Abstract
Nephronophthisis-related ciliopathies (NPHP-RCs) are developmental and degenerative kidney diseases that are frequently associated with extrarenal pathologies such as retinal degeneration, obesity, and intellectual disability. We recently identified mutations in a gene encoding the centrosomal protein SDCCAG8 as causing NPHP type 10 in humans. To study the role of Sdccag8 in disease pathogenesis, we generated a Sdccag8 gene-trap mouse line. Homozygous Sdccag8gt/gt mice lacked the wild-type Sdccag8 transcript and protein, and recapitulated the human phenotypes of NPHP and retinal degeneration. These mice exhibited early onset retinal degeneration that was associated with rhodopsin mislocalization in the photoreceptors and reduced cone cell numbers, and led to progressive loss of vision. By contrast, renal histologic changes occurred later, and no global ciliary defects were observed in the kidneys. Instead, renal pathology was associated with elevated levels of DNA damage response signaling activity. Cell culture studies confirmed the aberrant activation of DNA damage response in Sdccag8gt/gt-derived cells, characterized by elevated levels of γH2AX and phosphorylated ATM and cell cycle profile abnormalities. Our analysis of Sdccag8gt/gt mice indicates that the pleiotropic phenotypes in these mice may arise through multiple tissue-specific disease mechanisms.
Nephronophthisis-related ciliopathies (NPHP-RCs) (Online Mendelian Inheritance in Man [OMIM] 256100) are heterogenetic autosomal recessive disorders that feature nephronophthisis, a degeneration disorder of the kidney.1 To date, mutations in >20 NPHP-RC genes have been identified2 that manifest nephronophthisis as part of their pathogenesis in the context of ciliopathy syndromes such as Senior–Loken syndrome (OMIM 266900), Bardet–Biedl syndrome (BBS; OMIM 209900), Joubert syndrome (OMIM 213300), and orofaciodigital syndrome (OFD; OMIM 311200).
We recently showed that mutations in serologically defined colon cancer antigen 8 (SDCCAG8) cause nephronophthisis type 10, characterized by retinal and renal degeneration, mild intellectual disability, obesity, hypogonadism, and recurrent respiratory infections in humans.3,4 Because several of the clinical features are shared with BBS, with the exception of the absence of polydactyly, individuals with SDCCAG8 mutations are also considered as part of the BBS spectrum.3,4 SDCCAG8 encodes a coiled-coil domain protein with no additional conserved domains.5 The protein localizes to the centrioles throughout the cell cycle,3,5 to the basal body of cilia, and also to the spermatocytes in the rat testis.3,6 Immunohistochemical analysis of retina has shown SDCCAG8 colocalization with retinitis pigmentosa protein 1 (RP1), retinitis pigmentosa GTPase regulator (RPGR), and retinitis pigmentosa GTPase regulator interacting protein 1 (RPGRIP1) in the connecting cilium of the photoreceptors.3,7 Biochemical studies have demonstrated SDCCAG8 homodimerization and direct interaction with two ciliopathy proteins: (1) OFD1 and (2) family with sequence similarity 161, member A (FAM161A).3,5,8 Despite the data on SDCCAG8 protein localization and its interaction partners, the precise molecular function at centrosomes and cilia remains unknown.
We recently demonstrated that mutations in the gene encoding the centrosomal protein CEP164 cause NPHP-RC whose pathogenesis involves defects in the DNA damage response (DDR) signaling pathway.9 The same study also implicated SDCCAG8 in this pathway through its colocalization with CEP164 and Tat-interactive protein 60 in the cell nucleus.9 Indeed, there is a wealth of evidence in the literature that implicates centrosomal protein function in the regulation of genome stability, including the NPHP-RC proteins AHI1,10 NIMA-related kinase 8 (NEK8/NPHP9),11 and the SDCCAG8-interacting protein OFD1.12,13
To study the role of Sdccag8 in the pathogenesis of NPHP-RC, we generated a transgenic Sdccag8gt/gt mouse model. We demonstrate that Sdccag8gt/gt mice recapitulate aspects of the human disease phenotype. Furthermore, we show that Sdccag8 is involved in cell cycle S-phase progression and its loss leads to replication stress–related DDR activation.
Results
Generation of Sdccag8gt/gt Mice
To investigate the function of the Sdccag8 gene, the embryonic stem cell line OST40418 containing the gene-trap cassette VICTR24 in the intronic region downstream of Sdccag8 exon 1 (Supplemental Figure 1A) was microinjected and founders were bred. Allele-specific primers were used to genotype the mice (Supplemental Figure 1, A and B). Mice carrying the gene-trap allele are referred to as Sdccag8gt. The absence of Sdccag8 mRNA was verified by quantitative RT-PCR analysis using RNA isolated from embryonic day 13.5 (E13.5) Sdccag8gt/gt mouse embryonic fibroblasts (Supplemental Figure 1C). Immunoblotting (Supplemental Figure 1D) confirmed the absence of Sdccag8 protein from lung and kidney lysates of Sdccag8gt/gt mice. Two isoforms of the Sdccag8 protein (78 kD and 83 kD) were detected in Sdccag8wt/gt kidneys (Supplemental Figure 1D).3 Sdccag8gt/gt mice were present at Mendelian ratios at weaning age, indicating that the Sdccag8 gene-trap allele does not cause embryonic or early postnatal lethality.
Sdccag8 Is Expressed in Kidney and Lung Epithelia
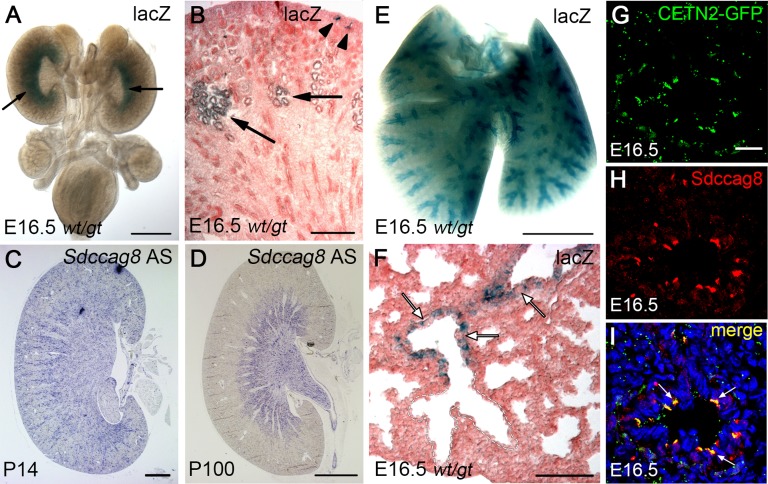
Mutations in SDCCAG8 were previously reported to affect two parenchymal organs in humans, the kidneys and the lungs, causing nephronophthisis and, infrequently, bronchiectasis.3,4 To understand the underlying pathogenetic mechanisms, we first examined the expression pattern of Sdccag8 in these organs by taking advantage of the lacZ cassette in the Sdccag8 gene-trap allele. β-Galactosidase activity staining of wild-type and Sdccag8wt/gt whole urogenital systems at E16.5 showed strong Sdccag8 expression in the corticomedullary region of the Sdccag8wt/gt kidneys (Figure 1A) and no staining in the wild-type control (Supplemental Figure 2A). Examination of the X-gal–stained kidney sections at higher resolution showed staining in the renal tubule epithelia in a pattern compatible with the distal convoluted tubule (DCT) and cortical collecting ducts (CCDs) (Figure 1B). Sdccag8 expression in the collecting ducts was also observed in postnatal P14 and P100 kidneys by in situ hybridization (Figure 1, C and D), whereas the sense probe showed no staining (Supplemental Figure 2, B and C). In the lung, X-gal staining in Sdccag8wt/gt mice at E16.5 showed Sdccag8 expression in the epithelium of the developing bronchi and bronchioles (Figure 1E). Examination of lung sections at higher resolution confirmed this observation and further showed that the blue lacZ+ cells were interspersed with lacZ− cells in the bronchioles (Figure 1F). No β-galactosidase staining was detected in the epithelial cells of alveoli—the terminal ends of the airways, which do not have cilia (Figure 1F, delineated with dashed line). To determine whether the lacZ+ cells in the lung epithelium represent the multiciliated cells, we performed immunofluorescence analysis on E16.5 CETN2-GFP mouse lung sections using the cilia marker anti-polyglutamylated tubulin antibody and anti-SDCCAG8 antibody. CETN2-GFP fusion protein localizes to centrioles,14 which are presented in hundreds of copies in the multiciliated cells of the respiratory epithelium.15 Because centriologenesis precedes multiciliogenesis, almost no cilia were detected in E16.5 distal bronchioles (Supplemental Figure 2, D–F), as previously described.16 However, we found that the CETN2-GFP–positive structures (Figure 1G) in the progenitors of the multiciliated cells fully overlapped with SDCCAG8 antibody staining in E16.5 bronchioles (Figure 1, H and I). Together, this analysis demonstrates that Sdccag8 is expressed in the embryonic and postnatal kidney in a pattern that partially overlaps with the localization of ciliated cells in these tissues. In lung Sdccag8 is expressed in the prospective multiciliated cells, whereas Sdccag8-negative cells most likely represent the nonciliated intercalating goblet cells.
