Abstract
Renal targets of autoimmunity in human lupus nephritis (LN) are unknown. We sought to identify autoantibodies and glomerular target antigens in renal biopsy samples from patients with LN and determine whether the same autoantibodies can be detected in circulation. Glomeruli were microdissected from biopsy samples of 20 patients with LN and characterized by proteomic techniques. Serum samples from large cohorts of patients with systemic lupus erythematosus (SLE) with and without LN and other glomerulonephritides were tested. Glomerular IgGs recognized 11 podocyte antigens, with reactivity varying by LN pathology. Notably, IgG2 autoantibodies against α-enolase and annexin AI were detected in 11 and 10 of the biopsy samples, respectively, and predominated over other autoantibodies. Immunohistochemistry revealed colocalization of α-enolase or annexin AI with IgG2 in glomeruli. High levels of serum anti–α-enolase (>15 mg/L) IgG2 and/or anti-annexin AI (>2.7 mg/L) IgG2 were detected in most patients with LN but not patients with other glomerulonephritides, and they identified two cohorts: patients with high anti–α-enolase/low anti-annexin AI IgG2 and patients with low anti–α-enolase/high anti-annexin AI IgG2. Serum levels of both autoantibodies decreased significantly after 12 months of therapy for LN. Anti–α-enolase IgG2 recognized specific epitopes of α-enolase and did not cross-react with dsDNA. Furthermore, nephritogenic monoclonal IgG2 (clone H147) derived from lupus-prone MRL-lpr/lpr mice recognized human α-enolase, suggesting homology between animal models and human LN. These data show a multiantibody composition in LN, where IgG2 autoantibodies against α-enolase and annexin AI predominate in the glomerulus and can be detected in serum.
Keywords: lupus nephritis, immunology and pathology, GN
Lupus nephritis (LN) is the most serious organ manifestation of systemic lupus erythematosus (SLE).1 It occurs in a relevant proportion of SLE patients and if not timely recognized and treated, may lead to renal failure and death. There is a general consensus that LN is mediated by antibody deposition in glomerular tuft,2–5 but the mechanisms leading to the formation of immune deposits and the development of renal lesions are not completely clarified. The glomerular targets of pathogenic antibodies, in particular, are not well defined. Consequently, no surrogate biomarker is available that could allow early identification of candidate patients to LN and/or drive the therapeutic approach.
Several theories have been developed over the years.6–12 They consider a pre-eminent role of circulating anti–double stranded DNA (dsDNA) antibodies that they can interact, for mimicry, with glomerular antigens expressed at the cell surface of podocytes and mesangial cells7,13–15 or in the glomerular basement membrane.12,16–20 Chromatin and nucleosomes deriving from ineffective fragmentation may interact with negatively charged constituents of the basement membrane7,16 and become the planted antigen for anti-dsDNA (and autoantibodies of other specificities).6,7,9,16
Other potentially nephritogenic antibodies different from anti-dsDNA have been proposed10,11,21–26; overall, it has been estimated that anti-DNA deposition in LN accounts for not more than 10%–20% of eluted IgG overall,27 implying that IgG not recognizing DNA represents the vast majority of antibodies in glomeruli. Renal targets of autoimmunity in human LN are, however, unknown.
An in vivo approach based on antibody microelution from glomeruli dissected from renal biopsies of LN patients and their characterization with proteomics seems potentially conclusive to elucidate main features of human LN. Studies published so far on direct analysis of antibodies eluted from glomeruli in patients with LN have been done only on autoptic samples of kidney tissue and focused only on renal targets of eluted anti-dsDNA.27,28 Ultraprecise techniques of dissection, such as laser capture, allow repetitive analysis of minute amounts of renal tissue and can be used routinely in vivo,29 which has already been done in membranous nephropathy.30,31
The aim of the present study was to evaluate the target antigens of antibodies eluted from glomeruli of LN patients to characterize their isotype and determine their serum levels in different cohorts of patients with SLE (with and without nephritis). Antigen targets in murine models of LN were characterized as well.
Results
Anti-Podocyte Antibodies in Glomerular Eluates and Serum
For the analysis of glomerular eluates, we used renal biopsies obtained from 20 SLE patients with proteinuria who underwent a renal biopsy for diagnostic purposes (Table 1). Glomeruli were dissected from frozen kidneys by laser capture, and eluted Igs were analyzed by Western blot using podocyte extracts as fixed antigens. An example showing laser capture efficiency and precision is shown in Supplemental Figure 1.
Table 1.
Clinical data and pathology characteristics relative to 20 patients with SLE who underwent a renal biopsy at the time of enrolment and had their renal tissue processed with laser capture for antibody characterization
| Patient | Sex | Age (yr) | Biopsy Year | LN Class | SCreat (mg/dl) | UProt (g/24 h) | C3 (mg/dl) | C4 (mg/dl) | ANA | Anti-DNA | Steroid | Cycloph | Cyclosp | MMF | Plaquenil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 16 | 2010 | IV | 0.5 | 2.2 | 103 | 16 | Pos | Pos | Yes | No | No | No | No |
| 2 | M | 22 | 2005 | III | 0.6 | 0.9 | 44 | 8 | Pos | Pos | Yes | No | No | No | No |
| 3 | M | 19 | 2010 | V | 2.4 | 0.9 | 78 | 12 | Pos | Pos | Yes | No | No | No | No |
| 4 | W | 18 | 2009 | IV | 0.4 | 1.4 | 65 | 10 | Pos | Pos | Yes | No | No | No | No |
| 5 | W | 25 | 2005 | V | 0.5 | 1.1 | 47 | 19 | Pos | Pos | Yes | No | No | No | Yes |
| 6 | W | 28 | 2005 | nd | 2.1 | 0.2 | 102 | 20 | Pos | Pos | Yes | No | Yes | Yes | No |
| 7 | W | 20 | 2007 | II | 0.4 | 0.6 | 60 | 10 | Pos | Pos | Yes | No | No | No | No |
| 8 | W | 17 | 2009 | IV and V | 0.7 | 0.8 | 47 | <3 | Pos | Pos | Yes | No | No | No | Yes |
| 9 | W | 25 | 2004 | IV | 0.9 | 1.8 | 5 | 20 | Pos | Pos | Yes | No | No | No | Yes |
| 10 | W | 43 | 2010 | IV | 0.8 | 1.4 | 53 | 6 | Pos | Pos | Yes | No | No | Yes | Yes |
| 11 | W | 44 | 2010 | IV and V | 0.7 | 0.9 | 46 | 4.8 | Pos | Pos | Yes | No | No | Yes | Yes |
| 12 | W | 53 | 2010 | III | 0.6 | 2.8 | 141 | 18 | Pos | Pos | Yes | No | No | Yes | No |
| 13 | W | 34 | 2009 | IV | 0.8 | 2.8 | 53 | 11.5 | Pos | Pos | Yes | Yes | No | No | Yes |
| 14 | W | 40 | 2009 | III | 0.5 | 1.4 | 71 | 10.7 | Pos | Pos | Yes | No | No | Yes | Yes |
| 15 | W | 25 | 2009 | V | 0.6 | 3.3 | 63 | 12.1 | Pos | Pos | Yes | No | No | Yes | Yes |
| 16 | W | 38 | 2009 | IV | 0.6 | 4.5 | 41 | 4.1 | Pos | Pos | Yes | Yes | No | No | Yes |
| 17 | W | 50 | 2009 | IV | 1.0 | 1.9 | 75 | 10 | Pos | Pos | Yes | No | No | Yes | Yes |
| 18 | W | 35 | 2010 | IV | 0.7 | 2.1 | 47 | 2.5 | Pos | Pos | Yes | Yes | No | No | Yes |
| 19 | W | 31 | 2011 | IV | 0.8 | 5.7 | 23 | 2.4 | Pos | Pos | Yes | No | No | Yes | No |
| 20 | W | 27 | 2011 | nd | 1.0 | 1.0 | 50 | 5 | Pos | Pos | Yes | No | No | No | No |
SCreat, serum creatinine; UProt, proteinuria; ANA, antinuclear antibody; Cycloph, cyclophosphimide; Cyclosp, cyclosporine; MMF, mycophenolate mofetil; M, man; Pos, positive; W, woman; nd, not determined.
