Abstract
Human cytomegalovirus infection in transplant recipients has been associated with adverse renal allograft outcome and with a large γδ T-cell response, but whether both mechanisms are connected is unknown. We previously showed that most expanded circulating cytomegalovirus-responsive γδ T cells express the Fcγ-receptor CD16, suggesting that γδ T cells may participate in allograft lesions mediated by donor-specific antibodies through antibody-dependent cellular cytotoxicity. Here, we show that cytomegalovirus-specific CD16pos γδ T cells can perform antibody-dependent cellular cytotoxicity against stromal cells coated with donor-specific antibodies in vitro. In vivo, graft-infiltrating γδ T cells localized in close contact with endothelial cells only in patients who experienced cytomegalovirus infection and were more frequent within peritubular capillaries and glomeruli from antibody-mediated acute rejections than within those from T cell–mediated acute rejections. Finally, a persistently increased percentage of circulating cytomegalovirus-induced γδ T cells correlated inversely with the 1-year eGFR only in kidney recipients with donor-specific antibodies. Collectively, these data support the conclusion that cytomegalovirus-induced γδ T cells are involved in, and may serve as a clinical biomarker of, antibody-mediated lesions of kidney transplants. Moreover, these findings offer a new physiopathologic link between cytomegalovirus infection and allograft dysfunction in recipients with donor-specific antibodies.
In kidney transplant recipients (KTRs), the importance of the recipient’s humoral response against the allograft has been recognized to play a key role in immunologic injuries contributing to graft deterioration.1–6 From an immunologic point of view, donor-specific antibody (DSA)–mediated lesions are considered to rely on complement-fixing DSA-mediated lysis, direct DSA-mediated apoptosis, or antibody-dependent cell-mediated cytotoxicity (ADCC) by natural killer (NK) cells. Until recently, complement was the most recognized way of leading to graft endothelial cell injury. Indeed, deposition of C4d, a breakdown product of complement component C4, in peritubular capillaries still represents the only specific tool providing the “immunopathologic evidence” of DSA interaction with graft tissue.7–11 However, it does not encompass all DSA-mediated lesions.12
Several groups reappraised the multiplicity of mechanisms leading to antibody-mediated rejections (AMR).13 Glomerulitis and peritubular capillaritis are defined by an accumulation of polymorphonuclear cells, macrophages, and lymphocytes around capillaries. These infiltrates are associated with DSA and indicate a poor prognosis.14–16 Among these infiltrates, NK cells have recently been shown to be involved in DSA-mediated lesions of kidney microcirculation,17,18 suggesting that ADCC could play a role in DSA-mediated lesions through DSA interaction with the low-affinity Fc receptor for IgG (FcγRIIIA-CD16) expressed on NK cells. Signaling through CD16 is instrumental for ADCC as it leads to cytolytic granule content release and subsequent death of target cells.
Beside NK cells, and unlike conventional αβ T cells, γδ T cells can also express CD16 at high levels, enabling them to efficiently mediate ADCC.19 In human transplantation, γδ T lymphocytes have been strongly linked to cytomegalovirus (CMV) infection, itself associated with rejection.20–22 A specific and persistent expansion of a γδ T-cell subset normally located in the epithelia (called Vδ2neg γδ T cells and mainly composed of Vδ1 and Vδ3 T cells) is observed in peripheral blood during CMV infection in all solid-organ transplantations.23–26 From our experience, CMV is the only clinical cause of Vδ2neg γδ T-cell expansion in KTR. This tight association between CMV infection and γδ T-cell expansion has been confirmed in many other pathophysiologic contexts.27–31 In vitro, clones of Vδ2neg γδ T cells display T-cell receptor (TCR)–dependent cytotoxicity against both CMV-infected cells and carcinoma cells.32 Accordingly, their expansion in kidney transplant recipients correlates with both reduced cancer risk33 and resolution of CMV infection, suggestive of their antiviral function.34
Interestingly, we recently observed that most (around 80%) Vδ2neg γδ T cells from CMV-infected individuals expressed CD16, whereas CMV-specific CD8+ αβ T cells or Vδ2pos T cells did not.35 CMV infection deeply reshapes the CD16+ lymphocyte compartment composition in CMV+ transplant recipients who exhibit an equal amount of CD16+ NK cells and CD16+ γδ T cells at the periphery.35 The latter are able to produce high levels of IFN-γ when recognizing IgG-opsonized CMV particles. This cooperation between γδ T cells and the humoral response could represent an interesting control mechanism of CMV reactivation in chronically infected tissues and of CMV spread in blood.35
Collectively these results raise the possibility that, in the context of transplantation and in the presence of DSA, reorganization of the CD16+ lymphocyte compartment following CMV infection could have a deleterious effect on the graft. The aim of the present study was to evaluate whether CMV-induced CD16+ γδ T cells were able to mediate ADCC against graft endothelial cells in the presence of DSA, a process that could participate in the association between CMV and DSA-mediated rejection.
Results
Model of KTR DSA Binding to Endothelial and Fibroblastic Cells
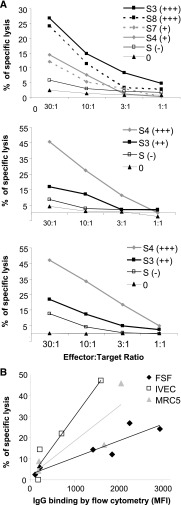
To assess the potential allocytotoxic effect of CMV-induced γδ T cells in the presence of DSA, we used allogeneic stromal cell lines recognized by DSA. To this purpose, we assessed the ability of sera from eight KTRs with DSA (sensitized KTRs, S3–S10) and from two nonsensitized KTRs (S1 and S2) to bind three allogeneic HLA-typed “stromal” cells lines: an endothelial cell line (IVEC), primary foreskin fibroblasts (FSF), and MRC5. Cell line–specific HLA antibodies (CLSA) levels in the sera were first evaluated using the HLA class I single antigen bead (SAB) assay (Tables 1, 2 and 3). As expected, control sera (S1 and S2) did not contain CLSA. Sera S3, S4, S7, and S8 contained high levels of CLSA. Although containing DSA, sera S5, S6, S9, and S10 contained low levels of CLSA. The capacity of CLSA to bind to the allogeneic cells was next confirmed by flow cytometry (Figure 1A). The most important stainings were obtained when the three cell lines were incubated with sera S3, S4, S7, and S8, which contained the highest levels of CLSA (Figure 1B). Accordingly, a strong correlation was observed between the CLSA mean fluorescence intensity (MFI) analyzed by SAB and the MFI of cell line staining analyzed by flow cytometry (r2=0.74; P<0.001) (Figure 1C). These results suggested that the stromal cell–binding activity of patient’s sera is mostly mediated by CLSA and that this assay is a reliable in vitro model to investigate DSA-dependent ADCC.
Table 1.
