Abstract
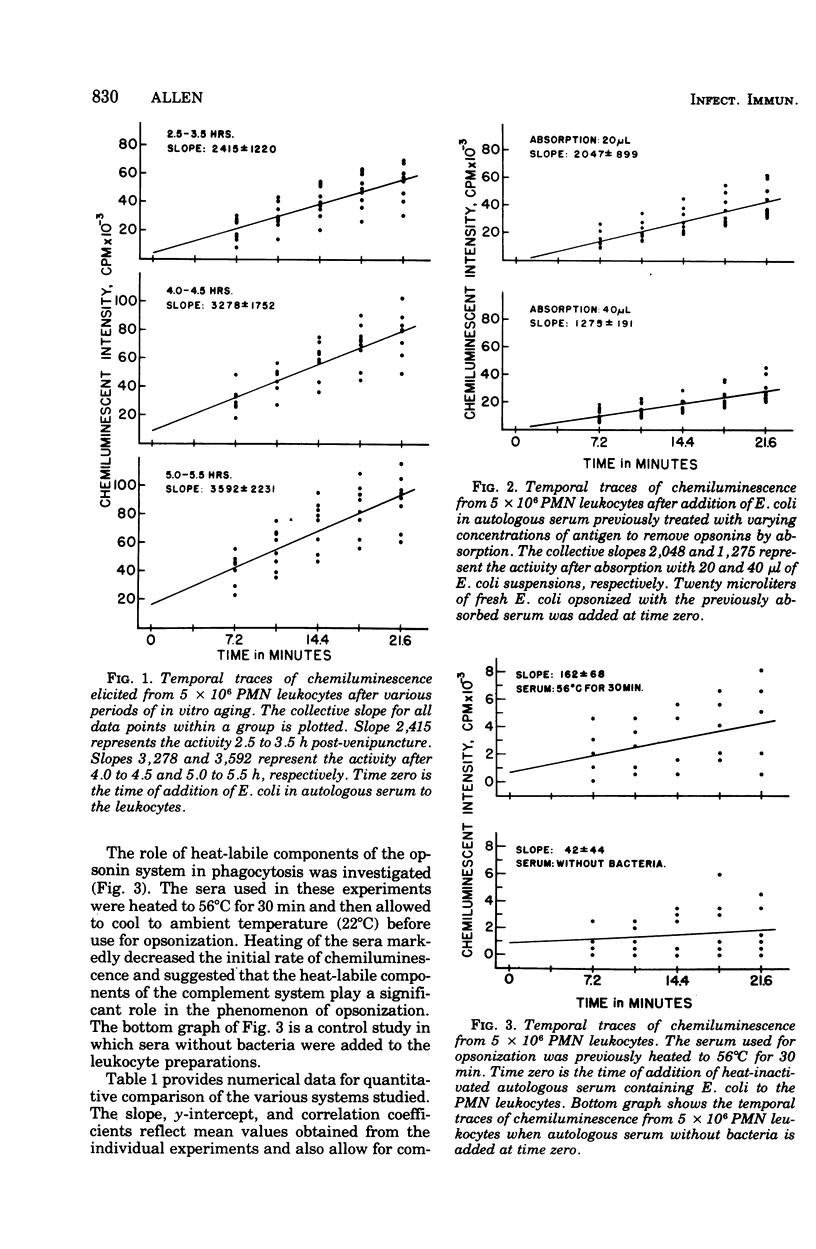
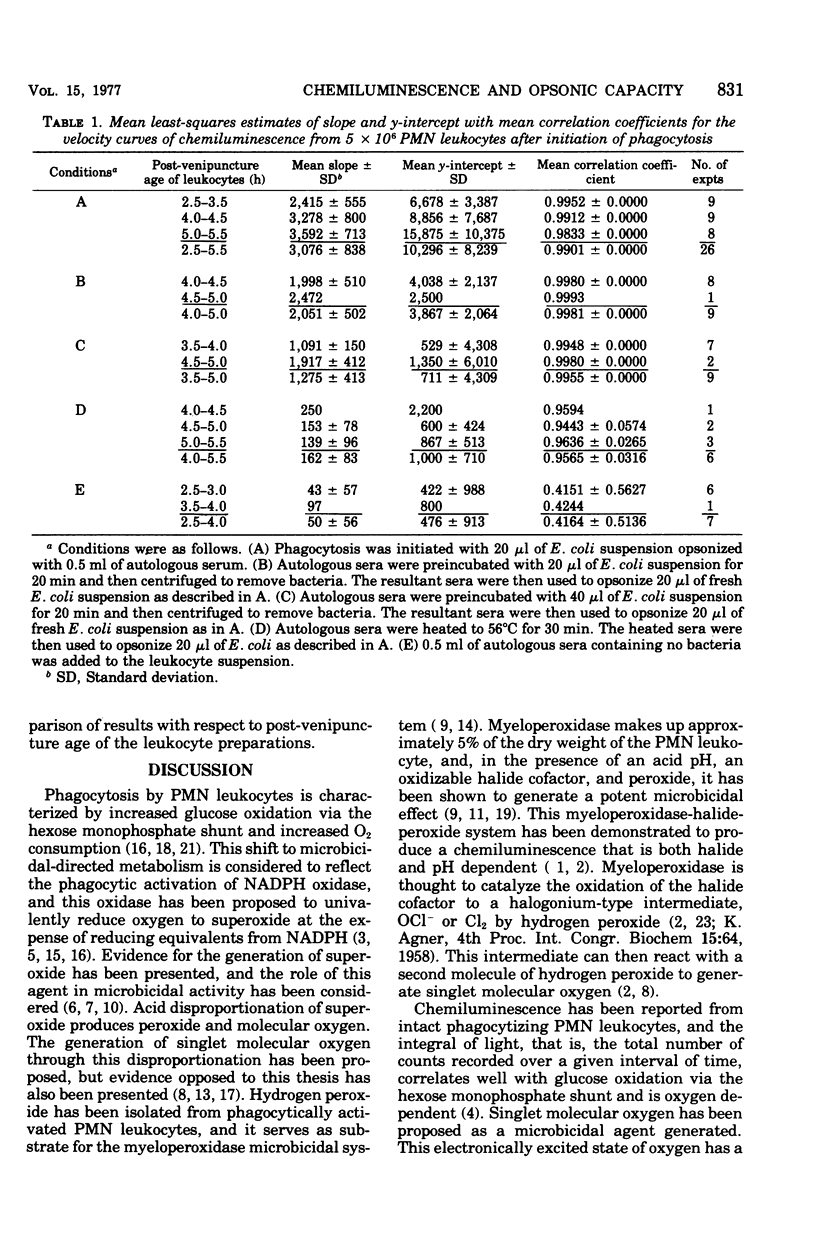
Phagocytically activated polymorphonuclear leukocytes produced a chemiluminescence that could be correlated metabolically with the stimulated oxidation of glucose via the hexose monophosphate shunt, The chemiluminescence observed was considered to originate from the relaxation of electronically excited carbonyl groups produced during singlet molecular oxygen-mediated microbicidal oxidation of the ingested microbe. With adequate adjustment of leukocyte and bacterial concentrations, the rate of chemiluminescence increase was nearly constant for the first minutes after initiation of phagocytosis. This rate was dependent on the quantity of bacteria phagocytized by the leukocytes. If both leukocytes and bacterial concentrations were held constant, this initial rate of chemiluminescence reflected the opsonic capacity of the sera used for opsonization. The prior absorption of opsonins from serum resulted in a decresed rate of chemiluminescence related to the quantity of bacteria used for absorption. Heating of sera to 56 degrees C for 30 min resulted in a great decrease in the chemiluminescent responses and may reflect the role of complement in the opsonization process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun. 1975 Apr 7;63(3):675–683. doi: 10.1016/s0006-291x(75)80437-2. