Abstract
It is increasingly appreciated that the properties of a biomaterial used in intramyocardial injection therapy influence the outcomes of infarcted hearts that are treated. In this report the extended in vivo efficacy of a thermally responsive material that can deliver dual growth factors while providing a slow degradation time and high mechanical stiffness is examined. Copolymers consisting of N-isopropyla-crylamide, 2-hydroxyethyl methacrylate, and degradable methacrylate polylactide were synthesized. The release of bioactive basic fibroblast growth factor (bFGF) and insulin-like growth factor 1 (IGF1) from the gel and loaded poly(lactide-co-glycolide) microparticles was assessed. Hydrogel with or without loaded growth factors was injected into 2 week-old infarcts in Lewis rats and animals were followed for 16 weeks. The hydrogel released bioactive bFGF and IGF1 as shown by mitogenic effects on rat smooth muscle cells in vitro. Cardiac function and geometry were improved for 16 weeks after hydrogel injection compared to saline injection. Despite demonstrating that left ventricular levels of bFGF and IGF1 were elevated for two weeks after injection of growth factor loaded gels, both functional and histological assessment showed no added benefit to inclusion of these proteins. This result points to the complexity of designing appropriate materials for this application and suggests that the nature of the material alone, without exogenous growth factors, has a direct ability to influence cardiac remodeling.

INTRODUCTION
Intramyocardial biomaterial injection therapy for the treatment of heart failure has shown promising results in recent years, leading to the continued investigation of both biological and synthetic materials for this application.1,2 It has been noted previously that the properties of the injected material may play a role in the overall benefit seen following injection. These properties include material degradation rate, bioactivity, and mechanical strength.3–8 It has been demonstrated, for example, that materials that degrade too quickly (<6 week) and those that are nondegradable both lead to decreased function similar to saline control groups at study end points.3–6 Likewise, theoretical models and empirical data suggest that stiffer materials may have a greater benefit than softer materials, though the upper limits on stiffness have not been investigated.7,8 Specifically, in Wall et al., a finite element model showed that simulated injection of materials with an initial modulus up to 20 kPa, which is twice as stiff as the native myocardium, decreased wall stress compared to softer materials. The combination of material stiffness and degradation rate are likely intertwined in improving myocardial performance after injection. It is hypothesized that the injected material provides mechanical support to the myocardium which reduces stress on the cardiac muscle cells and promotes a better healing response. Over time, as the material degrades it will transfer that load-bearing responsibility back to the cardiac muscle cells. The amount of mechanical support needed and the best timing for returning the load to the myocardium through material degradation is not known, but recent research using cardiac patch materials suggests there is likely an optimal range for these parameters.9 It has also been postulated that appropriate cell infiltration into the injected material may be important in eliciting sustainable improvements to cardiac function.5,10,11 While studying the effects of these material properties, it is important to provide adequate follow-up time after injection to see the lasting effects of the therapy. Few published studies extend beyond 2 months, making long-term efficacy difficult to assess.
Another approach that has shown promise to improve material injection therapy is the inclusion of appropriate growth factors with the bulking material to provide controlled release at the injection site. Growth factors have received the most attention to date, specifically those with known proangiogenic properties, such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor, platelet-derived growth factor, and hepatocyte growth factor.12–18 Insulin-like growth factor 1 (IGF1) has also been utilized due to its known benefits on the myocardium, including influencing cell size and metabolism, decreasing apoptosis, improving contractility, and encouraging cardiac stem cell proliferation.19–21 It has recently been demonstrated that controlled release of two or more growth factors, in some cases in a sequential manner, can have benefits beyond what is seen from single factor delivery in cardiovascular applications.22–24 For example, the delivery of complementary proteins sequentially has been shown to be more beneficial in angiogenesis than simultaneous delivery, presumably because it mimics the body’s own regenerative healing mechanisms.25,26 For cardiac applications, one useful combination to improve cardiac function may be to deliver relevant growth factors, such as bFGF and IGF1, to encourage angiogenesis and cardiac stem cell and cardiomyocyte proliferation, respectively.
We have previously reported on the benefits of injecting an N-isopropylacrylamide (NIPAAm)-based thermally responsive hydrogel in infarcted rat hearts, and have subsequently developed a stiffer material (90 kPa initial modulus) with a slower degradation time (>5 months), which may be more beneficial in cardiac injection therapy.27,28 The drug delivery-capabilities of this new material were subsequently studied with the release of model protein bovine serum albumin (BSA).29 Specifically, by loading the gel with one protein mixed into the bulk and a second protein contained within poly(lactide-co-glyoclide) (PLGA) microparticles, the two proteins could be delivered in a sequential fashion. The primary objective of this study was to evaluate the effects of intramyocardial injection of this new hydrogel material compared to saline in a rat model of ischemic cardiomyopathy. The material degrades slowly in vivo, is stiffer than other materials used in this application previously, and can deliver bFGF and IGF1 at different rates. Furthermore, after characterizing in vitro growth factor delivery from this gel, the effect of delivery of bFGF, IGF1 or both from the hydrogel on cardiac function and basic histological parameters was examined relative to the gel not loaded with growth factors. Animals were followed 16 weeks after the injection point to determine longer-term effects of this therapy.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. NIPAAm was purified by recrystallization from hexane and vacuum-dried. 2-Hydroxyethyl methacrylate (HEMA) was purified by vacuum distillation. Benzoyl peroxide (BPO), lactide, PLGA, sodium methoxide (NaOCH3), poly(vinyl alcohol) (PVA), and methacryloyl chloride were used as received. IGF1 and bFGF (Peprotech), BSA (Sigma), heparin (Sigma), and 125I-bFGF (Perkin-Elmer) were reconstituted according to manufacturer instructions. Protein extraction buffer (Bioo Scientific) and enzyme-linked immunosorbant assays (ELISA, R&D Systems) were used as received.
Material Synthesis
Methacrylate-poly(lactic acid) (MAPLA) was synthesized as reported previously.28 Briefly, after dissolving lactide in dichloromethane to which NaOCH3 initiator in methanol was added, polylactide-monomethyl ether (HOPLA-OCH3) was synthesized by ring-opening polymerization. The reaction persisted for 2 h at 0°C, after which the polymer solution was rinsed with 0.1 M HCl and deionized (DI) water. The organic phase was next dried with MgSO4 and then removed by rotary evaporation. HOPLA-OCH3 was dissolved again in dichloromethane with added triethylamine. MAPLA was formed by dropping methacryloyl chloride into this solution and allowing reaction overnight at 0 °C. Following reaction, precipitates were removed, the organic phase was dried and removed by rotary evaporation, leaving raw MAPLA product. MAPLA was purified by flash chromatography. NMR confirmed the synthesis of MAPLA with an average of 2.9 PLA units per MAPLA monomer.