Class I HLA typing of FSF and characterization of each CLSA for 10 sera, using the class I SAB assay
| Serum Sample | MFI SAB Typing Results for FSF | Σ Specific MFI | |||
|---|---|---|---|---|---|
| A*24 | A*29 | B*35 | B*51 | ||
| S1 | 0 | 0 | 0 | 0 | 0 |
| S2 | 0 | 0 | 0 | 0 | 0 |
| S3 | 10,200 | 7450 | 12,250 | 11,150 | 41,050 |
| S4 | 14,850 | 10,500 | 1030 | 1400 | 27,780 |
| S5 | 200 | 165 | 40 | 70 | 475 |
| S6 | 30 | 50 | 350 | 0 | 430 |
| S7 | 7600 | 1100 | 5670 | 0 | 14,370 |
| S8 | 15,400 | 2300 | 12,500 | 12,200 | 42,400 |
| S9 | 105 | 210 | 45 | 90 | 450 |
| S10 | 9100 | 0 | 0 | 0 | 9100 |
Table 2.
Class I HLA typing of MRC5 and characterization of each CLSA for 10 sera, using the class I SAB assay
| Serum Sample | MFI SAB Typing Results for MRC5 | Σ Specific MFI | |||
|---|---|---|---|---|---|
| A*02 | A*29 | B*07 | B*44 | ||
| S1 | 0 | 0 | 0 | 0 | 0 |
| S2 | 0 | 0 | 0 | 0 | 0 |
| S3 | 10,100 | 7450 | 12 | 3600 | 21,110 |
| S4 | 15,000 | 10,500 | 2869 | 0 | 28,350 |
| S5 | 100 | 165 | 1181 | 130 | 1500 |
| S6 | 0 | 50 | 9 | 3500 | 4000 |
| S7 | 4000 | 1100 | 3351 | 12,500 | 22,150 |
| S8 | 0 | 2300 | 7674 | 10,500 | 19,724 |
| S9 | 1000 | 210 | 10,324 | 5000 | 16,524 |
| S10 | 0 | 0 | 0 | 0 | 0 |
Table 3.
Class I HLA typing of IVEC and characterization of each CLSA for 10 sera, using the class I SAB assay
| Serum Sample | MFI SAB Typing Results for IVEC | Σ Specific MFI | |||
|---|---|---|---|---|---|
| A*02 | A*3001 | B*07 | B*4403 | ||
| S1 | 0 | 0 | 0 | 0 | 0 |
| S2 | 0 | 0 | 0 | 0 | 0 |
| S3 | 10,100 | 7301 | 12 | 2781 | 20,194 |
| S4 | 15,000 | 9021 | 2869 | 13 | 26,903 |
| S5 | 100 | 4 | 1181 | 18 | 1303 |
| S6 | 0 | 28 | 9 | 2143 | 2180 |
| S7 | 4000 | 3614 | 3351 | 11,469 | 22,434 |
| S8 | 0 | 4471 | 7674 | 9890 | 22,035 |
| S9 | 1000 | 21 | 10,324 | 4176 | 15,521 |
| S10 | 0 | 634 | 0 | 0 | 634 |
Figure 1.
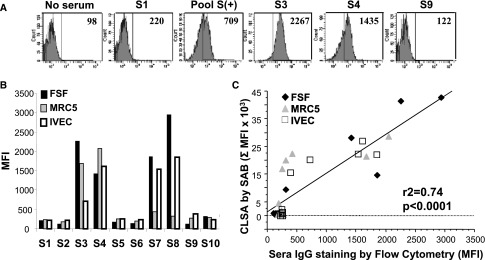
Anti-class I HLA antibodies from sensitized kidney transplant recipients sera bind on fibroblastic and endothelial cells. (A) Representative binding on FSF of IgG from different sera using a goat anti-human IgG antibody coupled to FITC and analyzed by flow cytometry (numbers indicate the MFI). Pool S+, pool of sensitized sera; S1, nonsensitized serum; S3 and S4, sera containing CLSA; S9, sensitized serum without CLSA. (B) MFI obtained with each serum on all three cell lines. Data are representative of three different experiments. (C) Linear regression analysis of CLSA MFI analyzed by SAB assay versus the MFI of the IgG binding analyzed by flow cytometry on the three cell lines.
γδ T Cell–Mediated ADCC against Endothelial and Fibroblastic Cells
We then tested the capacity of CMV-induced CD16pos γδ T-cells to mediate ADCC against endothelial and fibroblastic cell lines coated with CLSA. To this end, a CD16pos γδ T cell line was generated from a CMV–infected donor. FSF, IVEC, and MRC5 cell lines, preincubated with sera containing (S3, S4, S7, and S8) or not containing (S1 and S2) CLSA, were cocultured with CD16pos γδ T cells in a 4-hour chromium release assay. CD16pos γδ T cell line was able to kill the three allogeneic cell lines in the presence of CLSA-positive serum (Figure 2). Killing in the absence of serum or in the presence of CLSA-negative serum was equal or close to background, ruling out a direct allogeneic cytotoxicity of γδ T cells. Likewise, there was no direct cytotoxicity of CLSA on target cells in the absence of γδ T cells (data not shown). Finally, the percentage of chromium release at a 30:1 effector:target ratio by γδ T cells closely correlated with the IgG-binding MFI analyzed by flow cytometry (r2=0.44 [cumulated correlation for the three cell lines]; P=0.01) (Figure 2B). Collectively, these data demonstrated the capacity of CD16pos γδ T cells to mediate DSA-dependent ADCC against endothelial and fibroblastic cells.
Figure 2.
CD16pos γδ T cells can perform CLSA dependent cytotoxicity (ADCC) against endothelial and fibroblastic cells. (A) FSF, MRC5, and IVEC cell lines were labeled with 51Cr and pre incubated or not pre incubated with sera S3, S4, S7, and S8, which contained different levels of CLSA (indicated as + to +++) and sera that did not (indicated as −). Ability of the CD16pos γδ T cell line to induce lysis of preincubated cell lines was evaluated by 51Cr release in the supernatant. Results are the mean specific lysis of culture triplicates from three independent experiments. SD was always <15% of the mean value (not shown). (B) Linear regression analysis between the MFI of IgG binding analyzed by flow cytometry on the three cell lines and the percentage of specific lysis (FSF: r2=0.83 [P=0.01]; IVEC: r2=0.93 [P=0.04]; MRC5: r2=0.74 [P=0.14]).
