Abstract
Low birth weight is associated with ESRD. To identify specific growth patterns in early life that may be related to kidney function in later life, we examined the associations of longitudinally measured fetal and infant growth with kidney function in school-aged children. This study was embedded in a population-based prospective cohort study among 6482 children followed from fetal life onward. Fetal and childhood growth was measured during second and third trimesters of pregnancy, at birth, and at 6, 12, 24, 36, and 48 months postnatally. At the age of 6 years, we measured kidney volume by ultrasound. GFR was estimated using blood creatinine levels. Higher gestational age-adjusted birth weight was associated with higher combined kidney volume and higher eGFR (per 1 SD score increase in birth weight; 1.27 cm3 [95% confidence interval, 0.61 to 1.93] and 0.78 ml/min per 1.73 m2 [95% CI, 0.16 to 1.39], respectively). Fetal weight, birth weight, and weight at 6 months were positively associated with childhood kidney volume, whereas higher second trimester fetal weight was positively associated with higher GFR (all P values<0.05). Fetal and childhood lengths were not consistently associated with kidney function. In this cohort, lower fetal and early infant weight growth is associated with smaller kidney volume in childhood, whereas only lower fetal weight growth is associated with lower kidney function in childhood, independent of childhood growth. Whether these associations lead to an increased risk of kidney disease needs to be studied further.
Low birth weight is associated with higher risks of ESRD and hypertension in later life.1–3 Clearly, low birth weight is not the causal factor per se leading to kidney diseases in later life. Birth weight is the result of various exposures and growth patterns in fetal life and the starting point of childhood growth. It has been hypothesized that especially third trimester fetal growth restriction leads to persistently smaller kidneys with a reduced number of nephrons, which may predispose the individual to kidney disease in adulthood.4–6 This hypothesis is supported by both animal and human studies, showing that kidney volume and nephron number are reduced in fetal growth-restricted subjects and hypertensive subjects.7–9 Although nephrogenesis is known to continue until 36 weeks of gestation and cease thereafter, not much is known about the specific critical periods and early growth patterns related to kidney function in later life.10 Also, whether and to what extent the associations of low birth weight with CKD are explained by preterm birth are not known.1 Longitudinal studies suggested that the associations of low birth weight with hypertension were stronger in subjects with rapid weight gain in childhood, but results are inconclusive.11,12 A similar growth pattern has not been identified as a risk factor for kidney diseases yet.
Prospective studies linking fetal and early childhood growth patterns to kidney outcomes in later life might help to identify early critical periods for developing impaired kidney function in later life.
Therefore, we examined, in a population-based prospective cohort study among 6482 children followed from early fetal life onward (Figure 1), the associations of birth weight, gestational age, birth weight for gestational age, and longitudinally measured fetal and early childhood growth patterns with kidney size and function at school age. We used subclinical variations of kidney function in childhood as outcomes, because they relate to kidney disease in later life.13
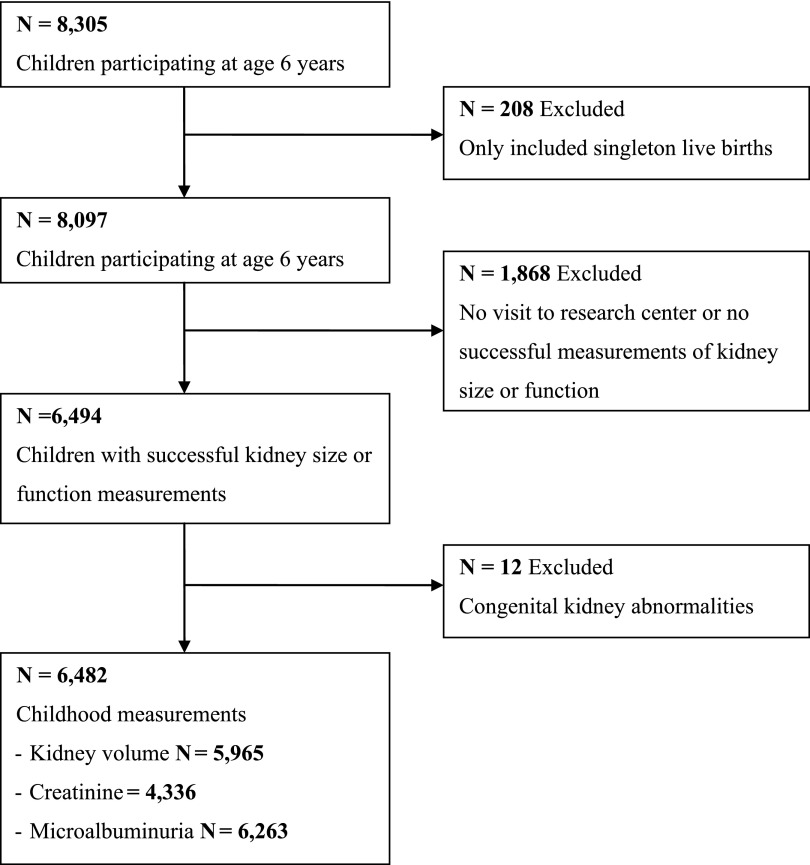
Figure 1.
