well-being ratings were calculated from scores at Wave 1 for the 91 macaques judged by two or more raters. We measured interrater reliabilities of personality and subjective well-being ratings using two intraclass correlations (Shrout & Fleiss, 1979): ICC(3,1) and ICC(3,k).
Principal-components analysis
To determine the factors underlying ratings, we conducted principal-components analyses1 using the principal procedure (Revelle, 2009) from the R statistics package (R Development Core Team, 2008) and determined the number of components by examining the scree plot and by using R's paran function (Dinno, 2008) to conduct a parallel analysis (Horn, 1965). We then used a varimax rotation to obtain orthogonal components and a promax procedure to obtain oblique components. The latter rotation was conducted because it enabled us to estimate correlations among components and to determine whether allowing components to correlate altered the structure.
The principal-components analysis of personality ratings was based on the mean scores across raters for the 52 personality items at Wave 1 which displayed some consistency across raters. For this analysis we dropped 13 subjects that were missing scores on 9 or more items. The principal-components analysis of subjective well-being items was based on the mean scores across raters for the three subjective well-being items.
We created personality domain scores using unit-weighting. This involved assigning items with salient loadings (defined as ≥ .40) on a component weights of +1 or −1 depending on whether the loading was positive or negative, respectively, and assigning a weight of 0 to items which did not have salient loadings. If an item had salient loadings on more than one component, it was assigned to the component on which it had the highest loading. The sum of the weighted item scores defined the domain score. The same procedure was used to create domain scores for any subjective well-being components.
It is important to note that creating scores via unit-weighting does not preserve the independence of varimax-rotated components. However, we chose to use unit-weighting because results derived from these scores are more generalizable than those derived from differentially-weighted scores (Gorsuch, 1983; p. 267). This advantage stems from the fact that, unless sample sizes are very large, the exact component loadings used to create differentially-weighted scores will reflect some degree of capitalization on chance (Gorsuch, 1983; p. 266).
To interpret rhesus macaque personality factors, we examined the items that had salient loadings on those factors in light of the content of those items, the relationship between those items and the five human dimensions, and the rhesus macaque personality literature (Bolig et al., 1992; Capitanio, 1999; Stevenson-Hinde, et al., 1980; Stevenson-Hinde & Zunz, 1978). To help interpret factors we also created unit-weighted domain scores for the rhesus macaques rated in Wave 1 using the definitions of the six chimpanzee (King & Figueredo, 1997) and five orangutan (Weiss et al., 2006) personality factors and examined the correlations between domain scores based on rhesus macaque personality structure and those based on the chimpanzee and orangutan personality structures.
Reliabilities of personality domains and subjective well-being
We measured interrater reliabilities of personality domain and subjective well-being scores using two intraclass correlation coefficients described by Shrout and Fleiss (1979): ICC(3,1) and ICC(3,k). Personality domain scores in these analyses were based on unit-weighted scores with mean substitution from individual judgments on the 81 subjects at Wave 1 and 49 subjects at Wave 2 that were assessed by multiple raters who did not miss more than 8 items. Subjective well-being scores for these analyses were generated by summing the three subjective well-being items from individual judgments of each animal. Internal consistency reliabilities for personality domains and subjective well-being were calculated via Cronbach's alpha on mean ratings of 111 macaques at Wave 1 and 71 at Wave 2. Retest reliabilities were measured using Pearson correlations between the Wave 1 and Wave 2 mean unit-weighted personality domain and subjective well-being scores for the 70 subjects assessed in both waves.
Personality and subjective well-being correlations
To examine the cross-sectional relationship between personality domains and subjective well-being we computed correlations between personality and subjective well-being correlations for the 111 subjects assessed in Wave 1 and the 71 subjects assessed in Wave 2 with fewer than 9 missing items. For the 70 subjects assessed at both time points we examined correlations between Wave 1 personality domains and Wave 2 subjective well-being as well as correlations between Wave 1 subjective well-being and Wave 2 personality domains.
Results
Interrater Reliabilities of Items
Personality
The reliability of single ratings (ICC[3,1]) ranged from -0.05 for autistic to 0.63 for dominant and had a mean of 0.26. The reliability of mean ratings (ICC[3,k]) ranged from -0.17 for autistic to 0.86 for dominant with a mean of 0.52. We excluded the items autistic and unperceptive from further analysis as unreliable because their interrater reliabilites were less than zero.
Subjective well-being
All three subjective well-being items were reliable. The ICC(3,1) for the items assessing balance of positive and negative moods, pleasure derived from social interactions, and how “happy” raters think they would be if they were the monkey were 0.41, 0.44, and 0.43, respectively. The ICC(3, k) values for the same three items were .72, .73, and .72, respectively.
Personality Structure
Principal-components analysis of the 52 reliable items indicated ten components with eigenvalues exceeding 1.00. Examination of the scree plot suggested there were six components and parallel analysis indicated that only the first six eigenvalues (10.47, 7.21, 7.05, 4.11, 2.74, and 2.24) were greater than expected by chance at the 95% confidence interval. We therefore extracted six components and subjected these components to a varimax rotation (see Table 1).
Table 1.