Figure 1.
Sdccag8 is expressed in kidney and lung epithelia. β-Galactosidase activity staining in the urogenital system at E16.5 demonstrates Sdccag8 expression in the corticomedullary region (arrows) and in the CCDs (arrowheads) in Sdccag8wt/gt kidneys (A and B). (C and D) Sdccag8 expression is maintained in the collecting ducts of the kidneys in two-week-old (C) and adult (D) mice. (E) β-Galactosidase activity staining on E16.5 whole lung demonstrates Sdccag8 expression in the bronchi and bronchioles. (F) Analysis of a cross-section through the X-gal–stained lung at higher resolution confirms Sdccag8 expression localization to the epithelial layer of the bronchioles and its absence from alveoli (delineated with a dashed line) and surrounding stromal mesenchyme. Notice the presence of lacZ-negative cells (white arrows) in the bronchioles. (G–I) CETN2-GFP expression in the bronchiole epithelium (G) overlaps (I) with SDCCAG8 antibody staining (H) in the E16.5 mouse lung section, indicating that Sdccag8-positive cells correspond to the progenitors of the multiciliated cells in the lung. Bar, 1 mm in A and B; 400 μm in C; 2 mm in D and E; 200 μm in F; 25 μm in G–I.
Sdccag8gt/gt Mice Develop Late-Onset Nephronophthisis
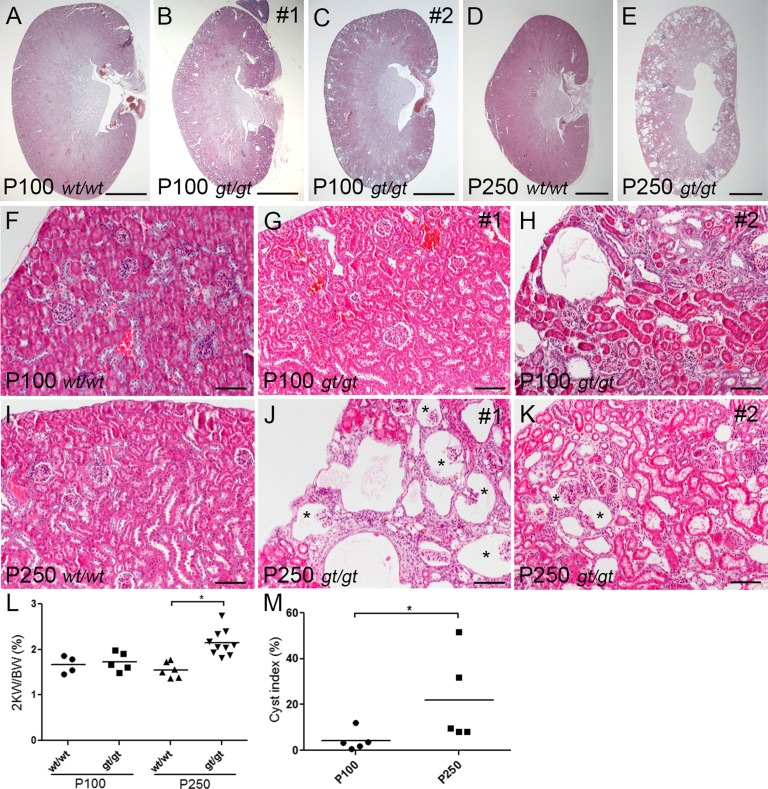
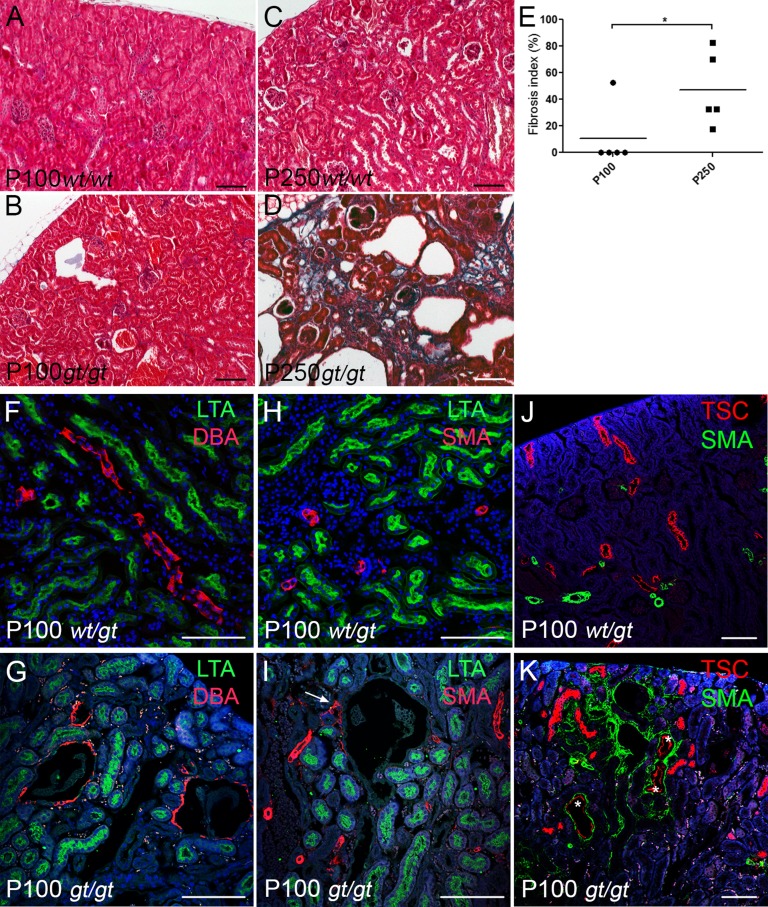
Given the Sdccag8 expression pattern in the kidney, we next examined whether loss of Sdccag8 causes nephronophthisis. The first histologic signs of cyst formation and interstitial infiltration, features that are characteristic of nephronophthisis, were detected at P100 in Sdccag8gt/gt kidneys (Figure 2, A–C). The initial cyst formation occurred primarily in the cortical region of the kidneys (Figure 2, B and C), with either no amount (Figure 2G) or a small amount (Figure 2H) of interstitial infiltrate surrounding the dilated tubules. At P250, the renal histology appeared progressively deteriorated with enlarged cortical cysts, cyst formation in the corticomedullary region, dedifferentiation of the corticomedullary junction, and replacement of the renal parenchyma by interstitial infiltrate (Figure 2, D and E). In addition to tubular cysts, glomerular cysts were also observed in Sdccag8gt/gt kidneys at P250 (Figure 2, I–K). There was no difference in kidney weight to body weight ratio in Sdccag8gt/gt mice at P100; however, the mutant kidneys were significantly enlarged by P250 (Figure 2L). Similarly, the mean value of the cystic index was significantly increased between P100 and P250 (Figure 2M). We next examined the extent of renal fibrosis in Sdccag8gt/gt kidneys using Masson trichrome staining (Figure 3, A–D). In a majority of P100 Sdccag8gt/gt kidneys, no collagen deposits were detected (Figure 3, B and E), which is in agreement with the limited amount of interstitial infiltrate seen by histologic staining (Figure 2G). By contrast, the P250 Sdccag8gt/gt kidneys displayed extensive fibrosis (Figure 3D), which was significantly increased between P100 and P250 kidneys (Figure 3E).