Flow chart: exclusion criteria and numbers of participants are given. Total numbers of available outcome measurements are given.
Results
Subject Characteristics
Maternal and child characteristics are shown in Table 1. At the age of 6.0 years (90% range=5.7–7.4 years), mean (SD) total kidney volume was 120.3 (23.5) cm3, and eGFR was 118.8 (16.4) ml/min per 1.73 m2. Microalbuminuria was present in 7.6% of all children. In Table 2, all fetal, birth, and childhood growth characteristics are presented. Observed data before multiple imputations are presented in Supplemental Table 1. Differences in subject characteristics between children with and without blood samples are shown in Supplemental Table 2.
Table 1.
Maternal and child characteristics (n=6482)
| Particpant Characteristics | Boys (n=3257) | Girls (n=3225) | P Value |
|---|---|---|---|
| Maternal characteristics | |||
| Age, yr | 31.1 (21.2–38.8) | 31.0 (21.2–38.5) | 0.28 |
| Height, cm | 167.6 (7.2) | 167.5 (7.6) | 0.13 |
| Prepregnancy body mass index, kg/m2 | 23.6 (4.2) | 23.8 (4.3) | 0.08 |
| Parity≥1, % | 44.6 (1452) | 43.1 (1390) | 0.30 |
| Ethnicity, % | 0.10 | ||
| European | 61.2 (3964) | 62.1 (2001) | |
| Non-European | 38.8 (2518) | 37.9 (1224) | |
| Educational level, % | 0.19 | ||
| No higher education | 53.9 (1756) | 55.4 (1788) | |
| Higher education | 46.1 (1501) | 44.6 (1437) | |
| Smoking, % | 0.22 | ||
| Nonsmoking | 73.5 (2066) | 75.9 (2133) | |
| Continued smoking | 26.5 (745) | 24.1 (678) | |
| Folic acid supplement use, % | 0.09 | ||
| No | 25.7 (570) | 24.5 (552) | |
| Preconceptional | 42.0 (929) | 44.5 (1001) | |
| Postconceptional | 32.3 (715) | 30.9 (696) | |
| Infant characteristics | |||
| Gestational age, wk | 40.1 (37.0–42.1) | 40.1 (36.9–42.0) | 0.07 |
| Birth weight, g | 3488 (569) | 3362 (534) | <0.001 |
| Breastfeeding, % | 0.81 | ||
| No | 7.5 (188) | 7.8 (252) | |
| Yes | 92.5 (2325) | 92.2 (2973) | |
| Child characteristics | |||
| Age, yr | 6.0 (5.7–7.5) | 6.02 (5.7–7.2) | 0.01 |
| Height, cm | 119.9 (6.1) | 119.0 (6.0) | <0.001 |
| Weight, kg | 23.4 (4.1) | 23.2 (4.5) | 0.01 |
| Body mass index, kg/m2 | 16.2 (1.8) | 16.3 (2.0) | 0.38 |
| Kidney volume combined, cm3 | 122.3 (24.2) | 118.3 (22.6) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 118.7 (16.2) | 118.9 (16.7) | 0.61 |
| Microalbuminuria, % | 6.8 (217) | 8.3 (256) | 0.20 |
Values are means (SDs), medians (90% ranges), or percentages (numbers); t tests were used for continuous variables, and chi-squared tests were used for categorical variables.
Table 2.
Fetal and early childhood growth characteristics (n=6482)
| Growth Characteristics | Boys (n=3257) | Girls (n=3225) | P Value |
|---|---|---|---|
| Fetal growth | |||
| Second trimester | |||
| Gestational age (wk) | 20.6 (18.9–22.9) | 20.5 (18.9–22.7) | <0.001 |
| Femur length (mm) | 33.5 (3.6) | 33.5 (3.6) | 0.82 |
| Estimated fetal weight (g) | 387 (98) | 378 (91) | <0.001 |
| Third trimester | |||
| Gestational age (wk) | 30.4 (29.0–32.4) | 30.3 (28.8–32.3) | 0.001 |
| Femur length (mm) | 57.4 (3.1) | 57.6 (3.1) | 0.002 |
| Estimated fetal weight (g) | 1633 (260) | 1618 (268) | 0.03 |
| Birth | |||
| Gestational age (wk) | 40.1 (37.0–42.1) | 40.1 (36.9–42.0) | 0.08 |
| Length (cm) | 50.6 (2.4) | 49.9 (2.3) | <0.001 |
| Weight (g) | 3488 (569) | 3362 (534) | <0.001 |
| Early childhood growth | |||
| 6 mo | |||
| Age (mo) | 6.2 (5.4–7.5) | 6.2 (5.5–7.5) | 0.65 |
| Length (cm) | 68.5 (2.5) | 66.7 (2.4) | <0.001 |
| Weight (g) | 8176 (903) | 7590 (832) | <0.001 |