Comparison of Rhesus Macaque Personality Domains to those of Humans, Chimpanzees, and Orangutans
| Rhesus macaque componentsa | Classification in other Speciesc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Cnf | Opn | Dom | Frd | Actb | Anxb | Humans | Chimpanzeesh | Orangutansi |
| Fearful | −0.86 | −0.13 | −0.14 | 0.03 | −0.08 | 0.21 | N+d | D- | N+ |
| Submissive | −0.81 | 0.10 | −0.28 | −0.03 | 0.06 | −0.06 | A+d | D- | D- |
| Timid | −0.73 | −0.03 | −0.07 | −0.09 | −0.17 | 0.13 | E-d | D- | N+ |
| Cautious | −0.73 | −0.22 | −0.36 | −0.21 | −0.01 | 0.06 | E-d | D- | N+ |
| Stable | 0.66 | 0.15 | −0.17 | 0.25 | −0.05 | −0.43 | N-d | N- | N- |
| Distractible | −0.56 | 0.01 | 0.45 | 0.05 | 0.20 | 0.16 | C-e | C- | --- |
| Disorganized | −0.55 | −0.14 | 0.41 | 0.02 | 0.04 | 0.05 | C-d | C- | I- |
| Dependent/Follower | −0.54 | 0.10 | −0.26 | 0.32 | −0.37 | 0.24 | N+d | D- | I- |
| Vulnerable | −0.50 | 0.43 | −0.35 | −0.10 | −0.06 | 0.39 | N+f | D- | N+ |
| Inquisitive | 0.17 | 0.85 | −0.06 | 0.14 | 0.14 | 0.09 | O+d | O+ | E+ |
| Thoughtless | −0.04 | 0.81 | −0.02 | −0.03 | −0.22 | 0.12 | C-g | C- | --- |
| Innovative | 0.15 | 0.66 | −0.09 | −0.05 | 0.50 | −0.06 | O+e | O+ | --- |
| Inventive | 0.18 | 0.64 | −0.17 | −0.07 | 0.49 | −0.05 | O+d | O+ | E+ |
| Curious | 0.10 | 0.64 | 0.13 | 0.42 | 0.22 | 0.05 | O+g | O+ | E+ |
| Imitative | −0.15 | 0.58 | −0.31 | 0.02 | −0.20 | 0.37 | O-d | E+ | E+ |
| Impulsive | −0.14 | 0.55 | 0.35 | 0.04 | 0.09 | 0.43 | E+d | C- | N+ |
| Bullying | 0.18 | −0.06 | 0.87 | 0.03 | 0.09 | −0.01 | A-d | D+ | D+ |
| Stingy/Greedy | 0.11 | −0.15 | 0.84 | 0.02 | −0.03 | −0.07 | A-d | D+ | D+ |
| Aggressive | 0.09 | 0.18 | 0.83 | 0.01 | 0.12 | 0.12 | A-d | C- | D+ |
| Irritable | 0.06 | −0.28 | 0.78 | −0.10 | −0.17 | 0.22 | A-d | C- | D+ |
| Manipulative | 0.01 | −0.17 | 0.75 | 0.14 | 0.10 | −0.23 | A-d | D+ | D+ |
| Defiant | −0.05 | 0.38 | 0.69 | 0.06 | 0.19 | −0.09 | A-d | C- | D+ |
| Excitable | −0.35 | 0.09 | 0.67 | 0.21 | 0.12 | 0.31 | N+d | N+ | N+ |
| Reckless | 0.20 | 0.33 | 0.61 | 0.00 | 0.22 | 0.37 | C-d | C- | D+ |
| Gentle | −0.28 | 0.08 | −0.60 | 0.40 | −0.12 | −0.44 | A+d | A+ | D- |
| Dominant | 0.55 | −0.23 | 0.57 | 0.20 | −0.11 | −0.14 | A-d | D+ | D+ |
| Independent | 0.37 | 0.05 | 0.51 | −0.19 | 0.34 | −0.22 | N-d | D+ | I+ |
| Individualistic | 0.19 | −0.10 | 0.41 | −0.09 | 0.32 | −0.07 | O+e | E- | --- |
| Helpful | −0.08 | −0.08 | 0.17 | 0.81 | 0.05 | 0.04 | A+d | A+ | A+ |
| Friendly | 0.00 | 0.24 | −0.30 | 0.73 | 0.12 | −0.26 | A+d | E+ | A+ |
| Affectionate | −0.05 | 0.12 | −0.28 | 0.73 | 0.05 | −0.12 | A+d | E+ | A+ |
| Sociable | 0.30 | 0.35 | 0.02 | 0.70 | 0.13 | −0.04 | E+d | E+ | A+ |
| Sensitive | −0.34 | 0.13 | −0.01 | 0.67 | −0.14 | 0.14 | A+d | A+ | A+ |
| Depressed | −0.48 | 0.11 | −0.13 | −0.64 | −0.31 | −0.04 | E-d | E- | E- |
| Protective | 0.11 | −0.21 | 0.27 | 0.63 | 0.13 | −0.06 | A+d | A+ | A+ |
| Solitary | −0.42 | −0.08 | 0.11 | −0.58 | −0.14 | −0.26 | E-d | E- | E- |
| Sympathetic | −0.06 | 0.45 | −0.42 | 0.55 | −0.01 | 0.08 | A+d | A+ | A+ |
| Intelligent | 0.22 | −0.17 | 0.30 | 0.50 | 0.06 | −0.06 | O+d | D+ | I+ |
| Persistent | 0.40 | 0.04 | 0.36 | 0.50 | −0.14 | 0.00 | C+d | D+ | D+ |
| Decisive | 0.39 | −0.15 | 0.41 | 0.44 | 0.03 | −0.20 | C+d | D+ | I+ |
| Conventional | −0.14 | −0.24 | −0.18 | 0.12 | −0.75 | 0.03 | O-g | A+ | E- |
| Lazy | 0.14 | 0.06 | 0.03 | −0.30 | −0.71 | −0.17 | C-d | E- | E- |
| Predictable | 0.11 | −0.10 | −0.25 | −0.06 | −0.72 | −0.23 | C+d | C+ | N- |
| Active | 0.01 | 0.11 | 0.26 | 0.37 | 0.69 | −0.05 | E+d | E+ | E+ |
| Clumsy | −0.33 | 0.36 | 0.18 | −0.25 | −0.52 | −0.20 | C-e | C- | I+ |
| Playful | −0.08 | 0.42 | −0.01 | 0.42 | 0.48 | 0.11 | E+d | E+ | E+ |
| Cool | 0.40 | −0.13 | 0.12 | 0.10 | −0.23 | −0.76 | N-e | N- | N- |
| Quitting | −0.05 | 0.13 | 0.02 | −0.02 | −0.03 | 0.66 | C-g | C- | --- |
| Anxious | −0.48 | 0.04 | −0.17 | −0.07 | −0.01 | 0.63 | N+f | D- | N+ |
| Erratic | 0.03 | 0.40 | 0.14 | −0.10 | 0.38 | 0.59 | C-d | C- | N+ |
| Unemotional | 0.22 | 0.16 | −0.10 | −0.18 | −0.21 | −0.45 | N-d | N- | E- |
| Jealous | −0.08 | 0.30 | 0.41 | 0.12 | −0.27 | 0.44 | A-d | C- | D+ |
Note. Salient loadings (≥ |.40|) are in boldface.
Cnf = Confidence, Opn = Openness, Dom = Dominance, Frd = Friendliness, Act = Activity, Anx = Anxiety.
Loadings were reflected.
N = Neuroticism, E = Extraversion, O = Openness, A = Agreeableness, C = Conscientiousness, D = Dominance, I = Intellect, + = positive loadings; - = negative loadings.
Classification from Goldberg (1990) as described in Table 1 of King and Figueredo (1997).
Classification from Weiss et al. (2006, 2009).
Classification from Costa and McCrae (1992).
Classification from McCrae and Costa (1985).
Classification from King and Figuredo (1997) and Weiss et al. (2009).
Classification from Weiss et al. (2006).
A promax rotation2 revealed that the correlations among components were modest with the mean of the absolute correlations being .14 (see Table 2) and correlations between the six components’ varimax- and promax-rotated loadings were all uniformly high (rs ≥ 0.97). Inspection of the loadings after promax rotation revealed some minor differences: three items (vulnerable, imitative, and intelligent) and eight items (stable, impulsive, gentle, dominant, solitary, playful, jealous, and persistent) loaded on different components. Of the latter eight items only one (persistent) loaded onto a component where it did not have a salient loading in the varimax structure. These changes in loadings did not appear to alter the nature of the components. Given these results, we decided to interpret and base our scores on the varimax solution.
Table 2.