CLSA-Dependent ADCC by γδ T Cells Relies on CD16 and Perforin
We next investigated the mechanisms by which γδ T-cells mediate CLSA-dependent ADCC. First, we demonstrated that CD16 was required as γδ T cell–mediated ADCC was prevented with use of a blocking anti-CD16 mAb or a CD16-negative γδ T cell line (Figure 3A for FSF preincubated with serum S8). Similar results were obtained using MRC5 and IVEC and using other CLSA-positive sera (data not shown). Second, we tested whether productive ADCC required the release of cytolytic granules. CD16pos γδ T-cells expressed high levels of granzyme B and perforin (Figure 3B). Concanamycin A, an inhibitor of cytotoxic granule content release, abrogated ADCC by CD16pos γδ T cell line in a dose-dependent manner (Figure 3C). In conclusion, the DSA-dependent γδ T cell–ADCC relies on CD16, the triggering of which elicited cytotoxic granule content release, similar to NK cell–mediated ADCC.
Figure 3.
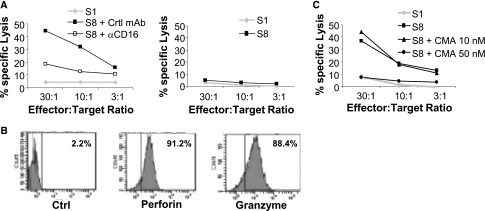
CLSA-dependent ADCC by γδ T cells depends on CD16 and perforin. (A) CD16pos (left panel) or CD16neg (right panel) γδ T cell–mediated lysis of the FSF primary cell line preincubated with the serum S8 (+++) or S1 (−) in the presence or absence of a blocking anti-CD16 mAb or control mAb. (B) Expression of intracellular granzyme B and perforin in CD16pos γδ T cells. Numbers indicate percentages of positive cells. (C) As in part A with the addition or no addition of indicated concentrations of concanamycin A (CMA). Viability of γδ T cells was >80% even after incubation of 7 hours with the highest concanamycin A concentration (data not shown). Data are representative of three different experiments. Ctrl, control.
γδ T Cells Are Present in AMR Microcirculation Lesions of CMV-Infected Patients
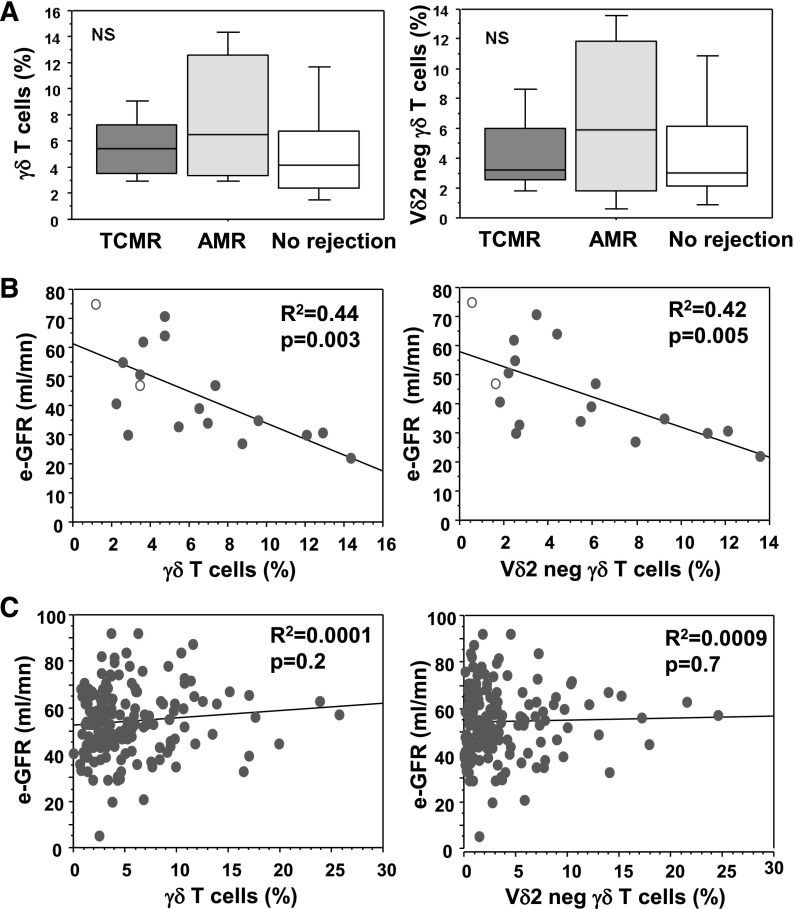
Peritubular capillaritis and glomerulitis are early signs of the microcirculation lesions characterizing AMR.36 To know whether γδ T cells could be involved in this process, we examined their potential colocalization with these organic lesions. Sections of kidney graft diagnosed with acute AMR (n=10) were stained with an anti-CD31 mAb (to localize the endothelial cells) and an anti-γδ TCR mAb. Interestingly, γδ T cells were found in close contact with endothelial cells in peritubular capillaritis and glomerulitis lesions associated with acute AMR (Figure 4A, Supplemental Figure 1). This association was specific, as it was not found for peritubular capillaries or glomeruli of allograft presenting with acute T cell–mediated rejection (acute TCMR, n=13). Furthermore, when we distinguished CMV-experienced patients (R− patients with postgraft CMV primary infection and all R+ patients) from CMV-naive patients (R− patients without postgraft CMV infection), γδ T cells were more frequent in peritubular capillaries and glomeruli of patients with AMR (mean, 34.8 cells/mm3 and 2.6 cells/mm3, respectively) than in those of patients with TCMR (3.8 cells/mm3 and 0.1 cells/mm3, respectively) only among CMV-experienced patients (Figure 4B, Supplemental Figure 1). Of note, two of the seven biopsy specimens from CMV+ patients were negative for C4d staining and showed the highest levels of γδ T cells/mm2, suggesting that γδ T-cell presence was independent of complement activation. Moreover, these seven biopsy specimens were negative for CMV antigens (not shown), suggesting that γδ T-cell presence in lesions was related more to the presence of DSA than to that of CMV. In CMV-naive patients, the rate of γδ T cells in peritubular capillaries and glomeruli was low in both TCMR and AMR (Figure 4B). γδ T cells could also be found in the interstitium but were more frequent during TCMR than during AMR, whatever the CMV status of the patient. Together, these results suggest that CMV-induced γδ T cells localize into the AMR microcirculation lesions, where they may mediate graft rejection through ADCC.
Figure 4.
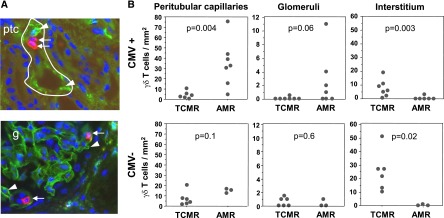
γδ T cells are present in antibody-mediated peritubular capillaritis and glomerulitis of CMV-infected patients. Triple-immunofluorescence staining for CD31 (green), γδ T cells (red), and nuclei (blue) on graft biopsy specimens from patients with acute AMR (n=10) and acute TCMR (n=13). (A) Representative staining of γδ T cells in microcirculation lesions associated with acute AMR in peritubular capillaritis (upper panel) and in glomerulitis (lower panel). Arrows indicate γδ T cells and arrowheads indicate endothelial cells of the microcirculation. Original magnification ×600. (B) Quantification of γδ T cells in peritubular capillaries and glomeruli of acute AMR or TCMR graft biopsy specimens in CMV-experienced (CMV+, upper panels) and CMV-naive (CMV−, lower panels) patients.