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Allen R. C. The role of PH in the chemiluminescent response of the myeloperoxidase-halide-HOOH antimicrobial system. Biochem Biophys Res Commun. 1975 Apr 7;63(3):684–691. doi: 10.1016/s0006-291x(75)80438-4. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Kearns D. R. Role of singlet oxygen in some chemiluminescence and enzyme oxidation reactions. J Phys Chem. 1974 Aug 15;78(17):1681–1683. doi: 10.1021/j100610a001. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Direct involvement of NADPH oxidase with the stimulated respiratory and hexose monophosphate shunt activities in phagocytizing leukocytes. Exp Cell Res. 1972 Aug;73(2):456–462. doi: 10.1016/0014-4827(72)90071-7. [DOI] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. 2. Incorporation of C14-labeled building blocks into lipid, protein, and glycogen of leukocytes during phagocytosis. J Biol Chem. 1960 Aug;235:2224–2229. [PubMed] [Google Scholar]
- SCHULTZ J., KAMINKER K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962 Mar;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Manak R. C. Carbohydrate metabolism in leukocytes. XIV. Regulation of pentose cycle activity and glycogen metabolism during phagocytosis. J Reticuloendothel Soc. 1970 Dec;8(6):550–560. [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Domański J., Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971 Jun 16;235(3):419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]