Component Intercorrelations Derived via Promax Rotation
| Component | Confidence | Openness | Dominance | Friendliness | Activity |
|---|---|---|---|---|---|
| Confidence | |||||
| Openness | 0.06 | ||||
| Dominance | 0.19 | 0.06 | |||
| Friendliness | 0.06 | 0.21 | 0.04 | ||
| Activity | 0.32 | 0.01 | 0.30 | 0.00 | |
| Anxiety | −0.36 | 0.15 | 0.03 | 0.11 | −0.25 |
The first component was defined by negative loadings on items such as fearful, which described adverse reactions to the physical or social environment and is related to human Neuroticism (Goldberg, 1990). The first component was also comprised of negative loadings on items such as disorganized, which suggest low focus and poor self controls and is thus related to low human Conscientiousness (Goldberg, 1990). High scoring individuals are thus seemingly more confident in the presence of potential threats and stressors as well as more directed and in control of their behavior. Low scoring individuals are thus more vigilant, highly reactive to these stressors, and exhibit poorer internal controls. The previously identified rhesus macaque Confidence domain (Stevenson-Hinde & Zunz, 1978) described variation along traits similar to or related to those which define this component. We therefore named this component Confidence. When the subjects were assigned unit-weighted scores as defined by the chimpanzee and orangutan structures (see Table 3), Confidence was positively correlated with chimpanzee Dominance and orangutan Intellect and negatively with orangutan Neuroticism.
Table 3.
Correlations Between Unit-Weighted Scores as Defined by the Rhesus Macaque, Chimpanzee, and Orangutan Structures
| Rhesus Macaque |
||||||
|---|---|---|---|---|---|---|
| Domains | Confidence | Openness | Dominance | Friendliness | Activity | Anxiety |
| Chimpanzee | ||||||
| Dominance | 0.85 (0.78, 0.89) | −0.10 (−0.28, 0.09) | 0.84 (0.77, 0.89) | 0.35 (0.18, 0.50) | 0.48 (0.32, 0.61) | −0.40 (−0.54, −0.23) |
| Extraversion | 0.26 (0.07, 0.42) | 0.53 (0.42, 0.67) | −0.01 (−0.18, 0.19) | 0.88 (0.83, 0.91) | 0.63 (0.51, 0.73) | 0.12 (−0.07, 0.30) |
| Agreeableness | −0.03 (−0.22, 0.15) | 0.28 (0.11, 0.45) | −0.19 (−0.36, 0.00) | 0.80 (0.72, 0.86) | 0.15 (−0.03, 0.33) | −0.01 (−0.19, 0.18) |
| Neuroticism | −0.51 (−0.64, −0.36) | 0.04 (−0.13, 0.24) | 0.29 (0.11, 0.45) | 0.00 (−0.19, 0.19) | 0.17 (−0.02, 0.35) | 0.73 (0.63, 0.80) |
| Openness | 0.24 (0.05, 0.41) | 0.84 (0.78, 0.89) | −0.07 (−0.26, 0.11) | 0.30 (0.12, 0.46) | 0.54 (0.39, 0.66) | 0.08 (−0.11, 0.26) |
| Conscientiousness | 0.11 (−0.08, 0.29) | −0.37 (−0.52, −0.20) | −0.67 (−0.76, −0.55) | −0.07 (−0.25, 0.12) | −0.38 (−0.53, −0.21) | −0.59 (−0.70, −0.45) |
| Orangutan | ||||||
| Dominance | 0.49 (0.34, 0.62) | −0.07 (−0.26, 0.11) | 0.97 (0.95, 0.98) | 0.19 (0.00, 0.36) | 0.37 (0.19, 0.52) | 0.00 (−0.19, 0.18) |
| Extraversion | 0.26 (0.07, 0.42) | 0.57 (0.46, 0.71) | 0.15 (−0.04, 0.33) | 0.58 (0.44, 0.69) | 0.82 (0.75, 0.87) | 0.25 (0.06, 0.42) |
| Agreeableness | 0.18 (0.00, 0.36) | 0.29 (0.11, 0.45) | 0.04 (−0.15, 0.22) | 0.92 (0.88, 0.94) | 0.33 (0.16, 0.49) | −0.05 (−0.23, 0.14) |
| Neuroticism | −0.83 (−0.88, −0.76) | 0.27 (0.08, 0.43) | −0.09 (−0.27, 0.10) | −0.21 (−0.38, −0.02) | −0.01 (−0.19, 0.18) | 0.90 (0.86, 0.93) |
| Intellect | 0.77 (0.68, 0.84) | −0.12 (−0.30, 0.07) | 0.37 (0.20, 0.52) | 0.34 (0.16, 0.49) | 0.50 (0.34, 0.63) | −0.49 (−0.62, −0.34) |
Note. N = 111. Correlations in boldface are significant at p < .001. 95% confidence intervals in parentheses.
The second component exhibited positive loadings on items related to exploratory and inquiring behavior, such as curious which is related to human Openness (McCrae & Costa, 1985). This component also loaded on items related to imprudence and poor behavioral controls, such as thoughtless and impulsive, which are markers of low human Conscientiousness (McCrae & Costa, 1985) and either high Neuroticism (Costa & McCrae, 1992) or low Conscientiousness (Goldberg, 1990), respectively. High scoring rhesus macaques would therefore be explorers of their physical and social surroundings, willing to engage in new behaviors, and curious. These same individuals were also perceived as being more forgetful and prone to act on impulse. Low scoring rhesus macaques would thus be less curious about their surroundings and conspecifics but less imprudent and impulse-ridden. Similar domains have not been previously described in rhesus macaques. However, human (Digman, 1990) and chimpanzee (King & Figueredo, 1997) Openness are comprised of sets of traits similar to or related to those making up this domain. Furthermore, the Openness domain in rhesus macaques closely resembled the one identified in chimpanzees (see Table 3). We therefore labeled this component Openness.
The third component was manifested by positive loadings on items indicating aggressive tendencies, such as bullying and items related to social potency and Machiavellianism (Maestripieri, 2007), such as dominant and manipulative, respectively. Such items are typically markers of low human Agreeableness (Goldberg, 1990). This component also positively loaded on items related to unpredictability in behavior or affect, such as reckless and excitable, which have been identified in human studies as indicators of low Conscientiousness and high Neuroticism, respectively (Goldberg, 1990). High scoring individuals would therefore be advantaged in social status competitions; low scoring individuals would be disadvantaged in social status competitions. Similar dimensions were not previously identified in studies of rhesus macaque personality. However, this component resembles the chimpanzee (King & Figueredo, 1997) and orangutan (Weiss, et al., 2006) Dominance domains (see Table 3). We therefore named this component Dominance.
The fourth component was made up of positive and negative loadings on items related to social engagement, including sociable and solitary, respectively. Similar items are related to high or low human Extraversion (Goldberg, 1990). Other loadings were on items indicating cooperative behavior and positive social interactions, such as helpful and friendly, respectively, which are indicative of Agreeableness in humans (Goldberg, 1990). This domain also positively loaded on persistent and intelligent, which are indicative of purposefulness and perception as they are markers of human Conscientiousness and Openness, respectively (Goldberg, 1990). High scorers would thus be sociable and cooperative. Low scoring macaques would be more solitary, unresponsive to social interaction, and inattentive to the dispositions and intentions of conspecifics. In that this domain captured traits related to sociality, it resembled the Sociable–Solitary (Stevenson-Hinde & Zunz, 1978) and Sociability (Capitanio, et al., 1999) domains previously identified in rhesus macaques. The domain score for this component was highly similar to the Extraversion and Agreeableness domains of chimpanzees and orangutans (see Table 3). We therefore labeled this component Friendliness.
