Abstract
Several genes, mainly involved in podocyte cytoskeleton regulation, have been implicated in familial forms of primary FSGS. We identified a homozygous missense mutation (p.P209L) in the TTC21B gene in seven families with FSGS. Mutations in this ciliary gene were previously reported to cause nephronophthisis, a chronic tubulointerstitial nephropathy. Notably, tubular basement membrane thickening reminiscent of that observed in nephronophthisis was present in patients with FSGS and the p.P209L mutation. We demonstrated that the TTC21B gene product IFT139, an intraflagellar transport-A component, mainly localizes at the base of the primary cilium in developing podocytes from human fetal tissue and in undifferentiated cultured podocytes. In contrast, in nonciliated adult podocytes and differentiated cultured cells, IFT139 relocalized along the extended microtubule network. We further showed that knockdown of IFT139 in podocytes leads to primary cilia defects, abnormal cell migration, and cytoskeleton alterations, which can be partially rescued by p.P209L overexpression, indicating its hypomorphic effect. Our results demonstrate the involvement of a ciliary gene in a glomerular disorder and point to a critical function of IFT139 in podocytes. Altogether, these data suggest that this homozygous TTC21B p.P209L mutation leads to a novel hereditary kidney disorder with both glomerular and tubulointerstitial damages.
Keywords: genetic renal disease, focal segmental glomerulosclerosis, podocyte, nephronophthisis
FSGS is a clinicopathologic entity leading to isolated proteinuria or steroid-resistant nephrotic syndrome,1 with progression to ESRD a few years later in half of the cases. Some cases are familial, with either an autosomal dominant or recessive inheritance,2 and are due to structural alterations of the glomerular filtration barrier. One of its principal actors, the podocyte, a highly specialized epithelial cell with an octopus-like shape, displays multiple foot processes that interdigitate on the glomerular basement membrane to form a modified junction called the slit-diaphragm. The identification of >20 causal genes, mainly expressed by podocytes,2 underlines the genetic heterogeneity and complexity of this disease.3 Nevertheless, in about half of the familial cases, the causal genes remain unknown.
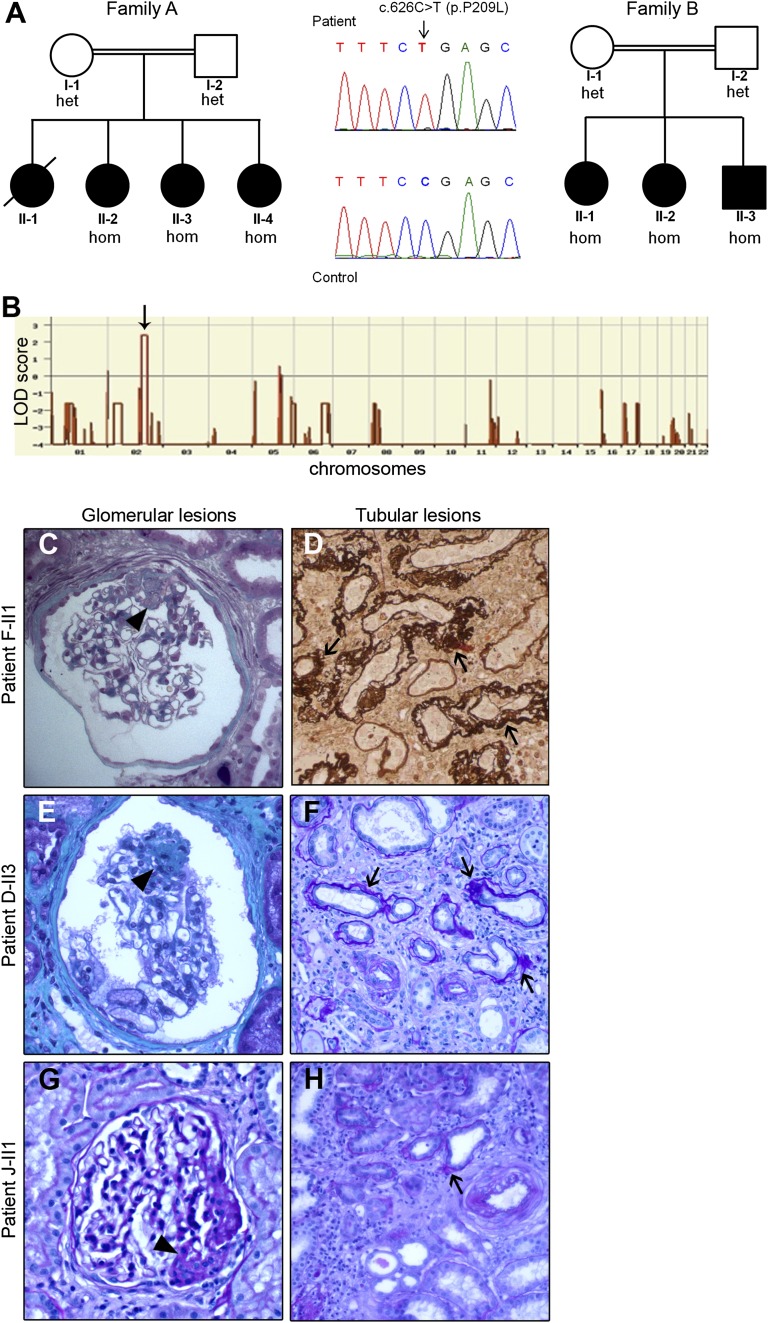
To identify novel genes, we performed whole exome sequencing (WES) combined with homozygosity mapping in two consanguineous families with FSGS (families A and B, Figure 1A), each of which had three affected siblings with available DNA. Their clinical phenotypes are summarized in Table 1. The patients had late-onset albuminuria (five of six patients age 9–23 years) with high BP (six of six patients) and FSGS on the three available kidney biopsy specimens and reached ESRD at 15–32 years of age, with no recurrence after transplantation. We identified one common region of homozygosity on chromosome 2 spanning a total of 18 Mb, with a maximum logarithm of odds (LOD) score of 2.4 for each family (Figure 1B). We then performed WES in one affected child from each pedigree. The mean sequencing coverage was 51 reads. Eighty-eight percent and 77% of the targeted regions were sequenced at 5× and at 15×depth or more, respectively. In the two patients, we identified the same homozygous missense mutation, p.P209L, in the TTC21B gene, located in the region of interest on chromosome 2. The proline-to-leucine change is predicted to be damaging by Polyphen2 and SIFT softwares (with scores of 0.916 and 0.03, respectively). The two patients did not share any other homozygous damaging mutation. Direct sequencing of TTC21B confirmed that the mutation segregated with the disease in both families (Figure 1A).
