Abstract
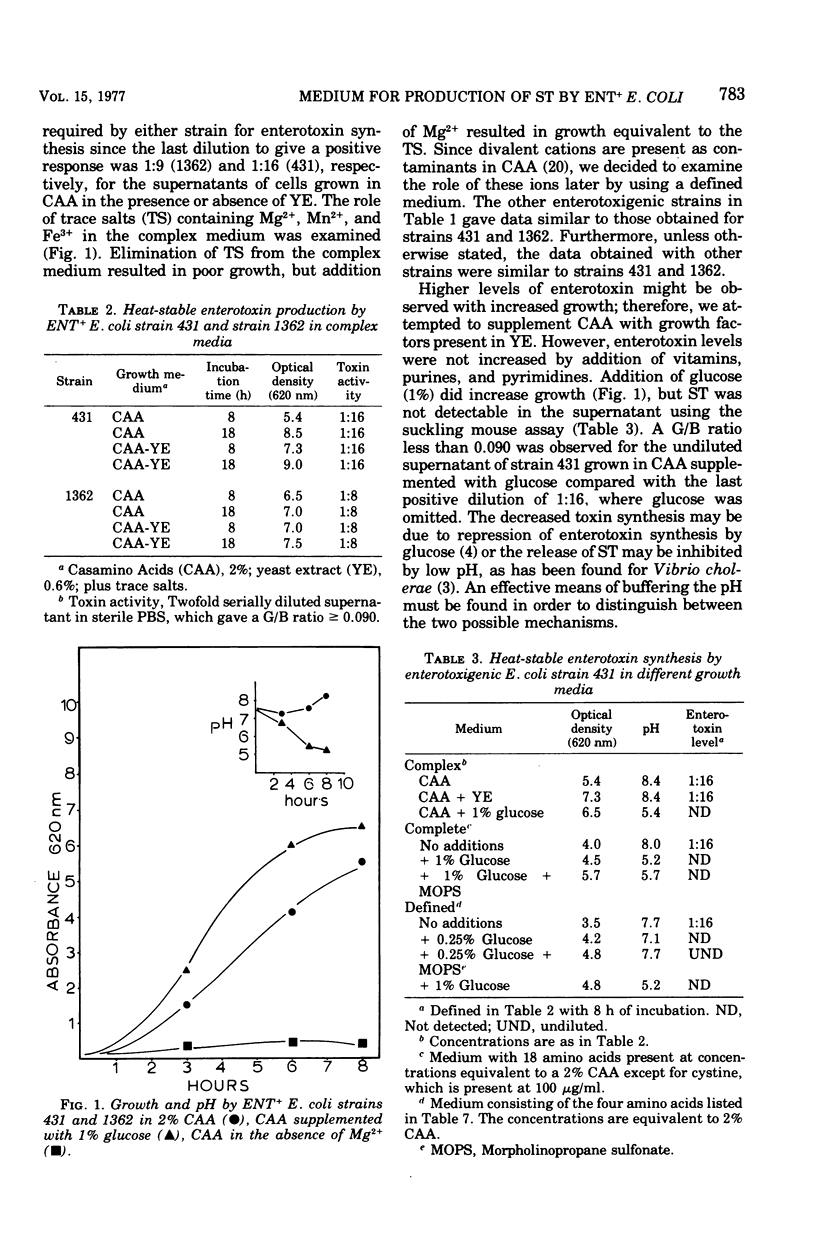
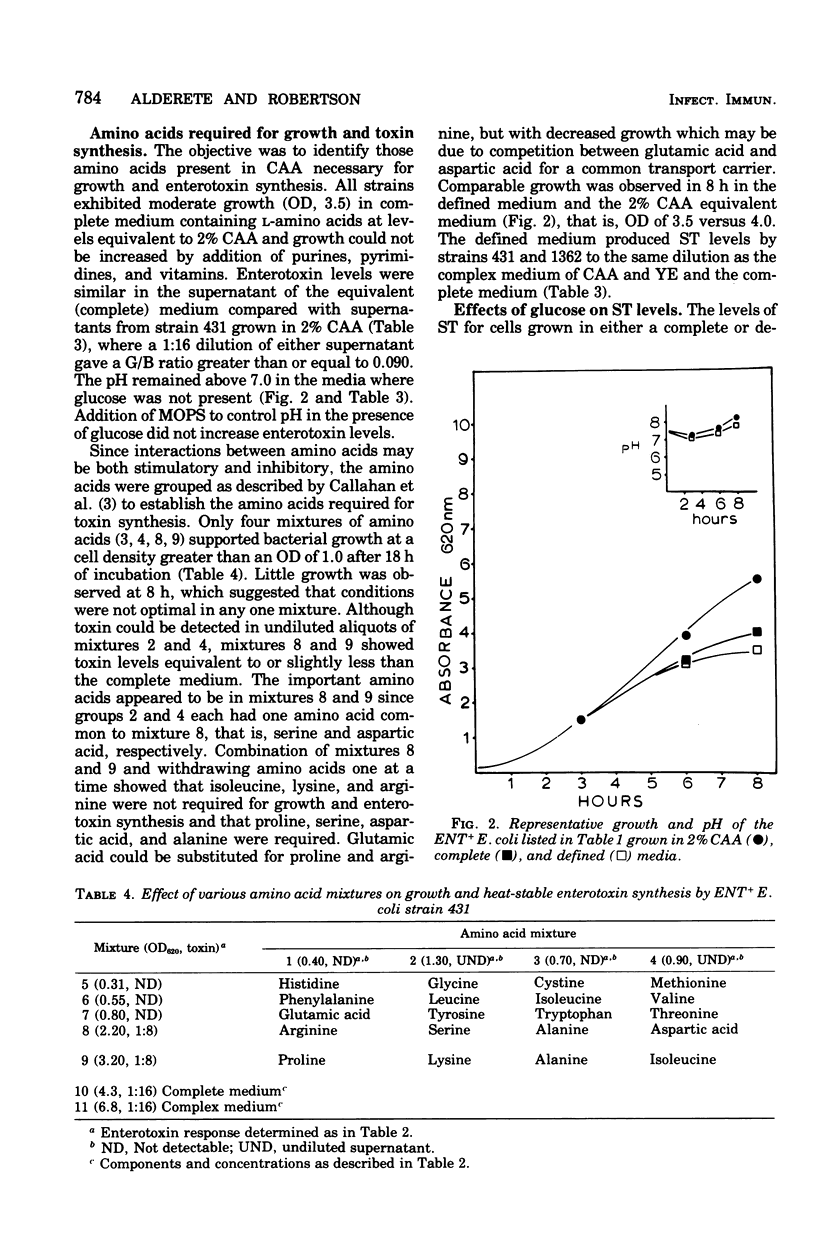
A defined medium has been developed which supports synthesis of heat-stable enterotoxin (ST) by porcine and bovine strains of enterotoxigenic (ENT+) Escherichia coli in levels equivalent or better than a complex Casamino Acids-salts medium. The medium components did not support production of heat-labile enterotoxin (LT) but were similar for ST synthesis by ENT+ strains producting only ST and those which produced ST in addition to LT. The amino acids in Casamino Acids found to be necessary for growth and enterotoxin synthesis were proline, serine, aspartic acid, and alanine. Maximal growth and toxin levels were obtained after 8 h of incubation. Improved growth, but not an increase in synthesis of ST, was observed in the presence of Mg2+, Mn2+ and Fe3+ compared with Mg2+ alone. A chelator, tricine, was necessary for maximal cell densities,, probably to solubilize trace ions and make them more available to the bacteria. Increased growth was observed upon addition of glucose to both complex and defined media; however, glucose as well as gluconate and pyruvate appeared to cause repression of toxin synthesis. Addition of vitamins, oleic acid, or DL-lactic acid to the defined medium slightly increased levels of ST.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bywater R. J. Dialysis and ultrafiltration of heat-stable enterotoxin from Escherichia coli. J Med Microbiol. 1972 Aug;5(3):337–343. doi: 10.1099/00222615-5-3-337. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Richardson S. H. Biochemistry of Vibrio cholerae virulence. 3. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973 Apr;7(4):567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Ryder R. C., Richardson S. H. Biochemistry of vibrio cholerae virulence. II. Skin permeability factor-cholera enterotoxin production in a chemically defined medium. Infect Immun. 