After reflecting the fifth component by multiplying loadings by −1, it had negative and positive loadings on items related to low (lazy) and high (active) energy, respectively. Items such as these are often markers of low human Extraversion (Costa & McCrae, 1992; Goldberg, 1990). This component also negatively loaded on items related to behavioral and social conformity and consistency, such as conventional and thus included aspects of low human Openness (McCrae & Costa, 1985). Individuals high on this component would be vigorous, playful, and spontaneous whereas low-scoring macaques would tend to be inactive, placid, and predictable. Comparable dimensions were not previously identified in studies of rhesus macaque personality. This domain is most similar to orangutan Extraversion though it also describes a blend of the six chimpanzee personality domains (see Table 3). We therefore named this domain Activity.
After reflecting the sixth component, it loaded positively on items reflecting high degrees of anxiety or distress (anxious) and negatively on items reflecting low degrees of anxiety or distress (cool). The former item is similar to a facet of human Neuroticism (Costa & McCrae, 1992) and, based on its behavioral description, the latter is likely a marker of low Neuroticism. This component also loaded positively on items reflecting low consistency, such as erratic, which is a low Conscientiousness marker in humans (Goldberg, 1990). Unlike Confidence, items loading on this component reflect general as opposed to situationally-determined levels of anxiety and distress. High scoring individuals would therefore be tense, anxious, and generally not calm whereas low scoring individuals would be relaxed, calm, and predictable. It is therefore not surprising that domain scores based on this component were highly similar to those based on definitions of chimpanzee and orangutan Neuroticism, though it was also similar to low chimpanzee Conscientiousness (see Table 3). Previous studies identified a rhesus macaque personality dimension named Excitable, which is made up of some similar and related traits (Stevenson-Hinde & Zunz, 1978). However, because the item excitable was, in fact, related to Dominance, we felt that naming this component Anxiety better captured its meaning.
Subjective Well-Being
Principal-components analysis indicated that only the first eigenvalue (2.50) was greater than 1.00. We therefore extracted a single component which described individual differences in subjective well-being. The loadings of this component on all three items exceeded 0.90.
Reliabilities of Personality Domains and Subjective Well-Being
The internal consistencies, interrater reliabilities, and stabilities of the personality domains and subjective well-being are presented in Table 4. For personality domains at both time points, interrater reliabilities ranged from poor (Anxiety) to good (Friendliness, Dominance, and Confidence) and internal consistencies were excellent. Retest reliabilities for the personality domains ranged from good (Activity) to excellent (Friendliness, Dominance, and Confidence).
Table 4.
Interrater Reliabilities, Internal Consistencies, and Stabilities of Personality Domains and Subjective Well-Being
| ICC(3,1) | ICC(3,k)a | alpha | r W1,W2 b | ||||
|---|---|---|---|---|---|---|---|
| Wave 1 | Wave 2 | Wave 1 | Wave 2 | Wave 1 | Wave 2 | ||
| Confidence | 0.35 | 0.40 | 0.54 | 0.60 | 0.90 | 0.87 | 0.75 |
| Openness | 0.32 | 0.20 | 0.50 | 0.36 | 0.86 | 0.76 | 0.63 |
| Dominance | 0.40 | 0.47 | 0.59 | 0.67 | 0.90 | 0.89 | 0.78 |
| Friendliness | 0.45 | 0.58 | 0.64 | 0.76 | 0.86 | 0.85 | 0.71 |
| Activity | 0.27 | 0.32 | 0.44 | 0.52 | 0.81 | 0.81 | 0.67 |
| Anxiety | 0.10 | 0.16 | 0.19 | 0.30 | 0.86 | 0.79 | 0.70 |
| Subjective well-being | 0.45 | 0.51 | 0.65 | 0.70 | 0.86 | 0.86 | 0.77 |
Note. rW1,W2 = Correlation between Wave 1 and Wave 2 (N = 70).
Based on a mean of 2.31 raters per subject.
All ps < .05.
For subjective well-being at both time points, interrater reliabilities and internal consistencies were excellent. The retest reliability of subjective well-being was also excellent.
Personality Predictors of Subjective Well-Being
Three of the personality domains were consistently related to subjective well-being in all four sets of analyses (see Table 5). Positive well-being was related to higher Confidence, and Friendliness,. Lower subjective well-being was related to higher Anxiety. One explanation for the high correlations among Friendliness and SWB scores is content similarity. Namely, the presence of the item depressed in Friendliness, because of its similarity in meaning to the items making up SWB, may inflate the correlations between scores. If this is the case, removing the depressed from the scale should substantially reduce the correlation. To test this, we recomputed Friendliness scores without depressed and re-ran the correlations with concurrent and prospective subjective well-being. The correlations did not change significantly (Hotelling-Williams tests, p = .76-.95).
Table 5.
Correlations Between Personality and Subjective Well-Being
| Wave 1 Personality | Wave 2 Personality | |||
|---|---|---|---|---|
| SWBW1 | SWBW2 | SWBW1 | SWBW2 | |
| Confidence | 0..59 (0.45, 0.70)*** | 0.48 (0.28, 0.64)*** | 0.58 (0.40, 0.71)*** | 0..51 (0.31, 0.66)*** |
| Openness | 0.11 (−0.07, 0.30) | 0.17 (−0.07, 0.39) | 0.23 (−0.01, 0.44) | 0.22 (−0.01, 0.43) |
| Dominance | 0.20 (0.00, 0.37)* | 0.19 (−0.05, 0.41) | 0.25 (0.01, 0.46)* | 0.08 (−0.15, 0.31) |
| Friendliness | 0.60 (0.46, 0.71)*** | 0.48 (0.28, 0.64)*** | 0.57 (0.39, 0.71)*** | 0.63 (0.47, 0.76)*** |
| Activity | 0.25 (0.06, 0.42)** | 0.12 (−0.11, 0.34) | 0.10 (−0.14, 0.33) | 0.14 (−0.10, 0.36)* |
| Anxiety | −0.46 (−0.60, −0.30)*** | −0.38 (−0.56, −0.15)** | −0.38 (−0.56, −0.16)*** | −0.46 (−0.63, −0.26)*** |
| N | 111 | 70 | 70 | 71 |
Note.
p < .05
p < .01
p < .001.
95% confidence intervals in parentheses. SWBW1 = Wave 1 subjective well-being, SWBW2 = Wave 2 subjective well-being.
Discussion
The results of our study suggest that ratings on 52 adjectival traits in a large sample of free-ranging rhesus macaques could be reduced to six components named Confidence, Openness, Dominance, Friendliness, Activity, and Anxiety. An oblique (promax) rotation indicated that the components were modestly correlated. However, the range of absolute correlations and their mean were in line with those of chimpanzee (M = .135; see Table 2 and p. 264 of King & Figueredo, 1997) and orangutan (M = .18; Weiss et al., 2006; p. 507) personality dimensions. Moreover, they were lower than the unweighted mean of absolute correlations from 14 studies of human personality (M = .29; computed from Appendix A in Digman, 1997). Finally, the components and loadings were highly similar to a varimax solution.