Figure 2.
Sdccag8gt/gt mice develop nephronophthisis. (A–C) H&E staining of sagittal sections of P100 kidneys shows the formation of cortical cysts in Sdccag8gt/gt kidneys (B and C). (D and E) Analysis of H&E staining on sagittal sections of P250 kidneys shows loss of corticomedullary differentiation and progressive cyst formation in Sdccag8gt/gt kidneys (E). (F–H) H&E-stained Sdccag8wt/wt (F) and Sdccag8gt/gt (G and H) kidney sections demonstrate the variation in tubular cyst size and amount of interstitial infiltrate in Sdccag8gt/gt kidneys at P100 (G and H). (I–K) By P250, kidney cysts and interstitial infiltrate have replaced most of the renal parenchyma in Sdccag8gt/gt kidneys (J and K). Glomerular cysts are marked with asterisks. (L) The kidney weight to body weight ratio is not changed in Sdccag8gt/gt mice at P100 (mean wt/wt 1.667±0.09516, gt/gt 1.732±0.09247); however, the ratio is significantly increased in P250 Sdccag8gt/gt mice (mean wt/wt 1.554±0.07007, gt/gt 2.151±0.08878; *P<0.05). (M) The kidney cyst index is low in P100 Sdccag8gt/gt mice (mean 4.270±2.024), but is greatly increased and with noticeable variation in P250 mice (mean 21.91±8.662; *P<0.05). H&E, hematoxylin and eosin; wt, Sdccag8 wild-type allele; gt, Sdccag8 gene-trap allele; KW/BW, kidney weight to body weight ratio. Bar, 2 mm in A–E; 100 μm in F–K.
Figure 3.
Sdccag8gt/gt mice develop fibrosis. (A–D) Masson trichrome staining reveals no collagen deposits in Sdccag8gt/gt kidneys at P100 (B); however, extensive fibrosis is visible in P250 Sdccag8gt/gt kidneys (D). (E) The fibrosis index is significantly increased in Sdccag8gt/gt mice between P100 (mean 10.50±10.50) and P250 (mean 47.00±12.41; *P<0.05). (F and G) LTA-FITC and DBA-rhodamine staining on kidney sections demonstrates the formation of cysts in the CCDs (DBA) and not in the proximal tubules (LTA) of Sdccag8gt/gt kidneys at P100. (H and I) Immunofluorescence staining with α-SMA-rhodamine and LTA-FITC demonstrate the presence of myofibroblasts in the proximity of a nascent cyst (I, white arrow). (J and K) Immunofluorescence staining with αSMA-FITC and DCT marker TSC show that besides CCDs, cysts also originate from DCTs (marked with white asterisks) in P100 Sdccag8gt/gt kidneys (K). αSMA-FITC staining shows the myofibroblasts surrounding the affected tubules. wt, Sdccag8 wild-type allele; gt, Sdccag8 gene-trap allele; α-SMA, α-smooth muscle actin. Bar, 100 μm in A–D; 100 μm in F–K.
To identify the tubular origin of the cysts, we stained P100 kidney sections using nephron segment-specific antibodies. Staining with a proximal tubule marker Lotus tetragonolobus agglutinin lectin (LTA; Figure 3, F–I), a DCT marker, and thiazide sensitive Na-Cl cotransporter (TSC) (Figure 3, J and K), a distal tubule/collecting duct marker Dolichos biflorus agglutinin (DBA) (Figure 3, F and G), revealed that the cysts in Sdccag8gt/gt kidneys originated from two sites—the CCD (Figure 3G) and the DCT (Figure 3K). Costaining with an antibody against α-smooth muscle actin that labels the fibrogenic myofibroblasts in Sdccag8gt/gt kidneys demonstrates that both CCD and DCT cysts were surrounded by interstitial fibrosis (Figure 3, I and K). We examined whether Sdccag8gt/gt mice have defective cilia in the cystic CCDs, by staining kidney sections with antibodies against the cilia marker acetylated tubulin and CCD marker aquaporin 2. No global ciliary abnormalities in the Sdccag8gt/gt CCDs were observed (Supplemental Figure 3, A and B). Deregulation of cytoskeletal rearrangements has been implicated in the pathomechanisms of polycystic kidney disease.17 We tested this hypothesis on Sdccag8gt/gt mouse embryonic fibroblasts using a wound healing assay, but found no changes in their ability to regulate cytoskeletal rearrangements when grown in two-dimensional culture (Supplemental Figure 3, C and D).
Taken together, our data show that Sdccag8gt/gt mice present late-onset nephronophthisis characterized by cyst formation in the DCT, CCD, and glomeruli. Cyst formation was not associated with global ciliary defects in the kidney. There was a marked fibrosis around the renal cysts.