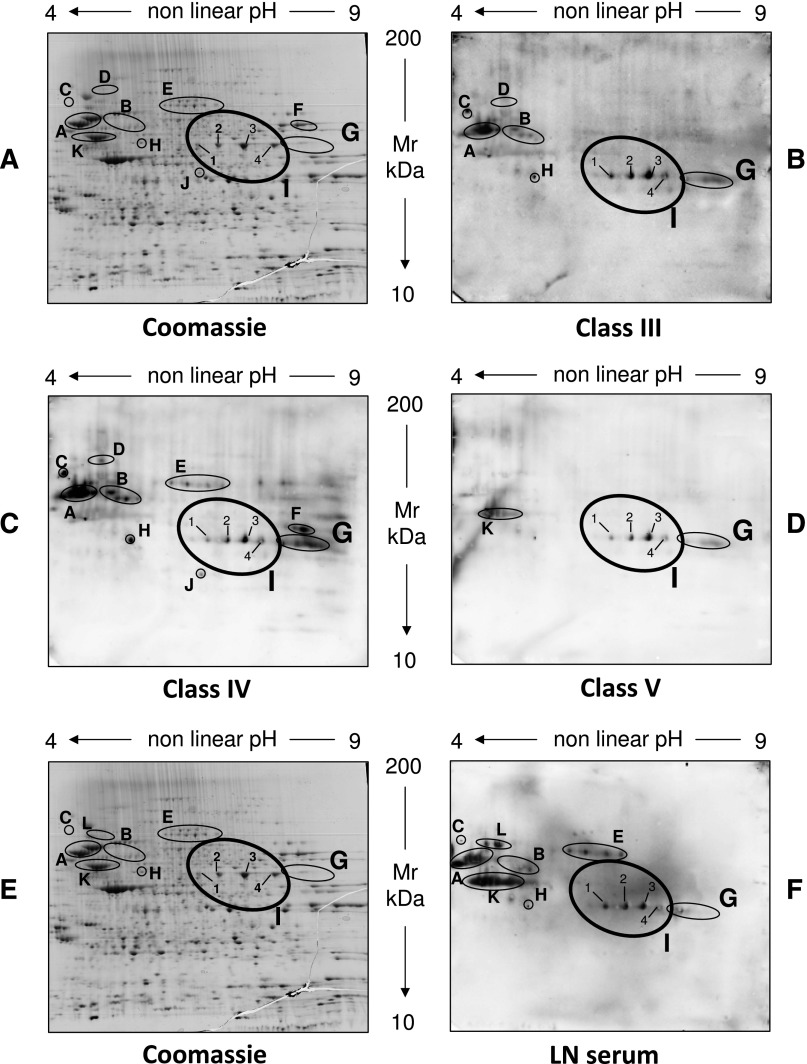
Pooled samples from different pathologic classes of LN (classes III–V) were first analyzed; a separate set of analysis of eluates from each renal biopsy was then carried out with dot blot after antigens had been characterized (see below for information on specific antibodies). As shown in Figure 1, glomerular IgGs recognized 11 proteins (Figure 1, A–D) that were characterized by matrix-assisted laser desorption ionization (MALDI)–mass spectrometry (MS) and liquid chromatography (LC)-MS. The predicted sequence for each recognized protein is reported in Table 2. The presence of specific autoantibodies in glomerular eluates versus this panel of proteins varied in different patients according to the pathology classes of LN, with only anti–α-enolase and anti-annexin AI being present in all eluates (Table 3). Five autoantibodies were detected in at least two pathology classes (anti-tubulin, anti–gluthatione synthetase, anti–heat shock cognate 71 kD, anti–Xaao dipeptidase, and anti–lactate dehydrogenase), and four autoantibodies were found in just one class (i.e., anti-ezirin/moesin, anti-transketolase, anti-vimentin, and heat shock protein/perodoxiiredoxin).
Figure 1.
Characterization of autoantibodies eluted from glomeruli of patients with LN and in corresponding serum. (A and E) Representative two-dimensional electrophoresis of podocyte cell extracts stained by colloidal Coomassie.57 The same cell extract was incubated with antibodies eluted from microdissected glomeruli obtained from patients with different classes of LN: (B) class III, (C) class IV, and (D) class V. Microeluted antibodies from normal kidneys did not react with any protein. Several spots were recognized by LN eluates and identified as 11 different proteins (A–K). One was characterized by LC-MS (spot I; α-enolase), and the remaining spots were characterized by MALDI-MS (Table 2). Their identity as predicted by MS is reported in Table 2. The same podocyte cell extracts separated by two-dimensional electrophoresis were incubated with normal sera (not shown) and (F) pooled LN sera (classes III–V) and then developed with anti-IgG (total) antibodies. Several proteins were detected, and most corresponded to proteins recognized in glomerular eluates (A–C and G–I); three proteins were recognized only in serum or a few glomerular eluates (K and L). All spots were characterized by LC-MS or MALDI-MS as above (Table 2, Supplemental Table 1). Their identity is reported in Table 2.
Table 2.
MALDI-MS/LC-MS spectra analyses of protein spots (A–L) from two-dimensional electrophoresis
| Spot | Gene Name | Protein Name | Technique | Score | Coverage |
|---|---|---|---|---|---|
| A | TBA1B | Tubulin α-1B chain | MALDI-MS | 1011 | 48.8 |
| TBB5 | Tubulin β-chain 2 | 961 | 42.6 | ||
| B | GSHB | Glutathione synthetase | MALDI-MS | 712 | 32.7 |
| C | HSP7C | Heat shock cognate 71 kD protein | MALDI-MS | 1087 | 36.1 |
| D | PEPD | Xaa-Pro dipeptidase | MALDI-MS | 208 | 13.4 |
| E | EZRI | Ezrin | MALDI-MS | 679 | 23.7 |
| MOES | Moesin | 1465 | 45.8 | ||
| F | TKT | Transketolase | MALDI-MS | 713 | 27.4 |
| G | ANXAI | Annexin AI | MALDI-MS | 1575 | 74.3 |
| H | LDHA | l-lactate dehydrogenase A chain | MALDI-MS | 507 | 32.8 |
| K | VIM | Vimentin | MALDI-MS | 512.3 | 57.5 |
| J | HSPB1 | Heat shock protein β-1 | MALDI-MS | 521 | 41.5 |
| PRDX6 | Peroxiredoxin-6 | MALDI-MS | 838 | 63.8 | |
| I.1 | ENOA | α-Enolase | LC-MS | 94.3 | 13.9 |
| I.2 | ENOA | α-Enolase | LC-MS | 178.4 | 18.2 |
| I.3 | ENOA | α-Enolase | LC-MS | 194.3 | 18.8 |
| I.4 | ENOA | α-Enolase | LC-MS | 99.2 | 14.2 |
| L | CNDP2 | Cytosolic nonspecific dipeptidase | MALDI-MS | 279 | 19.4 |
Proteins identified by two-dimensional electrophoresis from podocyte extracts were characterized by MALDI-MS in most cases (spots A–H and J–L) and LC-MS in one case (spot I). Structure prediction features (score and coverage) are reported.
Table 3.
Positivity of antibodies versus different podocyte antigens in serum and glomerular eluates of patients with different classes of LN as evaluated by two-dimensional electrophoresis/Western blot
| Spot | Protein Name | Serum | GN Class III | GN Class IV | GN Class V |
|---|---|---|---|---|---|
| A | Tubulin-α/β | + | + | + | − |
| B | Glutathione synthetase | + | + | + | − |
| C | Heat shock cognate 71 kD | + | + | + | − |
| D | Xaa Pro dipeptidase | − | + | + | − |
| E | Ezirin | + | − | − | − |
| Moesin | |||||
| F | Transketolase | − | − | + | − |
| G | Annexin AI | + | + | + | + |
| H | l-lactate dehydrogenase | + | + | + | − |
| K | Vimentin | + | − | − | + |
| I | α-Enolase | + | + | + | + |
| L | Cytosolic nonspecific dipeptidase | + | − | − | − |
| J | Heat shock protein β-1 | − | − | + | − |
| Perodoxiredoxin-6 |
Serum IgGs from the same LN patients recognized most of the antigens above (Figure 1, E and F) with the following exceptions: anti–Xaa Pro dipeptidase (spot D), transketolase (spot F), and anti-heat shock protein perodoxiiredoxin 6 (spot J) were not detected in serum, whereas anticytosolic dipeptidase antibodies were found in serum but not glomeruli. All other components were confirmed in both.
Based on the presence of anti–α-enolase and anti-annexin AI antibodies in all the LN pathology classes and serum, focused studies addressed isotypes of these antibodies in glomeruli and serum, single biopsy expression, antigen–antibody colocalization, and specificity.
Renal Anti–α-Enolase/Anti-Annexin AI Antibodies: Isotype, Glomerular Expression, and Colocalization
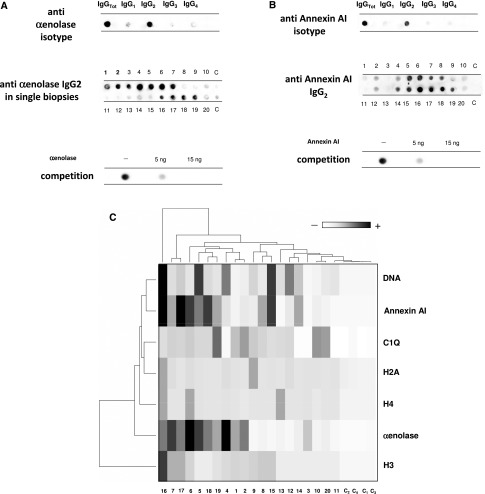
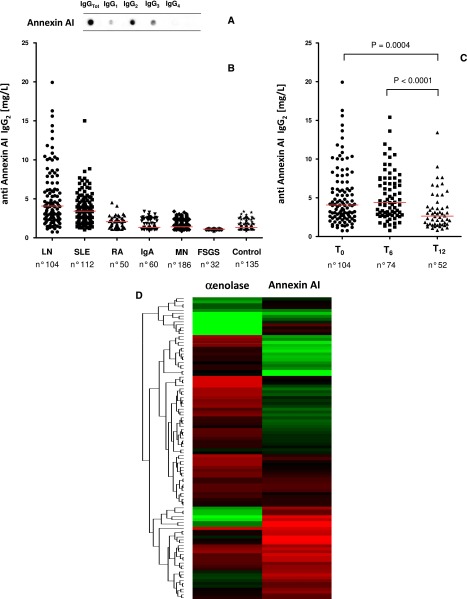
Isotype analysis of anti–α-enolase in glomerular eluates showed IgG2 and a minimal amount of IgG1 (Figure 2A); anti–α-enolase IgG3 and IgG4 were negative. Overall, specific anti–α-enolase IgG2 was detected in 11 of 20 renal biopsy samples processed with microdissection (Figure 2A).