The interrater reliabilities of the six components were acceptable, but somewhat lower than those in prior studies (Capitanio et al., 1999; King & Figueredo, 1997; Weiss, et al., 2006). This might be explained by the fact that each rater had to rate far more subjects (mean = 25.2) and knew subjects for a shorter period of time than raters in previous studies. On the other hand, the retest reliabilities were excellent.
Confidence, Dominance, Anxiety, and Friendliness were similar to dimensions identified in previous studies of rhesus macaque personality that used different questionnaires (Bolig, et al., 1992; Capitanio, et al., 1999; Stevenson-Hinde, et al., 1980; Stevenson-Hinde & Zunz, 1978). Specifically, Friendliness matched the Sociable dimension, though it was broader, incorporating traits related to chimpanzee (King & Figueredo, 1997) and orangutan (Weiss, et al., 2006) Agreeableness, such as protective. Traits indicative of aggression, vulnerability, and competitive prowess which were subsumed under Confidence dimensions in previous studies of rhesus macaques, defined Confidence, Dominance, and Anxiety in this study. Furthermore, we did not find components similar to the Equable and Excitable dimensions identified in previous studies (Bolig, et al., 1992; Capitanio, et al., 1999; Stevenson-Hinde, et al., 1980; Stevenson-Hinde & Zunz, 1978) as the traits making up these dimensions were markers of Dominance in the present study. We also found two previously unidentified dimensions. The first, Activity, consisted of items usually found in Extraversion domains in humans (Costa & McCrae, 1992), chimpanzees (King & Figueredo, 1997), and orangutans (Weiss, et al., 2006). The second, Openness, was similar to the Openness domain described in humans (Costa & McCrae, 1992) and chimpanzees (King & Figueredo, 1997).
The dimensions identified in this study also differed in five ways from those identified in studies of humans (Digman, 1990), chimpanzees (King & Figueredo, 1997), and orangutans (Weiss, et al., 2006). First, traits related to Neuroticism in these other species defined two dimensions in rhesus macaques: Confidence which was related to reactions to external stimuli and events, including social events; and Anxiety which could be described as general levels of distress and unease. These two dimensions can also be distinguished, as although both were similar to chimpanzee and orangutan Neuroticism, Confidence was composed of items that in chimps and orangutans make up Dominance. Second, traits related to Extraversion in these species defined a dimension related to sociability (Friendliness) and a dimension related to activity levels (Activity). These dimensions were thus similar to the Gregariousness and Activity facets of human Extraversion (Costa & McCrae, 1992). Third, we did not find a Conscientiousness dimension similar to that of humans or chimpanzees. Items composing chimpanzee Conscientiousness instead were part of the rhesus macaque Dominance and Anxiety dimensions. Fourth, unlike orangutans, but like chimpanzees and humans, we found a clear Openness dimension. Fifth, unlike humans, but like the great ape species we have data on, we found a Dominance dimension.
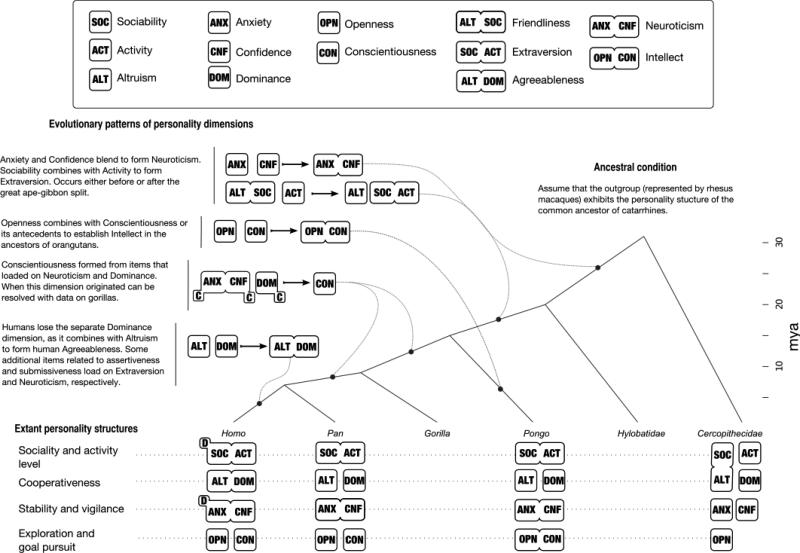
The similarities and differences between personality in rhesus macaques and those identified in hominoids are informative about personality phylogeny (see Figure 1). Why would it be useful to describe the origin and evolution personality dimensions in the same way that morphological or fixed behavioral traits are characterized, i.e. as being ancestral or derived? After all, personality dimensions describe differences between individuals and are not species-typical characteristics like thumbs or wings. Furthermore, if a personality trait is constructed out of the covariance of numerous items or behaviors, how does it evolve? Both of these issues arise out of a limitation in evolutionary theorizing rather than being problems unique to the evolution of personality structures. First, every biological trait, whether specific to a species or differing among individuals, is the result of a host of underlying genetic and environmental factors (Rice, 2004). Selection can act directly on the joint distribution of the factors contributing to each trait as well as to those among traits, allowing traits to become developmentally integrated or disassociated from one another. Genetic covariances between traits are both the result of natural selection and a constraint on evolution. Second, selection does not operate only on trait means, but can also influence higher moments of a trait's distribution, such as variance (Rice, 2004). Thus, personality differences can evolve within a species. Finally, our phylogeny, while parsimonious and consistent with the data, describes only one possible set of evolutionary patterns. Casting personality dimensions as explicitly ancestral or derived might hopefully motivate further work in this area, and lead to the exploration of personality and subjective well-being in additional, distantly related taxa, permitting formal, comparative analyses.
Figure 1.
Cladogram of the hypothesized patterns of personality evolution in the parvorder Catarrhini. Personality structures are described as a combination of “basic” or “blended” dimensions, e.g., Friendliness in rhesus macaques is a blend of Altruism and Sociability. The evolutionary transitions are interpreted as the integration or disintegration of these dimensions, shown by horizontal arrows between groups of dimensions. The possible transition points, indicated by the dashed lines, are placed according to phylogenetic parsimony. This picture is likely to change as more species are assessed. The structure of the genus Pan is represented by chimpanzees and the family Cercopithecidae by rhesus macaques. Figure by the authors, licensed under a Creative Commons Attribution Unported License and published under the terms of this license.