Poly(NIPAAm-co-HEMA-co-MAPLA) copolymer was synthesized by free radical polymerization as reported previously.28 Monomers NIPAAm (6 g, 0.053 mol), HEMA, and MAPLA at a molar ratio of 80/10/10 were dissolved in 200 mL of 1,4-dioxane containing 220 mg BPO. The polymerization was carried out at 70°C for 24 h under an argon atmosphere. The copolymer was purified by repeated precipitations in hexane, diethyl ether, and warm DI water. The final product was freeze-dried before being dissolved in cold phosphate buffered saline (PBS) at 15 wt % for use in all studies.
PLGA microspheres with a lactide:glycolide ratio of 75:25 (Mw 66 – 107 kD) were synthesized using a water-in-oil-in-water (W/O/W) double emulsion technique as described previously.30 IGF1 was mixed with BSA at a ratio of 1:10 and dissolved in 0.02× PBS to yield a final BSA concentration of 1 mg/mL. Initially, 200 mg of PLGA was dissolved in 4 mL of dichloromethane, after which 200 µL of IGF1 solution was added to the PLGA solution and vortexed for 1 min. This primary emulsion was immediately added to 60 mL of 2% PVA stirring at 1000 rpm. After 5 min, an additional 80 mL of 1% PVA solution was added and the stirring speed was decreased to 600 rpm. Dichloromethane evaporated from the mixture during 6 h of stirring and the microspheres were collected by centrifugation, rinsed with DI water and freeze-dried. The IGF1 content of the PVA and DI water supernatants was determined by ELISA to calculate the encapsulation efficiency of IGF1 in the particles.
Copolymer Characterization
Copolymer molecular weight was determined by gel permeation chromatography in tetrahydrofuran (Waters Breeze System). The change in mechanical properties of the hydrogels during sol–gel transition was characterized on a TA Instruments rheometer (AR2000). A temperature sweep from 5 to 30 °C (heating rate of 4 °C/min) was applied to gel solutions placed between two parallel plates. The shear storage modulus G′ and the loss modulus G″ were recorded as a function of temperature while at a fixed strain of 2% and a frequency of 1 Hz. The lower critical solution temperature (LCST) of the polymer was taken to be the temperature at which the storage modulus reached half of its maximum value.
In Vitro Growth Factor Release Studies
For quantification of the bFGF release rate from hydrogels, 125I-bFGF was loaded into 15 wt % gel solutions alone, or after being premixed with either BSA (1:350 bFGF/BSA molar ratio) or, because bFGF is a heparin-binding protein, with BSA and heparin (1:350 BSA, 1:1000 bFGF/heparin). These samples were gelled in PBS at 37 °C, and the releasate was periodically collected and measured with a gamma counter (Auto Gamma II, Perkin-Elmer) to determine the amount of bFGF released at each time point. For quantification by ELISA and to determine growth factor bioactivity, hydrogel solutions loaded with 16 µg/mL bFGF or 1 µg/mL IGF1 in loaded microparticles were injected in 0.25 mL aliquots into 24-well plates containing 37 °C basal medium (EMEM, Lonza). At designated time points the medium was removed, transferred to individual sterile microcentrifuge tubes, and replaced with fresh medium. The collected medium was stored at −80 °C until being used for bioactivity studies, at which point it was quickly thawed at 37 °C and transferred to cultured cells.
Rat smooth muscle cells (SMCs, isolated according to the method reported in31 were cultured at 5 × 103 cells per well in a 96-well plate in culture medium (Dulbecco’s Modified Eagle Medium, 5% fetal bovine serum, penicillin, and streptomycin). After 24 h, the cell culture media was removed and replaced with supernatant from the growth factor release studies. Cells were cultured with the supernatant for 48 h at which point a mitochondrial assay (CellTiter 96 AqueousOne, MTS assay, Promega) was used to represent cell numbers in terms of mitochondrial activity. Visual inspection qualitatively confirmed the results from the MTS assay. Results from the assay were compared against cells grown in basal medium without serum.
In Vivo Hydrogel Injection Studies
Adult female Lewis rats (Harlan Sprague–Dawley) weighing 160–210 g were used. The protocol followed National Institutes of Health guidelines for animal care and was approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee. Anesthesia was induced with 3.0% isoflurane inhalation with 100% oxygen followed by intubation and respiratory support with a rodent volume-controlled mechanical ventilator (683 Ventilator, Harvard Apparatus). Electrocardiogram and tail cuff blood pressure measurements were used to monitor vital signs in all animals. A left thoracotomy was performed to expose the heart after which the proximal left anterior descending coronary artery was ligated with a 7–0 polypropylene suture. The creation of myocardial ischemia was verified by regional cyanosis and ST segment elevation and the incision was closed in layers with 5–0 polypropylene continuous sutures.
Two weeks after induction of myocardial infarction, at which point LV remodeling begins,32 the rats were anesthetized and evaluated with echocardiography to measure infarct size in terms of the percentage of scar area (akinetic or dyskinetic regions) compared to the total left ventricular (LV) circumference. Rats with infarcts greater than 25% of the LV free wall were randomly divided into five groups: those that would receive hydrogel injections (hydrogel group, n = 9), control PBS injections (PBS group, n = 10), hydrogel with 25 µg/mL bFGF (gel+bFGF group, n = 10), hydrogel with 1 µg/mL IGF1 in PLGA microparticles (gel+IGF1 group, n = 10), and hydrogel with 25 µg/mL bFGF and 1 µg/mL IGF1 in PLGA microparticles (gel+bFGF/IGF1 group, n = 10). The amount of each growth factor that was injected in this study was based on amounts used in previous literature that were able to improve cardiac function following myocardial infarction.16,17,23,24 The infarcted anterior surface of the rat heart was exposed through a left thoracotomy. A 23 gauge needle connected to a 1 mL syringe was inserted, with the bevel up, just under the surface of the tissue at an angle of 10–20°. Injection was completed quickly, taking care not to inject material into the ventricular cavity or allow it to undergo phase transition inside the needle. For rats receiving hydrogel injection, a total volume of 400 µL of hydrogel solution in PBS was injected into four circumferentially distributed wall regions bordering the infarct as well as into the center of the infarct (5 injections, 80 µL per region). For rats in the PBS group, 400 µL PBS was injected into the same locations with the same volumes. The incision was closed in layers with 5–0 polypropylene continuous sutures for both groups. For prophylaxis of lethal ventricular arrhythmia, 10 mg/kg of lidocaine was administered intramuscularly once prior to surgery. For postoperative analgesic treatment, 0.1 mg/ kg of buprenorphine was administered subcutaneously 3 times per day for 3 days after surgery. For prophylaxis of surgical site infection, 100 mg/kg of cefuroxime was administered intramuscularly twice a day for 3 days after surgery.