1971 Nov;4(5):611–618. doi: 10.1128/iai.4.5.611-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Perlman R. L., Varmus H. E., Pastan I. Regulation of inducible enzyme synthesis in Escherichia coli by cyclic adenosine 3', 5'-monophosphate. J Biol Chem. 1969 Nov 10;244(21):5828–5835. [PubMed] [Google Scholar]
- Dorner F. Escherichia coli enterotoxin. Purification and partial characterization. J Biol Chem. 1975 Nov 25;250(22):8712–8719. [PubMed] [Google Scholar]
- Duncan J. L., Cho G. J. Production of staphylococcal alpha toxin. II. Glucose repression of toxin formation. Infect Immun. 1972 Nov;6(5):689–694. doi: 10.1128/iai.6.5.689-694.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Gorbach S. L. Identification of enterotoxigenic Escherichia coli and serum antitoxin activity by the vascular permeability factor assay. Infect Immun. 1973 Nov;8(5):731–735. doi: 10.1128/iai.8.5.731-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J. Biochemical properties of Escherichia coli low-molecular-weight, heat-stable enterotoxin. Infect Immun. 1974 Feb;9(2):342–347. doi: 10.1128/iai.9.2.342-347.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J., Braemer A. C., Bidlack D. E. Properties of the enterotoxic component in Escherichia coli enteropathogenic for swine. Infect Immun. 1973 Feb;7(2):178–189. doi: 10.1128/iai.7.2.178-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Partial purification and properties of Enterobacter cloacae heat-stable enterotoxin. Infect Immun. 1976 May;13(5):1307–1314. doi: 10.1128/iai.13.5.1307-1314.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Purification and properties of Klebsiella pneumoniae heat-stable enterotoxin. Infect Immun. 1976 Feb;13(2):373–381. doi: 10.1128/iai.13.2.373-381.