Abstract
Entomopathogenic nematodes (a.k.a. EPN) represent a group of soil-inhabiting nematodes that parasitize a wide range of insects. These nematodes belong to two families: Steinernematidae and Heterorhabditidae. Until now, more than 70 species have been described in the Steinernematidae and there are about 20 species in the Heterorhabditidae. The nematodes have a mutualistic partnership with Enterobacteriaceae bacteria and together they act as a potent insecticidal complex that kills a wide range of insect species.
Herein, we focus on the most common techniques considered for collecting EPN from soil. The second part of this presentation focuses on the insect-baiting technique, a widely used approach for the isolation of EPN from soil samples, and the modified White trap technique which is used for the recovery of these nematodes from infected insects. These methods and techniques are key steps for the successful establishment of EPN cultures in the laboratory and also form the basis for other bioassays that consider these nematodes as model organisms for research in other biological disciplines. The techniques shown in this presentation correspond to those performed and/or designed by members of S. P. Stock laboratory as well as those described by various authors.
Keywords: Environmental Sciences, Issue 89, Entomology, Nematology, Steinernema, Heterorhabditis, nematodes, soil sampling, insect-bait, modified White-trap
Introduction
Many nematodes are associated with insects, and their host interactions range from beneficial to detrimental. Herein, we focus on a group of nematodes that are pathogens of insects, also known as entomopathogenic nematodes (or EPN). Two nematode families belong to this group of nematodes: Steinernematidae and Heterorhabditidae11,13. Their pathogenic effect is in fact conferred by their interaction with facultative anaerobic enteric bacteria (Xenorhabdus bacteria associate with steinernematids and Photorhabdus bacteria associate with heterorhabditids)2. The bacteria are vectored from one insect host to another by the only free living nematode stage, the third-stage infective juvenile (also known as dauer juvenile, infective juvenile or IJ), which lives in the soil. Once inside the insect, the nematodes release the bacteria into the insect’s hemolymph, which kill the insect host by massive septicemia. The bacteria also degrade the insect’s tissues and become the nematodes’ food source, allowing them to mature and multiply. Usually, one or two generations of adult nematodes are produced within the insect cadaver. The progeny of the last adult generation reassociates with a few bacterial cells in a more specialize manner than their previous nutritional relationship, and nurture these cells in their intestines as they move out from the insect carcass into the soil where they will await another insect to parasitize6,11,14 (Figure 1).
Since their discovery in 1927, EPN have been considered valuable alternatives to chemical pesticides as they can parasitize a wide range of insects that are agricultural pests3,4,5. However, over the past two decades, these nematodes and their symbiotic bacteria have been recognized as a malleable model system for studying biological, ecological and evolutionary aspects of host-microbe interactions14.
The goal of this presentation is to show the methods and techniques most commonly used for soil sampling and isolation of EPN. A variety of tools is available and can be used for collecting soil samples including: soil corers, trowels, post-hole diggers, augers, sampling tubes, hand shovels, among others (Figure 2).
Depending on the purpose of the study, two sampling strategies can be considered: a) stratified, b) random sampling (Figure 3). The stratified sampling is usually used as part of an intensive study in a specific or demarked area over a given period of time. In general, a transect is demarked and soil samples are taken at stipulated intervals in the transect over a period of time (Figure 3A)15. The random sampling is generally employed when focusing on an extensive area. This sampling strategy has been used for studying the diversity of EPN from a large geographic area or region (Figure 3B)12. A number of factors can be considered depending on the focus of the study including a diverse range of elevations, soil textures and habitats (e.g., cultivated fields, forests, pastures, parks, seashores, riparian areas, etc.).
We show the insect-baiting technique, which was originally described by Bedding and Akhurst1 and represents a simple and selective alternative for recovering EPN from soil samples. This is a selective procedure that is based on the premise that the nematodes (IJ stage) will be attracted to an insect host and parasitize it. This technique can also be considered for isolation of other insect pathogens such as fungi, and bacteria8,10.
We also demonstrate the modified White trap technique which is used for retrieving nematode progeny from infected insect hosts7,14. This is an effortless method that offers the advantage of retrieving a ‘clean’ nematode progeny free of debris from the degrading insect cadavers.
Finally, the techniques described and shown in this article correspond to those conceived and/or performed in our laboratory as well as those described by various colleagues and collaborators.
Protocol
1. Soil Sample Collection
Consider covering a minimum area of 2 - 4 m2 for each sampling site.
Collect soil samples at a depth of at least 15 cm (Figure 4).
Take at least 5 random samples within this area.