Figure 2.
Glomerular α-enolase and annexin AI: isotypes and levels in single biopsies. The following studies were done to better characterized (A) anti–α-enolase and (B) anti-annexin AI antibodies: study 1, definition of isotype with dot blot analysis; study 2, single biopsy analysis; and study 3, competition experiment using the same glomerular eluates as above and increasing amounts of α-enolase/annexin AI from 5 to 15 ng to saturate antibodies. Results show complete inhibition and confirm the presence of anti–α-enolase/anti-annexin AI IgG2 in glomerular eluates. (C) Hierarchical cluster analysis heat map for a single antibody in each renal biopsy; antibody intensities (black, high; gray, medium; white, low) are reported in lines and refer to single patient biopsies that are reported at the bottom of the figure. Results are given for 20 LN patients of our study cohort and four normal kidneys.
Isotype analysis of glomerular anti-annexin AI showed IgG2 as a unique category (Figure 2B). Analysis in every separate renal biopsy sample showed the presence of anti-annexin AI IgG2 in 10 of 20 samples (Figure 2B); in other renal samples, the expression was scanty. In six cases, a double positivity for anti–α-enolase and anti-annexin AI IgG2 was observed. Multiple positivity for separate renal biopsy samples was analyzed with cluster analysis (heat map), and results are shown as intensity variation from black (maximum) to white (minimum) in Figure 2C. Other than results on anti–α-enolase and anti-annexin AI antibodies, in Figure 2C, it is also reported, for comparison, positivity of single biopsy for antibodies versus implanted antigens anti-C1q, anti-DNA, and anti-histones H2A, H3, and H4, which are typical findings in LN. With this type of approach, the intensity relative to all autoantibodies can be evaluated simultaneously in all biopsies. The results confirmed predominance of antibodies versus endogenous glomerular antigens followed by anti-DNA and anti-C1q.
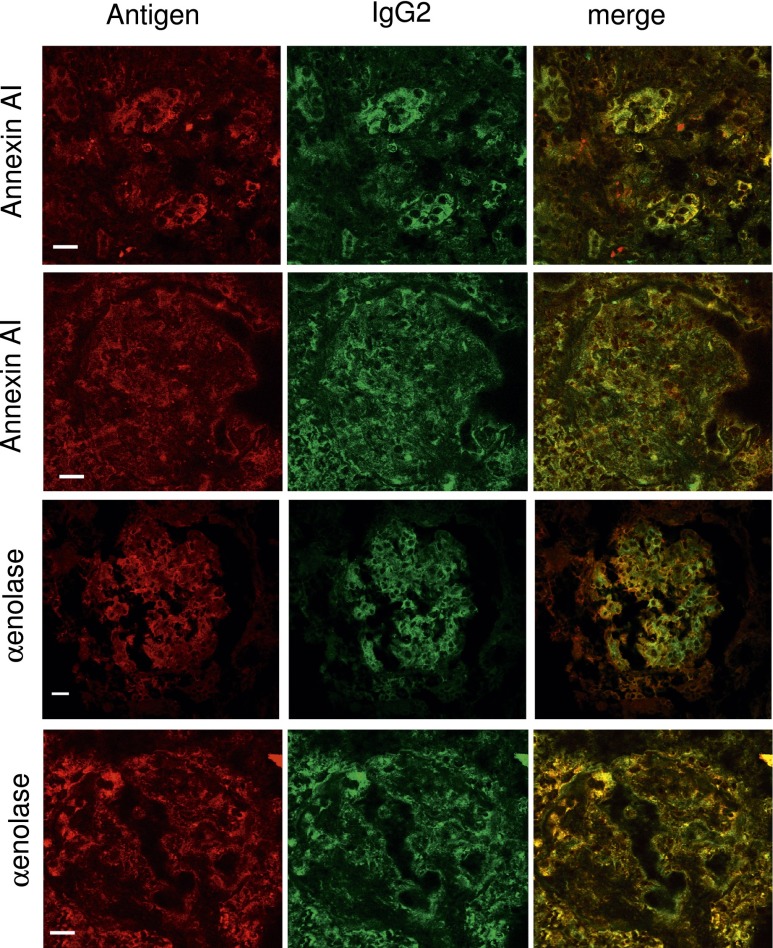
Other experiments showed colocalization of α-enolase and annexin AI with IgG2 in glomeruli of patients with LN. Figure 3 shows two examples of patients with LN classes III and V presenting with diffuse α-enolase expression and widespread IgG2 deposition. Vast areas of colocalization with IgG2 could be shown for both α-enolase and annexin AI that were observed also after DNase/RNase treatment (Supplemental Figure 2), implying that the target antigen is not a planted DNA or nucleosome structure.
Figure 3.
Colocalization of IgG2 with α-enolase and annexin AI. In this series, the colocalization of each endogenous antigen (α-enolase and annexin AI) with IgG2 within renal biopsy samples is reported. For each antigen/antibody, confocal images of two renal biopsy specimens are shown relative to patients with LN classes III and V. Double immunofluorescence staining was evaluated for each antigen (red) and IgG2 (green). Merged images are reported in yellow. RNase treatment of the same renal tissue did not modify colocalization of anti–α-enolase and IgG2 (Supplemental Figure 2). Magnification of this figure is in Supplemental Material. Original magnification, ×630. Scale bars, 20 µm.
Serum Anti–α-Enolase/Anti-Annexin AI: Isotype, Circulating Levels, and Correlations
The isotype of serum anti–α-enolase and anti-annexin AI was less selective than in glomeruli; even IgG2 was the major component. Minor amounts of anti-annexin AI IgG1 and IgG3 (Figures 4A and 5A) were observed.
Figure 4.
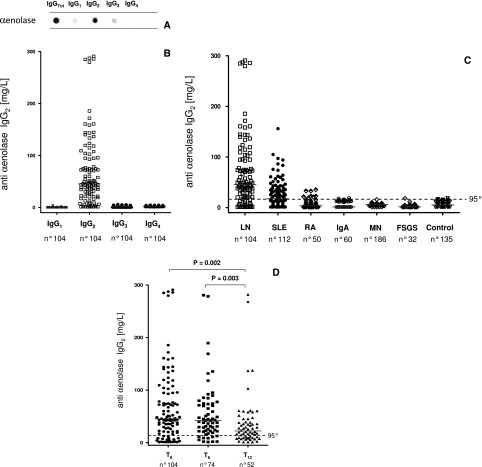
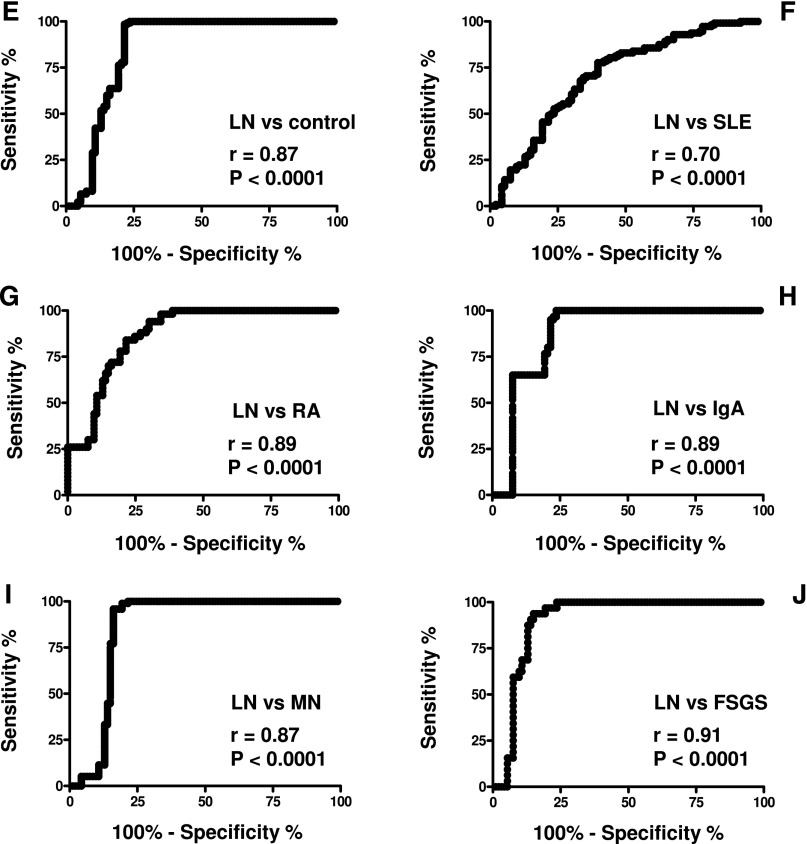
Serum anti–α-enolase isotype and levels. (A) Characterization of serum anti–α-enolase isotype with dot blot analysis. (B) The same technique was used to determine serum anti–α-enolase IgG1-IgG2-IgG3-IgG4 in patients with lupus erythematosus with (LN; n=104) and without (SLE; n=112) nephritis and several other control populations, including rheumatoid arthritis (RA; n=50), membranous nephropathy (MN; n=186), IgA nephropathy (IgA; n=60), FSGS (n=32), and normal controls (n=135). (C) Levels of anti–α-enolase IgG2 were very high in most patients with LN, whereas (D) other isotypes (IgG1, IgG3, and IgG4) were undetectable. (D) Patients with LN were evaluated at T0, and after 6 and 12 months of therapy, results showed a decrease of antibody levels after therapy. Dot blot analysis using recombinant α-enolase linked to nitrocellulose as antigen (Supplemental Figure 4); results (evaluated as the signal intensity of chemiluminescence detected by VersaDoc and computed with QuantyOne software; Bio-Rad) were transformed (milligrams per liter) using a standard curve of chemioluminescent IgG2. The horizontal line is set at the 95th percentile of levels titrated in normal controls. Receiver operating characterisitc curves for anti–α-enolase IgG2 were significantly greater in LN patients compared with other patient series: (E) normal subjects, (F) SLE, (G) RA, and (H–J) other nephropathies.