Loss of Sdccag8 Causes Photoreceptor Degeneration in Sdccag8gt/gt Mice
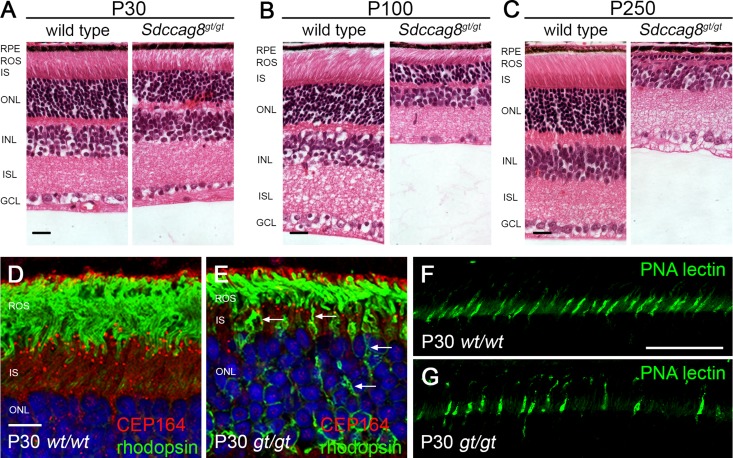
Mutations in SDCCAG8 cause retinal degeneration in humans.3 To study the disease progression in Sdccag8gt/gt mice, we performed retinal histologic analysis in mice aged P30, P100, and P250 days (Figure 4, A–C). Although the retina appeared to have formed normally, we observed a slight reduction of the photoreceptor layer at P30 (Figure 4A). No other retinal abnormalities were detected at this stage. The retinal phenotype became more severe at P100 (Figure 4B) and complete loss of the photoreceptor cell layer occurred by P250 (Figure 4C). We performed electroretinography measurements to study the physiologic consequences of the observed histologic changes in the retina. Sdccag8gt/gt mice displayed a progressive decline in electroretinography wave amplitudes from P100 to P250, which were in tight correlation with the degree of histologic defects at the respective ages (Supplemental Figure 4, A and B). SDCCAG8 localization to the photoreceptor connecting cilium is regulated by RPGRIP1.7 Because Rpgrip1−/− mice show a defect in photoreceptor protein trafficking,18,19 we hypothesized that loss of SDCCAG8 may also lead to impaired protein trafficking in the photoreceptors. To test this hypothesis, we stained Sdccag8gt/gt retinas with an antibody against rhodopsin that localizes to the photoreceptor outer segment and against CEP164 that labels the photoreceptor basal body. Consistent with a defect in protein trafficking, rhodopsin accumulated to the plasma membrane of the photoreceptor inner segment and cell bodies in Sdccag8gt/gt retina at P30 (Figure 4, D and E) and P100 (Supplemental Figure 4, D and E). Peanut agglutinin lectin staining of photoreceptor cone sheath at P30 revealed a reduced number of cones in Sdccag8gt/gt mice (Figure 4, F and G, Supplemental Figure 4C). Thus, our data indicate that Sdccag8 is not essential for retina development and photoreceptor formation, but is required for the maintenance of both rod and cone photoreceptor cells. Loss of Sdccag8 leads to progressive retinal degeneration and blindness.
Figure 4.
Sdccag8 is required for photoreceptor maintenance. (A–C) Histologic analysis of H&E-stained wild-type and Sdccag8gt/gt retinas at P30 (A), P100 (B), and P250 (C) show progressive degeneration of photoreceptor inner segments and outer segments, as well as severe reduction of the outer nuclear layer in Sdccag8gt/gt mice. (D and E) Immunolocalization of CEP164 and rhodopsin in the P30 retina shows rhodopsin accumulation (white arrows) at the photoreceptor inner segment plasma membrane and cell bodies in Sdccag8gt/gt retina (E). (F and G) PNA-FITC lectin staining of cone sheaths at P30 demonstrates a reduction in cone cell number in Sdccag8gt/gt retina (G). H&E, hematoxylin and eosin; RPE, retinal pigment epithelium; ROS, retinal outer segment; IS, inner segment; ONL, outer nuclear layer; INL, inner nuclear layer; ISL, inner plexiform layer; GCL, ganglion cell layer; wt, Sdccag8 wild-type allele; gt, Sdccag8 gene-trap allele; PNA, peanut agglutinin lectin. Bar, 100 μm in A–C; 10 μm in D and E; 50 μm in F and G.
Loss of Sdccag8 Leads to Impaired S-Phase Progression
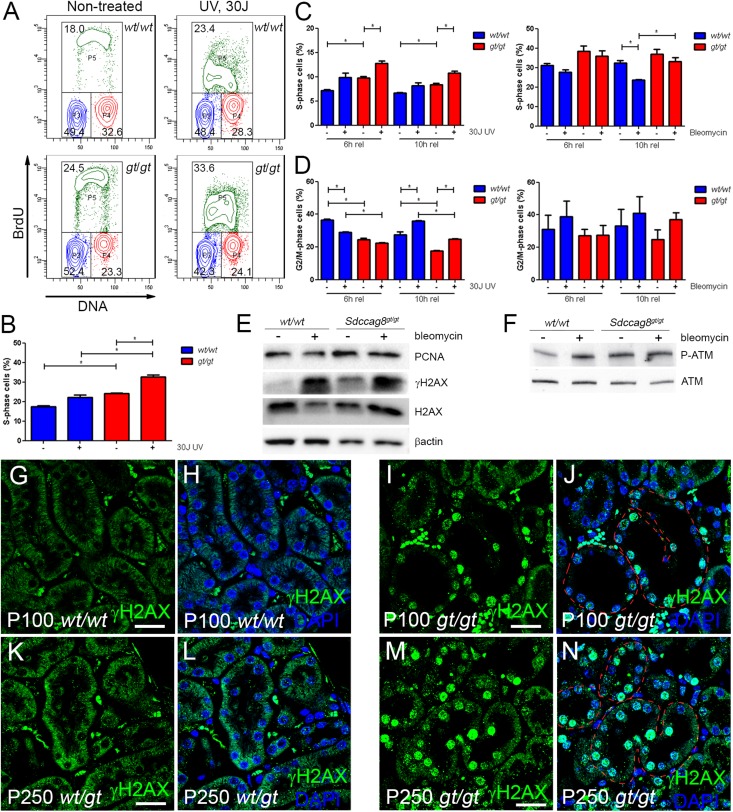
We and others have shown that the pathogenesis of renal ciliopathies and renal fibrosis may involve mutations in genes that modulate cellular response to DNA damage, such as ZNF423, CEP164, FAN1, and NEK8.9,11,20 We recently demonstrated that SDCCAG8 colocalizes with CEP164 and Tat-interactive protein 60, an activator of the DDR regulator ATM.9,21 However, it remained unaddressed whether SDCCAG8 functions in the context of DNA damage response signaling. To test this hypothesis, we first examined whether loss of Sdccag8 causes cell cycle abnormalities as a result of checkpoint activation by performing FACS analysis on conditionally immortalized adult kidney cells from Immorto;Sdccag8wt/wt and Immorto;Sdccag8gt/gt mice. To perform the experiments, cells were grown at 37°C without γ-IFN to exclude any interference from the large T antigen (Supplemental Figure 5A). Cell cycle analysis showed that Sdccag8gt/gt cells accumulated in the S phase. The number of bromodeoxyuridine (BrdU)-positive S-phase cells was increased from 18% in wild-type cells to 24.5% in Sdccag8gt/gt cells (Figure 5, A and B), and this figure was further increased from 23.4% in wild-type cells to 33.6% in Sdccag8gt/gt cells after treatment with 30 J ultraviolet (UV) light, indicating that loss of Sdccag8 affects primarily S-phase progression and makes the cells more sensitive to UV light–induced DNA damage. To study the cell cycle defect in more detail, we synchronized the cells with a double thymidine block and treated them with 4.5 U/ml bleomycin or 30 J UV light. After release from the block, Sdccag8gt/gt cells progressed in the S phase more slowly and remained in the S phase longer than wild-type cells (Figure 5C). Treatment with 30 J UV light further extended the S-phase duration, and bleomycin had a slightly milder effect (Figure 5C). Interestingly, G2/M checkpoint activation was not impaired in Sdccag8gt/gt cells because treatment with bleomycin or UV light arrested both wild-type and Sdccag8gt/gt cells in the G2/M checkpoint (Figure 5D). There was also no difference in the levels of apoptosis between wild-type and mutant cell lines after treatment with bleomycin or UV light, although treatment with 30 J UV increased the apoptotic index compared with nontreated cells (Supplemental Figure 5, B and C).