Echocardiography was performed immediately before injection (2 weeks postinfarction), as well as 4, 8, 12, and 16 weeks after injection. To perform the procedure, rats were anesthetized with 1.25–1.5% isoflurane inhalation. Standard transthoracic echocardiography was performed using the Acuson Sequoia C256 system with a 13-MHz linear ultrasonic transducer (Acuson Corporation) in a phased array format. The LV short axis view was studied using B-mode measurements. The end-systolic (ESA) and end-diastolic (EDA) inner LV areas were measured by outlining the endocardial surface during the systolic and diastolic phase. LV fractional area change (FAC) was calculated as FAC = [(LVEDA – LVESA)/LVEDA] × 100%. All measurements were performed using OsiriX image processing application v.3.7.1 (www.osirix-viewer.com).
Rats were sacrificed for histological analysis of the hearts 16 weeks after injection. Animals were anesthetized, and the heart was exposed and arrested at the diastolic phase by injection into the apex of 2 mL of a hypothermic arresting solution including 10 U/mL of heparin, 68 mM NaCl, 1 M KCl, 36 mM NaHCO3, 2.0 mM MgCl2, 1.4 mM Na2SO4, 11 mM dextrose, and 30 mM 2,3-butanedione monoxime. The excised hearts were fixed in 2% paraformaldehyde for 2 h before being embedded in optimal cutting temperature compound (Tissue-Tek) and frozen at −80 °C. LV tissues were serially sectioned to a thickness of 8 µm in the LV transverse direction. Masson’s trichrome staining and immunohistochemical staining were performed with antibodies against alpha-smooth muscle actin (α-SMA; 1:200, Abcam), CD 68 (1:100, Serotec), and CD 163 (1:100, Serotec). Nuclei were stained with 40′,6-diamidino-2-phenylindole (DAPI; 1:10000, Sigma). For evaluation of the extent of fibrosis of explanted hearts, percent fibrosis was determined using image analysis software (ImageJ v.1.41, National Institutes of Health, Bethesda, Maryland) of Masson’s trichrome stained samples. Specifically, the fraction of an image stained positively for Masson’s trichrome in the infarcted and noninfarcted myocardium was divided by the sum of all connective tissue and muscle areas. For quantification of the immunohistochemical staining, each LV from 6 different animals was photographed in 10 different microscopic fields at 200× magnification for all antibodies. For DAPI slides, images were recorded at 400× magnification. Vessels were identified as tubular structures positively stained for αSMA and arterioles were defined as αSMA-positive structures, having visible lumens, and more than 10 µm in diameter.10,34 All measurements and assessments were performed using a digital image analyzer (ImageJ v.1.41, National Institutes of Health, Bethesda, Maryland). LV cross sections were photographed in the region of the central material injection point. Infarction scar area was measured using computer-based planimetry. LV anterior wall thickness was expressed as follows: scar area/[(epicardial circumference + endocardial circumference)/2]. Measurement of each parameter (n = 6 per each group) was performed using ImageJ analysis software (version 1.41; NIH).
To test the residence time of bFGF and IGF1 in the heart, animals for each treatment group above were injected two weeks following MI as described above. At 5 min, 2 days, and 2 weeks after injection, three animals each from the hydrogel and hydrogel plus individual growth factor groups (n = 27) were euthanized and their complete hearts were immediately extracted and snap frozen in liquid nitrogen. The LV free wall of each heart was excised and digested using a tissue homogenizer and protein extraction solution followed by gentle mixing for 2 h at 4 °C. Samples were centrifuged for 10 min and the supernatant was decanted. Growth factor concentration in the supernatants was determined by ELISA. Healthy hearts (n = 3) and PBS-injected hearts (n = 9) were similarly digested and assayed for use as controls.
Statistical Analyses
Where three or more groups were being compared, one-way ANOVA was employed. Results are presented as the mean with standard deviation. To determine if changes in cardiac function over time varied among the experimental groups, a two-way repeated-measures ANOVA was used to determine the effects of treatment, time, and treatment-by-time interaction. The REGWQ post hoc testing was used when the necessary assumptions were met. When there was a lack of homogeneity of variance Games-Howell testing was used. Statistical significance was defined as p < 0.05.
RESULTS
Material Characterization
Poly(NIPAAm-co-HEMA-co-MAPLA) was successfully synthesized with properties similar to what has been reported previously.28 Specifically, the Mn was 34.1 kD, Mw was 67.1 kD, and PDI was 1.96, with an LCST of 19.4 °C. The maximum of the shear storage and loss moduli of the hydrogel were 11.1 and 22.3 kPa, respectively. When microparticles were added to the gel solution the maximum of the shear storage and loss moduli were 10.0 and 23.1 kPa, respectively, and the LCST was 21.3 °C. PLGA particles demonstrated an encapsulation efficiency of 56% for IGF1 leading to a concentration of 0.126 µg of IGF1 per mg of PLGA particle. The microparticles had an average diameter of 29 ± 20 µm.
In Vitro Growth Factor Release
The release rate of 125I-bFGF from the hydrogels is shown in Figure 1. During the initial gelation, which lasts approximately 3 h, there was significant release of bFGF from the gels. The extent of that initial loss was influenced by the presence of excipient with the protein. The release at 3 h for plain bFGF was nearly identical to the release when BSA was also present, with 41.5 ± 1.1% and 40.9 ± 1.2% released, respectively. When heparin was also combined with BSA and bFGF, the amount released after 3 h nearly doubled to 80.0 ± 2.8% (p < 0.05). Release profiles from the hydrogel after this early release were similar and characterized by a slow, steady release over several weeks. Specifically, after the first 3 h, protein was released at an average rate of 2.2%/week for the first 4 weeks and increased to an average of 3.5%/week for the next 5 weeks. Radioactivity measurements of the hydrogel samples at the end of the 35-day release study confirmed that all remaining protein was accounted for inside the hydrogel.
Figure 1.
Release profiles of 125I-bFGF from poly(NIPAAm-co-HEMA-co-MAPLA) hydrogels. The presence of heparin allowed more bFGF to be released during gel formation (A). Proliferation of rat smooth muscle cells in response to bFGF released from the hydrogel (B) or IGF1 released from microparticles inside the hydrogel (C). bFGF release caused proliferation in the first few weeks of release whereas IGF1 caused proliferation over a longer time with a peak after four weeks: *shows significance (p < 0.05) compared to controls (100%); †denotes value significantly higher than all other time points.