Figure 5.
Serum anti-annexin AI isotype and levels. (A) Characterization of serum anti-annexin AI isotype with dot blot analysis. (B) Serum levels of anti-annexin AI IgG2 were determined with a self-made ELISA in the same cohort of patients described in Figure 4; the technique is described in Supplemental Material. (C) Patients with LN were evaluated at T0, and after 6 and 12 months of therapy, results showed a decrease of antibody levels after therapy. (D) Double positivity for both anti–α-enolase and anti-annexin AI IgG2 in the different cohorts of patients with LN was analyzed with hierarchical cluster analysis. Results are presented as a heat map, in which color intensity (from red [high] to black [medium] to green [low]) indicates levels in separate patients. Two clusters could be observed: one cluster had high annexin AI and low anti–α-enolase IgG2 (bottom group; red), and one cluster had low annexin AI and high anti–α-enolase IgG2 (top group; green).
Circulating levels of anti–α-enolase IgG2 (Figure 4, B and C) and anti-annexin AI IgG2 (Figure 5B) were determined with dot blot analysis and ELISA in several SLE and non-SLE patients; antibodies of the other isotypes were evaluated as well (Figure 4B, Supplemental Figure 4). The following patient categories were considered (Table 4, Supplemental Table 1): 104 LN patients recruited at the time of renal biopsy, 112 SLE patients without LN, 50 patients with rheumatoid arthritis, and 278 patients with isolated glomerulonephrites.
Table 4.
Clinical data of LN and SLE patients
| Clinical Details | LN (n=104) | SLE (n=112) |
|---|---|---|
| Men | 11 (12%) | 17 (15%) |
| Age, yr | 34 (14–77) | 47 (16–79) |
| Age at SLE onset, yr | 26 (12–77) | 37 (7–79) |
| Disease duration, yr | 3 (0.1–24) | 7 (0–34) |
| Serum creatinine, mg/dl | 0.9 (0.3–6.4) | 0.7 (0.5–1.7) |
| C3, mg/dl | 63 (24–147) | 87 (42–154) |
| Anti-DNA ratio | 2.9 (0–26.7) | 0.8 (0–11.8) |
| Anti-C1q, units/ml | 166 (10–600) | 21 (10–257) |
| Proteinuria, g/d | 2.5 (0.1–20) | 0.1 (0–0.3) |
| Therapy | ||
| None | 19 (20%) | 30 (27%) |
| Steroids only | 39 (42%) | 20 (18%) |
| Multiple therapies | 35 (38%) | 62 (55%) |
| LN class | ||
| Proliferative (III and IV) | 52 (56%) | |
| Membranous (V) | 11 (12%) | |
| Mixed | 30 (32%) |
Data were collected from 216 SLE patients at the time of serum collection and presented as medians (ranges); 104 patients had overt LN, and in these cases, serum collection coincided with the time of renal biopsy. Anti-DNA antibodies were detected by different assays; to unify and analyze data, the anti-DNA ratio was conceived (Concise Methods). Briefly, an anti-DNA ratio of one indicates the lowest positive value of each method. Values smaller than one indicate a negative test. Data about anti-C1q antibodies were available for only 62 subjects in the non-nephritis group.
Serum levels of anti–α-enolase IgG2 were higher than the 95° limit of normal levels in 82% of the LN patients (Figure 4, B and C). Anti–α-enolase IgG1 and IgG3 serum levels were undetectable in all patient categories (Figure 4B); anti–α-enolase IgG4 was negative in LN and SLE patients but high, which is known, in patients with membranous nephropathy31 (Supplemental Figure 4B). Fewer SLE patients and no patients with rheumatoid arthritis or primary GN presented anti–α-enolase IgG2 levels exceeding normal values (Figure 4C). The median of serum levels of anti–α-enolase IgG2 was statistically higher in LN (45.37 mg/L) versus SLE (17.44 mg/L) patients and much more versus the other patient cohorts (Table 5). The area under the receiver operating characterisitc curves for anti–α-enolase IgG2 was significantly greater in LN patients compared with SLE patients, rheumatoid arthritis patients, and patients with other nephropathies (Figure 4, E–L). The results relative to anti–α-enolase IgG2 were confirmed by a self-made ELISA (Supplemental Figure 3). Some of the LN patients who had been evaluated at the beginning of disease were re-evaluated after 6 and 12 months, during which time they had received specific treatments (steroids and cyclophosphamide, mycophenolate mofetil, or tacrolimus): a significant decrease of anti–α-enolase IgG2 was reached at T12 (Figure 4D).
Table 5.
Serum levels of anti–α-enolase and anti-annexin AI IgG2 in LN and SLE patients and different cohorts of patients who were enrolled in the study compared with other cohorts presenting primary GN or rheumatoid arthritis
| Pathology Group | Number | Median (mg/L) | Interquartile Range | Probability Versus LN |
|---|---|---|---|---|
| Anti–α-enolase IgG2 | ||||
| LN T0 | 104 | 45.37 | 73.36 | |
| T6 | 74 | 41.95 | 52.52 | 0.09 |
| T12 | 52 | 22.21 | 31.45 | 0.002 |
| SLE | 112 | 17.44 | 32.13 | <0.001 |
| RA | 50 | 3.67 | 8.42 | <0.001 |
| IgAN | 60 | 1.21 | 10.71 | <0.001 |
| MN | 186 | 6.25 | 2.16 | <0.001 |
| FSGS | 32 | 1.51 | 3.54 | <0.001 |
| Controls | 135 | 5.24 | 9.09 (95°=15.62) | <0.001 |
| Anti-annexin AI IgG2 | ||||
| LN T0 | 104 | 4.077 | 4.250 | |
| T6 | 74 | 4.394 | 4.448 | |
| T12 | 52 | 2.633 | 2.251 | <0.001 |
| SLE | 112 | 3.422 | 2.549 | 0.01 |
| RA | 50 | 2.075 | 0.904 | <0.001 |
| IgA | 60 | 1.342 | 1.270 | <0.001 |
| MN | 184 | 1.416 | 0.944 | <0.001 |
| FSGS | 32 | 1.105 | 0.086 | <0.001 |
| Controls | 116 | 1.320 | 0.616 (95°=2.686) | <0.001 |
Results are given as milligrams per liter. Non-parametric Wilcoxon test for unpaired sample was used for comparison in different patient cohorts. Results are given as medians and interquartile ranges. RA, rheumatoid arthritis; IgAN, GN with mesangial IgA deposits; MN, membranous nephropathy.
Circulating anti-annexin AI IgG2 levels were determined with a self-made ELISA in the same patient cohorts. Results reported in Figure 5B and Table 5 indicate median levels one order of magnitude lower than anti–α-enolase IgG2 levels. Also, in this case, LN patients had higher levels than SLE patients (median of 4.250 versus 2.249 mg/L), and the difference was much more evident in other patient cohorts (Table 5). Twelve months after the start of therapy, LN patients presented a significant decrease of anti-annexin AI levels (2.251 mg/L).
Patients with double positivity for circulating anti–α-enolase and anti-annexin AI IgG2 were identified with heat map analysis (Figure 5D). A clear demarcation was observed among patients with LN regarding serum levels of the two antibodies, with one population presenting high anti–α-enolase in concomitance with low anti-annexin AI IgG2 (red indicates high levels and green indicates low levels in Figure 5D) and vice versa. Intermediate levels (Figure 5D, black) could be detected in a few patients. This result identifies two distinct patient cohorts with potentially different clinical characteristics and possibly, different mechanisms. Before definitive clinical considerations can be done, we need to have an overall picture of all antibodies present in glomeruli and serum of patients with LN, which also includes implanted antigens (G.M. Ghiggeri, unpublished data).