Figure 5.
Sdccag8 inactivation causes S-phase delay and replication stress. (A and B) The cell cycle profile of Immorto;Sdccag8wt/wt and Immorto;Sdccag8gt/gt nonsynchronized cells demonstrates significant accumulation of Sdccag8gt/gt cells in the S phase (P<0.05; n=3) compared with the wild-type control (P<0.05, n=3). The number of cells in the S phase is further increased after treatment with 30 J UV light. (C) Sdccag8gt/gt cells synchronized with double thymidine block show a delay in S-phase progression, which is further increased after treatment with UV light. (D) Sdccag8gt/gt cells are competent in G2/M check point activation, both in response to UV light and bleomycin treatment. (E) Sdccag8gt/gt cells have increased PCNA levels, reflecting their extended duration in the S phase. PCNA levels do not change in response to bleomycin treatment, which is in agreement with the cell cycle analysis data. Increased γH2AX levels in cultured Sdccag8gt/gt cells are a sign of replication stress. H2AX and β-actin are used as loading controls. (F) Phosphorylated ATM levels are low in cultured wild-type cells, which become elevated upon bleomycin treatment. By contrast, Sdccag8gt/gt cells display high phosphorylated ATM levels even without treatment. ATM is used as a loading control. (G–J) Immunofluorescence staining with anti-γH2AX antibody on P100 Sdccag8wt/wt and Sdccag8gt/gt kidneys shows no γH2AX staining in control kidneys (G and H), whereas the dilated tubules (red dashed lines) in Sdccag8gt/gt kidneys are positive for γH2AX (I and J). (K–N) P250 Sdccag8wt/gt kidneys are negative for gH2AX staining (K and L), while Sdccag8gt/gt kidneys show positive staining in dilated and nondilated nephron epithelium (red dashed lines) and fibrotic mesenchyme (M and N). wt, Sdccag8 wild-type allele; gt, Sdccag8 gene-trap allele; PCNA, proliferating cell nuclear antigen. Bar, 25 μm in G–J; 20 μm in K–N.
We next examined whether replication stress in Sdccag8gt/gt cells leads to the activation of DDR signaling. There was a significant increase in the levels of phosphorylated H2AX (γH2AX) and ATM (S1987) proteins in Sdccag8gt/gt cells, indicating activation of DDR signaling pathway in these cells (Figure 5, E and F). Treatment with bleomycin drastically increased the phosphorylation of either protein, both in wild-type and Sdccag8gt/gt cells. In addition, Sdccag8gt/gt cells demonstrated increased proliferating cell nuclear antigen protein levels, consistent with their prolonged stay in the S phase (Figure 5E).
We next asked whether the in vitro findings of abnormal DDR signaling activation in Sdccag8gt/gt cells can also be detected under in vivo conditions. We stained Sdccag8gt/gt and control kidneys with γH2AX antibody at P100 (Figure 5, G–J) and P250 (Figure 5, K–N) and indeed found increased nuclear γH2AX staining in the tubular and interstitial compartments of Sdccag8gt/gt kidneys. The γH2AX-positive nuclei were observed in the dilated tubules at P100 (Figure 5, I and J), whereas nondilated tubules were also positive for γH2AX staining at P250 (Figure 5, M and N), likely indicating a more general tubular stress in more fibrotic kidneys.20 Together, these data suggest that Sdccag8 has an important role in DNA damage signaling and its loss leads to replication stress and slower S-phase progression during the normal cell cycle. Furthermore, Sdccag8gt/gt kidneys display elevated levels of γH2AX, implicating impaired DDR in their pathogenesis.
Discussion
We generated Sdccag8gt/gt mice to study the role of Sdccag8 in nephronophthisis type 10. Sdccag8gt/gt mice faithfully recapitulate the human SDCCAG8 loss of function phenotypes of retinal degeneration and nephronophthisis. Through morphologic analysis of Sdccag8gt/gt mice and functional studies using cell culture assays, we show that SDCCAG8 is important for photoreceptor maintenance and cell cycle regulation, impairment of which leads to blindness and aberrant DDR signaling activation through replication stress.
Sdccag8 and Degenerative Phenotypes
Sdccag8 expression was previously localized to the retina and testis.3,6–8 Our studies using the Sdccag8 lacZ gene-trap allele and SDCCAG8 antibody staining show Sdccag8 expression in the multiciliated cells of the respiratory epithelium and in the DCTs and collecting ducts of the kidney. These expression domains are consistent with the recorded tissue pathologies in individuals with SDCCAG8 mutations.3 Our ability to detect lacZ expression only in the ciliated cells most likely reflects higher Sdccag8 promoter activity in ciliated cells. It does not, however, exclude that Sdccag8 is not expressed in nonciliated cells, whose levels may be below the detection limit of our β-galactosidase staining activity.3
Our histologic analysis of the kidneys showed that Sdccag8gt/gt mice displayed a classic nephronophthisis phenotype at P100, with the cystic dilations originating from the DCTs and CCDs. Compared with other mouse models of nephronophthisis,22–25 the onset of the renal phenotype in Sdccag8gt/gt mice occurred relatively late. The process of cyst formation appeared to coincide with interstitial fibrosis that surrounded the glomeruli and nephrons. In progressively later stages, tubular dilations were also observed in the Bowman’s capsule of glomeruli. Curiously, no global cilia defects were observed in the cystic renal tubule cells. Similar results were obtained in in vitro studies using a spheroid assay, in which loss of Sdccag8 did not affect cilia numbers.3,26 However, it remains to be studied whether the cilia in Sdccag8gt/gt collecting ducts have deficiencies in some aspects of cilia function, such as mechanosensation as shown for polycystic kidney disease or in loss of transition zone integrity.27
Retinal degeneration in Sdccag8gt/gt mice progresses slower than in the mouse model of its protein interactor RPGRIP1,19 but similarly to that of its other interaction partners RP1 and RPGR.28,29 This suggests that RPGRIP1 functions upstream of Sdccag8 in photoreceptor cilia, which is in agreement with a recent report of RPGRIP1-dependent ciliary localization of SDCCAG8.7 Although photoreceptor formation in Sdccag8gt/gt mice appeared intact, the absence of Sdccag8 led to accumulation of rhodopsin in the photoreceptor inner segments and cell bodies at P30. This study did not address whether rhodopsin mislocalization was caused by structural defects in the connecting cilium or whether SDCCAG8 is directly involved in rhodopsin trafficking. Rhodopsin mislocalization to photoreceptor inner segments is known to be toxic for the photoreceptors due to membrane crowding resulting in retinal degeneration.30,31 Our data are consistent with this mode of retinal pathogenesis and indicate a role for Sdccag8 in intraciliary trafficking. Immunohistochemical and functional analysis showed that both rod and cone photoreceptors were affected in Sdccag8gt/gt mice, demonstrating that Sdccag8 is essential for the survival and maintenance of both rods and cones, similar to RPGR function.29