Take 3 subsamples per sample. Depending on the objective of the study, either combine the subsamples or keep them separate.
Place each sample in a plastic bag. Consider double-bagging to avoid leaking of samples (Figure 4).
Label samples with a waterproof marker. Include the following information from each sampling site: Site information (including locality/area/site name and GPS coordinate information), date, habitat, associated vegetation, temperature, elevation, etc. NOTE: If applicable, collect and/or record the presence of insects or other invertebrates collected with the sample for subsequent identification. They may represent potential natural EPN hosts.
Clean collecting tools between samples by thoroughly washing with water and/or disinfecting them with 70% ethanol or 0.5% bleach solution.
Keep samples in a cooler (8 - 15 °C) during transport to the laboratory.
Take a portion of the soil sample for analysis to obtain information on soil composition, texture, moisture, electrical conductivity or other desired soil parameters.
2. Nematode Isolation from Soil Samples: Insect-baiting Technique
Remove any debris (i.e., rocks, pieces of wood or bark, leaves, etc.) collected with your samples to avoid contamination with saprobic microorganisms.
Add water to moisten the soil and facilitate the movement of nematodes. NOTE: Use a spray bottle to slowly increase the moisture level of the soil.
Place approximately 200 to 250 ml of moist soil in a clean plastic container with a lid.
Add insect baits. Consider 5 to 10 insects to the soil surface of each sample (Figure 5).
Cover the container with a lid and turn containers upside down.
Maintain containers in the dark and at RT (usually 22 - 25 °C).
Check containers every 2 - 3 days and remove dead insects. NOTE: add additional healthy insects to each container for further baiting of the soil sample.
Remove dead insects from the containers. NOTE: Cadavers with a brown or ochre coloration are usually parasitized by steinernematids, whereas brick red to dark purple cadavers are parasitized by heterorhabditids.
Rinse cadavers in sterile water.
Place cadavers in a modified White trap for recovery of nematode progeny (see procedure below).
3. Nematode Recovery from Infected Cadavers: Modified White Trap
Place the top of a 50 (or 60) mm diam. Petri dish inside a larger dish (100 mm).
Set one single circular filter paper (Whatman #1) inside the smaller dish.
Place cadavers on the filter paper of the smaller dish making sure cadavers do not touch each other to avoid any contamination.
Fill the outer (larger) Petri dish with ca. 20 ml of sterile distilled water. Do not add water in the dish that holds the cadavers.
Cover the large Petri dish and its contents with the lid (Figure 6).
Label dishes accordingly. Add the following information: Nematode name (species/isolate), infection date (date infection was set) and trap date (this is the date the trap is set up).
Keep trap at RT until emergence of infective juvenile stages (IJs) occurs. This process can take between 10 - 25 days depending on the nematode species and/or strain considered.
Harvest water with IJs by removing the larger dish of the trap and pouring water with nematodes into a beaker.
Allow nematodes to decant to the bottom of the beaker. This process may take a few minutes.
Pour water carefully, making sure nematodes remain in the bottom of the beaker.
Rinse nematodes by adding more water and allowing nematodes to decant. This step can be repeated 2 - 3 times until water is clean.
Place nematode suspension in a tissue culture flask (250 ml). Keep concentration of the suspension to 1,000 - 3,000 nematodes/ml.
Store flask with nematode suspension in a cold room or in incubator between 10 - 20 °C. NOTE: check stored flasks periodically as shelf life of EPN is variable. Usually steinernematids can be stored for 6 - 12 months without the need of subculturing, whereas heterorhabditids may require more periodic check-ups.
Representative Results
Soil Sample Collection
Third-stage infective juveniles of EPN are the only free living stages in these nematodes’ life cycle which reside in the soil (Figure 1). Therefore, collecting soil samples is a very efficient method to recover of these nematodes. Figure 4 shows a soil profile indicating the depth at which samples should be taken. Preserving the moisture of a sample is a key factor for survival of the nematodes. Consequently, it is important to keep soil samples in plastic bags and maintain them at cool temperatures during transit to the laboratory (Figure 4).
Nematode Recovery
In nature, chances of finding insects naturally parasitized by EPN are less than 3%, unless there is an epidemic and a larger sample of insects with EPN infestation is found. Therefore, baiting of soil samples with insects is a convenient approach to enhance the recovery of EPN from their natural habitat. Figure 5 depicts the soil baiting technique. In this case, a 250 ml plastic container with a screw top was used. Approximately 250 g of soil were placed in the container and 5 - 10 G. mellonella larvae were added on the surface of the soil (Figure 5 right). After 5 days, the container was opened to retrieve dead insects with signs of EPN infection. Notice nematode-infected larvae with red coloration (Figure 5 left).