Anti–α-Enolase Antibodies Antigen Binding Epitopes and Specificity
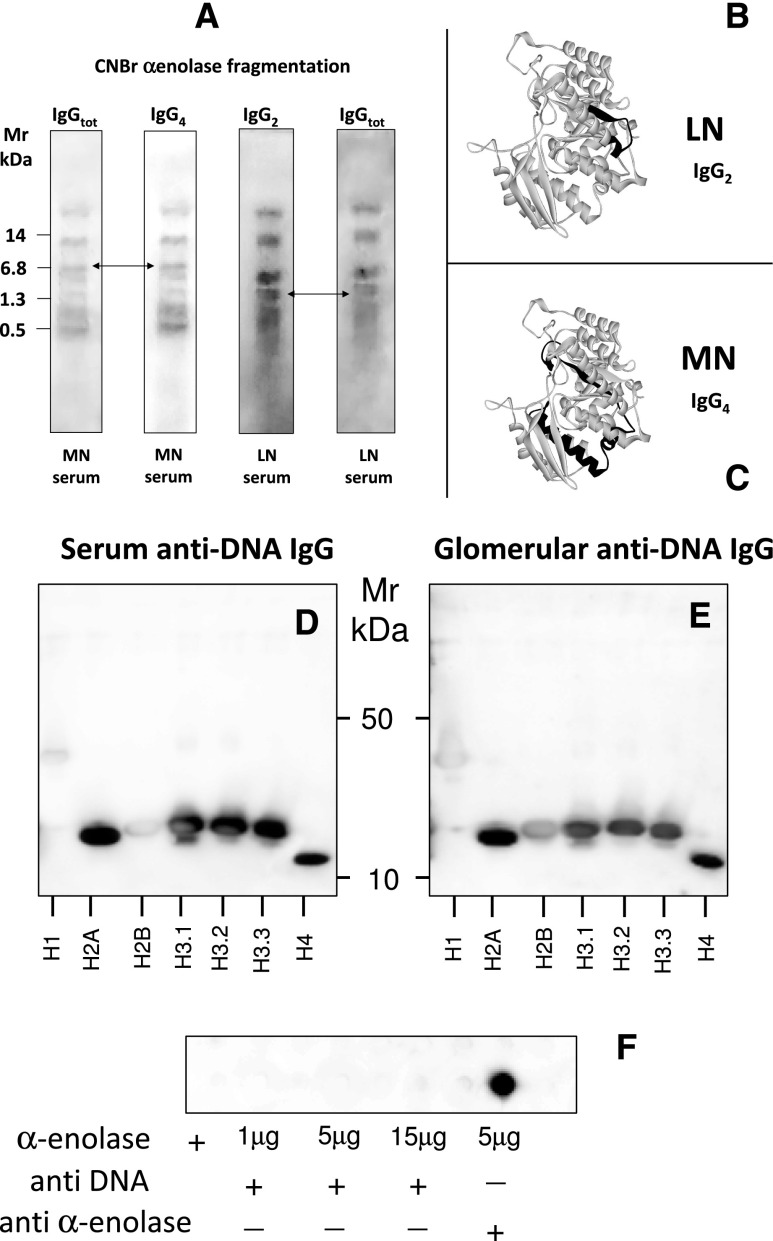
To better characterize anti–α-enolase antibodies, we analyzed the epitopes recognized by these antibodies: α-enolase cyanogen bromide (CNBr) digests were probed with IgG2 purified from glomeruli and serum of patients with LN and SLE or IgG4 eluted from glomeruli of patients with membranous nephropathy (a category of patients that has been recently recognized as having high levels of anti–α-enolase IgG4 in glomeruli and serum (Figure 6, A–C).30,31 IgG2 and IgG4 recognized different peptides derived from CNBr fragmentation with 1.3 kD (ILPVGAANFREAM) and 6.8 kD (DGTENKSKFGANAILGVSLAVCKAGAVEKGVPLYRHIADLAGNSEVILPVPAFNVINGGSHAGNKLAM), respectively (arrows in Figure 6A). Epitope mapping for the IgG2 and IgG4 recognition sites in the α-enolase model is reported in Figure 6A.
Figure 6.
Molecular features of anti–α-enolase antibody. (A–C) Anti–α-enolase IgG2 interacts with specific epitope protein. Characterization of α-enolase epitopes targets of anti–α-enolase IgG2 and IgG4; CNBr digests of anti–α-enolase were prepared as described in Concise Methods and then immunoblotted with IgG2 and IgG4 eluted from glomeruli and serum of patients with LN and MN, respectively. Arrows indicate two different peptides that are recognized by IgG2 (1.349 kD; ILPVGAANFREAM) and IgG4 (6.822 kD; DGTENKSKFGANAILGVSLAVCKAGAVEKGVPLYRHIADLAGNSEVILPVPAFNVINGGSHAGNKLAM); the second peptide contains a site for acetylation. The two epitopes above recognized by IgG2 and IgG4 have been localized in different regions of α-enolase. (D–F) Human anti-DNA IgG does not cross-react with α-enolase. Anti-dsDNA antibodies were purified from sera of LN patients by affinity chromatography; (D) they recognized several histones (only H1 and H2b were negative at Western blot) but (F) did not recognize α-enolase. Dot blot analysis was done by increasing the amount of fixed α-enolase up to 15 μg without showing any interaction. (E) The same histones separated by monodimensional electrophoresis were recognized by glomerular eluates. With the same dot blot assay, anti–α-enolase antibodies recognized the antigen at low concentration (5 μg).
A set of experiments was done to exclude interaction of anti-DNA antibodies with α-enolase. Anti-dsDNA antibodies were isolated from both serum and renal biopsies of several LN patients.32 On repeated experiments, purified anti-dsDNA recognized several histones (only H1 and H2b were negative) but did not recognize α-enolase, even when the amount of the protein in the dot blot assay was significantly increased (Figure 6, D–F). At variance, in the same dot blot essay using the same α-enolase amount, anti–α-enolase antibodies recognized the protein at low concentration (5 μg). Taken together with the results of colocalization after DNase/RNase treatment (Supplemental Figure 2), these findings exclude an interaction between anti–α-enolase/anti-annexin AI IgG2 with DNA and an interaction of anti-DNA antibodies with α-enolase.
Lupus-Prone Mice
LN is reproduced in mice by infusion of monoclonal anti-DNA IgG2 produced by cells derived from lupus-prone MRL-lpr/lpr mice.12 The prototype produced by clone H147 (an IgG2 encoded by 7183/81X VH gene)33 induces the formation of glomerular or tubular basement membrane, mesangial immune deposits, and proliferative GN after passive transfer to normal mice.12 The target antigen of these antibodies was recognized as a protein of 46 kD, which is the same molecular mass of α-enolase in the work by D’Andrea and colleagues,12 but it has not been characterized until now. We attempted to fill this gap by characterizing the target protein of the mouse IgG2 above (a gift from M.M.) and found that these antibodies at various dilutions recognize α-enolase at dot blot (Figure 7A). This finding shows important similarities in LN in humans and mice and strongly supports the implication of anti–α-enolase antibodies in murine LN.
Figure 7.
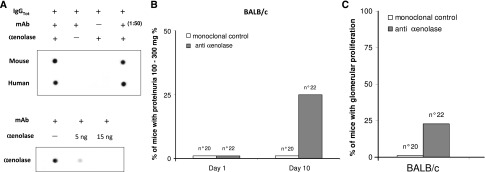
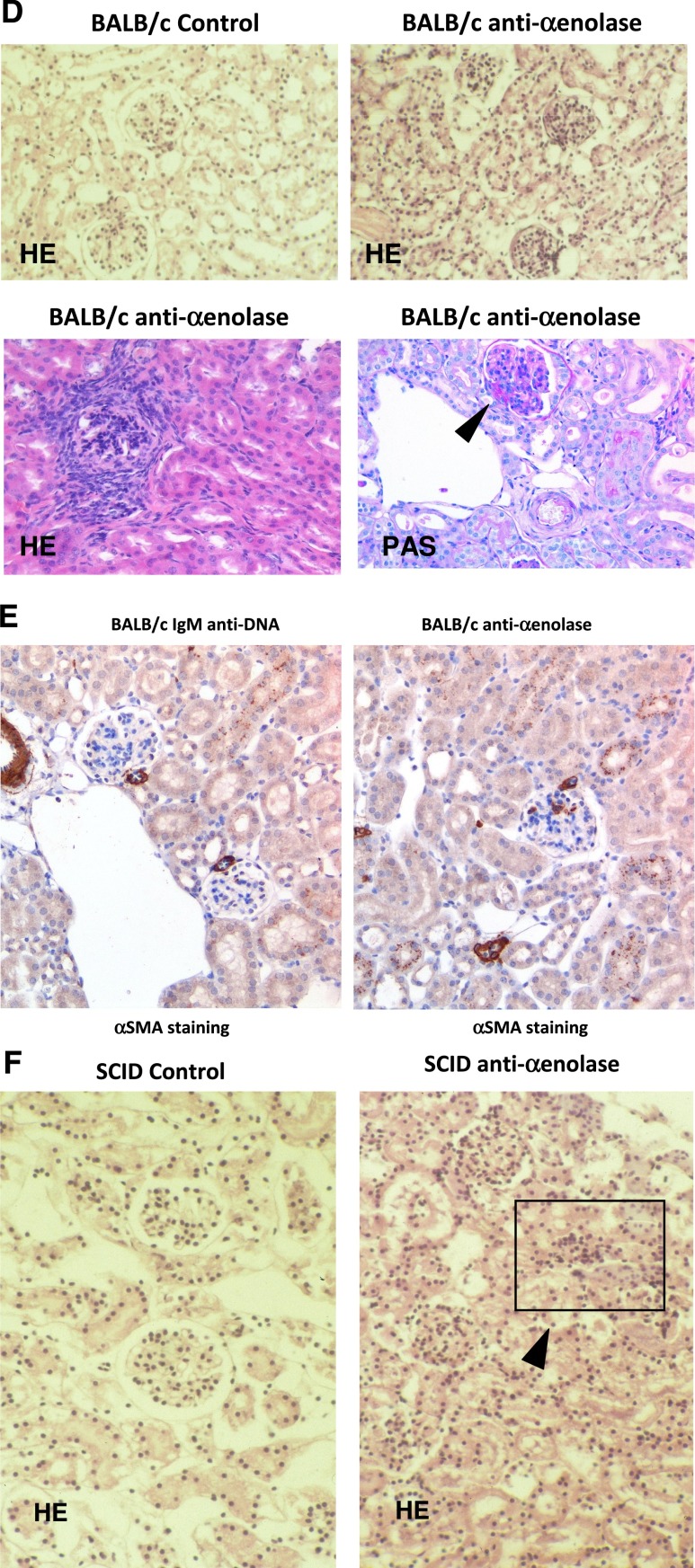
Experimental lupus nephritis. (A) mAbs from nephritogenic hybridomas recognize α-enolase. Nepritogenic monoclonal anti-DNA IgG2 clone (H147) derived from lupus-prone MRL-lpr/lpr mice was furnished by M.M. On dot blot using dilutions of the original hybridoma, a specific reaction with α-enolase was found. Techniques for developing hybridomas have been reported in detail in previous works.17,45 (B–F) Anti–α-enolase IgG infusion produced proliferative renal lesions in BALB/c and SCID mice. Twenty-two BALB/c mice were injected intraperitoneally with 1×106 hybridoma cells producing anti–α-enolase mAbs58; hybridoma cells producing IgM anti-DNA antibodies were injected into three BALB/c mice as controls. After 10 days, six mice developed proteinuria (100–300 mg%). (B and C) Controls and other mice had proteinuria less than 1 mg/ml. Gross pathology is reported in D, and it shows hematoxylin/eosin (HE; ×100 and ×200) and periodic–acid Schiff (PAS) staining. In E, immunostaining for nonmuscle myosin (αSMA) is shown; in mice infused with anti–α-enolase antibodies, mesangial deposits of αSMA were seen in glomeruli and small vessels (where αSMA is physiologic), whereas no mesangial staining could be seen in mice injected with anti-DNA IgM (×200). (F) Four of six SCID mice similarly injected with the same hybridomas developed proliferative GN with crescents and tubulointerstitial lesions.