Correlation of γδ T-Cell Counts with eGFR at 12 Months after Transplant in Patients with DSA
If the assumption that CMV-induced γδ T cells are involved in AMR is true, their expansion in patients with DSA should be associated with renal dysfunction. In a recent study, we observed that among sensitized KTRs, those who had the highest risk for graft rejection and the lowest eGFR 12 months after transplantation were the patients who had anti-HLA DSA the day of the graft.37 We tested a potential link between circulating γδ T cells at 12 months after transplant and DSA-mediated rejection in this cohort of 21 KTRs with DSA. For a control group, we used 162 KTRs without DSA, whose characteristics are summarized in Table 4. Among the 21 consecutive sensitized KTRs, baseline donors' and recipients' characteristics, as well as DSA MFI levels, did not significantly differ between those with rejection and those without rejection (Table 5). Notably, 19 of these 21 patients had experienced CMV infection before or after the transplantation. No statistical differences of the percentage of total γδ or Vδ2neg γδ T cells were observed between patients with TCMR, AMR, or without rejection (Figure 5A). However, the circulating γδ T cells and Vδ2neg γδ T cells were strongly and inversely correlated with the eGFR 12 months after transplant (r2=0.44 [P=0.003] and r2=0.43 [P<0.01], respectively) (Figure 5B). Conversely, in the control group of 162 nonsensitized KTRs, no correlation between γδ or Vδ2neg γδ T cells and post-transplant eGFR (r2<0.001 [P=0.2] and r2<0.001 [P=0.7], respectively) (Figure 5C) was observed, even when we focused on the 98 CMV-experienced patients (r2=0.02; P=0.2 [data not shown]). In summary, high percentages of CMV-induced Vδ2neg γδ T cells were associated with a poor eGFR only in KTRs with DSA, suggesting cooperation between CMV, γδ T cells, and DSA to induce graft dysfunction.
Table 4.
Characteristics of the 162 KTRs without DSA according to occurrence of TCMR (no AMR was observed in this cohort)
| Variable | TCMR (n=23) | No Rejection (n=139) | P Value | Total |
|---|---|---|---|---|
| Recipient age at time of graft (yr) | 47 (16–70) | 45 (20–58) | 0.7 | 47 (16–70) |
| Men/women (n/n) | 17/6 | 98/41 | 0.6 | 115/47 |
| Transplantation condition | ||||
| Donor age (yr) | 38 (7–68) | 44 (21–64) | 0.08 | 39 (7–68) |
| Expanded-criteria donor (%) | 5 (22) | 21 (15) | 0.06 | 26 (16) |
| Ischemia time (hr) | 21 (5–35) | 21 (8–37) | 0.9 | 21 (5–37) |
| Delayed graft function, n (%) | 7 (30) | 24 (17) | 0.1 | 31 (19) |
| Immunologic variables at grafting | ||||
| HLA mismatch: A-B-DR | 3 (1–5) | 3 (0–6) | 0.7 | 3 (0–6) |
| Induction immunosuppressive treatment (n/n) | ||||
| Tacrolimus/cyclosporine | 8/15 | 69/70 | 0.2 | 77/85 |
| Mycophenolate mofetil/azathioprine | 20/3 | 121/18 | 0.9 | 141/21 |
| ATG/anti–IL-2R antibodies/no induction | 2/14/7 | 11/67/61 | 0.6 | 13/81/68 |
| CMV status | ||||
| Pregraft CMV infection | 11 (48) | 71 (51) | 0.6 | 82 (51) |
| Postgraft CMV infection | 4 (17) | 35 (26) | 0.5 | 39 (24) |
| Median γδ TL, % (minimum–maximum) | 3 (0–16.9) | 3.8 (0.6–25.7) | 0.3 | 3.75 (0–25.7) |
| Median Vδ2neg γδ TL, % (minimum–maximum) | 1.79 (0–10.3) | 2.2 (0.1–24.6) | 0.3 | 2.15 (0–24.6) |
Unless otherwise noted, values are expressed as the median (range) or number (percentage). ATG, antithymocyte polyclonal antibodies; TL, T lymphocytes.
Table 5.
Characteristics of the 21 KTRs with DSA according to occurrence of rejection
| Variable | Acute TCMR (n=7) | Acute AMR (n=6) | No Rejection (n=8) |
|---|---|---|---|
| Recipient age at time of graft (yr) | 55 (22–70) | 46 (33–61) | 52 (25–64) |
| Men/women (n/n) | 2/5 | 4/2 | 4/4 |
| Risk factors of HLA sensitization (n) | |||
| History of blood transfusion | 4 | 6 | 6 |
| History of pregnancy | 4 | 2 | 2 |
| >1 kidney transplant | 4 | 6 | 6 |
| Transplantation conditions | |||
| Donor age (yr) | 56 (18–70) | 46 (22–59) | 48 (18–63) |
| Expanded-criteria donor (n) | 4 | 2 | 1 |
| Ischemia time (hr) | 23 (13–33) | 25 (23–34) | 20 (14–34) |
| Delayed graft function (n) | 3 | 1 | 2 |
| Immunologic variables at grafting | |||
| HLA mismatch: A-B-DR-DQ | 4 (1–7) | 4.5 (1–5) | 4.5 (2–7) |
| MFI ratio of T cell–positive FCXM | 2.20 (0.28–2.46) | 1.98 (0.97–7.69) | 1.47 (1.18–2.22) |
| MFI ratio of B cell–positive FCXM | 2.83 (1.38–33.02) | 3.84 (0.74–25.06) | 3.84 (1.38–8.32) |
| Day 0 anti–class I DSA | 4 | 3 | 4 |
| Day 0 anti–class II DSA | 3 | 3 | 4 |
| MFI of day 0 anti–class I DSA | 600 (0–7500) | 1250 (0–7900) | 300 (0–9700) |
| MFI of day 0 anti–class II DSA | 0 (0–15,800) | 550 (0–3000) | 500 (0–8600) |
| MFI of day 0 anti–class I+II DSA | 2400 (600–15,800) | 2750 (1100–7900) | 2200 (600–9700) |
| Induction immunosuppressive treatment (n) | |||
| Tacrolimus/mycophenolate mofetil | 7 | 5 | 8 |
| ATG/ anti–IL-2R antibodies | 1 | 6 | 5 |
| Induction with IVIG | 7 | 6 | 6 |
| CMV status | |||
| Pregraft CMV infection (n) | 6 | 5 | 6 |
| Postgraft CMV infection (n) | 4 | 1 | 2 |
| Pre- or postgraft CMV infection (n) | 6 | 6 | 7 |
| Median γδ TL, % (minimum–maximum) | 5.4 (2.8–9,5) | 6.5 (2.9–14.3) | 4.2 (1.2–12.9) |
| Median Vδ2neg γδ TL, % (minimum–maximum) | 3.19 (1.6–9.3) | 5.9 (0.6–13.6) | 3.0 (0.5–12.1) |
Unless otherwise noted, values are expressed as the median (range) or number of patients. FCXM, flow cytometric cross-match.