| 12 mo | |||
| Age (mo) | 11.1 (10.2–12.3) | 11.1 (10.2–12.3) | 0.62 |
| Length (cm) | 75.1 (2.5) | 73.5 (2.5) | <0.001 |
| Weight (g) | 9970 (1061) | 9326 (991) | <0.001 |
| 24 mo | |||
| Age (mo) | 24.8 (23.5–27.6) | 24.8 (23.6–27.4) | 0.35 |
| Length (cm) | 88.9 (3.3) | 87.7 (3.4) | <0.001 |
| Weight (kg) | 13.2 (1.5) | 12.7 (1.5) | <0.001 |
| 36 mo | |||
| Age (mo) | 36.7 (35.6–39.8) | 36.7 (35.5–39.6) | 0.17 |
| Length (cm) | 97.9 (3.8) | 96.8 (3.8) | <0.001 |
| Weight (kg) | 15.4 (1.8) | 15.0 (1.9) | <0.001 |
| 48 mo | |||
| Age (mo) | 45.8 (44.8–48.0) | 45.8 (44.7–47.9) | 0.33 |
| Length (cm) | 103.7 (4.1) | 102.8 (4.2) | <0.001 |
| Weight (kg) | 17.1 (2.2) | 16.7 (2.3) | <0.001 |
| 72 mo | |||
| Age (mo) | 72.6 (44.7–48.0) | 72.6 (69.0–87.3) | 0.01 |
| Length (cm) | 119.9 (6.1) | 119.0 (6.0) | <0.001 |
| Weight (kg) | 23.4 (4.1) | 23.1 (4.5) | 0.01 |
Values are means (SDs), medians (90% ranges), or percentages (numbers); t tests were used for comparison between boys and girls.
Birth Outcomes and Childhood Kidney Outcomes
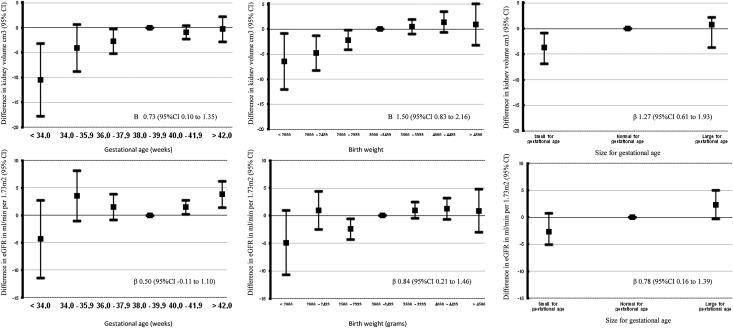
Figure 2 shows that a 1 SD longer duration of gestational age at birth was associated with a larger combined kidney volume in childhood (P value for trend<0.05). Compared with term-born children (38.0–39.9 weeks), children born very preterm (<34 weeks) had a smaller kidney volume (difference=−10.48 cm3; 95% confidence interval [95% CI], −17.74 to −3.22). Post-term birth (>42 weeks) was associated with higher eGFR in childhood (difference=3.80 ml/min per 1.73 m2; 95% CI, 1.39 to 6.21). Gestational age at birth was not associated with the risk of childhood microalbuminuria. Birth weight not adjusted for gestational age was positively associated with combined kidney volume and eGFR (P values for trend<0.01) in childhood. Gestational age-adjusted birth weight was positively associated with childhood combined kidney volume and eGFR (P values for trend<0.05). Compared with children with size appropriate for gestational age, children born small for gestational age had smaller kidney volume (−3.74 cm3; 95% CI, −6.89 to −0.89). We performed a sensitivity analysis using the lower 10% and upper 10% as the definitions for small and large sizes for gestational age of children. These analyses showed the same results compared with a 5% cutoff (Supplemental Figure 1). Birth weight was not associated with risk of microalbuminuria. Results from models adjusted for sex and age only were similar, with some stronger effect estimates (Supplemental Table 3). Results from analyses on the data before imputation are shown in Supplemental Table 4. After additional adjustment for childhood kidney volume, the associations of birth outcomes with kidney function attenuated to nonsignificant (results are presented in Supplemental Table 5).
Figure 2.
Associations of birth outcomes with kidney outcomes (n=6482). Bars represent regression coefficients (95% CI) based on multiple regression models and reflect the difference for each outcome for the birth weight or gestational age group compared with the reference group. Models are adjusted for maternal age, body mass index, parity, ethnicity, educational level, folic acid supplementation, and smoking during pregnancy as well as child sex, breastfeeding, current age, and body surface area. β-Value for trend (95% CI) is shown. Results from models adjusted for sex and age only are given in Supplemental Table 3. Results for models additionally adjusted for kidney volume are given in Supplemental Table 5.