Figure 1.
The TTC21B homozygous p.P209L mutation is identified in patients with glomerular and tubular lesions. (A) Pedigrees of families A and B carrying the homozygous p.P209L mutation in the TTC21B gene. The allele status is given below each tested individual. Heterozygous (het); homozygous (hom). Family studies confirmed the segregation of the mutant allele as an autosomal recessive trait. (B) Linkage analysis in family A. One region of interest spanning 24 Mb was identified on chromosome 2 with a maximum logarithm of odds (LOD) score of 2.4. (C–H) Histologic lesions of kidney biopsy specimens from patients F-II1, D-II3, and J-II1: trichrome (C and E), periodic acid-Schiff (F–H) and Jones methenamine silver (D) staining. Left panel (magnification ×100): Patients displayed a broad spectrum of glomerular lesions from minimal changes to FSGS lesions (arrowhead). Right panel (magnification ×40): Tubulointerstitial lesions consisted of thickening and duplicated tubular basement membranes (arrows) around atrophic tubules within interstitial fibrosis.
Table 1.
Clinical and pathologic phenotypes of patients bearing the homozygous p.P209L mutation.
| Family | Origin | Patient | Sex | Age at Onset of Proteinuria (yr) | Proteinuria (g/d) | Glomerular Lesions | Tubular Lesions | Age at ESRD/CKD (Stage) (yr) | Other Features |
|---|---|---|---|---|---|---|---|---|---|
| A | Tunisia | II-2 | F | 15 | NA | ND | ND | 15 | HBP |
| II-3 | F | 23 | 1.5 | FSGS | Stripes of tubulointerstitial fibrosis, atrophic tubules | 32 | HBP, myopia | ||
| II-4 | F | 15 | Non-nephrotic proteinuria | ND | ND | 27 | HBP | ||
| B | Tunisia | II-1 | F | 18 | Positive | ND | ND | 27 | HBP |
| II-2 | F | 18 | Positive | FSGS | Tubulointerstitial fibrosis | 26 | HBP, cerebral aneurysm, deafness | ||
| II-3 | M | 9 | 3.0 | FSGS | NA | 16 | HBP, cerebral aneurysm | ||
| C | Algeria | II-1 | M | 22 | 2.5 | ND | ND | 22 | HBP |
| II-4 | F | 19 | FSGS | Stripes of tubulointerstitial fibrosis, atrophic tubules | 23 | HBP | |||
| D | Tunisia | II-3 | M | 18 | 2.9 | FSGS | Tubulointerstitial fibrosis | 27 | HBP |
| II-11 | F | 26 | 7.0 | MCNS | Foci of atrophic tubules, thickened TBM | 35 | HBP | ||
| E | Tunisia | II-1 | F | 16 | Nephrotic-range proteinuria | FSGS | NA | 17 | NA |
| F | Algeria | II-1 | M | 26 | 1.0 | FSGS | Severe tubulointerstitial lesions, thickened TBM | CKD (IV) 26 | HBP |
| G | Algeria | II-1 | F | 30 | 2.2 | FSGS | Tubulointerstitial fibrosis | 34 | Primary biliary cirrhosis |
| H | Portugal | II-2 | M | 14 | Non-nephrotic proteinuria | FSGS | Tubulointerstitial lesions, dedifferentiated tubules, thickened and multilayered TBM, atrophic tubules | 20 | HBP, severe scoliosis |
| II-3 | F | 11 | NA | ND | ND | 12 | Bilateral hip osteotomy | ||
| I | Morocco | II-1 | F | 10 | 0.5 | Global sclerosis | Severe tubulointerstitial fibrosis, atrophic tubules, thickened TBM, medullar cysts | 14 | HBP |
| 14 | 2.1 | ||||||||
| II-2 | M | 26 | 1.5 | ND | ND | 32 | HBP | ||
| J | Portugal | II-1 | M | 11 | 0.25 | FSGS | Tubulointerstitial fibrosis, foci of atrophic tubules, thickened TBM | CKD (III) 11 | HBP, elevated liver enzymes |
Families A–G had a primary diagnosis of FSGS, whereas families H–J had a primary diagnosis of nephronophthisis. F, female; NA, not available; ND, not determined; HBP, high BP; M, male; MCNS, minimal-change nephrotic syndrome; TBM, tubular basement membrane.