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Mehlman I. J., Fishbein M., Gorbach S. L., Sanders A. C., Eide E. L., Olson J. C., Jr Pathogenicity of Escherichia coli recovered from food. J Assoc Off Anal Chem. 1976 Jan;59(1):67–80. [PubMed] [Google Scholar]
- Miller R. D., Fung D. Y. Amino acid requirements for the production of enterotoxin B by Staphylococcus aureus S-6 in a chemically defined medium. Appl Microbiol. 1973 May;25(5):800–806. doi: 10.1128/am.25.5.800-806.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan R. A. Amino acids and growth factors in vitamin-free casamino acids. Mycologia. 1971 Nov-Dec;63(6):1231–1234. [PubMed] [Google Scholar]
- Nolan R. A., Nolan W. G. Elemental analysis of vitamin-free casamino acids. Appl Microbiol. 1972 Aug;24(2):290–291. doi: 10.1128/am.24.2.290-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Sandefur P. D., Peterson J. W. Isolation of skin permeability factors from culture filtrates of Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):671–679. doi: 10.1128/iai.14.3.671-679.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein I., Green R. F., Santos D. S., Maas W. K. Partial purification and characterization of a heat-labile enterotoxin of Escherichia coli. Infect Immun. 1976 Jun;13(6):1710–1720. doi: 10.1128/iai.13.6.1710-1720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The effect of cell-free fluids prepared from cultures of human and animal enteropathogenic strains of Escherichia coli on ligated intestinal segments of rabbits and pigs. J Med Microbiol. 1970 Aug;3(3):403–409. doi: 10.1099/00222615-3-3-403. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. Studies on Escherichia coli enterotoxin. J Pathol Bacteriol. 1967 Apr;93(2):531–543. doi: 10.1002/path.1700930212. [DOI] [PubMed] [Google Scholar]