Fetal and Early Childhood Growth and Kidney Outcomes
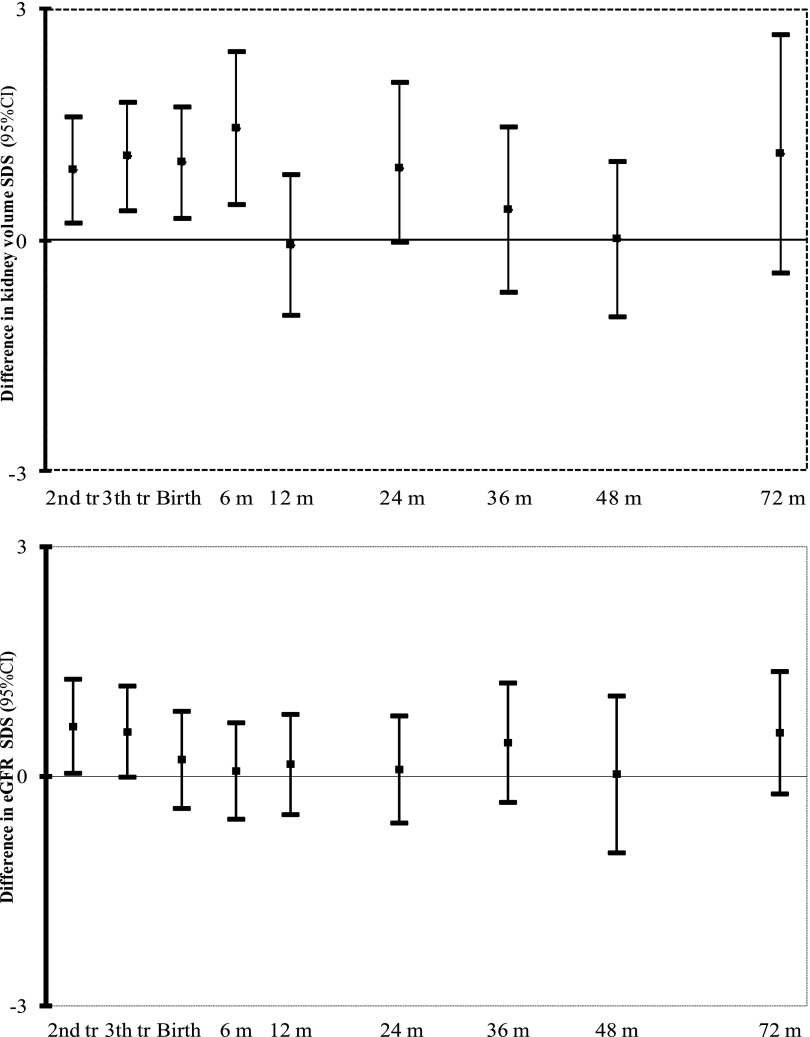
We explored whether the associations of fetal and early childhood growth characteristics with childhood kidney function outcomes were independent from growth measures at other ages using conditional growth analyses. Figure 3 shows that higher second and third trimester fetal weights, birth weight, and weight at the age of 6 months were all independently associated with a larger combined kidney volume in childhood (all P values<0.05). Also, higher second trimester and third trimester fetal weights tended to be independent from weight at other ages (associated with higher eGFR; P value<0.05 and P value=0.05, respectively). When we additionally adjusted the models focused on eGFR for childhood kidney volume, we observed that these associations attenuated to nonsignificant (results are shown in Supplemental Figure 3). Conditional analyses for fetal and childhood length growth did not show consistent associations with childhood kidney volume and function outcomes (results are shown in Supplemental Figure 2).
Figure 3.
Associations of fetal and childhood weight with kidney outcomes at the age of 6 years (n=6482). Effect estimates (95% CI) represent regression coefficients based on multiple regression models and reflect the difference per 1 SD increase in standardized residual score for (estimated) weight measures at different time points (details on conditional regression models are in Supplemental Material). Models are adjusted for maternal age, body mass index, parity, ethnicity, educational level, folic acid supplementation, and smoking during pregnancy as well as child sex, breastfeeding, current age, and body surface area. Results for models additionally adjusted for kidney volume are given in Supplemental Figure 3. SDS, SD score.
Results from the normal multivariate regression models, which do not take into account growth measures at other ages, showed that longer length at different ages is associated with higher eGFR and an increased risk of microalbuminuria (all P values<0.05). Higher weight at different ages is associated with larger kidney volume and higher eGFR (all P values<0.05) but with the risk of microalbuminuria (basic and adjusted models are shown in Supplemental Tables 6 and 7).
Discussion
In this population-based prospective cohort study, we aimed to identify critical periods during fetal life and childhood for development of impaired kidney function. We observed that preterm birth was associated with smaller kidney volume and that smaller size for gestational age at birth was associated with a smaller kidney volume and lower eGFR. Higher fetal weight, birth weight, and weight at 6 months were independently positively associated with childhood kidney volume, whereas only higher fetal weight was independently positively associated with higher eGFR. Fetal life and early infancy may be critical periods for kidney function in later life.