Given the known primate personality structures, Openness appears to be an ancestral characteristic present in the ancestor of Hominoidea and Cercopithecoidea. Finding Openness in rhesus macaques is not surprising given their relatively large neocortex, which probably evolved in response to their large group sizes and complex social system (Dunbar, 1998). The presence of Openness may have enabled rhesus macaques to survive in a wide range of habitats including different ecological conditions (Seth & Seth, 1986). Future studies of less widely-distributed macaque species could be used to test whether the presence of Openness is one explanation for the successful distribution of rhesus macaques as a species. In contrast, Openness traits in orangutans are more closely related to traits defining Conscientiousness with which they form the Intellect domain and to traits defining Extraversion. This pattern of loadings is likely derived, having evolved in response to their semi-solitary social structures (Galdikas, 1985) as encountering unrelated conspecifics would be highly novel experiences. Future studies should test this hypothesis by comparing the personalities of related solitary and social species. These findings also support earlier suggestions that Conscientiousness is a derived domain (Gosling & John, 1999), possibly exclusive to African apes or at least Pan and Homo (Weiss, et al., 2006). To address this requires studying gorilla and bonobo personality with a similar questionnaire, which remains to be done.
These findings also suggest that the unidimensional Neuroticism and Extraversion domains found in humans, chimpanzees, and orangutans are derived from multidimensional ancestral variants: Confidence and Anxiety in the case of Neuroticism; Friendliness and Activity in the case of Extraversion. The presence of lower-order facets in the human Five-Factor Model (Costa & McCrae, 1992) is consistent with this possibility. However, research on other macaque species is needed to determine whether the configuration of traits responsible for this multidimensionality are a derived characteristic of rhesus or ancestral and shared with other macaque species.
These results also affirm perhaps the key characteristic of human personality that sets it apart from that of nonhuman primates, i.e., the absence of a specific Dominance domain in five-factor personality space (Gosling & John, 1999). This does not mean humans do not have dominance relationships. In fact, some aspects of human personality such as the assertiveness facet of Extraversion (Costa & McCrae, 1992), social potency (Patrick, Curtin, & Tellegen, 2002), and the Dominance factor of Cattell's 16 PF (Conn & Rieke, 1994) clearly indicate that individual differences in human dominance can be assessed. Moreover, a personality style (Leaders) defined by a combination of high Extraversion and low Agreeableness (Costa & McCrae, 1998) describes a similar construct. One possible reason for this difference between humans on the one hand and nonhuman primates on the other was hinted at by Hinde (1978) who argued that human dominance may be context-specific, i.e., humans dominant in one domain of life or society are not necessarily dominant in another. However, this explanation is unlikely as the dominance rank a rhesus macaque occupies also varies across contexts (Bernstein & Gordon, 1980). A second possibility is that the absence of a dominance-related domain is a consequence of selection for increasing egalitarianism in human evolution (Boehm, 1999). One way to test this last possibility hypothesis is by comparing macaque species that, while occupying similar ecological niches, vary to the extent to which they are egalitarian as opposed to despotic (Matsumura, 1999; Sterck, Watts, & van Schaik, 1997; Thierry, 1985, 2000). A third possibility is that humans might be unique in achieving dominance by different means, such as intelligence or accrued resources, which could tap into different personality facets across different domains, rather than a singular one. A fourth possibility is that human societies may offer multiple dominance hierarchies in which individuals can participate (Gosling & John, 1999).
Ratings related to the balance of moods, pleasure derived from social interactions, and global well-being were highly intercorrelated and described a domain similar to human (Diener, Suh, Lucas, & Smith, 1999), chimpanzee (King & Landau, 2003), and orangutan (Weiss, et al., 2006) subjective well-being. At Waves 1 and 2 this domain displayed high interrater reliabilities; the stability of this domain was also high. Higher Confidence and Friendliness and lower Anxiety were consistently related to greater subjective well-being. Thus, like humans (Steel, et al., 2008), chimpanzees (King & Landau, 2003; Weiss, et al., 2009), and orangutans (Weiss, et al., 2006), rhesus macaques that are more social, sympathetic, and equable and less anxious, timid, and erratic exhibit more positive affect. It therefore appears that the nexus of positive affect, low Neuroticism, and high Extraversion are phylogenetically ancestral, and may have been present in the common ancestor of old world monkeys, great apes, and humans some 31 mya (Steiper & Young, 2006).
We also found differences in how positive affect is related to personality in rhesus macaques. Unlike chimpanzees (King & Landau, 2003; Weiss, et al., 2009) though like orangutans (Weiss, et al., 2006), Dominance and Openness were not consistently related to rhesus macaque subjective well-being. These results suggest that, ancestrally, there was no relationship between personality dimensions similar to Dominance or Openness and positive affect and that the interrelationship between chimpanzee Dominance, Openness, and subjective well-being may be evolutionarily more recent.
In addition to possibly being a product of environmental consistency across time, the high correlations between Wave 1 personality domains and Wave 2 subjective well-being may reflect, as they do in humans (Lykken, Bouchard, McGue, & Tellegen, 1990; Lykken & Tellegen, 1996; Nes, Roysamb, Tambs, Harris, & Reichborn-Kjennerud, 2006), common genetic influences at both time points. Similarly, as is the case for humans (Weiss, Bates, & Luciano, 2008) and chimpanzees (Weiss, King, & Enns, 2002), the relationships between personality and subjective well-being may also be genetically mediated in rhesus macaques.
One potential limitation of the present study is that, like other studies of nonhuman primate personality (e.g., King & Figueredo, 1997), the ratio of sample size to items (2.13) is considerably smaller than that typically recommended for principal-components analysis (5.00 or 10.00). However, in a series of simulation studies Guadagnoli and Velicer (1988) demonstrated that there was little support for the sample size to item ratio rules. Instead they found that the stability of structures was mostly a function of the size of component or factor loadings and absolute sample size with absolute sample sizes of 150 yielding stable structures in most cases. While the sample size in the present study fell slightly below 150, items were based on multiple ratings, thus making them more reliable. However, as in studies of chimpanzee personality (King et al., 2005; Weiss et al., 2007; Weiss et al., 2009), there should be attempts to replicate this structure in other samples.
Our findings also speak to the validity of using questionnaires to assess animal personality and subjective well-being. Critics may argue that questionnaire-based measures are compromised by anthropomorphic projection, but we found domains similar to those found in studies of rhesus macaques using different questionnaires which also differed from those identified in other species rated on the same questionnaire. Critics might further claim that differences reflect situational artefacts, such as the background of the raters or the environment in which the rhesus macaques were observed. However, previous research has shown that the same species-typical domains emerge even in differing environments or when ratings are made by raters with different cultural backgrounds (King, Weiss, & Farmer, 2005; Weiss, et al., 2009; Weiss, King, & Hopkins, 2007). Thus, these findings build upon a rich set of findings indicating that personality ratings probably do not reflect anthropomorphic projections or other artefacts (Capitanio, 1999; Konečná, et al., 2008; Uher & Asendorpf, 2008; Uher, et al., 2008; Weinstein & Capitanio, 2008).
In light of findings in humans (Digman, 1990), chimpanzees (King & Figueredo, 1997), and orangutans (Weiss, et al., 2006), our results indicate that intense sociality may have been a potent selective pressure driving personality trait evolution and co-evolution. On the other hand, these same forces appear not to have impacted the evolution of positive affect or its co-evolution with personality.
Assessing multiple related species using similar instruments is an effective tool with which to better understand the phylogeny of personality as well as the relationship of personality domains to affect. In the future, these measures, together with similarly standardized measures of behavior, ecology, and social structure, could help develop a fuller understanding of personality in nonhuman primates such as macaques, and even ourselves.