Figure 5.
γδ T-cell levels correlate with eGFR at 12 months after transplant in patients with DSA. (A) Median, 25th–75th percentiles and 10th–90th percentiles of γδ T cell (right panel) or Vδ2neg γδ T cell (left panel) percentages in patients with acute TCMR, with acute AMR, or without rejection. (B) Linear regression analysis between circulating γδ T cell percentages (right panel) or Vδ2neg γδ T-cell percentages (left panel) and the 12 month post-transplant eGFR in KTRs with DSA the day of the graft. Plain circles are CMV-experienced patients and open circles are CMV-naive patients. (C) Same analysis as in part B in anti-HLA nonsensitized KTRs.
Discussion
Allogeneic reaction is considered the result of direct or indirect recognition of foreign MHC molecules by alloreactive T lymphocytes. Therefore, γδ T cells are usually viewed as nonalloreactive because they do not recognize peptides bound to MHC molecules. However, γδ T cells express a broad range of activatory molecules that allow them to respond to stressed cells and that could potentially participate in a different type of reactivity against allografts. These molecules include the γδ TCR itself, which can recognize self-stress antigens,38–40 or NKG2D, which binds stress-induced MHC-like ligands (MHC class I–related chain and UL16-binding protein).41 Here, we describe the implication of CD16 in the alloreactive potential of γδ T cells responding to CMV infection.
We first present a model of anti-HLA ADCC, using sera from patients sensitized to HLA-A/B antigens expressed by “model” stromal cell lines. Using this model, we demonstrated that CMV-induced γδ T cells can make DSA dose-dependent ADCC. The lytic capacity of γδ T cells was strictly dependent on patients’ sera presence in the assay, excluding direct recognition of putative stromal cell line stress antigens by γδ T-cell activatory receptors (e.g., TCR, NKG2D, etc.). Of note, slight IVEC lysis can be observed with sera from nonsensitized KTRs (15% at a 30:1 effector:target ratio), suggesting the presence of antibodies to other antigens specifically expressed on IVEC. Antibodies to endothelial cell antigens other than HLA antigens have been previously detected in KTRs, particularly in relation to CMV infection.42–45 However, because no binding of IgG from nonsensitized sera was found by flow cytometry on IVEC, lysis of these cells with this serum suggests the presence of unknown factors other than IgG able to induce γδ T-cell cytolytic potential. This assay could therefore be interesting to identify new factors involved in endothelial cell damage. γδ T-cell ADCC relies on the release of perforin and granzyme from the cytotoxic vacuole, but other molecules, such as granulysin, cannot be excluded.46 Conversely, death receptor pathways do not seem to play any role in Vδ2neg γδ T-cell ADCC because expression of neither Fas-L nor TNF-related apoptosis-inducing ligand was found on Vδ2neg γδ CD16+ T cells (data not shown). Thus, Vδ2neg γδ T cells use the same cytotoxic arsenal when activated via CD16 to mediate ADCC as when they are activated via the TCR to kill CMV-infected or tumor cells (previously shown by Halary et al.32).
In situ visualization of γδ T cells within allografts has not been reported before. Tissue staining of γδ T cells is challenging because of their low number and because specific mAbs are not suitable for paraffin-embedded tissue staining. Nevertheless, using frozen-tissue immunofluorescence staining, we could visualize γδ T cells in kidney grafts undergoing acute rejection. In combination with endothelial cell staining through CD31, we could precisely define γδ T-cell localization within the graft. T cells were observed in close contact with peritubular capillaritis and glomerulitis associated with acute AMR in CMV-experienced patients. This localization is consistent with γδ T-cell involvement in antibody-mediated microcirculation injuries, such as that reported for NK cells17 and macrophages.47–49 The fact that higher numbers of γδ T cells can be found in these areas in grafts from CMV-experienced patients compared with grafts from CMV-naive patients suggests that only CMV-induced γδ T cells contribute to DSA-mediated microcirculation injuries, in agreement with their expansion and CD16 overexpression in response to CMV. Because of the limited choice in antibodies against CD16 and γδ TCR suitable for tissue immunofluorescence double-staining, CD16 expression by γδ T cells in the graft remains to be established. Staining of CD16 alone was positive but might also be attributed to NK cells or polymorphonuclear cells (data not shown). However, because higher γδ T-cell numbers were found in peritubular capillaries and glomeruli in acute AMR compared with acute TCMR, we can postulate that DSA and their likely binding to CD16 were required for this specific localization. In addition, there was no increase (and even a significant decrease, P=0.01 using a Mann–Whitney comparison) in γδ T-cell number within the interstitium lymphocyte infiltrate in CMV-experienced patients compared with CMV-naive patients during TCMR, suggesting that CMV-mediated γδ T-cell blood expansion is not the only reason for their migration into the graft.
This suspected γδ T-cell role in acute AMR is supported by the association of decreased graft function in DSA-positive patients with a high percentage of circulating CMV-induced γδ T cells. This is in marked contrast with CMV-specific CD8 αβ T cells, which have been shown to associate with less alloreactivity and improved graft function,50 consistent with their absence of CD16 expression.35 γδ T-cell ADCC could represent a new physiopathologic contribution to the well known but poorly understood association between CMV infection and the increased occurrence of rejection,20,51 poor long-term graft function,21,52–54 and low graft survival.55,56 Hence, both CMV and DSA are required to see a correlation between circulating γδ T cells and graft function deterioration, consistent with a model where CMV infection evokes activation and expansion of CD16pos γδ T cells in undetermined anatomic sites, and DSA triggers their ADCC function within graft microcirculation. This implication of γδ T cells in graft rejection seemingly contrasts with the previously reported Vδ1 T-cell expansion in the peripheral blood and grafts of operationally tolerant liver transplant recipients.57–59 However, Sánchez-Fueyo et al. recently revised their interpretation of Vδ1 expansions, confirming that they relate to CMV infection and are not restricted to tolerant liver recipients.24
Even though the Banff diagnostic criteria of antibody-mediated rejection requires positive C4d peritubular capillary staining, it is now well accepted that C4d does not explain all antibody-mediated lesions.12,60 Complement-independent pathways have been proposed, and their characterization could help define novel markers of DSA-mediated microcirculation damage. NK cells are suspected to play a role because NK-cell transcripts or transcripts induced by IFN-γ suspected to be produced by NK cells are increased in AMR biopsy specimens.18,61 Moreover, peripheral blood NK cells from HLA-sensitized patients produce IFN-γ in a CD16-dependent manner when exposed in vitro to allogeneic cells plus alloantibodies.62 CMV-responsive γδ T cells share many features with NK cells, such as common expression of activating (e.g., NKp80, NKG2D, CD16,) and inhibiting (e.g., CD158, CD85j) receptors;26,31 expression of specific cytotoxic molecules, such as granulysin (J. Déchanet-Merville, unpublished data); and production of IFN-γ.32,35 Transcripts for some of these molecules have been found in kidney biopsy specimens from patients with DSA and have been attributed to NK cells.17 We know, however, from previous studies that engagement of CD16 can also induce IFN-γ production by γδ T cells,35 and that this production depends on a Th1 cytokinic environment. Transplant glomerulopathies were associated with a predominantly Th1 and cytotoxic profile, which could provide this context.63 It is then conceivable that both NK cells and γδ T cells could contribute to AMR through ADCC.