Strengths and Limitations
A major strength of our study is its prospective design from fetal life onward within a large population-based cohort. Our analyses were based on more than 6000 children with kidney volume and function measurements available. The study population comprised a multiethnic group, with almost 40% being non-Western children. The largest non-Western groups were Moroccan, Surinamese, and Turkish, which are the largest ethnic minority groups in The Netherlands.14 Whether these results are generalizable to other populations should be further studied. Repeated fetal and childhood growth measures were available, which enabled us to identify critical growth periods that might influence kidney volume and function. We did take account the repeated growth measures by performing conditional growth analyses. Of all children, more than 80% did participate in the kidney follow-up studies. Because not all participants in the study gave consent for collecting blood samples, 67% of all children provided useful blood samples for measurements of creatinine levels. There were no differences in birth outcomes and fetal and early childhood growth measures between children with and without blood samples. However, children without blood samples had smaller kidney dimensions at the age of 6 years. These differences might have led to an underestimation of the observed associations. Furthermore, statistical power might have been reduced because of the missing data. We used kidney size as a measure of kidney development, because nephron number cannot be studied in vivo. Kidney size is correlated with the number of glomeruli and can be used in epidemiologic studies as a measure of kidney development.4 However, glomerular enlargement caused by hyperfiltration may attenuate the differences in childhood kidney volume and lead to an underestimation of the associations of interest.15 In the present study, eGFR was based on one random creatinine value, which is a limitation of the study. However, measurement error because of only one creatinine value is likely to be random and might have underestimated the observed differences. Mean values for eGFR and overall prevalence of microalbuminuria are in line with results of previous population-based studies in children of the same age range.16,17 We used the urine albumin to creatinine ratio to evaluate albuminuria in a random urine sample.18 Because the within-subject variation in urinary albumin excretion is large, the variability would probably be lower if we collected first morning void samples instead of random samples during the day.19 Finally, although we had information about a large number of confounders, residual confounding might still be an issue because of the observational design of the study.
Fetal and Early Childhood Growth and Childhood Kidney
To the best of our knowledge, the current study is the largest population-based prospective cohort study from fetal life onward focused on the associations of early growth with kidney function at school age. Several studies showed associations of low birth weight with renal disease and hypertension in later life.1–3 Results from a systematic review based on 31 studies among 49,387 subjects showed that low birth weight is associated with a 1.73 higher risk of kidney disease.1 Studies focused on the associations of birth weight with predictors of renal disease at younger ages are scarce. A Norwegian study among 7457 subjects showed that young adults born with a small size for gestational age had an increased risk of low-normal creatinine clearance compared with children with appropriate birth weight for gestational age.20 In contrast, a Dutch study among 82 severely growth-restricted children did not show associations of birth weight with renal function in young adulthood.8 A study among 86 children ages 9–12 years found no differences in kidney volume or function between preterm-born children, children born small for gestational age, and children born appropriate for gestational age.21 A study among 73 children aged 9.5 years showed a positive association of birth weight with eGFR.22 An observational cohort study among 426 children with congenital kidney disease showed that low birth weight and being small for gestational age are risk factors for poor growth outcomes in children with mild and moderate CKD.23 We observed that younger gestational age and lower gestational age-adjusted birth weight are associated with both a lower kidney volume and a lower eGFR in school-aged children. These findings are in line with previous studies showing that low birth weight for gestational age is associated with kidney function in childhood.13 We are not aware of other large population-based studies focused on the associations of birth weight taking into account gestational age with kidney function at young age.
Because birth weight is the result of various exposures and growth patterns in fetal life and the starting point of childhood growth, longitudinal fetal and early childhood growth patterns might be strongly associated with increased risk of renal disease in later life.13 Longitudinal studies linking fetal and early childhood growth patterns to kidney outcomes in later life might help to identify critical periods in later life. A study among 50 children 7.6 years old showed that children with low birth weight and slow growth rates had slightly lower GFR compared with children with appropriate growth.24 It has been postulated that rapid weight gain and obesity in childhood are associated with an increased risk of hypertension and type 2 diabetes in adulthood, which are risk factors for kidney disease.11,12 In a retrospective cohort study among 80 children with proteinuric kidney disease, obese children who were born preterm had an increased risk of progression of kidney disease compared with obese children who were born at term, suggesting an additive risk of obesity and prematurity in the risks for progression of kidney disease.25 Experimental studies have shown that adequate feeding of low birth weight rats could restore nephron numbers to normal and that overfeeding of these rats led to low nephron numbers, hypertension, and renal injury. Overfeeding of normal birth weight rats also had adverse effects.26–28 In line with these findings, we observed that lower fetal weight gain and lower early infancy weight gain led to impaired kidney growth, whereas only lower fetal weight gain led to impaired kidney function. The results from our present study suggest that both fetal life and early infancy may be critical periods for the development of kidney diseases in later life. To our knowledge, this study is the first population-based study that shows that fetal growth and early growth are associated with kidney function in childhood. The present study was focused on the associations of fetal and childhood growth in relation to kidney outcomes. Previous studies, including those studies from the same cohort as the present study, suggested that children with fetal growth restriction followed by infant growth acceleration have higher BP.29,30 Both fetal growth in later pregnancy and growth in early infancy seem to be critical periods for childhood BP.