Infusion of Anti–α-Enolase Antibodies in Mice
For anti–α-enolase antibodies, 22 BALB/c mice were injected intraperitoneally with hybridomas producing IgG anti–α-enolase antibodies or IgM anti-dsDNA antibodies as a negative control. Proteinuria (100–300 mg%) was detected in 25% of injected animals after 10 days from injection (Figure 7B); proteinuria in control mice was constantly less than 10 mg%. Renal histology showed diffuse glomerular proliferative lesions in 5 of 22 mice injected with anti–α-enolase monoclonals (Figure 7D). In two mice, proliferative lesions coexisted with basement membrane thickening, and in one case, proliferative lesions coexisted with FSGS. Few tubular infiltrates were observed. In mice infused with anti–α-enolase antibodies, mesangial deposits of nonmuscle myosin were seen in glomeruli and small vessels (where nonmuscle myosin is physiologic), whereas no mesangial staining could be found in mice injected with anti-DNA IgM (Figure 7E). Mice injected with monoclonal IgM anti-DNA developed only focal glomerular infiltrates.
Four of six SCID mice similarly injected with the same hybridomas developed proliferative GN with crescents and tubulointerstitial lesions (Figure 7F). Proteinuria was, in these cases, minimal, which is in agreement with the basic finding of cell proliferation within Bowman cells and interstitial infiltration.
Discussion
This study is the first study attempting to define the identity of glomerular targets of autoantibodies in human LN in vivo. New technologies based on laser capture microdissection and proteomics made this evolution possible. The crucial point of the program was to use human biopsies from a relevant number of patients with LN and cross-match the presence of microeluted antibodies versus renal and implanted antigens with the homolog sera. The strength of our study is the in vivo approach based on renal biopsies and the reproducibility of results. The novelty of our findings is the characterization of an autoantibody panel in kidneys of human LN, which defines IgG2 as a major isotype. The potential benefit is linked to the definition of mechanisms and the characterization of specific surrogate biomarkers of LN.
The characterization in vivo of specific autoantibodies versus podocyte antigens in glomeruli of LN patients was a main objective. Considering concomitance of autoantibodies in glomeruli and serum as a criterion to define importance, anti–α-enolase and anti-annexin AI emerged among a list of 11 proteins and were further evaluated. Actually, antibodies versus these two proteins were detected in more than 50% of biopsies that were relevant, because other autoantibodies, including antibodies versus implanted antigens, were less expressed. It is also worth noting that we could exclude any interaction of α-enolase with anti-DNA antibodies purified from serum and renal biopsies. Finally, characterization of a single autoantibody in glomeruli indicated IgG2 as the unique deposited component; this crucial finding influenced the search for circulating biomarkers, posing the basis for the definition of specific antibodies. The implication of anti–α-enolase IgG2 has been, in particular, addressed on the basis of homology with experimental models of LN and will be discussed below. Anti-annexin AI antibodies in SLE are not new,34–36 because they have been proposed as markers of discoid lupus erythematosus,34 and more generally, high serum levels have been detected in small series of patients with systemic autoimmune diseases,14 particularly anti-phospholipid syndrome.35 The demonstration of specific anti-annexin AI IgG2 in glomeruli emerges as a major finding of this work and strengthens their implication in LN. Other than anti–α-enolase and anti-annexin AI, the panel of antibody versus podocyte antigens included proteins of the cytoskeleton, such as vimentin and ezirin/moesin, or cytosol enzymes, such as glutathione synthetase, lactate dehydrogenase, and transketolase. It is of note that other antibodies previously proposed as markers of disease, such as anti–α-actinin and antilaminin antibodies,37–39 could not be detected in glomeruli with our approach. We cannot exclude that sensitivity problems of our methods caused the failure to detect these antibodies in glomeruli of LN patients, because α-actinin and laminin are expressed by cell lysates at very low levels. However, both anti–α-actinin and antilaminin antibodies have been never be documented in the kidney of human LN and data of the literature indicate that both proteins cross-react with anti-DNA antibodies.38,40 Therefore, additional studies must address the role of anti–α-actinin and antilaminin antibodies before concluding on this aspect.
A special focus of our study was α-enolase, because its serum levels are very high (in the range of 40–50 mg/L, with peaks up to 300 mg). Indeed, anti–α-enolase IgG has been detected in clinical conditions involving the kidney other than LN, and with the methodology limitations that characterized the preproteomics era, it has been generically associated with different autoimmune and inflammatory pathologies.25,26,41–43 Previous works on anti–α-enolase did not characterize the isotype of antibodies and reported variable results, overall suggesting a limited specificity for various clinical conditions, such as SLE (data on LN not reported), mixed cryoglobulinemia, systemic sclerosis, and ANCA vasculitis.41 Recently, we eluted anti–α-enolase IgG4 from glomeruli of patients with primary membranous nephropathy31 and detected specific antibodies of the same isotype in their serum.44 It is noteworthy that IgG2 was constantly absent in both glomerular eluates and circulating enolase antibodies of patients with membranous nephropathy.31,44 In our hand, patients with rheumatoid arthritis and other GNs were frankly negative for circulating anti–α-enolase IgG2.
It is of interest that anti–α-enolase IgG2 and IgG4 (purified from patients with membranous nephropathy) recognize different fragments deriving from the α-enolase CNBr digestion, suggesting that different parts of the protein become immunogenic in different clinical conditions. This basic finding opens up consideration of different mechanisms with determinant structural basis for autoantibody formation.
Analysis of target renal antigens in spontaneous animal models of LN strengthens the results from the human study. Actually, there are many factors influencing the development (variable genetics and environment) or progression (diet, lifestyle, sensitivity to therapies, etc.) of LN in humans that are controlled in animal models, suggesting that any comparison should be considered with circumspection. However, data on α-enolase in lupus-prone mice and anti–α-enolase in BALB/c mice go in the direction of a direct mechanism linked to anti–α-enolase and anti-annexin AI antibodies. In fact, it is shown here that α-enolase is recognized by nephritogenic mAbs derived from lupus-prone MRL-lpr/lpr mice12 (clone H147) that produce renal lesions reminiscent of human LN in mice. Data in other animal models are weaker because of the difficulty to develop unbiased controls (i.e., antibodies anti-DNA IgM). Overall, data obtained in BALB/c mice suggested, however, that anti–α-enolase antibodies produce diffuse renal proliferation, highly reminiscent of LN, in only a percentage of mice. Once again, the limited nephrotoxic potential of anti–α-enolase antibodies in mice is in agreement with the finding reported above in human LN showing 50% positivity of anti–α-enolase antibodies in renal biopsies.
Overall, these data implicate a multicomposition of renal autoantibodies in LN with a significant presence of anti–α-enolase and anti-annexin AI antibodies. This concept has been proposed over the years21–24,27 and is strengthened here by the finding of a panel of autoantibodies versus endogenous and implanted antigens, such as anti-DNA, antihistones, and anti-C1q. It is of interest that positivity for a single antibody did not exceed 50% of biopsies (see heat map analysis) for any case, implying a potential multiorigin of renal lesions. Confirmatory data and new results should constitute a sound start to approach pathogenesis of LN.17–20,45 It seems, in particular, that the key aspect is related to an implication of anti-dsDNA/histone antibodies as a starter of LN, because these antibodies could not be detected in a few biopsies. Our results, excluding an interaction of α-enolase with anti-dsDNA antibodies, suggest that other mechanisms and in vivo studies in the near future may modify the prevailing view of anti-dsDNA as a main player in LN.