The cellular assays showed that the bFGF and IGF1 released from gels retained bioactivity as demonstrated by increased rat SMC proliferation – Figure 1. Gels containing bFGF elicited increased muscle cell proliferation for 4 weeks (p < 0.05) compared to control gels in medium, with a peak effect at 9 days. In contrast, IGF1 released from microparticles in the gel was able to increase SMC proliferation above control gels continuously for 3 months (p < 0.05). The peak effect of IGF1 was seen at 4 weeks demonstrating the delayed IGF1 release from this carrier system.
In Vivo Injection Results
The infarction procedure was performed in a total of 114 rats, weighing 186 ± 11 g. Within 36 h of infarction, 12 rats died, for a mortality rate of 10.5%. Among the surviving 102 rats, 9 rats (8.8%) with <25% functional infarct based on echocardiographic assessment were excluded from the study. The intraoperative mortality rate for the second surgery was 8.6% (n = 8), and these animals were excluded from the study. There was no late mortality or morbidity after the second surgery. There was no difference between the groups in terms of body weight (230 ± 15 g) and tibial length (49 ± 1 mm) at the 16 week end point.
There were no differences between treatment groups for geometric (EDA and ESA) or functional (%FAC) parameters preinjection. Compared to preinjection, FAC was significantly reduced in hearts injected with PBS after 16 weeks (Figure 2; representative videos can be found in the Supporting Information). By contrast, there was no significant decrease in FAC for any hearts receiving hydrogel injection. The inclusion of growth factors did not change the results seen from injection of the hydrogel without growth factors. Cardiac dilation was markedly inhibited after gel injection compared to PBS controls (Figure 3). At 16 weeks, the ESA and EDA of hearts receiving hydrogel was 0.42 ± 0.15 and 0.62 ± 0.13 cm2, respectively, compared to 0.70 ± 0.10 and 0.88 ± 0.11 cm2 for hearts injected with PBS (p < 0.05). There was no difference in dilation when the hydrogel was loaded with growth factors compared to hydrogel without these factors. In all cases, the dilation was statistically larger at 16 weeks for all experimental groups than at baseline (p < 0.05). In the case of gel-injected groups, the majority of the change occurred in the first 4 weeks, and subsequently, there was no significant difference for either of these parameters over time from 4 to 16 weeks. In comparison, following PBS injection the area at end systole and diastole continued to increase at each time point.
Figure 2.
Effect of gel injection on fractional area change 16 weeks after treatment (top). Gel injection prevented the decreased function observed in hearts receiving PBS injection. Addition of growth factors did not improve upon the results of the plain hydrogel: *denotes a significant change from preinjection; †denotes differences between groups over time. Representative M-mode echocardiographic images of the left ventricle for each group (bottom). An image from an age-matched healthy heart is from the data set used in ref 9. Scale bar: 1 cm and 0.2 s.
Figure 3.
Measurements of dilation in hearts receiving injection of hydrogels with or without growth factors: *denotes a significant change from preinjection; †denotes differences between groups over time.
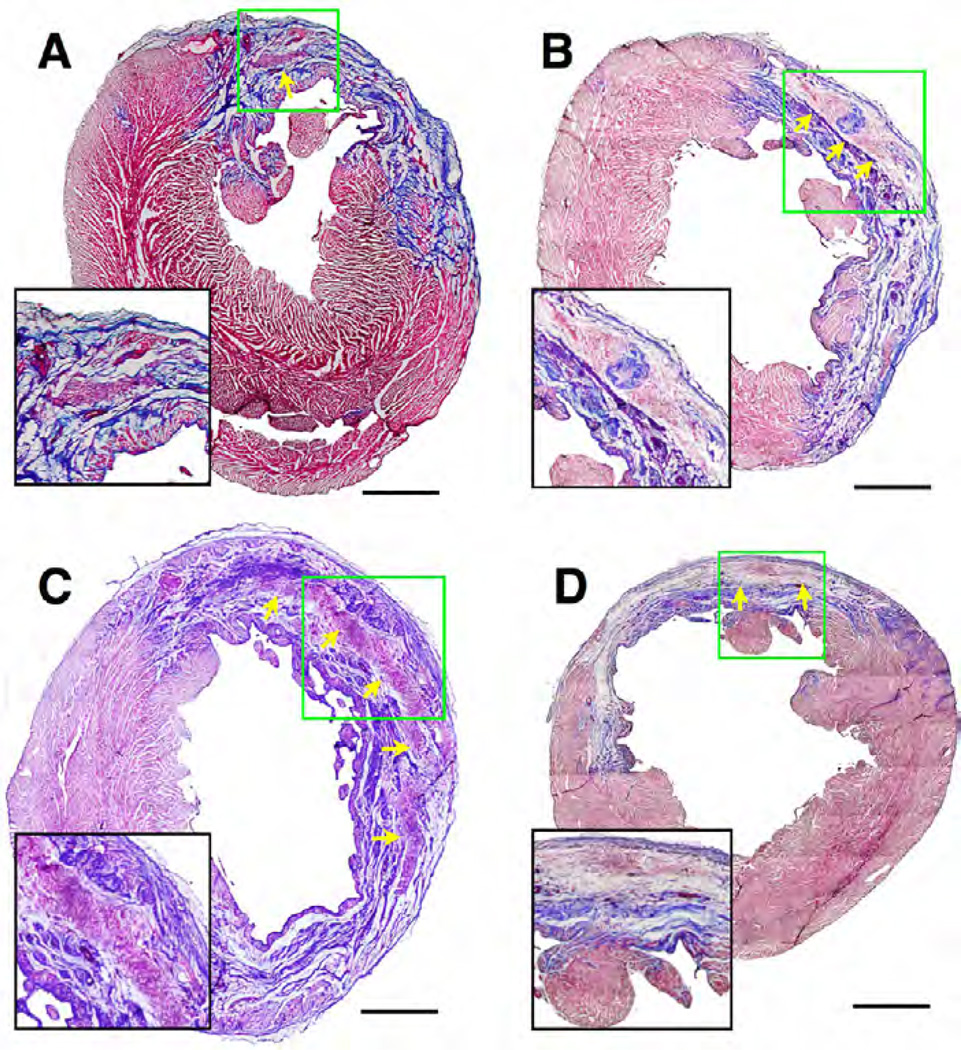
Hearts explanted after 16 weeks showed regions where the material was still present and infiltrated with cells, as confirmed by nuclear staining of serial sections – Figure 4 and Figure 5. The percent fibrosis for all groups at 16 weeks was not statistically different from each other (hydrogel only 17.4 ± 2.5, bFGF 19.4 ± 2.0%, IGF-1 19.7 ± 4.5%, bFGF+IGF-1 17.9 ± 2.0%, PBS 13.9 ± 1.2%). LV free wall thicknesses for hearts injected with hydrogel, hydrogel + bFGF, hydrogel + IGF1, and hydrogel + bFGF + IGF1 were 1.60 ± 0.22, 1.84 ± 0.17, 1.68 ± 0.21, and 1.72 ± 0.36 mm, respectively. These values were not statistically different from each other but they were each significantly larger than for PBS control hearts (0.79 ± 0.09 mm, p < .0.05) and significantly smaller than healthy controls (3.25 ± 0.19 mm, p < 0.05). Immunohistochemical evaluation of the LV walls at 16 weeks showed that all hearts injected with hydrogel showed CD68 macrophage cell infiltration, αSMA-positive cells near the injected material and neighboring infarct regions, and evidence of vascularization (Figure 5). Statistical analyses on all histological observations did not reveal any differences in the amount of vascularization or the number or type of cells infiltrating the infarct zone at 16 weeks following injection of hydrogels with or without different growth factors (Table 1). At 16 weeks, the material was clearly infiltrated by cells staining positive for CD163, which is a marker for macrophages demonstrating an M2 phenotype. The relative degree of positive staining for nuclei and αSMA(+) cells associated with injected material compared to PBS injected hearts is presented in Table 2.