We then screened 44 families with similar late-onset FSGS for the p.P209L mutation. Interestingly, five additional unrelated individuals were homozygous for this mutation (families C–G) (Supplemental Figure 1, Table 1), whereas the p.P209L mutation was absent in 226 ethnically matched controls (Supplemental Figure 2A).
These results were unexpected because TTC21B encodes a ciliary protein, and until now no ciliary gene has been found to be involved in glomerular diseases. Moreover, in a collaborative effort, we have previously reported the p.P209L mutation in the homozygous state in patients with nephronophthisis,4 an autosomal recessive chronic tubulointerstitial nephritis, belonging to the group of ciliopathies.5 Main clinical features of nephronophthisis are polyuria and polydipsia related to reduced urinary concentrating ability and urinary sodium wasting. Hematuria and proteinuria are absent or minimal, and BP is usually normal before ESRD. Histologically, nephronophthisis is characterized by irregular alternation of dilated and atrophic tubules with thickened basement membrane, associated with interstitial fibrosis and corticomedullary cysts. Glomeruli are mostly normal, although secondary sclerosis is observed in advanced disease.6 The finding of the p.P209L mutation in patients with FSGS led us to carefully re-examine their kidney biopsy specimens. Strikingly, in addition to the typical FSGS lesions, all patients displayed tubular lesions as severe as that found in nephronophthisis (Figure 1, C–F, Table 1). Among our cohort of patients initially referred for nephronophthisis, five patients from three families were found to be homozygous for the TTC21B p.P209L mutation (families H–J) (Supplemental Figure 1, Table 1). Interestingly, three patients for whom the information was available had non-nephrotic proteinuria and FSGS lesions that had been initially regarded as secondary to tubular changes (Figure 1, G and H, Table 1). We further demonstrated that patients bearing the TTC21B p.P209L mutation are of North African or Portuguese descent and share a common haplotype, indicating a founder effect (Supplemental Figure 2B). Altogether these data suggest that the homozygous TTC21B p.P209L mutation leads to a novel hereditary kidney disorder characterized by the association of glomerular and tubulointerstitial involvement.
TTC21B encodes IFT139, a component of the intraflagellar transport-A complex that is associated with the motor protein dynein2 to regulate retrograde trafficking in the primary cilium.7 The cilium is a microtubule-based organelle present on the surface of most cells, sensing flow changes and mediating signaling pathways involved in the establishment of cell polarity during development.8 Knockdown or missense mutations of IFT139 impair ciliogenesis in mouse tubular renal cells, as well as in mice and zebrafish, and lead to severe developmental alterations in animal models.4,9 Defects in cilia length and body axis curvature during zebrafish development can be partially rescued by re-expression of the p.P209L mutant, indicating that p.P209L is likely a hypomorphic variant.4
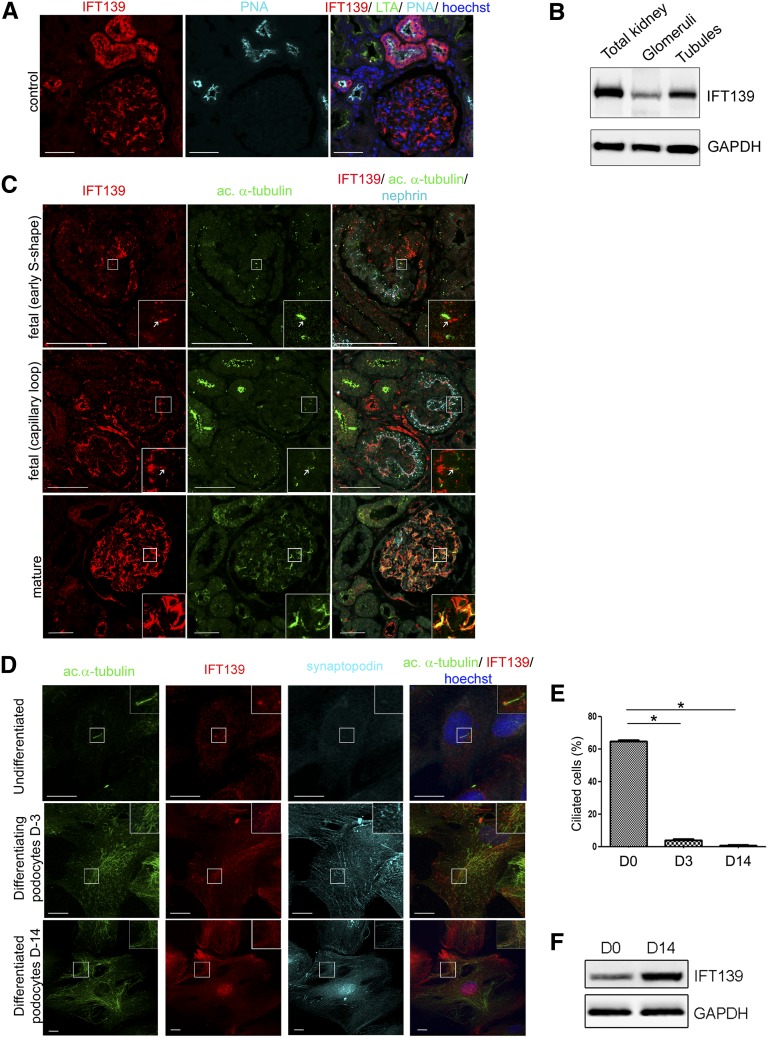