A final point of interest is to define whether and how the present findings would modify the clinical approach to SLE patients. Indeed, treating LN is a possible task given that this condition is rapidly recognized and possibly, anticipated. A number of tests have been already been proposed as surrogate biomarkers of disease activity in LN.46 The list includes anti-dsDNA, antihistones,47–52 and antibodies versus endogenous antigens, such as antiactinin.10,13,53,54 Actually, definition of specific renal and serum levels of the above antibodies are in progress and will be reported very soon (G.M. Ghiggeri, unpublished data). To know the renal isotypes of these implanted components, it is crucial to look at the serum levels. Until definite data are available, it is difficult to conclude about sensitivity and correlation among different components, in which anti–α-enolase and anti-annexin AI IgG2 should be included in the list of candidates.
In conclusion, we describe here the presence of several specific antibodies versus endogenous glomerular antigens in glomeruli and sera of a relevant proportion of patients with LN. Anti–α-enolase and anti-annexin AI IgG2 seem to be the prevalent autoantibody components in glomeruli in vivo. Anti–α-enolase IgG2 seems to also be implicated in experimental LN. A multiantibody panel should be developed as a surrogate biomarker of LN.
Concise Methods
Patients
Overall, 216 SLE patients were included in the study (Table 2), and their sera were used for studies on circulating autoantibodies; 103 patients with LN were recruited when they underwent a renal biopsy for diagnostic purposes. Sera were obtained from all patients at the time of diagnosis and after months of therapy (T6 and T12) from a significant portion of patients (63 and 68 patients, respectively, after 6 and 12 months). All patients had a diagnosis of LN based on typical renal lesions. Fresh frozen renal samples were available for 20 patients and used for the proteomic approach (Table 1). For histologic evaluation of kidney disease, Dubosq–Bresil solution-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin/eosin, Masson’s trichrome, silver methenamine, and periodic–acid Schiff. Routine immunofluorescence studies on frozen sections were performed using anti-human IgG, IgA, IgM, C1q, C3, and fibrinogen antibody.
Normal Kidneys
Noncarcinomatous portions of kidneys removed for renal cancer were obtained from four patients and processed as negative tissue controls with laser capture.
Other Diseases
Serum was obtained from 50 patients with rheumatoid arthritis and 278 patients with isolated nephritis (186 patients with membranous nephropathy, 32 patients with FSGS, and 60 patients with IgA nephritis). One hundred thirty-five sera from normal people were also obtained and analyzed.
Ethical Committee
Permission for the study was given on June 10, 2010, by the Ethical Committee of San Carlo Borromeo Hospital in Milan, Italy. Informed consent to the study was obtained from all participants.
Antibodies
Antibody sources are reported as Supplemental Material.
Laser Capture Microdissection and Elution of Antibodies from Renal Biopsy Tissue
Laser capture microdissection and elution of antibodies were done as already described29,31; details are given in Supplemental Material.
Two-Dimensional Electrophoresis
Two-dimensional electrophoresis of podocyte extracts (a gift from M.A. Saleem) was performed in soft gels as described.55 A detailed description is given in Supplemental Material.
Monodimensional Electrophoresis
Gradient PAGE was done according to the work by Laemmli.56
Gel/Membrane Staining and Image Analysis
After separation in SDS-PAGE gels, proteins were visualized by a double staining procedure. The methyltrichloroacetate negative staining was followed by the blue silver colloidal Coomassie57 staining for preparative MS analysis. Images of stained gels were digitized using a GS800 photometer, and Western blots were acquired using a Versa DOC 400. All images were analyzed with PD Quest software (Bio-Rad, Hercules, CA).
Western Blot
Western blot with glomerular eluates and sera was done with podocyte cell line whole extracts separated by either mono- or two-dimensional electrophoresis. Equipment and technique of analysis are described in Supplemental Material.
Tryptic Digestion and Protein Identification by MALDI-MS and LC-MS
For the identification of proteins from two-dimensional electrophoresis, we used MALDI-MS and LC-MS as described in Supplemental Material. For MALDI-MS, we used a Voyager-DE PRO mass spectrometer (Applied Biosystems, Framingham, MA). For LC-MS, we used an Orbitrap mass spectrometer (Thermo Fisher Scientific, San Jose, CA) coupled to an HPLC Surveyor (Thermo Fisher Scientific) and equipped with a Jupiter C18 column of 250×1 mm (Phenomenex). Details of the analysis procedure are given in Supplemental Material.
Classic Immunofluorescence and Colocalization
Renal biopsy specimens were embedded in optimal cutting temperature (Tissue Tek; Miles Inc., Elkhart, IN) and stored in liquid nitrogen. Three-micrometer sections were cut by a cryostat (Leica CM1850; Leica Mycrosystems) and placed on poly-l-lysine–coated glass slides for indirect immunostaining. Details of staining are given in Supplemental Material.
Characterization of Autoantibody Isotype and Levels in Single Glomerular Eluates and Serum
Autoantibody isotype characterization and single biopsy levels were evaluated with dot blot using a Bio-Dot apparatus (Bio-Rad) as described in Supplemental Material. The same technique was used for the determination of anti–α-enolase serum levels; an ELISA was also developed to confirm results.
Anti–α-Enolase ELISA
A method for determining serum α-enolase levels in serum was developed. Main characteristics and steps of the procedure are illustrated in Supplemental Material.
Anti-Annexin AI ELISA
For anti-annexin AI, we used a self-made ELISA (Supplemental Material).
CNBr Digestion of α-Enolase and Analysis of Fragmentation Binding
For digestion with CNBr, α-enolase (10 μg) methods are illustrated in Supplemental Material.
Isolation of Serum Anti-DNA Antibodies
Isolation of anti-dsDNA antibodies from sera of patients with SLE was done according to the work by Chan and colleagues.32 Details are given in Supplemental Material.
Nepritogenic Monoclonal Anti-DNA IgG2 Clone (H147) Derived from Lupus-Prone MRL-lpr/lpr Mice
Nepritogenic monoclonal anti-DNA IgG2 clone (H147) derived from lupus-prone MRL-lpr/lpr mice was furnished by M.M. Techniques for developing hybridomas have been reported in detail in previous works.17,45 mAbs were purified from hybridoma supernatants by affinity chromatographys.18
Anti–α-Enolase IgG in BALB/c Mice
Twenty-two BALB/c and six SCID mice were injected intraperitoneally with 1×106 hybridoma cells producing anti–α-enolase mAbs; proteinuria and renal pathology were tested at weekly intervals (Supplemental Material).
Statistical Analyses
Serum levels of antibodies were expressed as median and interquartile range. A nonparametric Wilcoxon test for unpaired samples was used for comparison of anti–α-enolase IgG2 or anti-annexin AI IgG2 serum titer in different patient cohorts, whereas a nonparametric Wilcoxon test for paired samples was used for comparison of anti–α-enolase IgG2 or anti-annexin AI IgG2 LN serum titer at different times. Statistical analysis was performed using the R software. Differences were considered statistically significant at two-tailed P values <0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by funds from Cinque per mille of Personal Income Tax (IRPEF)-Finanziamento della ricerca sanitaria and the Italian Ministry of Health Ricerca Corrente contributo per la ricerca intramuraria. The Institute Giannina Gaslini provided financial and logistic support to the trial. This work was also supported by the Renal Child Foundation and Fondazione La Nuova Speranza (Progetto integrato per la definizione dei meccanismi implicati nella glomerulo sclerosi focale).
The present study was investigator-initiated and -driven. All members of the study steering committee are listed as authors of the present report, had access to the study data, and vouch for the accuracy and completeness of the data reported. A patent on the potential use of anti–α-enolase IgG2 antibodies as surrogate biomarkers of lupus nephritis is pending.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090987/-/DCSupplemental.