Sdccag8 and DDR Signaling
Examination of the molecular defects underlying the pathogenesis in Sdccga8gt/gt mice using cell culture revealed abnormalities in cell cycle progression as a result of activation of DDR signaling, characterized by hyperactivation of ATM and elevation of γH2AX levels. Increased DDR signaling activity was also observed in Sdccag8gt/gt kidneys, implicating DDR signaling in the pathogenesis of NPHP-RCs. Impaired DDR signaling is associated with degenerative diseases such as Seckel syndrome, which is caused by mutations in genes encoding for other centrosomal and DDR proteins such as ATR,32 pericentrin,33 CEP152,34 or CEP63.35 Pathologically, Seckel syndrome shares with NPHP-RC the phenotype of premature degeneration and fibrosis of parenchymal organs, including the kidney.36 Because kidneys are constantly exposed to genotoxins, they are especially prone to lesions caused by impaired DDR signaling. Increased susceptibility to genotoxins was previously reported for the Ahi1−/− mouse model of NPHP-RC,10 and genome instability because of the loss of survivin was reported in Pkd1−/−, Pkd2−/−, and Tg737Orpk/Orpk isolated primary cells.37 Mutations in a related ciliopathy NEK8/NPHP9 were recently shown to lead to replication stress–related DDR signaling activation.11 Our data are consistent with a model in which Sdccag8 has an important role in S-phase progression during the normal cell cycle, in which it could facilitate DNA replication from the stalled replication forks; however, the molecular analysis required to answer this question is extensive and will be the focus of future work. It remains to be tested whether treatment of Sdccag8gt/gt mice with cyclin-dependent kinase inhibitors, such as R-roscovitine or S-CR8, reverses or attenuates the renal cyst progression in Sdccag8gt/gt mice as has been shown for jck/Nphp9−/− and Pkd1−/− mice.38,39
Concise Methods
Mouse Breeding and Maintenance
The experimental protocol was reviewed and approved by the Animal Care Committee of the University of Michigan. Embryonic stem cell line OST40418 containing gene-trap vector VICTR24 in intron 1 of Sdccag8 was obtained from Texas Institute for Genomic Medicine and cultured as described with the use of ESGRO (EMD Millipore).40 Chimeric mice were prepared by blastocyst microinjection and bred with C57BL/6J mice to obtain germline transmission. Genotyping primers and PCR conditions are available upon request.
CETN2-GFP mice were purchased from The Jackson Laboratory (stock number 008234). ImmortoMouse mice were purchased from Charles River Laboratories (strain code 238). Sdccag8 wild-type or heterozygous littermates were used as controls for mutant mice. For timed matings, noon on the day a plug was found was designated as E0.5.
Histologic Analyses
Tissues were fixed in 4% (w/v) paraformaldehyde in PBS at 4°C. All tissues were then dehydrated through an ethanol series and embedded in paraffin. Sections were taken at 5 μm. Hematoxylin and eosin staining followed standard protocols. β-Galactosidase activity staining was carried out on whole tissues as previously described.41 β-Galactosidase–stained tissues were cleared in glycerol for imaging or embedded in paraffin and sectioned at 10 μm.
Cystic Index and Fibrosis Index Calculation
Representative images of hematoxylin and eosin–stained or Masson trichrome–stained kidneys were acquired. A grid was placed over the images, and the cystic index or fibrosis index was calculated as the percentage of grid intersection points that bisect cystic and noncystic or fibrotic and nonfibrotic areas.
Electroretinography
To assess rod- and cone-mediated function, electroretinograms were performed using the Espion e2 recording system (Diagnosys) as previously described.42 After overnight dark adaptation, mice were anesthetized with an intraperitoneal injection of ketamine (93 mg/kg) and xylazine (8 mg/kg). Body temperature was maintained at 37°C with a heating pad. After pupil dilation with topical phenylephrine (2.5%) and tropicamide (1.0%), corneal electroretinograms were recorded from both eyes using gold wire loops and a drop of 2% methylcellulose for corneal hydration. A gold wire loop placed in the mouth was used as reference, and the ground electrode was placed on the tail. The dark-adapted electroretinogram was recorded from −5.8 to +1.09 log cd⋅s⋅m−2/flash in steps of 0.5 log units. After 10 minutes of light adaptation to a white 32 cd⋅m−2 rod-suppressing background, light-adapted electroretinograms were recorded from −0.91 to +2.0 log cd⋅s ⋅m−2. Ten to 25 responses were recorded at 3–60 seconds depending upon the stimulus intensity intervals.
Antibodies
For immunostaining, tissues were deparaffinized and hydrated through a graded ethanol series. Antigen retrieval was carried out in heated antigen retrieval buffer (pH 8, SIG-31910-50; Covance). Primary antibodies used were as follows: rabbit polyclonal anti-CEP164 (gift from Erich Nigg, University of Basel, Switzerland), rabbit polyclonal anti-SDCCAG8 (13471-1-AP; Proteintech), rabbit polyclonal anti-SDCCAG8 (ab101969; Abcam, Inc.), mouse monoclonal anti–γ-tubulin (GTU-88; Sigma-Aldrich), anti-acetylated tubulin (6-11B-1; Sigma-Aldrich), rabbit anti-Aquaporin 2 (ab15081; Abcam, Inc.), rabbit polyclonal anti-TSC (AB3553; EMD Millipore), peanut agglutinin lectin-FITC (Vector Laboratories), lectin Lotus tetragonolobus agglutinin (Vector Laboratories), anti–αSMA-FITC (1A4; Sigma-Aldrich), anti-γH2AX (9718; Cell Signaling), rabbit anti-ATM (S1981, 5883; Cell Signaling Technology), mouse monoclonal anti-rhodopsin (R5403; Sigma-Aldrich), rat anti–proliferating cell nuclear antigen (ABIN334654; Antibodies Online), mouse anti–β-actin (AC-15, A5441; Sigma-Aldrich), rabbit anti-histone H2A.X (070627; EMD Millipore), and mouse monoclonal anti-polyglutamylated tubulin (T9822; Sigma-Aldrich). Secondary antibodies were goat anti-mouse Alexa Fluor 488 (Molecular Probes) and goat anti-rabbit Alexa Fluor 594 (Molecular Probes). Samples were mounted in ProlongGold (Molecular Probes) and images were captured on a Leica TSC 5SP X confocal microscope (Leica Microsystems).
Generation of Mouse Embryonic Fibroblasts and Immortalized Adult Kidney Cells
Mouse embryonic fibroblasts were established from wild-type and Sdccag8gt/gt E13.5 embryos and cultured in DMEM with 10% FBS and penicillin/streptomycin. Isolation of immortalized adult kidney cells from P50 Immorto;Sdccag8wt/wt and Immorto;Sdccag8gt/gt mouse kidneys was performed as previously described.43
RNA Extraction and Quantitative RT-PCR
RNA was isolated from wild-type, Sdccag8wt/gt, and Sdccag8gt/gt mouse embryonic fibroblast cells and adult kidney cells using the RNeasy Mini Kit (Qiagen), and RT was performed using Superscript III (Invitrogen). Quantitative real-time PCR was carried out using SYBR Green (Qiagen) and run on a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). Data were normalized to Gapdh. Primer sequences are available upon request.