Because the p.P209L mutation may affect both tubular and glomerular compartments of the kidney, we first analyzed the endogenous expression of IFT139 in control adult human kidneys by immunofluorescence microscopy. IFT139 was expressed predominantly in distal tubules, as expected for a nephronophthisis-causing gene, but was also strongly expressed in glomerular podocytes (Figure 2A), as evidenced by costaining with synaptopodin (Supplemental Figure 3A). Western blot analyses confirmed IFT139 expression in both tubules and glomeruli (Figure 2B). In patient kidney sections, IFT139 staining in glomeruli appeared weaker than in control sections, but the severity of the lesions precluded drawing any conclusion with respect to putative effect of the p.P209L mutation (Supplemental Figure 3B).
Figure 2.
IFT139, expressed at the base of primary cilium in tubules, fetal and undifferentiated podocytes, relocalizes along the microtubule network in mature and differentiated podocytes. (A) Immunolocalization of IFT139 in adult control human kidney using lotus tetragonolobus (LTA) and peanut agglutinin lectin (PNA) to identify proximal and distal tubules, respectively, revealed that IFT139 is expressed predominantly in distal tubules and glomeruli. Scale bar: 50 µm. (B) Western blot showing expression of IFT139 in total kidney and isolated glomeruli or tubules from adult wild-type mice of mixed background. (C) Immunolabeling of IFT139; acetylated α-tubulin, a ciliary component; and nephrin, a podocyte marker, in fetal (14 weeks) and mature kidneys. IFT139 is expressed in developing glomeruli, notably at the base of primary cilia (arrow). In contrast, we observed a rearrangement of the acetylated α-tubulin network in mature glomeruli, and IFT139 colocalizes with this network. Scale bar: 50 µm. (D) Immunolocalization of IFT139 in podocytes after 3 and 14 days of differentiation (D-3 and D-14). IFT139 localizes at the base of the cilium in undifferentiated podocytes and is redistributed along the acetylated α-tubulin network in differentiated podocytes, labeled by synaptopodin. Scale bar: 20 µm. (E) Percentage of ciliated cells in cultured podocytes demonstrated that differentiated podocytes lose their primary cilium. For statistical analysis, four independent experimentations were performed. A t test was conducted; the mean±SEM is shown (*P<0.05). (F) Western blot of IFT139 in differentiated and undifferentiated podocytes showed an increase of its expression after differentiation.
The role of the primary cilium in podocyte physiology is unclear. Ichimura et al. demonstrated in the rat kidney that most fetal podocytes present a primary cilium at the S-shaped body and capillary loop stages that gradually disappears during glomerular maturation.10 In human fetal kidney sections, we showed that podocytes, labeled by nephrin, also display cilia at the S-shaped and capillary loop stages (Figure 2C, upper and middle panels, Supplemental Figure 3C). Moreover, we detected IFT139 staining in the cytoplasm of fetal podocytes, notably at the base of the primary cilium (Figure 2C, upper and middle panels). Similar to findings in rats, human podocytes seemed to lose their cilium after maturation (Figure 2C, lower panel, Supplemental Figure 3C). In mature podocytes, IFT139 expression appeared enhanced and it localized to the extended microtubule network, as shown by costaining with acetylated α-tubulin that presents a wide distribution within cell body and primary processes (Figure 2C, lower panel, Supplemental Figure 3D).