The underlying mechanisms for the associations between early growth and kidney function are not known. Adverse fetal growth may lead to a persistently reduced congenital nephron number and smaller kidney volume with glomerular hyperfiltration and subsequent glomerulosclerosis. These adaptations may predispose individuals to impaired renal function and hypertension.4,5 Specifically, third trimester growth is important in kidney development, because approximately 60% of the total nephron number develops during the third trimester of gestation.31 In line with this hypothesis, we observed that fetal growth was positively associated with kidney growth and eGFR. We also observed that, after additional adjustment for kidney volume, most associations of birth outcomes focused on kidney function outcomes attenuated. Other mechanisms may also be involved. Experimental studies showed alterations in the renin angiotensin system in experimentally induced intrauterine growth-restricted individuals at adult age, and these differences were not present at younger age.32 Several markers of the renin angiotensin system were increased in intrauterine growth-restricted subjects with hypertension.32 Future studies are needed to identify possible underlying mechanisms. The observed effect estimates in the present study are small and without clinical significance at young age. However, they are important from an etiological developmental perspective. The presented results suggest that birth characteristics, fetal growth, and early childhood growth influence kidney function throughout the life course. Whether and to what extent the observed variations in kidney function relate with kidney disease in later life are not known yet. Tracking of BP from childhood to adulthood has been described previously33 but is less clear for kidney function. Studies showing that lower eGFR relates with renal disease development many decades later suggest that subclinical variation in kidney function precedes the developmental of renal disease.13
In conclusion, lower fetal weight gain and lower early infant weight gain led to smaller kidneys, whereas only lower fetal weight gain led to a lower eGFR. Although the observed effect estimates are small and without direct individual clinical consequence, they suggest that suboptimal early growth affects kidney function in later life. Future studies are needed to evaluate the long-term consequences of the observed associations.
Concise Methods
Design and Study Population
This study was embedded in the Generation R Study, a population-based prospective cohort study from fetal life onward in Rotterdam, The Netherlands.14 All children were born between April of 2002 and January of 2006. Written informed consent was obtained from all parents. The study has been approved by the Medical Ethics Committee of the Erasmus Medical Center. In total, 8305 children participated in the follow-up measurements at the age of 6 years; of these children, 6494 (78%) children visited the research center with successful measurements of kidney size. We excluded children with kidney abnormalities (n=12). Blood samples for kidney function measurements were successfully obtained in 4336 (67%) children (Figure 1). Missing blood samples were mainly because of nonconsent.
Fetal and Childhood Growth Measurements
Gestational age was established by first trimester ultrasound measurements.34 Second and third trimester fetal growth examinations were performed at median (90% range) gestational ages of 20.6 (18.9–22.9) and 30.4 (29.0–33.1) weeks, respectively. Fetal head circumference (HC), abdominal circumference (AC), and femur length (FL) were measured, and estimated fetal weight was calculated using the formula by Hadlock et al.35: log10 estimated fetal weight=1.5662−0.0108(HC)+0.0468(AC)+0.171(FL)+0.00034(HC)2−0.003685(AC×FL). At birth, gestational age, length, and weight were obtained from community midwife and hospital registries. Small and large sizes for gestational age were defined as the lower 5% and the upper 5% of birth weight SD score, respectively.
We measured childhood length and weight using standardized methods at the median (90% range) ages of 6.2 (5.4–7.5), 11.1 (10.2–12.3), 24.8 (23.5–27.6), 36.7 (35.6–39.7), 45.8 (44.8–48.0), and 72.6 (68–95.5) months. All growth characteristics were converted into SD score using fetal,34 birth weight,36 and childhood37 reference growth charts (Growth Analyzer 3.5; Dutch Growth Research Foundation, Rotterdam, The Netherlands).
Childhood Kidney Outcomes
Left and right kidney biometrics were measured at the median age of 6.0 (90% range=5.7–7.5) years. We identified the left and right kidneys in the sagittal plane along the longitudinal axis. We performed measurements of maximal bipolar kidney length, width, and depth. Kidney width and depth were measured at the level of the hilum. The cross-sectional area in which the kidney appeared symmetrically round at its maximum width was used. Kidney volume was calculated using the equation of an ellipsoid: volume (centimeters3)=0.523×length (millimeters)×width (millimeters)×depth (millimeters).38 Combined kidney volume was calculated by summing right and left kidney volumes. We previously reported good intraobserver and interobserver correlation coefficients.39
Blood creatinine levels were measured with an enzymatic method on a Cobas c 502 analyzer (Roche Diagnostics). Quality control samples showed intra- and interassay coefficients of variation ranging from 0.51% to 1.37%. eGFR was calculated according to the revised 2009 formula by Schwartz et al.40: eGFR=36.5×(height [centimeters]/creatinine [micromoles per liter]).40 Urine creatinine (micromoles per liter) and urine albumin (milligrams per liter) levels were determined on a Beckman Coulter AU analyzer, and creatinine levels were measured according to the Jaffe method. We calculated the albumin-to-creatinine ratio. For boys, microalbuminuria was defined as an albumin-to-creatinine ratio between 2.5 and 25 mg/mmol; for girls, we used a ratio between 3.5 and 25 mg/mmol.41
Covariates
Information on maternal age, prepregnancy weight, parity, ethnicity, educational level, smoking during pregnancy, folic acid supplementation during pregnancy, and breastfeeding was obtained by questionnaires.14 Maternal height was measured without shoes, and prepregnancy body mass index was calculated (kilograms per meter2). Infant sex was obtained from midwife and hospital registries. At the age of 6 years, child height and weight were measured without shoes and heavy clothing, and body surface area was calculated.