In conclusion, CMV elicits the emergence of an unconventional T-cell subpopulation that does not have intrinsic alloreactivity but has the potential to be activated by alloantibodies through CD16. These γδ T cells are able to control CMV and to limit tumor occurrence but also appear as new players in complement-independent AMR. Because they are found selectively in peritubular capillaries in acute AMR, their detection could be used as a clinical marker to aid in diagnosis, particularly in C4d-negative humoral rejection. More easily, CD16pos Vδ2neg γδ T-cell expansion in peripheral blood can also be monitored and considered as a poor prognostic factor in DSA-positive patients.
Concise Methods
This study was approved by the Institutional Review Board of the Bordeaux Hospital.
Cell Lines and HLA Typing
FSFs were prepared in the laboratory with the help of Catherine Pain (National Institute of Health and Medical Research U1035, Bordeaux) by a classic tissue-dissociation procedure. Fetal lung fibroblasts (MRC5) were purchased from Eurobio. IVECs were obtained from human umbilical vein endothelial cells immortalized with SV40 virus and kindly provided by the Pasteur Institute.64 DNA HLA class I typing of cell lines was performed using the routine LabType SSO HR assay (One Lambda, Canoga Park, CA) following the manufacturer’s instructions. Results of HLA typing were as follows: FSF, A*24,*29; B*35,*51; for MRC5, A*02,*29; B*07,*44; and for IVEC, A*02,*30; B*07,*44.
Characterization of Anti-HLA Antibodies in Patient Sera
Sera from sensitized KTRs were tested for HLA class I antibodies using the SAB assay (LabScreen single antigen LS1A04 and LS2A01; One Lambda) on a Luminex platform (Luminex BV). Manufacturer’s instructions were followed. For any given antigen, the MFI was the average of all beads harboring the different alleles of the same antigen (Tables 1–3). For any given serum, we calculated the global MFI of the antibodies specific for the HLA-A and HLA-B expressed by each cell line used in the in vitro assay (CLSA).
Analysis of DSA Binding to Cell Lines by Flow Cytometry
Ten microliters of sera from sensitized KTRs made up to 50 µl with PBS was incubated with the fibroblastic or endothelial cell lines at 4°C for 20 minutes. A secondary FITC-conjugated goat anti-human anti-Fc IgG (Immunotech, 1/200) was added for 15 minutes. Cells were then washed and analyzed with the FACScanto Cytometer (BD Biosciences). A pool of immunized sera (pool S+) covering all HLA specificities was used as a positive control.
Generation of Polyclonal γδ T Cell Lines
A long-term CD16pos Vδ2neg γδ T cell line was selected on the basis of persistent expression of CD16 in culture as follows: Vδ2neg γδ T cells from PBMCs of a CMV-seropositive blood donor were sorted on a FACSAria (BD Biosciences) using a combination of anti-CD3, anti-Vδ2, and anti-TCRγδ. The sorted Vδ2neg γδ T cells were expanded in culture in RPMI medium supplemented with 10% human serum, 1000 U/mlrIL2 (Chiron), 1 μg/ml leuco-agglutinin (Sigma-Aldrich), and irradiated allogeneic PBMCs (35 Gy). After 1 month in culture, the Vδ2neg T cell line was phenotyped for CD16 expression. CD16neg and CD16pos cells were sorted by flow cytometry, expanded as previously described, and then used in the experiments.
Phenotyping of γδ T Cells by Flow Cytometry
mAbs directed against the following molecules were purchased from Beckman-Coulter: TCR-Cδ, TCR-Vδ2, and CD16. mAbs directed against CD3, perforin, and granzyme B were from BD Biosciences. Staining was performed on γδ T cell lines as previously described23 and analyzed on a FACScanto cytometer (BD Biosciences) with the use of the FACSDiva software, version 6.1.2 (BD Biosciences). At least 10,000 T lymphocytes were analyzed for six-color staining. Permeabilization and fixation of cells for intracellular staining were performed as described elsewhere.32
Cell Cytotoxicity Assays
Cytolytic activity of γδ T cells was measured by a standard 51chromium release assay.32 Target cells (FSF, MRC5, IVEC) were labeled with 51Cr (1.85 MBq/106 cells) for 1 hour at 37°C, washed, and incubated with selected sera for 20 minutes. After washes, 3000 cells/well were incubated in triplicate with γδ T cells at the indicated effector:target ratio. After 4 hours at 37°C, 51Cr released in the supernatant was measured. The percentage of specific lysis was calculated as ([experimental release–spontaneous release)/(maximum release–spontaneous release])×100. The spontaneous 51Cr release from target cells in medium alone was always <15% of the control maximal 51Cr release obtained with 1% Triton X-100. In some experiments, γδ T-effector cells were preincubated in plates at 37°C for 2.5 hours in 50 µl of culture medium with different concentrations of concanamycin A (Calbiochem; Sigma-Aldrich), which is known to prevent cytotoxic granule content release through a pH increase of the vacuoles.65
In Situ Immunofluorescence Studies
Renal tissues were from selected graft biopsy fragments exhibiting different type of acute rejections (acute AMR) and acute TCMR). Kidney biopsy specimens were distinguished according to the status of the kidney transplant recipients related to CMV: those coming from patients who had never experienced CMV infection (seronegative on the day of the graft and no infection after transplantation) and those from patients who had been in contact with CMV before (R+) or after (R− with CMV primary infection) transplantation. All kidney biopsy specimens were graded according to the last Banff criteria meeting report.4 Seven biopsy specimens with AMR lesions and from CMV-experienced patients were also assessed for the presence of CMV antigens using the mAb 810–500 from Abcys. Immunofluorescence was performed on frozen graft-biopsy fragments using rabbit anti-CD31 mAb (clone ab28364, 1/75; Abcam, Inc.) to stain endothelial cells, and mouse pan-γδ mAb (clone IMMU510, 1/50, Beckman-Coulter). Differential staining revelation was performed using Alexa-488–coupled anti-rabbit antibody (green staining) and Alexa-533–coupled anti-mouse (red staining), both from Invitrogen. Nuclei were stained with 0.5 µg/ml DAPI (blue staining). Staining analysis was done on an inverted Leica DMI 6000 microscope (Leica Microsystems) equipped with a Quantum camera (Roper Scientific, Evry, France). The objective used was a HCX PL Fluotar 40× dry 0.75 NA. Cumulated images of the entire cortex of the biopsy (defined as the region inside the renal capsule and outside the medulla) were obtained and analyzed by MetaMorph software. Cellular infiltrate (expressed in number of stained cells per mm2) was assessed in high-power fields on the entire cortical region of the biopsy specimens by observers blinded to the origin of the slides. Cells were considered to be part of the capillaritis using the Banff criteria.9
Patients
Twenty-one sensitized KTRs transplanted with anti-HLA DSA the day of the graft (determined by both the SAB assay and the flow cytometric cross-match) were analyzed retrospectively for this study to appraise the potential association between γδ T cells and the incidence of biopsy-proven acute rejection and 1-year eGFR (calculated using the Modification of diet in Renal Disease formula66). A control group of patients without DSA was selected in our cohort. From 1998 to 2002, we retrospectively identified 162 consecutive nonsensitized KTRs who underwent transplantation with a negative flow cytometric cross-match, who had not developed DSA at 1 year after transplant. For the purpose of the study, d0 and year 1 sera were retested for class I and class II antibodies using SAB assays (LabScreen single antigen LS2A01 for class II, One Lambda). γδ T-cell subsets are measured routinely in our center in all transplant recipients.