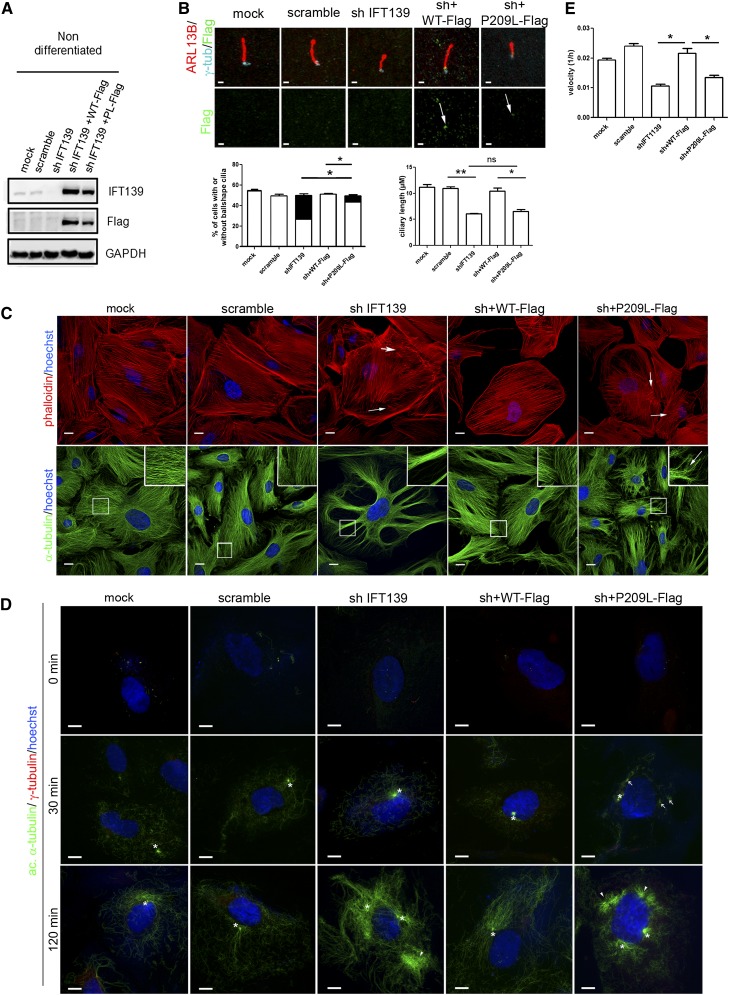
In human immortalized undifferentiated podocytes,11 we confirmed the endogenous IFT139 localization at the base of the primary cilium (Figure 2D, upper panel). As in in vivo studies, during podocyte differentiation, the cilia disappeared (Figure 2E), cells underwent microtubule rearrangements concomitant with IFT139 relocalization along the microtubule network (Figure 2D, middle and lower panel). In differentiated cells expressing synaptopodin, IFT139 expression was increased, as confirmed by Western blot and quantitative PCR (Figure 2, D and F, Supplemental Figure 3E). We further showed that IFT139 depletion in undifferentiated podocytes resulted in cells with short and ball-shaped cilia, with accumulation of IFT-B components at the tip (Figure 3, A and B, Supplemental Figure 4, A–C), matching with the ciliary phenotype observed in other models of altered intraflagellar retrograde transport.12 Re-expression of the p.P209L protein in knockdown cells led to its correct localization at the cilium base and to the partial rescue of the ball-shaped phenotype (Figure 3B, Supplemental Figure 5). Because the primary cilium plays a major role in Hedgehog signaling, we also investigated the role of p.P209L mutation on this pathway using a Smoothened agonist. No alteration of the ciliary translocation of Smoothened was observed (Supplemental Figure 4D). Although our in vitro studies in undifferentiated podocytes showed that p.P209L has a hypomorphic effect on podocyte ciliogenesis, this does not seem sufficient to inhibit podocyte differentiation, as indicated by the equal proportion of synaptopodin-positive cells in controls, knockdown and IFT139 re-expressing podocytes (Supplemental Figure 6).
Figure 3.
The p.P209L mutant partially rescues the cilia defects in undifferentiated podocytes, but does not rescue the cytoskeleton alterations in differentiated cells, indicating a hypomorphic effect. (A) Western blot analysis in IFT139 knocked-down undifferentiated podocytes re-expressing Flag-tagged IFT139 wild-type or mutant forms. (B) IFT139 staining using Flag antibodies on undifferentiated podocytes revealed no mislocalization of the mutant protein (arrow). Knocked-down and p.P209L podocytes display shorter and ball-shaped primary cilia, stained with ARL13B. Scale bars: 1 µm. Left graph represents the proportion of ciliated cells: normally shaped cilia (in white) and ball-shaped cilia (in black). The percentage of normal cilia of depleted cells versus p.P209L re-expressing cells was significantly different (*P<0.005). Right graph shows the quantification of the ciliary length of cells without ball-shaped cilia. (C) Differentiated podocytes labeled with α-tubulin (lower panel) or phalloidin (upper panel). Knockdown and p.P209L re-expressing cells display microtubule organization defects and alterations of actin stress fibers. (D) Differentiated podocytes were treated with nocodazole for 4 hours, washed, and placed at 37°C to assess microtubule repolymerization. Cells were fixed at the indicated time and stained with γ-tubulin and acetylated α-tubulin to visualize nucleation. In controls and wild-type cells, nucleation starts at the centriole (white asterisks). IFT139 depleted cells show an increase of acetylated α-tubulin, dissociated centrioles, and clusters of microtubules suggesting anchoring alteration (white arrowheads). pP209L re-expressing podocytes present ectopic nucleation sites (arrows), separated centrioles, indicating an alteration of both nucleation and anchoring. Scale bars: 20 µm. (E) Real-time migration assay. The graph represents migration velocities 10 hours after wounding using xCELLigence technology. Migration velocity is decreased in IFT139 knockdown and p.P209L re-expressing podocytes. For statistical analysis, at least three independent experimentations were performed. A t test was conducted; the mean±SEM is shown (*P<0.05; **P<0.01).