Figure 4.
Representative Masson’s trichrome stained cardiac cross sections 16 weeks after injection of unloaded material (A), or material loaded with bFGF (B), IGF1-loaded microparticles (C), or both (D). Arrows point to residual hydrogel infiltrated with cells, which are presented in the insets at a higher magnification. Scale bars = 500 µm.
Figure 5.
Representative images of αSMA (green), macrophage CD68 (red), and DNA (blue) staining from hearts receiving injection of hydrogel with and without growth factors at 16 wk. There is marked infiltration of macrophages into the hydrogel and presence of smooth muscle cells near the gel. Scale bar = 100 µm for all images; epi designates the epicardial surface; end designates the endocardial surface; dashed line shows edge of hydrogel material; *shows which side of the line has the gel.
Table 1.
Quantification of Immunohistochemical Staining of Infarct Zones in Hearts
| hydrogel type | nuclei (per HPF)a |
αSMA (×10000 pixels per HPF) |
CD68 (×10000 pixels per HPF) |
CD 163 (×10000 pixels per HPF) |
vessels (per HPF) |
arterioles (per HPF) |
|---|---|---|---|---|---|---|
| plain gel | 126 ± 34 | 3.6 ± 1.8 | 3.4 ± 0.6 | 2.0 ± 0.5 | 4.1 ± 0.9 | 0.3 ± 0.1 |
| gel + bFGF | 136 ± 28 | 6.0 ± 3.0 | 5.1 ± 2.7 | 3.5 ± 1.2 | 3.8 ± 0.7 | 0.7 ± 0.4 |
| gel + IGF1 | 216 ± 79 | 4.0 ± 1.2 | 3.9 ± 1.7 | 3.5 ± 1.4 | 3.1 ± 0.8 | 0.5 ± 0.4 |
| gel + bFGF + IGF1 | 157 ± 55 | 3.9 ± 1.6 | 3.6 ± 2.5 | 2.8 ± 1.7 | 5.1 ± 2.0 | 0.7 ± 0.4 |
HPF = high-power field.
Table 2.
Relative Degree of Immunohistochemical Labeling of Cells Associated with Injected Hydrogela
| inside hydrogel |
outer layer of the liydrogel |
infarct area adjacent to liydrogel |
infarct area in PBS injected heart |
|
|---|---|---|---|---|
| nuclei | + | + | ++ | + |
| αSMA(+) cells |
− | + | ++ | −b |
− = not present; + = present; ++ = strongly present.
αSMA in the vascular wall has been excluded.
The in vivo detection of elevated levels of the growth factors loaded into the gels for treated animals are shown in Figure 6. Hearts injected with the bFGF-loaded gel demonstrated a 590-fold, 10-fold, and 2-fold increase of bFGF content 5 min, 2 days, and 2 weeks after injection, respectively, compared to injection of gel without growth factor at the same time points. Similarly, there was a 7-fold, 3-fold, and 4-fold increase in detected IGF1 for hearts receiving hydrogel mixed with IGF1-loaded microparticles. There was no difference in detectable bFGF or IGF1 between infarct control hearts and those receiving injection of hydrogel without growth factors.
Figure 6.
Total content of bFGF (A) and IGF1 (B) in the left ventricular free wall of rat hearts injected with either bFGF-loaded hydrogel or hydrogel loaded with microparticles encapsulating IGF1, respectively. Increased levels for both growth factors are shown up to 2 weeks after injection: *denotes significance compared to injection of plain gel at the same time point.
DISCUSSION
The continued development of intramyocardial biomaterial injection therapy requires that the mechanisms by which benefits are obtained be further elucidated. Many parameters may contribute to successful treatment, including timing and location of injections, distribution of material in the heart wall, and physical and biological properties of the injected material. For example, it has already been demonstrated by Wall et al. and Burdick et al. that, when controlling for all other material properties, stiffer materials have a greater capacity to maintain cardiac function than softer materials when injected after infarction.7,8 Informed by the finite element model of Wall et al., our lab reported on the injection of a degradable hydrogel with an initial modulus higher than those used previously which demonstrated that cardiac function and geometry were maintained for at least 8 weeks after injection in infarcted hearts. The current report presents the application of a material that has been designed to include features that are increasingly appreciated to be beneficial for intramyocardial injection therapy including the ability to provide substantial mechanical strength, deliver bioactive growth factors, and degrade slowly. Specifically, the material has a higher stiffness than materials used previously in this application, with an initial modulus of 90 kPa.28 Because the hydrogel used in this study undergoes rapid sol–gel transition upon injection into the heart, it will form distinct regions within the tissue. This presents regions of mechanical strength similar to what has been studied in finite element models.8,35 Additionally, the material degrades over approximately 5 months in vivo and is able to deliver dual bioactive growth factors to the injection site. The effects of treatment with this current material were observed for the extended period of 4 months after injection, to better elucidate the long-term benefits of this therapy.
Using 125I-bFGF it was shown that the release profile of bFGF was similar to what has been previously shown with BSA delivery from this material: particularly a substantial burst release during the first 3 h of gel formation followed by slow release afterward.29 It was expected that, similar to the previous study, the release of protein would accelerate as material degradation progressed over time. While there is very slow release after the initial burst in the in vitro study, the dynamic environment and increased potential for material degradation in vivo may accelerate the protein release after gel formation. This was shown by measuring the growth factor content of the LV for two weeks after injection of growth factor loaded gels. At each subsequent time point measured there was an order of magnitude reduction in detected protein, suggesting a much more rapid release.