Statistical Analyses
First, we explored differences in characteristics between boys and girls by t tests for continuous variables and chi-squared tests for categorical variables. Second, we performed multiple linear or logistic regression models to explore the associations of birth outcomes (gestational age at birth, birth weight, and gestational age adjusted birth weight) with childhood combined kidney volume, eGFR, and microalbuminuria. These models were adjusted for sex and age only, and additionally, they were adjusted for potential confounders. Potential confounders were based on their associations with kidney outcomes or a change in effect estimate of more than 10%. The associations with kidney function outcomes were additionally adjusted for kidney volume to explore whether any association was explained by kidney growth. We performed a sensitivity analysis using the lower 10% and upper 10% as the definitions for small and large size, respectively, for gestational age of children. Third, we assessed the associations of fetal (second and third trimesters and birth) and childhood (6, 12, 24, 36, 48, and 72 months) weight and length measures with kidney outcomes at the age of 6 years using multiple linear regression models. Because fetal and childhood growth measurements at different ages are strongly correlated, we additionally performed conditional regression analyses to explore the independent associations of fetal and early childhood growth with kidney outcomes, taking account for their correlation.42 For these analyses, we constructed length and weight variables for each time point, which are statistically independent from each other, using standardized residuals obtained from regression of growth measures at a specific time point on prior growth measures.42 Because conditional growth measures are statistically independent of each other, this approach allows inclusion of growth measures simultaneously in one linear regression model. Thus, the associations of fetal and childhood growth measures at specific ages with kidney outcomes can be assessed, adjusted for, and compared with fetal and childhood growth measures at other ages.43,44 Results from these datasets were pooled and presented in the conditional growth results. All statistical analyses were performed using the Statistical Package for the Social Sciences version 20.0 for Windows (SPSS Inc., Chicago, IL).
Disclosures
None.
Supplementary Material
Acknowledgments
The Generation R study is being conducted by the Erasmus Medical Center and Erasmus University Rotterdam in close collaboration with the Municipal Health Service Rotterdam area, Rotterdam, The Netherlands and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond, Rotterdam, The Netherlands. We acknowledge the contributions of children and their parents, general practitioners, hospitals, midwives, and pharmacies in Rotterdam, The Netherlands.
The Generation R Study receives financial support from the Erasmus Medical Center and The Netherlands Organization for Health Research and Development (ZonMw). Additional support was provided by Dutch Kidney Foundation Grant C08.2251. V.W.V.J. received an additional grant from The Netherlands Organization for Health Research and Development (ZonMw Grant VIDI 016.136.361).
O.H.F. works in ErasmusAGE, a center for aging research across the life course funded by Nestlé Nutrition (Nestec, Ltd.), Metagenics, Inc. and AXA. Nestlé Nutrition (Nestec, Ltd.), Metagenics, Inc. and AXA had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Life Cycle of the Kidney: Implications for CKD,” on pages 2388–2390.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013091003/-/DCSupplemental.
References
- 1.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR: Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54: 248–261, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ: Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med 160: 1472–1476, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Neil A, Collins R: Unravelling the fetal origins hypothesis: Is there really an inverse association between birthweight and subsequent blood pressure? Lancet 360: 659–665, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Luyckx VA, Brenner BM: The clinical importance of nephron mass. J Am Soc Nephrol 21: 898–910, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Chertow GM: Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 7.Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frölich M, van der Heijden BJ, Dutch POPS-19 Collaborative Study Group : Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 16: 2762–2768, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Keijzer-Veen MG, Kleinveld HA, Lequin MH, Dekker FW, Nauta J, de Rijke YB, van der Heijden BJ: Renal function and size at young adult age after intrauterine growth restriction and very premature birth. Am J Kidney Dis 50: 542–551, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D: Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991 [PubMed] [Google Scholar]
- 11.Andersen LG, Angquist L, Eriksson JG, Forsen T, Gamborg M, Osmond C, Baker JL, Sørensen TI: Birth weight, childhood body mass index and risk of coronary heart disease in adults: Combined historical cohort studies. PLoS ONE 5: e14126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fall CH, Sachdev HS, Osmond C, Lakshmy R, Biswas SD, Prabhakaran D, Tandon N, Ramji S, Reddy KS, Barker DJ, Bhargava SK, New Delhi Birth Cohort : Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: Data from the New Delhi Birth Cohort. Diabetes Care 31: 2349–2356, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE: Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382: 273–283, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, van der Lugt A, Mackenbach JP, Moll HA, Raat H, Rivadeneira F, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A: The Generation R Study: Design and cohort update 2012. Eur J Epidemiol 27: 739–756, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD: Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens 17: 258–265, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L: Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol 6: 552–560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rademacher ER, Sinaiko AR: Albuminuria in children. Curr Opin Nephrol Hypertens 18: 246–251, 2009 [DOI] [PubMed] [Google Scholar]