These data suggest that the glomerular defects observed in patients with this mutation are, rather, due to a nonciliary alteration of IFT139 in mature podocytes, uncovered by the hypomorphic p.P209L mutation, which is consistent with the late-onset phenotype observed in the patients, and the absence of extrarenal developmental disorders associated with ciliopathies.
We and others have shown that actin and microtubule cytoskeletons are key regulators of the delicate architecture, plasticity, and contractility of podocytes.13–15 Because IFT139 is redistributed along the microtubule network in mature podocytes, we studied the effect of the p.P209L mutant on cytoskeleton organization in differentiated podocytes. Interestingly, the depletion of IFT139 led to increased cell surface associated with actin cytoskeleton alterations, such as short and misorganized stress fibers and microtubule rearrangement into bike wheel–like shape. All these defects were fully rescued by the wild-type protein. In contrast, p.P209L re-expression led to the rescue of the cell-size defect, but actin and microtubule networks remained severely altered (Figure 3C), even though no obvious mislocalization of IFT139 was detected (Supplemental Figure 6). Furthermore, microtubule repolymerization after nocodazole treatment revealed that controls and wild-type–expressing cells displayed a unique nucleation site at the centrioles close to the nucleus, as visualized by acetylated α-tubulin staining. Despite a normal nucleation at 30 minutes, the knockdown of IFT139 induced typical microtubule anchoring defects at 2 hours, namely separated centrioles and ectopic microtubule clusters, as well as microtubule hyperacetylation, perhaps giving rise to the extended cell body. In p.P209L mutant cells, multiple ectopic nucleation sites around the nucleus revealed nucleation defects at 30 minutes in addition to dissociated centrioles observed at 2 hours (Figure 3D). These abnormalities were present only in differentiated podocytes (Supplemental Figure 7B). Altogether, this suggests a partial alteration of the microtubule network, thereby supporting the hypothesis of a hypomorphic effect of the p.P209L mutation.
Cytoskeleton alterations may affect cellular motility, a suggested mechanism underlying podocyte foot process effacement.15 Despite no obvious cytoskeleton alteration in undifferentiated cells, here we showed that in challenging conditions, undifferentiated podocytes depleted for IFT139 exhibited a decrease in cell migration that could not be rescued by expressing the p.P209L mutant (Figure 3E). Altogether, these results suggest that the p.P209L mutant may destabilize mature podocyte architecture by affecting cytoskeleton dynamics.
In conclusion, by WES combined with homozygosity mapping, we identified a homozygous missense mutation (p.P209L) in the ciliary gene TTC21B in seven of 46 families with a primary diagnosis of late-onset FSGS. This is the first gene encoding a ciliary protein involved in a hereditary glomerulopathy, and our data show that IFT139 function is not restricted to the cilium but extends to the regulation of podocyte cytoskeleton architecture as reported for most proteins implicated in FSGS. Whether the p.P209L mutation is the unique TTC21B mutation responsible for this new entity combining glomerular and tubular involvement remains to be determined. It is also expected that other ciliary proteins expressed in mature podocytes and sharing additional functions in microtubule maintenance may be involved in FSGS. Nevertheless, the data presented herein modify our understanding of hereditary kidney diseases previously classified as “primary glomerular” or “primary tubulointerstitial” disorders and open a new chapter in nephrology textbooks on “primary tubuloglomerular” diseases.
Concise Methods
Patients
Genomic DNA specimens were isolated from peripheral blood using standard procedures. Written informed consent was obtained from participants or their parents, and the study was approved by the Comité de Protection des Personnes “Ile-De-France II.”
Homozygosity Mapping, WES, and Mutation Calling
Homozygosity mapping in families A and B was performed using the Human Mapping 250k NspI array (Affymetrix), and parametric LOD scores were calculated with Multipoint Engine for Rapid Likelihood Interference16 software, assuming autosomal recessive inheritance. WES of DNA from families A and B was performed using the Agilent SureSelect All Exon 50Mb V3 capture kit and SOLiD 5500XL (Life Technologies) sequencer (paired-end reads: 50 and 25 bases in the forward and reverse orientation, respectively). Obtained sequences were aligned to the human genome (National Center for Biotechnology build 37/hg19) using the Lifescope suite from Life Technologies. Substitution and variation calls were made with the Genome Analysis Toolkit pipeline (mpileup, bfctools, vcfuitil). Variants were then annotated with an inhouse software (Polyweb), which allows users to set up bioinformatic filters in order to identify the putative mutation. Detailed filtering strategy is available in the Supplemental Material.
p.P209L Mutation Screening and Haplotype Analysis
PCR fragments covering TTC21B exon 6 were amplified using specific primers N12-ex6F-CTTAAAAAGTGATAACTGCTCC and N12-ex6R-AGTATTTCCTCGGTTCCA on a 2700 thermal cycler (Life Technologies). For detection of the p.P209L mutation, PCR products were incubated with 3 U of DdeI enzyme (Biolabs). After digestion, the analyses were completed by electrophoresis in a 2% agarose gel.
Patients with nephronophthisis were screened by applying exon-enriched next-generation sequencing of 1209 ciliary candidate genes, including TTC21B (“ciliome sequencing”).17 Exon 6 Sanger sequencing using the above primers was performed to validate the next-generation sequencing findings and the segregation of the mutation within all the families.