There is no accepted standard that defines the mass of a given growth factor that must be delivered over a set interval in order to show a biological effect in vivo. Looking to the prior literature, some broad ranges for necessary protein delivery rates can be found. First, amounts of bFGF that have been injected into infarcted rat hearts within a carrier for controlled release and that have shown an ability to improve cardiac function range from 2 µg to 100 µg.16,17,36,37 The release rate of bFGF from these carriers is not often investigated making it difficult to know the amount of protein released over a given time period, but the broad range of loading amounts suggests a broad range of delivered protein masses over time. In the case of Garbern et al., 5 µg of bFGF was delivered per rat heart in a pH and temperature-responsive synthetic hydrogel.16 Tissue digestion of hearts at 1, 2, and 7 days after injection demonstrated the in vivo delivery rate. In this case, >80% of the bFGF (4 µg) was released in the first day, leaving a subsequent average rate of delivery of 0.15 µg/day over the rest of the first week and <0.2 µg total available to release beyond 1 week. This mass of protein delivery was associated with maintenance of cardiac function versus controls at the 4 week study end point. Through ELISA measurements on digested LV tissue, the protein delivery rate in vivo in this study was shown to be faster than in vitro, with little elevation of bFGF measured in the LV at the 2 week measurement. On average this would equate to a two-week release rate of 0.9 µg bFGF/ day for the 12.5 µg of bFGF injected per animal. This number is above the amount that has been associated with a benefit in other in vivo studies as described above. From this perspective, it is reasonable to expect that the amount and rate of bFGF delivered in this study would have been adequate to show an improvement to cardiac function.
The bioactivity of bFGF released beyond the first few weeks in vitro cannot be determined from these data. The absence of rat SMC proliferation beyond 4 weeks in vitro may be due to the slow rate of release leading to an inadequate concentration to elicit a response. It may also be an indication that there is a loss of bioactivity in the protein that resides in the gel for extended periods. It was interesting to note that when growth factor was loaded into the gel in the presence of BSA it released at the same rate as when it was loaded by itself. However, when heparin was mixed into the solution nearly twice as much protein was released during gelation. It was because of this high release rate in the presence of heparin that BSA was chosen as the excipient for the in vivo studies. The differences in release rates suggest that there may be a significant interaction between bFGF and the polymer chains in the gel which was disrupted once heparin, which can bind bFGF, was added. This highlights the importance of proper in vitro investigation of each polymer–protein combination for injection therapy before in vivo use.
The dual protein delivery system described here also agrees well with previous release studies from this material. The in vitro bioactivity assays show that bFGF, which was loaded free into the gel phase, was released early as manifest by increased rat SMC proliferation in vitro for the first two weeks of release. Beyond this, there was no observed effect of bFGF. Likewise, the fact that IGF1 release was able to increase SMC proliferation in vitro over a long period with a peak at 4 weeks is in agreement with the hypothesis that encapsulation would decrease the burst and allow a delayed, yet sustained delivery. Visual inspection of growing cells in this assay qualitatively agreed with the MTS results, however a direct measurement of cell number would be the most accurate method to determine SMC proliferation. Although IGF1 was not quantitatively measured in this study, the effect that protein encapsulation in microparticles has on protein release rates within this hydrogel was shown previously.29 In that report, the initial release of BSA was attenuated, and the continued release was more prolonged, when the protein was encapsulated within microparticles loaded into the gel. While it is logical that placing a second protein within a separate compartment (the microparticles) within the hydrogel would delay the release, there may be differences in the degree to which the release is delayed for the model protein BSA and IGF1.
The in vitro degradation rate of this polymer was investigated previously and reported by Ma et al.28 While the exact rate of in vivo degradation is difficult to quantify it is generally appreciated that degradation will be increased in vivo due to the complex milieu of cells and enzymes that are acting on the material. This material has a degradable side chain containing ester bonds that provides a time-dependent solubility of the polymer backbone as the ester bonds are cleaved. While water can cleave these ester bonds, it is well-known that enzymes such as esterase and lipase, both of which are produced by macrophages, can significantly speed cleavage of that bond.38,39 A basic comparison between the in vitro and in vivo degradation speeds can be gained by comparing the published in vitro degradation results to what is seen histologically from hearts explanted 16 weeks after injection in this study. After 16 weeks in vitro, this hydrogel maintained approximately 80% of its mass, with rapid decreases in mass occurring over the subsequent 2 months. Looking at the histological images from explanted hearts in this study 16 weeks after injection, it is difficult to distinguish regions of hydrogel remaining. This highlights the fact that in vivo degradation will be much faster than in vitro testing.
Injection of poly(NIPAAm-co-HEMA-co-MAPLA) in two-week-old infarcts is remarkable for the ability to maintain cardiac function for at least 16 weeks after injection. Not only is the improvement compared to PBS injections evident, this result demonstrates the potential long-term benefits of this approach. This study uses the same animal model and injection regimen employed previously by our lab for injection of a poly(NIPAAm-co-HEMA-co-trimethylene carbonate) hydrogel.27 While a direct comparison between studies is not possible due to surgical and imaging variables, we demonstrated a similar ability to maintain cardiac function as was seen in that earlier study, but in this case the benefits were studied and evident for 16 weeks compared to just 8 weeks seen previously. Additional testing is needed to determine how long beyond 16 weeks this benefit can be maintained.
During the 16 week follow up time after injection cellular infiltration was observed in all of the injected material mass as was the presence of αSMA-positive cells adjacent to the injection sites. The presence of these smooth muscle-like cells was previously observed following injection of a different thermoresponsive gel with initial modulus of 6 kPa that degraded over a few months in vivo.27 While not completed here, in that study the contractile nature of the smooth muscle cells was demonstrated by colocalized staining for αSMA, h-caldesmon, and smooth muscle myosin. Interesting research into the biological response following application of a stiff and elastomeric degradable epicardial patch has also shown a similar aSMA-positive zone below the implant.40 That muscular zone was shown to be similar to the myocardium during fetal growth and differentiation and may be a sign that the observed cells are on a pathway of tissue regeneration. The exact mechanisms that are involved in the growth and survival of these muscle cells near the injected material are unknown but may be related to a combination of appropriate cellular infiltration, material degradation, and mechanical support.