Statistical Analyses
Graphs of linear regression were performed using Statview software (Abacus Concepts, Berkeley, CA). Cellular infiltrates are expressed as means and ranges. The statistical differences between groups of AAMR and ACMR were tested with the unpaired Mann–Whitney test. A P value<0.05 represents statistical differences.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the Bordeaux Imaging Center for microscopy analysis, the SFR TransBioMed Flow Cytometry facility for phenotyping analysis and cell sorting, the technicians from the Laboratory of Immunology and Immunogenetics at Bordeaux University Hospital for their expert assistance, Catherine Rio for clinical data collection, the nurses of the Department of Nephrology for patient care, the Etablissement Français du Sang Aquitaine Limousin for providing blood samples, and M. Capone and C. Behr for thoughtful discussions.
This work was supported by the Fondation pour la Recherche Médicale (DEQ20110421287) and the Agence Nationale de la Recherche (ANR-12-BSV3-0024-02). TB was supported by CNRS and CHU Bordeaux.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101052/-/DCSupplemental.
References
- 1.Colvin RB, Smith RN: Antibody-mediated organ-allograft rejection. Nat Rev Immunol 5: 807–817, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Lee PC, Zhu L, Terasaki PI, Everly MJ: HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation 88: 568–574, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D, Banff meeting report writing committee : Banff 2011 Meeting report: New concepts in antibody-mediated rejection. Am J Transplant 12: 563–570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bentall A, Cornell LD, Gloor JM, Park WD, Gandhi MJ, Winters JL, Chedid MF, Dean PG, Stegall MD: Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant 13: 76–85, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmüller G, Land W, Albert E: Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int 43: 1333–1338, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Mengel M, Husain S, Hidalgo L, Sis B: Phenotypes of antibody-mediated rejection in organ transplants. Transpl Int 25: 611–622, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Loupy A, Hill GS, Suberbielle C, Charron D, Anglicheau D, Zuber J, Timsit MO, Duong JP, Bruneval P, Vernerey D, Empana JP, Jouven X, Nochy D, Legendre CH: Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant 11: 56–65, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES: Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Cosio FG, Lager DJ, Lorenz EC, Amer H, Gloor JM, Stegall MD: Significance and implications of capillaritis during acute rejection of kidney allografts. Transplantation 89: 1088–1094, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, Mengel M, Matas A, Halloran PF: A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant 12: 1168–1179, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Méjean A, Charron D, Duong van Huyen JP, Bruneval P, Legendre C, Nochy D: Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant 9: 2561–2570, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Hidalgo LG, Sis B, Sellares J, Campbell PM, Mengel M, Einecke G, Chang J, Halloran PF: NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: Evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant 10: 1812–1822, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo LG, Sellares J, Sis B, Mengel M, Chang J, Halloran PF: Interpreting NK cell transcripts versus T cell transcripts in renal transplant biopsies. Am J Transplant 12: 1180–1191, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Braakman E, van de Winkel JG, van Krimpen BA, Jansze M, Bolhuis RL: CD16 on human gamma delta T lymphocytes: Expression, function, and specificity for mouse IgG isotypes. Cell Immunol 143: 97–107, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Pouteil-Noble C, Ecochard R, Landrivon G, Donia-Maged A, Tardy JC, Bosshard S, Colon S, Betuel H, Aymard M, Touraine JL: Cytomegalovirus infection—an etiological factor for rejection? A prospective study in 242 renal transplant patients. Transplantation 55: 851–857, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Humar A, Gillingham KJ, Payne WD, Dunn DL, Sutherland DE, Matas AJ: Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation 68: 1879–1883, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Dzabic M, Bojakowski K, Kurzejamska E, Styczynski G, Andziak P, Söderberg-Nauclér C, Religa P: Significance of cytomegalovirus infection in the failure of native arteriovenous fistula. Clin Microbiol Infect 18: E5–E7, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, Michelson S, Méric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF: Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 103: 1437–1449, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puig-Pey I, Bohne F, Benítez C, López M, Martínez-Llordella M, Oppenheimer F, Lozano JJ, González-Abraldes J, Tisone G, Rimola A, Sánchez-Fueyo A: Characterization of γδ T cell subsets in organ transplantation. Transpl Int 23: 1045–1055, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Couzi L, Lafarge X, Pitard V, Neau-Cransac M, Dromer C, Billes MA, Lacaille F, Moreau JF, Merville P, Déchanet-Merville J: Gamma-delta T cell expansion is closely associated with cytomegalovirus infection in all solid organ transplant recipients. Transpl Int 24: e40–e42, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Couzi L, Pitard V, Netzer S, Garrigue I, Lafon ME, Moreau JF, Taupin JL, Merville P, Déchanet-Merville J: Common features of gammadelta T cells and CD8(+) alphabeta T cells responding to human cytomegalovirus infection in kidney transplant recipients. J Infect Dis 200: 1415–1424, 2009 [DOI] [PubMed] [Google Scholar]
- 27.de Villartay JP, Lim A, Al-Mousa H, Dupont S, Déchanet-Merville J, Coumau-Gatbois E, Gougeon ML, Lemainque A, Eidenschenk C, Jouanguy E, Abel L, Casanova JL, Fischer A, Le Deist F: A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest 115: 3291–3299, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, Kühr J, Mascart F, Schmitt-Graeff A, Niemeyer C, Fisch P: A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest 115: 3140–3148, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M, Twité N, Goldman M, Marchant A, Willems F: Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med 207: 807–821, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW: The role of Vdelta2-negative gamma-delta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplants [published online ahead of print June 24, 2010]. Blood 10.1182/blood-2010-01-255166 [DOI] [PubMed] [Google Scholar]