Extended haplotype analysis using eight microsatellite markers flanking the TTC21B locus was performed in the 10 families bearing the p.P209L mutation in the homozygous state. Three markers were specifically designed for this study in the vicinity of TTC21B and named according to their genomic position (CA-166771200: Fwd-GTATGCTTCTATGTTTACCCTT and Rev-CTGATGCCCTTATGAGTTA; CA-166845500: Fwd-TGTCAACGTGGGTATGCC and Rev-TTTTATTGTTTGCCCAGTCACT; CA-166888700: Fwd-TAATAGAGGCCATGAAAGGTAA and Rev-ATGCCAGGGAGACTAGATAAA). PCR products with fluorescent primers were separated by capillary electrophoresis and analyzed using GeneMapper analysis software (Life Technologies).
Plasmids, Cell Culture, and Establishment of Lentiviral Cell Lines
Human Flag-TTC21B clone was purchased from Origene (clone accession BC055424.1) and subcloned into the lentiviral pRRLSIN. cPPT.PGK/WPRE18 vector. The missense mutation p.P209L was created using the QuickChange site-directed mutagenesis kit according to the manufacturer protocol (Stratagene).
A small hairpin RNA construct targeting the 3′-untranslated region of the human TTC21B mRNA (sh: 5′-TCTGTGGTAAAGACTATAAT-3′) was cloned into the lentiviral pLKO.1 vector, and lentiviral particles were produced in HEK 293T cells as previously described.19 Stable human podocyte cell lines were obtained by transduction of the shIFT139 lentiviral particles, followed by puromycin selection (2 µg/ml). For rescue experiments, IFT139 depleted cells were transduced with the wild-type or mutant IFT139 constructs.
A conditionally immortalized human podocyte cell line was kindly provided by M.A. Saleem (University of Bristol, Southmead Hospital, Bristol, United Kingdom).11 Briefly, cells were grown at the permissive temperature (33°C) in RPMI 1640 medium supplemented with 10% fetal bovine serum, insulin-transferrin-selenium, glutamine, and penicillin/streptomycin (all from Life Technologies). To induce differentiation, cells were switched to the nonpermissive temperature (37°C) for 14 days. To induce ciliogenesis in undifferentiated podocytes, cells were serum-starved for 24 hours.
Protein Extraction and Western Blot
Isolated glomeruli from wild-type mice were obtained as previously described.20 Proteins from glomeruli, tubules, total kidney, or cultured podocytes were extracted in 50 mM Tris-HCl, 150 mM NaCl, 0.5% sodium deoxycholate, 2 mM EDTA, 1% Triton X-100, and 0.1% sodium dodecyl sulfate. Protein dosage was then performed using the BCA protein assay kit (Thermo Scientific). Fifty micrograms of proteins were loaded on a 4%–20% acrylamide gel (Bio-rad), and Western blot was conducted using the indicated antibodies.
Antibodies
Antibodies used for Western blot and immunostaining were goat anti–γ-tubulin and goat anti-synaptopodin (Santa Cruz), guinea pig anti-nephrin and mouse anti-synaptopodin (Progen), mouse anti–α-tubulin, mouse anti-acetylated α-tubulin, mouse anti-Flag M2, rhodamine phalloidin and rabbit anti-IFT139 (Sigma-Aldrich), rabbit anti-IFT139, rabbit anti-Smoothened (Abcam), rabbit anti-ARL13B (Proteintech), Rhodamin PNA lectin and fluorescein LTA lectin (Vector Laboratories), and rabbit anti-IFT46 (gift from F. Mallein-Gerin).
Immunofluorescence
Podocytes were plated on rat tail collagen type I (Corning) coated coverslips, fixed in cold 100% methanol or in 4% paraformaldehyde, and treated with 50 mM NH4Cl for paraformaldehyde-fixed cells. Cells were then incubated with blocking solution (PBS, 1% BSA, 0.1% Tween 20) and probed with proper primary antibodies followed with appropriate Alexa Fluor–conjugated secondary antibody (Life Technologies) incubation, and nuclei were stained using Hoechst stain (Life Technologies).
Paraffin-embedded sections were deparaffinized in Bioclear solution (Bio-Optica) and rehydrated in decreasing concentrations of ethanol baths. Antigen retrieval was achieved in citrate buffer (Dako) and microwave boiling. Blocking and antibody incubations were performed as described for cells. Confocal images were obtained using a Leica SP8 microscope, and post-treatment analysis was performed with the ImageJ software.
Cilia Length Measurements and Quantification of Ciliated Cells
For quantification of ciliated cells, 10 fields were randomly chosen to determine the number of ciliated and nonciliated cells. For each condition, cilia of 100 cells were measured from three different experiments using the ImageJ software. Statistical analyses were conducted as described below.
Nocodazole Assay
To assess microtubule repolymerization, cells were incubated with 30 μM nocodazole (Sigma-Aldrich) for 4 hours at 37°C, washed with PBS, let grown at 37°C, and fixed at different time points (0 minutes, 30 minutes, and 120 minutes). Immunofluorescence stainings were then conducted as described above.