PBS-injected control animals had functional and geometric parameters measured through the 16 week end point of the study, but no histological characterization was performed except for Masson’s trichrome staining. This limitation prevents conclusions from being made on the effect that hydrogel injection has over saline injection in terms of tissue remodeling. Previous work using the same animal model has demonstrated that, in conjunction with decreased functional capacity, infarcted rat hearts showed decreased wall thickness, significant scar formation, and decreased vessel density compared to injection of a similar thermally responsive, degradable hydrogel at 8 weeks postinjection.27
A central observation in this study was the lack of additional benefit to either cardiac function or cellular environment when either or both growth factors were included in the material. While aspects of angiogenesis and the immune response to the hydrogel were studied here, there may be other cellular behaviors such as cardiomyocyte proliferation or cardiac stem cell recruitment that could be influenced by the presence of bFGF or IGF1 and which could be studied in this model. This may provide better clarity on what role included growth factors play in the tissue response after delivery. The amounts of growth factor that were delivered were similar to amounts that have shown benefits in other intramyocardial injection studies (2–100 µg bFGF and 250–250 ng IGF1).16,17,23,36,37,41 However, one main benefit of IGF1 is in protecting cardiomyocytes from apoptosis when administered early after infarction.19 Given that infarcts were two weeks old before this growth factor was delivered, the ability of IGF1 to improve cardiac function may have been limited in this animal model. Beyond acute myocyte survival benefits, IGF1 is known to provide cardiac benefits relating to cardiomyocyte size, regulation of cellular glucose metabolism, increased Akt cellular signaling, and inotropic effects that could be improved from long-term delivery and studied in future work.19 PBS-injected control animals were studied for functional and geometric parameters through the 16 week end point of the study, but no histological characterization was performed. This limitation prevents conclusions from being made on the effect that hydrogel injection has over PBS injection. Previous work using the same animal model has demonstrated that in conjunction with decreased functional capacity, infarcted rat hearts showed decreased wall thickness, significant scar formation, and decreased vessel density compared to injection of a similar thermally responsive, degradable hydrogel.
It was observed that the tissue had elevated levels of both bFGF and IGF1 for at least two weeks following injection of growth factor loaded hydrogel, which would be consistent with growth factor being locally released from the gel at the site of injury. However, ELISA assay measurement of growth factor content from digested tissue is not able to differentiate between growth factor that had been released from the gel to the surrounding tissue and the growth factor that remained in the gel for future release. In vitro, the bioactivity of growth factors released from the gel was demonstrated by cellular proliferation assays. In this study the frequency of ELISA assays used to detect growth factor content in explanted hearts did not provide high resolution of the rates of delivery over time or the profile of the sequential release of bFGF and IGF1 in vivo. While the increase in IGF1 in the digested LV tissue was mostly gone by 2 weeks, it is possible that more IGF1, which may not have been removed from the particles during tissue digestion, remained loaded in the microparticles to be released slowly over a longer term.
These results may provide important information about the mechanisms by which benefits are observed following hydrogel injection. It is well-known that myriad inflammatory processes are at work following injection of a material, especially a synthetic one, into the body.42,43 One aspect of that response is the recruitment of macrophages to the site of injury, which are known to excrete many cytokines and growth factors that aid in new ECM formation and angiogenesis.44 We demonstrated that even 16 weeks after hydrogel injection, macrophage infiltration to the site of injury persisted. Many of these cells stained positively as those of a M2 phenotype, which encourages tissue regeneration as opposed to scar formation.43,45 It may be that a continued release of angiogenic and pro-healing factors from these cells at the implant site is adequate to provide robust angiogenesis that is not further augmented by exogenous delivery of these factors from the material. However, the exact effect that material injection had on the cellular response compared to PBS injection was not fully studied. While immunohistochemical staining of PBS injected hearts was not performed here, Fujimoto et al. previously showed that injection of a similar thermoresponsive material resulted in higher capillary density in the infarct zone than injection of PBS.27 The lack of detectable differences in bFGF and IGF1 levels between injection of material without growth factors and control hearts may be the result of the ELISA analysis having been done on full LV tissue digests. The local growth factor milieu at the infarct and material injection sites may be lost in this compounding, which would mask the elevation in these factors that are critical for local angiogenesis.
The degradation period of the material was also a likely contributor to the overall success of this material, especially as it relates to the cellular environment at the injection site. It has been shown previously that the benefits of material injection seen soon after injection can be altogether lost if the material degrades too quickly.3 On the other hand, nondegradable materials, whether they are infiltrated with cells or not, do not appear to provide any long-term benefits.4,5 The material used in this research is one that degrades in vivo over approximately 4–5 months. This time frame will be intimately tied to the cellular response in the native tissue. The material may remain present locally long enough to maintain cell recruitment to the injection site where the cells may provide continued positive tissue remodeling as described above. While the optimum timing of degradation in the myocardium remains to be determined, there is evidence that a duration beyond 2 months should be considered if the material is also providing mechanical support.6 The support provided during that time and the slow shifting of load-bearing responsibilities to the newly formed tissue may also play a role in the overall health and function of the cellular environment.
The inability of growth factor delivery to improve the functional results with hydrogel delivery is not unprecedented. Whereas in many cases intramyocardial delivery of a growth factor with a biomaterial has shown functional improvements, in other reports the benefits are seen only histologically in terms of cellular milieu and vascularization. For example, Shao et al. demonstrated that delivery of a bFGF gelatin showed a higher EF than injection of material alone 2 weeks after treatment, but the increase was not maintained for 4 weeks despite higher vascular density, decreased infarct expansion and fewer TUNEL-positive cells throughout the study.37 Likewise, delivery of IGF1 tethered to self-assembling peptides demonstrated bioactivity in the form of cellular Akt signaling, but was unable to improve functional or geometric cardiac parameters.41
Taken together, these and other data suggest that there may be a continuum between bioactivity, degradation, and mechanical strength of materials as it relates to improving cardiac function after injection. Whereas there are often demonstrated benefits to inclusion of growth factors in soft materials, there was no added benefit when growth factors were added to the stiff material in this report. In the absence of mechanical strength, the increased cellular response that comes from injection of growth factor loaded materials or some extracellular matrix materials may be necessary to elicit cardiac improvement. As strength of the material increases, the mechanical benefits that are known to exist in this application may overshadow the biological benefits of additional growth factors, at least those examined in this study. Appropriate material degradation, which is associated with a natural cellular response, may also be critical to success as a lack of degradation has been as ineffective at achieving long-term benefits as quickly degrading materials.