Acknowledgments
We thank Amanda K. Accamando, Lauren J. N. Brent, Julie Cascio, Camille Guillier, Doreen Hess, Constance Dubuc, Veronique Martel, Jennifer L. Danzy, Joyce Moewius, Maggie Chivavetta, Veronica Gutierrez, Claude Richer, Agnieszka Sukiennik Koniec, and Akie Yanagi for rating the monkeys. Without their cooperation, this project would not have been possible. We also thank Edgar Davíla and Julio Resto for maintaining a daily census of the rhesus macaques of Cayo Santiago. We thank Jana Uher and Michael Tomasello for their helpful comments on an earlier draft. Finally, we would like to thank one anonymous reviewer for suggesting the analyses depicted in Table 3 and both anonymous reviewers for several helpful comments which have improved the manuscript.
This study complies with the current laws of Puerto Rico and the United States. The IACUC of the University of Puerto Rico, Medical Sciences Campus approved this investigation. The rhesus macaque population of Cayo Santiago is supported by the University of Puerto Rico, Medical Sciences Campus and by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) (NCRR grant P40RR003640 award to the CPRC). The content of this publication is solely the responsibility of all of the authors and does not necessarily represent the official views of NCRR, NIH, or the United States Government.
Footnotes
Previous studies have found that principal-components analysis yields nearly identical structures as those derived from principal axis factor analysis (Velicer, 1977; Weiss et al., 2006, footnote 4). For the present study, use of principal axis factor analysis yielded a virtually identical personality structure as that derived via principal-components analysis.
The table of loadings derived via promax rotation is available from the first author upon request.
Contributor Information
Alexander Weiss, Department of Psychology, School of Philosophy, Psychology, and Language Sciences, The University of Edinburgh..
Mark James Adams, Department of Psychology, School of Philosophy, Psychology, and Language Sciences, The University of Edinburgh..
Anja Widdig, Junior Research Group of Primate Kin Selection; Department of Primatology, Max-Planck Institute for Evolutionary Anthropology and Institute of Biology, University of Leipzig..
Melissa S. Gerald, Caribbean Primate Research Center and Department of Internal Medicine, University of Puerto Rico - Medical Sciences Campus. Center for Scientific Review, National Institutes of Health. Melissa S. Gerald contributed to this article as an employee of the University of Puerto Rico - Medical Sciences Campus.
References
- Andrews P. Fossil evidence on human origins and dispersal. Cold Spring Harbor Symposium on Quantitative Biology. 1986;51:419–428. doi: 10.1101/sqb.1986.051.01.050. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The social component of dominance relationships in rhesus monkeys (Macaca mulatta). Animal Behaviour. 1980;28:1033–1039. [Google Scholar]
- Boehm C. Hierarchy in the forest: The evolution of egalitarian behavior. Harvard University Press; Cambridge, MA: 1999. [Google Scholar]
- Bolig R, Price CS, O'Neill PL, Suomi SJ. Subjective assessment of reactivity level and personality traits of rhesus monkeys. International Journal of Primatology. 1992;13:287–306. [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Baroncelli S. The relationship of personality dimensions in adult male rhesus macaques to progression of simian immunodeficiency virus disease. Brain Behavior and Immunity. 1999;13:138–154. doi: 10.1006/brbi.1998.0540. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Bentson KL. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta). Psychoneuroendocrinology. 2004;29:1300–1308. doi: 10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Chimpanzee Sequencing and Analysis Consortium Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- Colvin JD. Proximate causes of male emigration at puberty in rhesus monkeys. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago Macaques. SUNY Press; Albany, NY: 1986. pp. 131–157. [Google Scholar]
- Conn SR, Rieke ML. The 16PF fifth edition technical manual. Institute for Personality and Ability Testing; Champaign, IL: 1994. [Google Scholar]
- Costa PT, Jr., McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- Diener E. Subjective well-being: The science of happiness and a proposal for a national index. American Psychologist. 2000;55:34–43. [PubMed] [Google Scholar]
- Diener E, Suh EM, Lucas RE, Smith HL. Subjective well-being: Three decades of progress. Psychological Bulletin. 1999;125:276–302. [Google Scholar]
- Digman JM. Personality structure: Emergence of the Five-Factor Model. Annual Review of Psychology. 1990;41:417–440. [Google Scholar]
- Digman JM. Higher-order factors of the Big Five. Journal of Personality and Social Psychology. 1997;73:1246–1256. doi: 10.1037//0022-3514.73.6.1246. [DOI] [PubMed] [Google Scholar]
- Dinno A. paran: Horn's test of principal components/factors. 2008 [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;6:178–190. [Google Scholar]
- Dutton DM. Subjective assessment of chimpanzee (Pan troglodytes) personality: Reliability and stability of trait ratings. Primates. 2008;49:253–259. doi: 10.1007/s10329-008-0094-1. [DOI] [PubMed] [Google Scholar]
- Foley RA, Lee PC. Finite social space, evolutionary pathways and reconstructing hominid behavior. Science. 1989;243:901–906. doi: 10.1126/science.2493158. [DOI] [PubMed] [Google Scholar]
- Galdikas BMF. Orangutan sociality at Tanjung-Puting. American Journal of Primatology. 1985;9:101–119. doi: 10.1002/ajp.1350090204. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. An alternative “description of personality”: the Big-Five factor structure. Journal of Personality and Social Psychology. 1990;59:1216–1229. doi: 10.1037//0022-3514.59.6.1216. [DOI] [PubMed] [Google Scholar]
- Gorsuch RL. Factor analysis. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Gosling SD, Graybeal A. Tree thinking: A new paradigm for integrating comparative data in psychology. The Journal of General Psychology. 2007;134:259–277. doi: 10.3200/GENP.134.2.259-278. [DOI] [PubMed] [Google Scholar]
- Gosling SD, John OP. Personality dimensions in nonhuman animals: A cross-species review. Current Directions in Psychological Science. 1999;8:69–75. [Google Scholar]
- Gouzoules S, Gouzoules H. Kinship. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 299–305. [Google Scholar]
- Guadagnoli E, Velicer WF. Relation of sample size to the stability of component patterns. Psychological Bulletin. 1988;103:265–275. doi: 10.1037/0033-2909.103.2.265. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford University Press; Oxford, England: 1991. [Google Scholar]
- Hinde RA. Dominance and role---two concepts with dual meanings. Journal of Social and Biological Structures. 1978;1:27–38. [Google Scholar]
- Horn JL. A rationale and test for the number of factors In factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- King JE, Figueredo AJ. The Five-Factor Model plus Dominance in chimpanzee personality. Journal of Research in Personality. 1997;31:257–271. [Google Scholar]
- King JE, Landau VI. Can chimpanzee (Pan troglodytes) happiness be estimated by human raters? Journal of Research in Personality. 2003;37:1–15. [Google Scholar]