- 18.de Jong PE, Curhan GC: Screening, monitoring, and treatment of albuminuria: Public health perspectives. J Am Soc Nephrol 17: 2120–2126, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, Itoh Y, Lieske JC, Seccombe DW, Jones G, Bunk DM, Curhan GC, Narva AS, National Kidney Disease Education Program-IFCC Working Group on Standardization of Albumin in Urine : Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 55: 24–38, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW: Effect of intrauterine growth restriction on kidney function at young adult age: The Nord Trøndelag Health (HUNT 2) Study. Am J Kidney Dis 51: 10–20, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Rakow A, Johansson S, Legnevall L, Sevastik R, Celsi G, Norman M, Vanpée M: Renal volume and function in school-age children born preterm or small for gestational age. Pediatr Nephrol 23: 1309–1315, 2008 [DOI] [PubMed] [Google Scholar]
- 22.López-Bermejo A, Sitjar C, Cabacas A, Vázquez-Ruíz M, García-González MM, Mora C, Soriano P, Calvo M, Ibáñez L: Prenatal programming of renal function: The estimated glomerular filtration rate is influenced by size at birth in apparently healthy children. Pediatr Res 64: 97–99, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Greenbaum LA, Muñoz A, Schneider MF, Kaskel FJ, Askenazi DJ, Jenkins R, Hotchkiss H, Moxey-Mims M, Furth SL, Warady BA: The association between abnormal birth history and growth in children with CKD. Clin J Am Soc Nephrol 6: 14–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacchetta J, Harambat J, Dubourg L, Guy B, Liutkus A, Canterino I, Kassaï B, Putet G, Cochat P: Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int 76: 445–452, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Abitbol CL, Chandar J, Rodríguez MM, Berho M, Seeherunvong W, Freundlich M, Zilleruelo G: Obesity and preterm birth: Additive risks in the progression of kidney disease in children. Pediatr Nephrol 24: 1363–1370, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM: Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18: 1688–1696, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Boubred F, Buffat C, Feuerstein JM, Daniel L, Tsimaratos M, Oliver C, Lelièvre-Pégorier M, Simeoni U: Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am J Physiol Renal Physiol 293: F1944–F1949, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Boubred F, Daniel L, Buffat C, Feuerstein JM, Tsimaratos M, Oliver C, Dignat-George F, Lelièvre-Pégorier M, Simeoni U: Early postnatal overfeeding induces early chronic renal dysfunction in adult male rats. Am J Physiol Renal Physiol 297: F943–F951, 2009 [DOI] [PubMed] [Google Scholar]
- 29.van Houten VA, Steegers EA, Witteman JC, Moll HA, Hofman A, Jaddoe VW: Fetal and postnatal growth and blood pressure at the age of 2 years. The Generation R Study. J Hypertens 27: 1152–1157, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R: First trimester fetal growth restriction and cardiovascular risk factors in school age children: Population based cohort study. BMJ 348: g14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung MY: Oligonephropathy, developmental programming and nutritional management of low-gestation newborns. Acta Paediatr 95: 263–267, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT: Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Wang Y: Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation 117: 3171–3180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW, Witteman JC: New charts for ultrasound dating of pregnancy and assessment of fetal growth: Longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol 31: 388–396, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK: Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 150: 535–540, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P: An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr Scand 80: 756–762, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Fredriks AM, van Buuren S, Burgmeijer RJ, Meulmeester JF, Beuker RJ, Brugman E, Roede MJ, Verloove-Vanhorick SP, Wit JM: Continuing positive secular growth change in The Netherlands 1955–1997. Pediatr Res 47: 316–323, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Geelhoed JJ, Taal HR, Steegers EA, Arends LR, Lequin M, Moll HA, Hofman A, van der Heijden AJ, Jaddoe VW: Kidney growth curves in healthy children from the third trimester of pregnancy until the age of two years. The Generation R Study. Pediatr Nephrol 25: 289–298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geelhoed JJ, Kleyburg-Linkers VE, Snijders SP, Lequin M, Nauta J, Steegers EA, van der Heijden AJ, Jaddoe VW: Reliability of renal ultrasound measurements in children. Pediatr Nephrol 24: 1345–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K, International Society for Pediatric and Adolescent Diabetes : ISPAD Clinical Practice Consensus Guidelines 2006–2007. Microvascular and macrovascular complications. Pediatr Diabetes 8: 163–170, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC: A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol 58: 1320–1324, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Jones A, Charakida M, Falaschetti E, Hingorani AD, Finer N, Masi S, Donald AE, Lawlor DA, Smith GD, Deanfield JE: Adipose and height growth through childhood and blood pressure status in a large prospective cohort study. Hypertension 59: 919–925, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey NC, Mahon PA, Kim M, Cole ZA, Robinson SM, Javaid K, Inskip HM, Godfrey KM, Dennison EM, Cooper C, SWS Study Group : Intrauterine growth and postnatal skeletal development: Findings from the Southampton Women’s Survey. Paediatr Perinat Epidemiol 26: 34–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.