Figure 6A shows a schematic representation of the modified White trap. Figure 6B demonstrates the set-up of the trap. Notice that EPN infected cadavers do not touch each other and that filter paper in the small petri dish remains dry. Figure 6C shows IJs emerging from cadavers. Note the ‘trails’ of nematodes (IJs) that are formed on the Petri dish as they move towards the water. The time for IJ emergence from cadavers is variable depending on nematode species and also dependent on the temperature and moisture at which the White trap is kept. Figure 6D shows IJs already in the water of the larger dish of this trap. Observe that the nematodes in the water look clean and free of insect tissues.
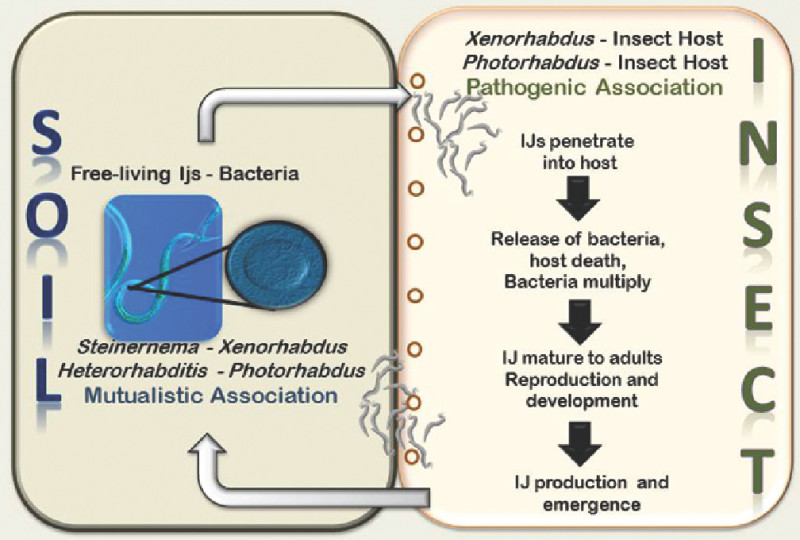 Figure 1. Life cycle of entomopathogenic nematodes.
Figure 1. Life cycle of entomopathogenic nematodes.
 Figure 2. Shovels and other devices used for collecting soil samples.(A) Classic point-shovel. (B) hand shovel. (C) From left to right: Viehmeyer tube, trowel, oakfield tubes, soil corers.
Figure 2. Shovels and other devices used for collecting soil samples.(A) Classic point-shovel. (B) hand shovel. (C) From left to right: Viehmeyer tube, trowel, oakfield tubes, soil corers.
 Figure 3. Soil sampling strategies.(A) Stratified. (B) Random.
Figure 3. Soil sampling strategies.(A) Stratified. (B) Random.
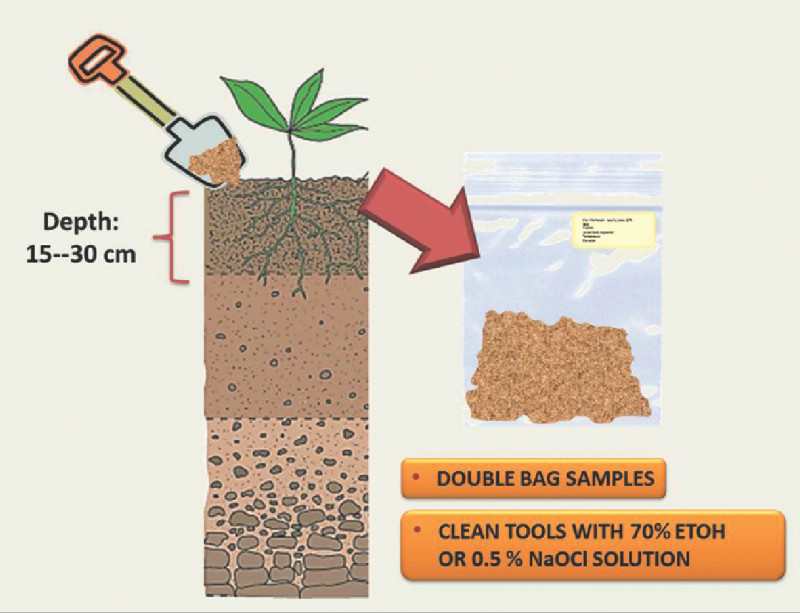 Figure 4. Schematic representation of the soil sampling strategy. Image on the left shows a soil profile indicating desired depth at which samples should be taken. Image of the right shows a soil sample in a plastic bag with appropriate labeling.