References
- 1.Cameron JS: Lupus nephritis. J Am Soc Nephrol 10: 413–424, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Madaio MP: The relevance of antigen binding to the pathogenicity of lupus autoantibodies. Kidney Int 82: 125–127, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Waldman M, Madaio MP: Pathogenic autoantibodies in lupus nephritis. Lupus 14: 19–24, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bagavant H, Fu SM: Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol 21: 489–494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanrotel-Saliou C, Segalen I, Le Meur Y, Youinou P, Renaudineau Y: Glomerular antibodies in lupus nephritis. Clin Rev Allergy Immunol 40: 151–158, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Kramers C, Hylkema MN, van Bruggen MC, van de Lagemaat R, Dijkman HB, Assmann KJ, Smeenk RJ, Berden JH: Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest 94: 568–577, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalaaji M, Mortensen E, Jørgensen L, Olsen R, Rekvig OP: Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol 168: 1779–1792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortensen ES, Rekvig OP: Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol 20: 696–704, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Mjelle JE, Rekvig OP, Van Der Vlag J, Fenton KA: Nephritogenic antibodies bind in glomeruli through interaction with exposed chromatin fragments and not with renal cross-reactive antigens. Autoimmunity 44: 373–383, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Renaudineau Y, Croquefer S, Jousse S, Renaudineau E, Devauchelle V, Guéguen P, Hanrotel C, Gilburd B, Saraux A, Shoenfeld Y, Putterman C, Youinou P: Association of alpha-actinin-binding anti-double-stranded DNA antibodies with lupus nephritis. Arthritis Rheum 54: 2523–2532, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Deocharan B, Zhou Z, Antar K, Siconolfi-Baez L, Angeletti RH, Hardin J, Putterman C: Alpha-actinin immunization elicits anti-chromatin autoimmunity in nonautoimmune mice. J Immunol 179: 1313–1321, 2007 [DOI] [PubMed] [Google Scholar]
- 12.D’Andrea DM, Coupaye-Gerard B, Kleyman TR, Foster MH, Madaio MP: Lupus autoantibodies interact directly with distinct glomerular and vascular cell surface antigens. Kidney Int 49: 1214–1221, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Kalaaji M, Sturfelt G, Mjelle JE, Nossent H, Rekvig OP: Critical comparative analyses of anti-alpha-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum 54: 914–926, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Yung S, Cheung KF, Zhang Q, Chan TM: Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol 21: 1912–1927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster MH, Sabbaga J, Line SR, Thompson KS, Barrett KJ, Madaio MP: Molecular analysis of spontaneous nephrotropic anti-laminin antibodies in an autoimmune MRL-lpr/lpr mouse. J Immunol 151: 814–824, 1993 [PubMed] [Google Scholar]
- 16.Kalaaji M, Fenton KA, Mortensen ES, Olsen R, Sturfelt G, Alm P, Rekvig OP: Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int 71: 664–672, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Vlahakos DV, Foster MH, Adams S, Katz M, Ucci AA, Barrett KJ, Datta SK, Madaio MP: Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int 41: 1690–1700, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Madaio MP, Carlson J, Cataldo J, Ucci A, Migliorini P, Pankewycz O: Murine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune deposits. J Immunol 138: 2883–2889, 1987 [PubMed] [Google Scholar]
- 19.Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, Tung KS, Fu SM: Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med 199: 255–264, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ: Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med 202: 321–331, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pankewycz OG, Migliorini P, Madaio MP: Polyreactive autoantibodies are nephritogenic in murine lupus nephritis. J Immunol 139: 3287–3294, 1987 [PubMed] [Google Scholar]
- 22.Sabbaga J, Pankewycz OG, Lufft V, Schwartz RS, Madaio MP: Cross-reactivity distinguishes serum and nephritogenic anti-DNA antibodies in human lupus from their natural counterparts in normal serum. J Autoimmun 3: 215–235, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Jacobi AM, Wang T, Berlin R, Volpe BT, Diamond B: Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun 33: 270–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C, Mohan C: Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest 115: 3428–3439, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabbatini A, Dolcher MP, Marchini B, Chimenti D, Moscato S, Pratesi F, Bombardieri S, Migliorini P: Alpha-enolase is a renal-specific antigen associated with kidney involvement in mixed cryoglobulinemia. Clin Exp Rheumatol 15: 655–658, 1997 [PubMed] [Google Scholar]
- 26.Migliorini P, Pratesi F, Bongiorni F, Moscato S, Scavuzzo M, Bombardieri S: The targets of nephritogenic antibodies in systemic autoimmune disorders. Autoimmun Rev 1: 168–173, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Mannik M, Merrill CE, Stamps LD, Wener MH: Multiple autoantibodies form the glomerular immune deposits in patients with systemic lupus erythematosus. J Rheumatol 30: 1495–1504, 2003 [PubMed] [Google Scholar]
- 28.Winfield JB, Faiferman I, Koffler D: Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest 59: 90–96, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murtas C, Bruschi M, Carnevali ML, Petretto A, Corradini E, Prunotto M, Candiano G, degl’Innocenti ML, Ghiggeri GM, Allegri L: In vivo characterization of renal auto-antigens involved in human auto-immune diseases: The case of membranous glomerulonephritis. Proteomics Clin Appl 5: 90–97, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Prunotto M, Carnevali ML, Candiano G, Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto A, Santucci L, Mattei S, Gatti R, Scolari F, Kador P, Allegri L, Ghiggeri GM: Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 21: 507–519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruschi M, Carnevali ML, Murtas C, Candiano G, Petretto A, Prunotto M, Gatti R, Argentiero L, Magistroni R, Garibotto G, Scolari F, Ravani P, Gesualdo L, Allegri L, Ghiggeri GM: Direct characterization of target podocyte antigens and auto-antibodies in human membranous glomerulonephritis: Alfa-enolase and borderline antigens. J Proteomics 74: 2008–2017, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Chan TM, Leung JK, Ho SK, Yung S: Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus. J Am Soc Nephrol 13: 1219–1229, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Vargas MT, Gustilo K, D’Andrea DM, Kalluri R, Foster MH, Madaio MP: Structural features of nephritogenic lupus autoantibodies. Methods 11: 62–69, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Kretz CC, Norpo M, Abeler-Dörner L, Linke B, Haust M, Edler L, Krammer PH, Kuhn A: Anti-annexin 1 antibodies: A new diagnostic marker in the serum of patients with discoid lupus erythematosus. Exp Dermatol 19: 919–921, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Salle V, Mazière JC, Smail A, Cévallos R, Mazière C, Fuentes V, Tramier B, Makdassi R, Choukroun G, Vittecoq O, Goëb V, Ducroix JP: Anti-annexin II antibodies in systemic autoimmune diseases and antiphospholipid syndrome. J Clin Immunol 28: 291–297, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Zhou D, Luo N, Wu Q, You Y, Zhai Z, Mou Z, Wu Y, Hao F: Transcellular distribution heterogeneity of Annexin A5 represents a protective response to lupus-related thrombophilia: A pilot Proteomics-based study. Biochem Biophys Res Commun 420: 357–363, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Renaudineau Y, Deocharan B, Jousse S, Renaudineau E, Putterman C, Youinou P: Anti-alpha-actinin antibodies: A new marker of lupus nephritis. Autoimmun Rev 6: 464–468, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Zhao Z, Weinstein E, Tuzova M, Davidson A, Mundel P, Marambio P, Putterman C: Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum 52: 522–530, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Kootstra CJ, Bergijk EC, Veninga A, Prins FA, de Heer E, Abrahamson DR, Bruijn JA: Qualitative alterations in laminin expression in experimental lupus nephritis. Am J Pathol 147: 476–488, 1995 [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Yehuda A, Rasooly L, Bar-Tana R, Breuer G, Tadmor B, Ulmansky R, Naparstek Y: The urine of SLE patients contains antibodies that bind to the laminin component of the extracellular matrix. J Autoimmun 8: 279–291, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Moodie FD, Leaker B, Cambridge G, Totty NF, Segal AW: Alpha-enolase: A novel cytosolic autoantigen in ANCA positive vasculitis. Kidney Int 43: 675–681, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Wakui H, Imai H, Komatsuda A, Miura AB: Circulating antibodies against alpha-enolase in patients with primary membranous nephropathy (MN). Clin Exp Immunol 118: 445–450, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L: Alpha-enolase: A target of antibodies in infectious and autoimmune diseases. Autoimmun Rev 6: 176–182, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Murtas C, Bruschi M, Candiano G, Moroni G, Magistroni R, Magnano A, Bruno F, Radice A, Furci L, Argentiero L, Carnevali ML, Messa P, Scolari F, Sinico RA, Gesualdo L, Fervenza FC, Allegri L, Ravani P, Ghiggeri GM: Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol 7: 1394–1400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP: Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol 2: 1345–1354, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Moroni G, Radice A, Giammarresi G, Quaglini S, Gallelli B, Leoni A, Li Vecchi M, Messa P, Sinico RA: Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis 68: 234–237, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Kiss E, Lakos G, Szegedi G, Poor G, Szodoray P: Anti-nuscleosome antibody, a reliable indicator for lupus nephritis. Autoimmunity 42: 393–398, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Gómez-Puerta JA, Burlingame RW, Cervera R: Anti-chromatin (anti-nucleosome) antibodies: Diagnostic and clinical value. Autoimmun Rev 7: 606–611, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Hung WT, Chen YM, Lan JL, Chen HH, Chen YH, Chen DY, Hsieh CW, Wen MC: Antinucleosome antibodies as a potential biomarker for the evaluation of renal pathological activity in patients with proliferative lupus nephritis. Lupus 20: 1404–1410, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Sui M, Lin Q, Xu Z, Han X, Xie R, Jia X, Guo X, Zhang W, Guan X, Ren H: Simultaneous positivity for anti-DNA, anti-nucleosome and anti-histone antibodies is a marker for more severe lupus nephritis. J Clin Immunol 33: 378–387, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Rubin RL, Bell SA, Burlingame RW: Autoantibodies associated with lupus induced by diverse drugs target a similar epitope in the (H2A-H2B)-DNA complex. J Clin Invest 90: 165–173, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costa O, Monier JC: Antihistone antibodies detected by ELISA and immunoblotting in systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol 13: 722–725, 1986 [PubMed] [Google Scholar]
- 53.Croquefer S, Renaudineau Y, Jousse S, Gueguen P, Ansart S, Saraux A, Youinou P: The ananti-alpha-actinin test completes ananti-DNA determination in systemic lupus erythematosus. Ann N Y Acad Sci 1050: 170–175, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Becker-Merok A, Kalaaji M, Haugbro K, Nikolaisen C, Nilsen K, Rekvig OP, Nossent JC: Alpha-actinin-binding antibodies in relation to systemic lupus erythematosus and lupus nephritis. Arthritis Res Ther 8: R162, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruschi M, Musante L, Candiano G, Ghiggeri GM, Herbert B, Antonucci F, Righetti PG: Soft immobilized pH gradient gels in proteome analysis: A follow-up. Proteomics 3: 821–825, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 57.Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG: Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25: 1327–1333, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Moscato S, Pratesi F, Sabbatini A, Chimenti D, Scavuzzo M, Passatino R, Bombardieri S, Giallongo A, Migliorini P: Surface expression of a glycolytic enzyme, alpha-enolase, recognized by autoantibodies in connective tissue disorders. Eur J Immunol 30: 3575–3584, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.