Western Blotting
Lung and kidney tissues were lysed in radioimmunoprecipitation assay lysis buffer (Pierce) and homogenized with a douncer. Cleared tissue lysates were produced by centrifugation of the resulting samples at 16,000×g for 30 minutes at 4°C. Gel electrophoresis of tissue lysates and immunoprecipitation eluents were performed using the NuPAGE system (Invitrogen). Samples were resolved on 4%–12% Bis-Tris gels in 3-(N-morpholino)propanesulfonic acid buffer and transferred to a nitrocellulose membrane that was then probed for the protein of interest using antibodies diluted in Tris-buffered saline containing 5% milk and 0.1% Tween-20 (Sigma-Aldrich).
Apoptosis FACS
To quantify apoptosis, wild-type or Sdccag8gt/gt cells were plated and incubated with or without thymidine. After two overnight thymidine blocks, cells were released and damage was induced by 1-hour 4.5 U/ml bleomycin or 30 J UV exposure. After 24 hours, cells were harvested and washed once with 1% BSA-PBS. Cells were collected in FACS tubes in 200 µl 1% BSA-PBS containing Vybrant DyeCycle Violet Stain (V35003, 1:1000; Invitrogen) to stain living and apoptotic cells (7 minutes at 37°C) and 7-AAD viability stain (00-6993, 1:60; eBioscience) to stain dead cells (10 minutes on ice). Cells were measured (20,000 events) with a BD FACSCanto II flow cytometer and analyzed using BD FACSDiva Software.
BrdU FACS
To quantify cell cycle phase distribution, wild-type or Sdccag8gt/gt cells were plated and incubated with or without thymidine. After double overnight thymidine block, cells were released and damage was induced by 30 J UV exposure. After 6- or 10-hour release of thymidine block, cells were incubated with 10 μM BrdU for 30 minutes and fixed in 70% EtOH. Samples were stained for FACS analysis with BrdU mouse mAb (clone MoBU-1), Alexa Fluor 647 conjugate (1:200; Invitrogen) in 0.1% BSA-PBS-T for 1 hour on ice, and 4′,6-diamidino-2-phenylindole (1:2000) in PBS, and were measured (10,000 events) with a BD FACSCanto II flow cytometer and analyzed using BD FACSDiva Software.
Statistical Analyses
The t test was used to compare data between two groups. Significance was determined at P<0.05. Where appropriate, data are presented as the mean±SEM.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Erich Nigg for providing the CEP164 antibody. We also thank Thomas L. Saunders, Elizabeth Hughes, Keith Childs, Galina Gavrilina, and Debra Vanheyningen for preparation of the embryonic stem cell mouse chimeras from gene-trapped embryonic stem cell clone OST40418 and the Transgenic Animal Model Core of the University of Michigan’s Biomedical Core Facilities.
Core support was provided by the Diabetes Research and Training Center and the O’Brien Renal Core Center, which are funded by grants from the National Institutes of Health (NIH) (DK20572 and P30-DK08194, respectively). This research was also supported by grants from the NIH (1K99-DK099434-01 to R.A., and DK068306 and RC4-DK090917 to F.H.) and the European Commission Seventh Framework Programme (HEALTH-F4-2008-201648/PROSPECTS to J.S.A.). G.G.S. and R.H.G. were supported by grants from the European Union FP7/2009 Consortium “SYSCILIA” (241955) and the Dutch Kidney Foundation Kouncil Consortium (CP11). A.-C.W. and A.K. were supported by a grant from the German Research Foundation (DFG Ki728/9-1). F.H. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050565/-/DCSupplemental.
References
- 1.Hildebrandt F, Benzing T, Katsanis N: Ciliopathies. N Engl J Med 364: 1533–1543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurd TW, Hildebrandt F: Mechanisms of nephronophthisis and related ciliopathies. Nephron, Exp Nephrol 118: e9–e14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, van Reeuwijk J, Letteboer SJ, Sang L, Giles RH, Liu Q, Coene KL, Estrada-Cuzcano A, Collin RW, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, Macdonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford LM, Neumann HP, Obermüller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Dias MB, Zhang X, Cavalcoli JD, Nürnberg G, Nürnberg P, Pierce EA, Jackson PK, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F: Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet 42: 840–850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer E, Zaloszyc A, Lauer J, Durand M, Stutzmann F, Perdomo-Trujillo Y, Redin C, Bennouna Greene V, Toutain A, Perrin L, Gérard M, Caillard S, Bei X, Lewis RA, Christmann D, Letsch J, Kribs M, Mutter C, Muller J, Stoetzel C, Fischbach M, Marion V, Katsanis N, Dollfus H: Mutations in SDCCAG8/NPHP10 cause Bardet-Biedl syndrome and are associated with penetrant renal disease and absent polydactyly. Mol Syndromol 1: 273–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenedy AA, Cohen KJ, Loveys DA, Kato GJ, Dang CV: Identification and characterization of the novel centrosome-associated protein CCCAP. Gene 303: 35–46, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kamio T, Asano A, Hosaka YZ, Khalid AM, Yokota S-i, Ohta M, Ohyama K, Yamano Y: Expression of the centrosomal colon cancer autoantigen gene during spermatogenesis in the maturing rat testis. Biosci Biotechnol Biochem 74: 1466–1469, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Patil H, Tserentsoodol N, Saha A, Hao Y, Webb M, Ferreira PA: Selective loss of RPGRIP1-dependent ciliary targeting of NPHP4, RPGR and SDCCAG8 underlies the degeneration of photoreceptor neurons. Cell Death Dis 3: e355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Gioia SA, Letteboer SJF, Kostic C, Bandah-Rozenfeld D, Hetterschijt L, Sharon D, Arsenijevic Y, Roepman R, Rivolta C: FAM161A, associated with retinitis pigmentosa, is a component of the cilia-basal body complex and interacts with proteins involved in ciliopathies. Hum Mol Genet 21: 5174–5184, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A, Gee HY, Ramaswami G, Hong CJ, Hamilton BA, Cervenka I, Ganji RS, Bryja V, Arts HH, van Reeuwijk J, Oud MM, Letteboer SJ, Roepman R, Husson H, Ibraghimov-Beskrovnaya O, Yasunaga T, Walz G, Eley L, Sayer JA, Schermer B, Liebau MC, Benzing T, Le Corre S, Drummond I, Janssen S, Allen SJ, Natarajan S, O’Toole JF, Attanasio M, Saunier S, Antignac C, Koenekoop RK, Ren H, Lopez I, Nayir A, Stoetzel C, Dollfus H, Massoudi R, Gleeson JG, Andreoli SP, Doherty DG, Lindstrad A, Golzio C, Katsanis N, Pape L, Abboud EB, Al-Rajhi AA, Lewis RA, Omran H, Lee EY, Wang S, Sekiguchi JM, Saunders R, Johnson CA, Garner E, Vanselow K, Andersen JS, Shlomai J, Nurnberg G, Nurnberg P, Levy S, Smogorzewska A, Otto EA, Hildebrandt F: Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 150: 533–548, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG: Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med 15: 1046–1054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi HJ, Lin JR, Vannier JB, Slaats GG, Kile AC, Paulsen RD, Manning DK, Beier DR, Giles RH, Boulton SJ, Cimprich KA: NEK8 links the ATR-regulated replication stress response and S phase CDK activity to renal ciliopathies. Mol Cell 51: 423–439, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dollé P, Franco B: Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet 38: 112–117, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Giorgio G, Alfieri M, Prattichizzo C, Zullo A, Cairo S, Franco B: Functional characterization of the OFD1 protein reveals a nuclear localization and physical interaction with