- King JE, Weiss A, Farmer KH. A chimpanzee (Pan troglodytes) analogue of cross-national generalization of personality structure: Zoological parks and an African sanctuary. Journal of Personality. 2005;73:389–410. doi: 10.1111/j.1467-6494.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- King JE, Weiss A, Sisco MS. Aping humans: Age and sex effects in chimpanzee (Pan troglodytes) and human (Homo sapiens) personality. Journal of Comparative Psychology. 2008;122:418–427. doi: 10.1037/a0013125. [DOI] [PubMed] [Google Scholar]
- Konečná M, Lhota S, Weiss A, Urbánek T, Adamová T, Pluháček J. Personality in free-ranging Hanuman langur (Semnopithecus entellus) males: Subjective ratings and recorded behavior. Journal of Comparative Psychology. 2008;122:379–389. doi: 10.1037/a0012625. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: Some initial findings. Acta Geneticae Medicae Et Gemellologiae. 1990;39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Tellegen A. Happiness is a stochastic phenomenon. Psychological Science. 1996;7:186–189. [Google Scholar]
- Maestripieri D. Macachiavellian intelligence: How rhesus macaques and humans have conquered the world. University of Chicago Press; Chicago: 2007. [Google Scholar]
- Maninger N, Capitanio JP, Mendoza SP, Mason WA. Personality influences tetanus-specific antibody response in adult male rhesus macaques after removal from natal group and housing relocation. American Journal of Primatology. 2003;61:73–83. doi: 10.1002/ajp.10111. [DOI] [PubMed] [Google Scholar]
- Manson JH. Do female rhesus macaques choose novel males? American Journal of Primatology. 1995;37:285–296. doi: 10.1002/ajp.1350370403. [DOI] [PubMed] [Google Scholar]
- Matsumura S. The evolution of “Egalitarian” and “Despotic” social systems among macaques. Primates. 1999;40:23–31. doi: 10.1007/BF02557699. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr. Updating Norman's “adequate taxonomy”: Intelligence and personality dimensions in natural language and in questionnaires. Journal of Personality and Social Psychology. 1985;49:710–721. doi: 10.1037//0022-3514.49.3.710. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr. Personality in adulthood: A Five-Factor Theory perspective. Guilford Press; New York, NY: 2003. [Google Scholar]
- Melnick DJ, Pearl MC. Cercopithecines in multimale groups: Genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 121–134. [Google Scholar]
- Nes RB, Roysamb E, Tambs K, Harris JR, Reichborn-Kjennerud T. Subjective well-being: Genetic and environmental contributions to stability and change. Psychological Medicine. 2006;36:1033–1042. doi: 10.1017/S0033291706007409. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire (MPQ). Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pederson AK, King JE, Landau VI. Chimpanzee (Pan troglodytes) personality predicts behavior. Journal of Research in Personality. 2005;39:534–549. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Rawlins RG, Kessler MJ. The history of the Cayo Santiago colony. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago Macaques: History, behavior and biology. SUNY Press; Albany, NY: 1986. pp. 13–45. [Google Scholar]
- Revelle W. psych: Procedures for psychological, psychometric, and personality research. 2009 [Google Scholar]
- Rice SH. Evolutionary Theory: Mathematical and Conceptual Foundations. Sinauer Associates; Sunderland, MA.: 2004. [Google Scholar]
- Seligman MEP, Csikszentmihalyi M. Positive psychology: An introduction. American Psychologist. 2000;55:5–14. doi: 10.1037//0003-066x.55.1.5. [DOI] [PubMed] [Google Scholar]
- Seth PK, Seth S. Ecology and behaviour of rhesus monkeys in India. In: Else JG, Lee PC, editors. Primate ecology and conservation. Vol. 2. Cambridge University Press; Cambridge, UK: 1986. pp. 89–103. [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Steel P, Schmidt J, Shultz J. Refining the relationship between personality and subjective well-being. Psychological Bulletin. 2008;134:138–161. doi: 10.1037/0033-2909.134.1.138. [DOI] [PubMed] [Google Scholar]
- Steiper ME, Young NM. Primate molecular divergence dates. Molecular Phylogenetics and Evolution. 2006;41:384–394. doi: 10.1016/j.ympev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Sterck EHM, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology. 1997;41:291–309. [Google Scholar]
- Stevenson-Hinde J, Stillwell-Barnes R, Zunz M. Individual differences in young rhesus monkeys: Consistency and change. Primates. 1980;21:498–509. [Google Scholar]
- Stevenson-Hinde J, Zunz M. Subjective assessment of individual rhesus monkeys. Primates. 1978;19:473–482. [Google Scholar]
- Thierry B. Patterns of agonistic interactions in three species of macaque (Macaca mulatta, M. fascicularis, M. tonkeana). Aggressive Behavior. 1985;11:223–233. [Google Scholar]
- Thierry B. Covariation of conflict management patterns across macaque species. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. University of California Press; Berkeley, CA: 2000. pp. 106–128. [Google Scholar]
- Uher J. Comparative personality research: Methodological approaches. European Journal of Personality. 2008;22:475–496. [Google Scholar]
- Uher J, Asendorpf JB. Personality assessment in the Great Apes: Comparing ecologically valid behavior measures, behavior ratings, and adjective ratings. Journal of Research in Personality. 2008;42:821–838. [Google Scholar]
- Uher J, Asendorpf JB, Call J. Personality in the behaviour of great apes: Temporal stability, cross-situational consistency and coherence in response. Animal Behaviour. 2008;75:99–112. [Google Scholar]
- Velicer WF. An empirical comparison of the similarity of principal component, image, and factor patterns. The Journal of Multivariate Behavioral Research. 12:3–22. doi: 10.1207/s15327906mbr1201_1. [DOI] [PubMed] [Google Scholar]
- Weinstein TAR, Capitanio JP. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Animal Behaviour. 2008;76:455–465. doi: 10.1016/j.anbehav.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Bates TC, Luciano M. Happiness is a personal(ity) thing - The genetics of personality and well-being in a representative sample. Psychological Science. 2008;19:205–210. doi: 10.1111/j.1467-9280.2008.02068.x. [DOI] [PubMed] [Google Scholar]
- Weiss A, Inoue-Murayama M, Hong K-W, Inoue E, Udono S, Ochiai T, et al. Assessing chimpanzee personality and subjective well-being in Japan. American Journal of Primatology. 2009;71:283–292. doi: 10.1002/ajp.20649. [DOI] [PubMed] [Google Scholar]
- Weiss A, King JE, Enns RM. Subjective well-being is heritable and genetically correlated with dominance in chimpanzees (Pan troglodytes). Journal of Personality and Social Psychology. 2002;83:1141–1149. [PubMed] [Google Scholar]
- Weiss A, King JE, Hopkins WD. A cross-setting study of chimpanzee (Pan troglodytes) personality structure and development: Zoological parks and Yerkes National Primate Research Center. American Journal of Primatology. 2007;69:1264–1277. doi: 10.1002/ajp.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, King JE, Perkins L. Personality and subjective well-being in orangutans (Pongo pygmaeus and Pongo abelii). Journal of Personality and Social Psychology. 2006;90:501–511. doi: 10.1037/0022-3514.90.3.501. [DOI] [PubMed] [Google Scholar]
- Wrangham RW. The evolution of social structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 282–296. [Google Scholar]