- 31.Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, Merville P, Moreau JF, Déchanet-Merville J: Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 112: 1317–1324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, Dromer C, Emilie D, Moreau JF, Déchanet-Merville J: Shared reactivity of Vdelta2(neg) gammadelta T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 201: 1567–1578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couzi L, Levaillant Y, Jamai A, Pitard V, Lassalle R, Martin K, Garrigue I, Hawchar O, Siberchicot F, Moore N, Moreau JF, Dechanet-Merville J, Merville P: Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol 21: 181–188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafarge X, Merville P, Cazin MC, Bergé F, Potaux L, Moreau JF, Déchanet-Merville J: Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis 184: 533–541, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O, Merville P, Moreau JF, Déchanet-Merville J: Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 119: 1418–1427, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Sis B, Einecke G, Chang J, Hidalgo LG, Mengel M, Kaplan B, Halloran PF: Cluster analysis of lesions in nonselected kidney transplant biopsies: Microcirculation changes, tubulointerstitial inflammation and scarring. Am J Transplant 10: 421–430, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Couzi L, Araujo C, Guidicelli G, Bachelet T, Moreau K, Morel D, Robert G, Wallerand H, Moreau JF, Taupin JL, Merville P: Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation 91: 527–535, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau JF, Hayday AC, Willcox BE, Déchanet-Merville J: Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol 13: 872–879, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, Strong RK: Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A 108: 2414–2419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH: Antigen recognition determinants of gammadelta T cell receptors. Science 308: 252–255, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF: MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity 15: 83–93, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Toyoda M, Petrosian A, Jordan SC: Immunological characterization of anti-endothelial cell antibodies induced by cytomegalovirus infection. Transplantation 68: 1311–1318, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Costa C, Touscoz GA, Bergallo M, Terlizzi ME, Astegiano S, Sidoti F, Sinesi F, Segoloni GP, Cavallo R: Non-organ-specific and anti-endothelial antibodies in relation to CMV infection and acute rejection in renal transplant recipients. Clin Transplant 24: 488–492, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Sigdel TK, Li L, Tran TQ, Khatri P, Naesens M, Sansanwal P, Dai H, Hsieh SC, Sarwal MM: Non-HLA antibodies to immunogenic epitopes predict the evolution of chronic renal allograft injury. J Am Soc Nephrol 23: 750–763, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Dragun D, Philippe A, Catar R: Role of non-HLA antibodies in organ transplantation. Curr Opin Organ Transplant 17: 440–445, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Clayberger C: Cytolytic molecules in rejection. Curr Opin Organ Transplant 14: 30–33, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Lipták P, Kemény E, Morvay Z, Szederkényi E, Szenohradszky P, Marofka F, Toldi J, Exner M, Iványi B: Peritubular capillary damage in acute humoral rejection: an ultrastructural study on human renal allografts. Am J Transplant 5: 2870–2876, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Papadimitriou JC, Drachenberg CB, Munivenkatappa R, Ramos E, Nogueira J, Sailey C, Klassen DK, Haririan A: Glomerular inflammation in renal allografts biopsies after the first year: Cell types and relationship with antibody-mediated rejection and graft outcome. Transplantation 90: 1478–1485, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Papadimitriou JC, Drachenberg CB, Ramos E, Kukuruga D, Klassen DK, Ugarte R, Nogueira J, Cangro C, Weir MR, Haririan A: Antibody-mediated allograft rejection: Morphologic spectrum and serologic correlations in surveillance and for cause biopsies. Transplantation 95: 128–136, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Nickel P, Bold G, Presber F, Biti D, Babel N, Kreutzer S, Pratschke J, Schönemann C, Kern F, Volk HD, Reinke P: High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl Immunol 20: 238–242, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Reischig T, Jindra P, Hes O, Svecová M, Klaboch J, Treska V: Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant 8: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Tong CY, Bakran A, Peiris JS, Muir P, Herrington CS: The association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation 74: 576–578, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F: Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: Results of a randomized clinical trial. Am J Transplant 8: 975–983, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, McDonald RA: Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol 21: 1579–1586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degré M, Fauchald P, Rollag H: Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int 66: 329–337, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Helanterä I, Koskinen P, Finne P, Loginov R, Kyllönen L, Salmela K, Grönhagen-Riska C, Lautenschlager I: Persistent cytomegalovirus infection in kidney allografts is associated with inferior graft function and survival. Transpl Int 19: 893–900, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Koshiba T, Yoshizawa A, Yonekawa Y, Masuda K, Ito A, Ueda M, Mori T, Kawamoto H, Tanaka Y, Sakaguchi S, Minato N, Wood KJ, Tanaka K: Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant 4: 2118–2125, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benítez C, Pons JA, Parrilla P, Ramírez P, Bruguera M, Rimola A, Sánchez-Fueyo A: Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest 118: 2845–2857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X, Li Y, Ohe H, Nafady-Hego H, Uemoto S, Bishop GA, Koshiba T: Intragraft Vδ1 γδ T cells with a unique T-cell receptor are closely associated with pediatric semiallogeneic liver transplant tolerance. Transplantation 95: 192–202, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF: Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, Halloran PF: Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant 13: 971–983, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Toyoda M, Ge S, Suviolahti E, Pichurin P, Shin B, Pao A, Vo A, Deer N, Aguiluz A, Karasyov A, Jordan SC: IFNγ production by NK cells from HLA-sensitized patients after in vitro exposure to allo-antigens. Transpl Immunol 26: 107–112, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Homs S, Mansour H, Desvaux D, Diet C, Hazan M, Buchler M, Lebranchu Y, Buob D, Badoual C, Matignon M, Audard V, Lang P, Grimbert P: Predominant Th1 and cytotoxic phenotype in biopsies from renal transplant recipients with transplant glomerulopathy. Am J Transplant 9: 1230–1236, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Vicart P, Testut P, Schwartz B, Llorens-Cortes C, Perdomo JJ, Paulin D: Cell adhesion markers are expressed by a stable human endothelial cell line transformed by the SV40 large T antigen under vimentin promoter control. J Cell Physiol 157: 41–51, 1993 [DOI] [PubMed] [Google Scholar]
- 65.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K: Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol 156: 3678–3686, 1996 [PubMed] [Google Scholar]
- 66.Klahr S: The modification of diet in renal disease study. N Engl J Med 320: 864–866, 1989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.