subunits of a chromatin remodeling complex. Mol Biol Cell 18: 4397–4404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higginbotham H, Bielas S, Tanaka T, Gleeson JG: Transgenic mouse line with green-fluorescent protein-labeled Centrin 2 allows visualization of the centrosome in living cells. Transgenic Res 13: 155–164, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Vladar EK, Stearns T: Molecular characterization of centriole assembly in ciliated epithelial cells. J Cell Biol 178: 31–42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL: Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol 43: 731–739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boca M, D’Amato L, Distefano G, Polishchuk RS, Germino GG, Boletta A: Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3β-dependent cell cell mechanical adhesion. Mol Biol Cell 18: 4050–4061, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Hong D-H, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T: The retinitis pigmentosa GTPase regulator (RPGR)-interacting protein: Subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci U S A 100: 3965–3970, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Won J, Gifford E, Smith RS, Yi H, Ferreira PA, Hicks WL, Li T, Naggert JK, Nishina PM: RPGRIP1 is essential for normal rod photoreceptor outer segment elaboration and morphogenesis. Hum Mol Genet 18: 4329–4339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Otto EA, Cluckey A, Airik R, Hurd TW, Chaki M, Diaz K, Lach FP, Bennett GR, Gee HY, Ghosh AK, Natarajan S, Thongthip S, Veturi U, Allen SJ, Janssen S, Ramaswami G, Dixon J, Burkhalter F, Spoendlin M, Moch H, Mihatsch MJ, Verine J, Reade R, Soliman H, Godin M, Kiss D, Monga G, Mazzucco G, Amann K, Artunc F, Newland RC, Wiech T, Zschiedrich S, Huber TB, Friedl A, Slaats GG, Joles JA, Goldschmeding R, Washburn J, Giles RH, Levy S, Smogorzewska A, Hildebrandt F: FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet 44: 910–915, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciccia A, Elledge SJ: The DNA damage response: Making it safe to play with knives. Mol Cell 40: 179–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, Seelow D, Nürnberg G, Becker C, Chudley AE, Nürnberg P, Hildebrandt F, Treier M: Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet 39: 1018–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Atala A, Freeman MR, Mandell J, Beier DR: Juvenile cystic kidneys (jck): A new mouse mutation which causes polycystic kidneys. Kidney Int 43: 1081–1085, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, Nürnberg G, Becker C, Grimm T, Girschick G, Lynch SA, Kelehan P, Senderek J, Neuhaus TJ, Stallmach T, Zentgraf H, Nürnberg P, Gretz N, Lo C, Lienkamp S, Schäfer T, Walz G, Benzing T, Zerres K, Omran H: Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 82: 959–970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama N, Yokoyama T: Sustained cell proliferation of renal epithelial cells in mice with inv mutation. Genes Cells 11: 1213–1224, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, Huntzicker EG, Sfakianos MK, Sandoval W, Bazan JF, Kulkarni P, Garcia-Gonzalo FR, Seol AD, O’Toole JF, Held S, Reutter HM, Lane WS, Rafiq MA, Noor A, Ansar M, Devi AR, Sheffield VC, Slusarski DC, Vincent JB, Doherty DA, Hildebrandt F, Reiter JF, Jackson PK: Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145: 513–528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantiello HF: A tale of two tails: Ciliary mechanotransduction in ADPKD. Trends Mol Med 9: 234–236, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Cheon K, Nusinowitz S, Liu Q, Bei D, Atkins K, Azimi A, Daiger SP, Farber DB, Heckenlively JR, Pierce EA, Sullivan LS, Zuo J: Progressive photoreceptor degeneration, outer segment dysplasia, and rhodopsin mislocalization in mice with targeted disruption of the retinitis pigmentosa-1 (Rp1) gene. Proc Natl Acad Sci U S A 99: 5698–5703, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang WC, Wright AF, Roman AJ, Cideciyan AV, Manson FD, Gewaily DY, Schwartz SB, Sadigh S, Limberis MP, Bell P, Wilson JM, Swaroop A, Jacobson SG: RPGR-associated retinal degeneration in human X-linked RP and a murine model. Invest Ophthalmol Vis Sci 53: 5594–5608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P: Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet 15: 216–219, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Deretic D: A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Res 46: 4427–4433, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Murga M, Bunting S, Montaña MF, Soria R, Mulero F, Cañamero M, Lee Y, McKinnon PJ, Nussenzweig A, Fernandez-Capetillo O: A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet 41: 891–898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffith E, Walker S, Martin C-A, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, Hamel B, Earnshaw WC, Jeggo PA, Jackson AP, O’Driscoll M: Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet 40: 232–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, Bicknell LS, Kayserili H, Li Y, Tüysüz B, Nürnberg G, Kiess W, Koegl M, Baessmann I, Buruk K, Toraman B, Kayipmaz S, Kul S, Ikbal M, Turner DJ, Taylor MS, Aerts J, Scott C, Milstein K, Dollfus H, Wieczorek D, Brunner HG, Hurles M, Jackson AP, Rauch A, Nürnberg P, Karagüzel A, Wollnik B: CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet 43: 23–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sir J-H, Barr AR, Nicholas AK, Carvalho OP, Khurshid M, Sossick A, Reichelt S, D’Santos C, Woods CG, Gergely F: A primary microcephaly protein complex forms a ring around parental centrioles. Nat Genet 43: 1147–1153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ: Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1: 113–126, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.AbouAlaiwi WA, Ratnam S, Booth RL, Shah JV, Nauli SM: Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation. Hum Mol Genet 20: 354–367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O: Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444: 949–952, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Bukanov NO, Moreno SE, Natoli TA, Rogers KA, Smith LA, Ledbetter SR, Oumata N, Galons H, Meijer L, Ibraghimov-Beskrovnaya O: CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD. Cell Cycle 11: 4040–4046, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen GM, Markesich DC, Burnett MB, Zhu Q, Dionne KM, Richter LJ, Finnell RH, Sands AT, Zambrowicz BP, Abuin A: Large-scale gene trapping in C57BL/6N mouse embryonic stem cells. Genome Res 18: 1670–1679, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Airik R, Bussen M, Singh MK, Petry M, Kispert A: Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest 116: 663–674, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson DA, Khan NW, Othman MI, Chang B, Jia L, Grahek G, Wu Z, Hiriyanna S, Nellissery J, Li T, Khanna H, Colosi P, Swaroop A, Heckenlively JR: Rd9 is a naturally occurring mouse model of a common form of retinitis pigmentosa caused by mutations in RPGR-ORF15. PLoS ONE 7: e35865, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grupp C, Begher M, Cohen D, Raghunath M, Franz H-E, Müller GA: Isolation and characterization of the lower portion of the thin limb of Henle in primary culture. Am J Physiol 274: F775–F782, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.