Figure 4. Schematic representation of the soil sampling strategy. Image on the left shows a soil profile indicating desired depth at which samples should be taken. Image of the right shows a soil sample in a plastic bag with appropriate labeling.
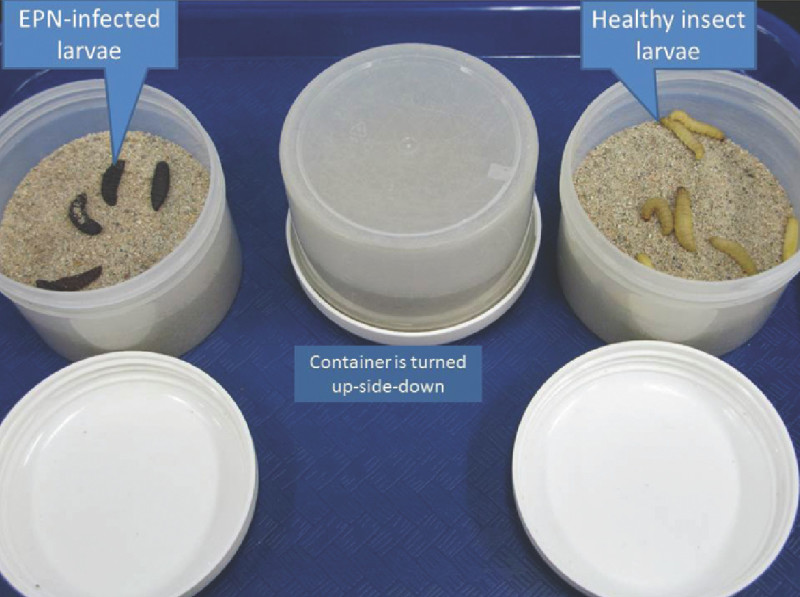 Figure 5. Bating of soil samples with insect larvae. Image on the right shows healthy larvae, whereas image on the left shows dead larvae after a 5 day baiting period.
Figure 5. Bating of soil samples with insect larvae. Image on the right shows healthy larvae, whereas image on the left shows dead larvae after a 5 day baiting period.
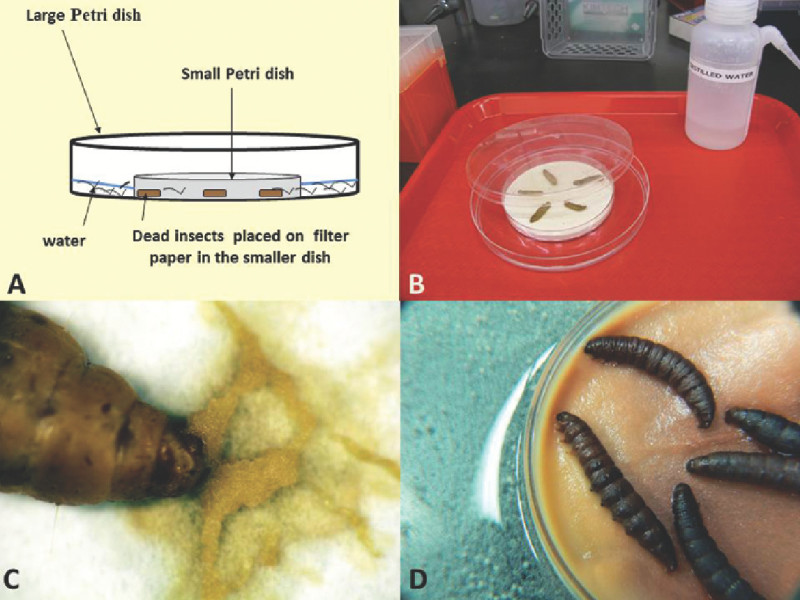 Figure 6. Schematic representation (A) and photographs (B-D) of a modified White trap. (B) Trap set up; (C) close up of infected cadaver showing IJ emergence; (D) close-up showing massive emergence of IJs from cadavers into the water.
Figure 6. Schematic representation (A) and photographs (B-D) of a modified White trap. (B) Trap set up; (C) close up of infected cadaver showing IJ emergence; (D) close-up showing massive emergence of IJs from cadavers into the water.
Discussion
This demonstration is the first of two presentations that describe a number of techniques that are commonly used for studying EPN and their symbiotic bacteria. These techniques represent key steps for the successful establishment of EPN cultures in the laboratory and also form the basis for other bioassays that employ EPN as model organisms for research.