CONCLUSION
A synthetic, slowly degrading hydrogel was injected into two-week-old infarcted rat hearts. The material was able to maintain cardiac function for a period of at least 16 weeks. The injected material exhibited marked infiltration of macrophages in the infarct zone. Cells staining positive for αSMA were found throughout the infarct region near the injected material. Growth factors bFGF and IGF1 were successfully loaded into the gel directly or in polyester microparticles, respectively. Despite demonstration that injected growth factor was present at elevated levels in the LV for at least two weeks after injection, and that released growth factor was bioactive in vitro, there was no added benefit to cardiac function or histology when growth factors were present. While adding to the understanding of how benefits may be achieved from intramyocardial injection therapy, these results suggest that biomaterials with appropriate properties can ameliorate cardiac damage following intramyocardial injection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH), Grant No. HL105911. D.M.N. was supported by NIH Training Grant No. T32-HL076124.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Video sequences of representative M-mode echocardiograms of the left ventricle at the 16 week end point for each group demonstrate the size and function of each heart. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Acta Biomater. 2011;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rane AA, Christman KL. J. Am. Coll. Cardiol. 2011;58:2615–2629. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Christman KL, Chin E, Sievers RE, Saeed M, Lee RJ. J. Thorac. Cardiovasc. Surg. 2009;137:180–187. doi: 10.1016/j.jtcvs.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. J. Card. Failure. 2009;15:629–636. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Rane AA, Chuang JS, Shah A, Hu DP, Dalton ND, Gu Y, Peterson KL, Omens JH, Christman KL. PLoS ONE. 2011;6:e21571. doi: 10.1371/journal.pone.0021571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tous E, Ifkovits JL, Koomalsingh KJ, Shuto T, Soeda T, Kondo N, Gorman JH, Gorman RC, Burdick JA. Biomacromolecules. 2011;12:4127–4135. doi: 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, Gorman RC, Burdick JA. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11507–11512. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume R, Hong Y, Takanari K, Fujimoto KL, Tobita K, Wagner WR. Biomaterials. 2013;34:7353–7363. doi: 10.1016/j.biomaterials.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singelyn JM, Dequach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, DeMaria AN, Dib N, Christman KL. J. Am. Coll. Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes GC, Biswas SS, Yin B, Coleman RE, DeGrado TR, Landolfo CK, Lowe JE, Annex BH, Landolfo KP. Ann. Thorac. Surg. 2004;77:812–818. doi: 10.1016/j.athoracsur.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 13.Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD, Simons M, Hung D. J. Pharmacol. Exp. Ther. 2000;292:795–802. [PubMed] [Google Scholar]
- 14.Richardson TP, Peters MG, Ennett AB, Mooney DJ. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 15.Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, Matsumoto K, Nakamura T, Kaneda Y, Higaki J, Ogihara T. Gene Ther. 2000;7:417–427. doi: 10.1038/sj.gt.3301104. [DOI] [PubMed] [Google Scholar]
- 16.Garbern JC, Minami E, Stayton PS, Murry CE. Biomaterials. 2011;32:2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Zhang X, Li Y, Ma Y, Zhang Y, Liu Z, Zhou J, Lin Q, Wang Y, Duan G, Wang C. J. Heart Lung Trans. 2010;29:881–887. doi: 10.1016/j.healun.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Zeng F, Huang X-P, Chung JCY, Konecny F, Weisel RD, Li R-K. Biomaterials. 2011;32:579–586. doi: 10.1016/j.biomaterials.2010.08.098. [DOI] [PubMed] [Google Scholar]
- 19.Suleiman MS, Singh RJ, Stewart CE. Pharmacol. Ther. 2007;114:278–294. doi: 10.1016/j.pharmthera.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Circ. Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 21.Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, Purushothaman S, Galuppo V, Iaconetti C, Waring CD, Smith A, Torella M, Cuellas Ramon C, Gonzalo-Orden JM, Agosti V, Indolfi C, Galinanes M, Fernandez-Vazquez F, Nadal-Ginard B. J. Am. Coll. Cardiol. 2011;58:977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Cheng K, Malliaras K, Shen D, Tseliou E, Ionta V, Smith J, Galang G, Sun B, Houde G, Marban E. J. Am. Coll. Cardiol. 2012;59:256–264. doi: 10.1016/j.jacc.2011.10.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruvinov E, Leor J, Cohen S. Biomaterials. 2011;32:565–578. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 24.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Wardell E, Brodin LA, Mooney DJ, Sylven C. Cardiovasc. Res. 2007;75:178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Tengood JE, Kovach KM, Vescovi PE, Russell AJ, Little SR. Biomaterials. 2010;31:7805–7812. doi: 10.1016/j.biomaterials.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tengood JE, Ridenour R, Brodsky R, Russell AJ, Little SR. Tissue Eng., Part A. 2011;17:1181–1189. doi: 10.1089/ten.tea.2010.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, Wagner WR. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Z, Nelson DM, Hong Y, Wagner WR. Biomacromolecules. 2010;11:1873–1881. doi: 10.1021/bm1004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson DM, Ma Z, Leeson CE, Wagner WR. J. Biomed. Mater. Res., Part A. 2012;100A:776–785. doi: 10.1002/jbm.a.34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFail AJ, Chu CR, Izzo N, Marra KG. Biomaterials. 2006;27:1579–1585. doi: 10.1016/j.biomaterials.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Lynn Ray J, Leach R, Herbert J-M, Benson M. Methods Cell Sci. 2001;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 32.Holmes JW, Borg TK, Covell JW. Annu. Rev. Biomed. Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Zhang JQ, Zhang J, Lamparter S. J. Lab. Clin. Med. 2000;135:316–323. doi: 10.1067/mlc.2000.105971. [DOI] [PubMed] [Google Scholar]
- 34.Zhao ZQ, Puskas JD, Xu D, Wang NP, Mosunjac M, Guyton RA, Vinten-Johansen J, Matheny R. J. Am. Coll. Cardiol. 2010;55:1250–1261. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 35.Wenk JF, Wall ST, Peterson RC, Helgerson SL, Sabbah HN, Burger M, Stander N, Ratcliffe MB, Guccione JM. J. Biomech. Eng. 2009;131:10111–10117. doi: 10.1115/1.4000165. [DOI] [PubMed] [Google Scholar]
- 36.Iwakura A, Fujita M, Kataoka K, Tambara K, Sakakibara Y, Komeda M, Tabata Y. Heart Vessels. 2003;18:93–99. doi: 10.1007/s10380-002-0686-5. [DOI] [PubMed] [Google Scholar]
- 37.Shao ZQ, Takaji K, Katayama Y, Kunitomo R, Sakaguchi H, Lai ZF, Kawasuji M. Circulation. 2006;70:471–477. doi: 10.1253/circj.70.471. [DOI] [PubMed] [Google Scholar]
- 38.Peng H, Ling J, Liu J, Zhu N, Ni X, Shen Z. Polym. Degrad. Stab. 2010;95:643–650. [Google Scholar]
- 39.Labow RS, Meek E, Santerre JP. J. Biomed. Mater. Res. 2001;54:189–197. doi: 10.1002/1097-4636(200102)54:2<189::aid-jbm5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Fujimoto KL, Tobita K, Guan J, Hashizume R, Takanari K, Alfieri CM, Yutzey KE, Wagner WR. J. Card. Failure. 2012;18:585–595. doi: 10.1016/j.cardfail.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson JM, Rodriguez A, Chang DT. Semin. Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunderkötter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. J. Leukocyte Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 45.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Tissue Eng., Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.