Various soil surveys of EPN have shown that their abundance and distribution can vary across seasons, habitats and geographic regions4, 5,9,15. Factors such as soil texture, moisture content, and temperature as well as host availability may play a key role in their distribution. Therefore, it is critical that a preliminary data on the survey. Once samples are taken, it is important they are processed as soon as possible. However, if this is not a possibility, they should be stored in a cold room or other appropriate temperature controlled unit. Different temperatures may be considered for storing insect baiting containers depending on the origin of the samples. Usually, cooler temperatures (e.g., 15 °C) are considered for temperate samples and for samples from tropical regions, warmer temperatures (e.g., 30 °C) are for contemplated.
The insect baiting technique is the most commonly used method for recovery of EPN from soil samples. It is a convenient strategy that allows the retrieval of EPN in the lab. Last instar larvae of the greater wax moth, Galleria mellonella (Lepidoptera: Pyralidae) are usually considered; however, other insect hosts (i.e., crickets, mealworms, beetle larvae, etc.) and/or suitable stages can be considered and are recommended to use. A few studies have shown that a single extraction cycle of the Galleria-bait method is often insufficient for determining presence of EPN in a particular sample or for extracting all of the EPN from soil samples15. Usually, recovery rates vary between 20 - 40%12,15.
An alternative to the baiting of soil samples in the laboratory is the consideration of baiting in situ. This is the placement of insect baits in the sampling site. In this case, the insects are placed in cages (usually made of a metal or plastic mesh) and are left in demarked sites in the study area for a given period of time. The cages with the insects are then retrieved and examined for EPN infection. However, this approach is labor intensive and time consuming15.
The nematode trapping technique was originally designed by White10 and later modified by Kaya and Stock6. The technique utilizes the attraction of nematodes to water (i.e., positive hygrotropism) and successfully recovers infective stages of EPN free of insect tissues and/or debris.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors wish to thank past members of the Stock lab: Sam-Kyu Kim, Kathryn Plichta and Victoria Miranda-Thompson for their contributions to the improvement of many of these protocols. We acknowledge the National Science Foundation support (grants IOS-0840932 and IOS-0724978) to S. P. Stock.
References
- Bedding RA, Akhurst RJ. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- Boemare NE, Akhurst RA. In: The Genera Photorhabdus and Xenorhabdus. In The Prokaryotes. Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. New York, N.Y: Springer; 2007. pp. 473–488. [Google Scholar]
- Gaugler R. Entomopathogenic Nematology. Wallingford, UK: CABI Publishing; 2002. [Google Scholar]
- Gaugler R, Kaya HK. Entomopathogenic Nematode s in Biological Control. Boca Raton, Florida: CRC Press, Inc; 1990. [Google Scholar]
- Hazir S, Keskin N, Stock SP, Kaya HK, Özcan S. Diversity and distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Turkey. Biodiversit., & Conservation. 2003;12:375–386. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock SP. Techniques in insect nematology. In Manual of techniques in insect pathology. London: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Lacey LA. Manual of techniques in invertebrate pathology. London: Academic Press; 2012. [Google Scholar]
- McCoy CW, Shapiro-Ilan D, Duncan L, Nguyen K. Entomopathogenic nematodes and other natural enemies as mortality factors for larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) Biological Control. 2000;19:182–190. [Google Scholar]
- Meyling NV, Eilenberg J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agriculture, ecosystem., & environment. 2006;113:336–341. [Google Scholar]
- Poinar GO. Taxonomy and biology of Steinernematidae and Heterorhabditidae. In: Entomopathogenic Nemtaodes in Biological. Boca Raton: CRC Press; 1990. pp. 23–61. [Google Scholar]
- Stock SP, Gress JC. Diversity and phylogenetic relationships of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from the Sky Islands of southern Arizona. Journal of invertebrate pathology. 2006;92:66–72. doi: 10.1016/j.jip.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Stock SP, Hunt DJ. Nematode morphology and systematics. In: Grewal PS, Ehlers RU, Shapiro-Ilan DI, editors. Nematodes as biological control agents. CAB International Publishing. Wallingford, UK: CAB International Publishing; 2005. pp. 3–43. [Google Scholar]
- Stock SP, Goodrich–Blair H. Entomopathogenic nematodes and their bacterial symbionts: The inside out of a mutualistic association. Symbiosis. 2008;46:65–75. [Google Scholar]
- Stuart RJ, Gaugler R. Patchiness in populations of entomopathogenic nematodes. Journal of Invertebrate Pathology. 1994;64:39–45. [Google Scholar]
- White GF. A method for obtaining infective nematode larvae from cultures. Science. 1927;66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]