Podocyte Migration
The xCELLigence real time cell analysis (RTCA) technology (Roche Applied Science) was used to perform real-time migration assay according to the manufacturer’s protocol. Briefly, 3×105 cells per well were plated in triplicates. Cells were allowed to grow until they reached the confluent state, scratches were performed using a 10-µl tip, and migration was monitored over a 10-hour period. Data obtained were processed using RTCA system software. Cell index values were normalized against the cell index values corresponding to the time point of the scratch. Each cell index value corresponds to the average of triplicates±SD. Migration velocity is represented by the slope of the curves over a 10-hour time frame from the scratch.
Statistical Analyses
Results are presented as mean±SEM or SD. Statistical analyses were performed by using a t test for two-group comparisons with GraphPad Prism software. P<0.05 was considered to represent a statistically significant difference. All experimentations were performed at least three times.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank M.A. Saleem for kindly providing us a conditionally immortalized human podocyte cell line. We greatly acknowledge the Necker cell imaging facility for providing expert knowledge on confocal microscopy. We thank A. Raia and G. Pivert (Pathology Department, Necker Hospital) for their excellent technical assistance. We are grateful to all the individuals with FSGS or nephronophthisis and their family members for their participation.
This work was supported by grants from the “Agence Nationale de la Recherche” (ANR) to C. Antignac (GenPod project: ANR-12-BSV1-0033.01) and to S.S. (ANR-09-GENO-022-01 and 2010-BLAN112202) and “Investments for the Future” program (ANR-10-IAHU-01) to C.A. and S.S., the “Fondation pour la Recherche Médicale” (FRM) to C.A. (project DMP 2010-11-20-386) and to S.S. (DEQ20071210558), the GIS Institut des maladies rares to C.A., the European Community’s 7th Framework program grant to C.A. (2012-305608 Eurenomics) and the Ministère de l'Education Nationale de la Recherche et de la Technologie (MRT) to E.H.C.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Dysfunction of Intraflagellar Transport Proteins beyond the Primary Cilium,” on pages 2385–2386.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101126/-/DCSupplemental.
References
- 1.D’Agati V: Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23: 117–134, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Machuca E, Benoit G, Antignac C: Genetics of nephrotic syndrome: Connecting molecular genetics to podocyte physiology. Hum Mol Genet 18[R2]: R185–R194, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Büscher AK, Weber S: Educational paper: The podocytopathies. Eur J Pediatr 171: 1151–1160, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, Muzny DM, Young AC, Wheeler DA, Cruz P, Morgan M, Lewis LR, Cherukuri P, Maskeri B, Hansen NF, Mullikin JC, Blakesley RW, Bouffard GG, Gyapay G, Rieger S, Tönshoff B, Kern I, Soliman NA, Neuhaus TJ, Swoboda KJ, Kayserili H, Gallagher TE, Lewis RA, Bergmann C, Otto EA, Saunier S, Scambler PJ, Beales PL, Gleeson JG, Maher ER, Attié-Bitach T, Dollfus H, Johnson CA, Green ED, Gibbs RA, Hildebrandt F, Pierce EA, Katsanis N, NISC Comparative Sequencing Program : TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 43: 189–196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas-Rodriguez M, Badano JL: Ciliary biology: Understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet 151C: 263–280, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Salomon R, Saunier S, Niaudet P: Nephronophthisis. Pediatr Nephrol 24: 2333–2344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taschner M, Bhogaraju S, Lorentzen E: Architecture and function of IFT complex proteins in ciliogenesis. Differentiation 83: S12–22, 2012 [DOI] [PMC free article] [PubMed]
- 8.Ishikawa H, Marshall WF: Ciliogenesis: Building the cell’s antenna. Nat Rev Mol Cell Biol 12: 222–234, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR: THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet 40: 403–410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichimura K, Kurihara H, Sakai T: Primary cilia disappear in rat podocytes during glomerular development. Cell Tissue Res 341: 197–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Liem KF, Jr, Ashe A, He M, Satir P, Moran J, Beier D, Wicking C, Anderson KV: The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol 197: 789–800, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, Cong EH, Arrondel C, Tête MJ, Montjean R, Richard L, Karras A, Pouteil-Noble C, Balafrej L, Bonnardeaux A, Canaud G, Charasse C, Dantal J, Deschenes G, Deteix P, Dubourg O, Petiot P, Pouthier D, Leguern E, Guiochon-Mantel A, Broutin I, Gubler MC, Saunier S, Ronco P, Vallat JM, Alonso MA, Antignac C, Mollet G: INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med 365: 2377–2388, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsh GI, Saleem MA: The podocyte cytoskeleton—key to a functioning glomerulus in health and disease. Nat Rev Nephrol 8: 14–21, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Abecasis GR, Cherny SS, Cookson WO, Cardon LR: Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30: 97–101, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY, McInerney-Leo AM, Krug P, Filhol E, Davis EE, Airik R, Czarnecki PG, Lehman AM, Trnka P, Nitschké P, Bole-Feysot C, Schueler M, Knebelmann B, Burtey S, Szabó AJ, Tory K, Leo PJ, Gardiner B, McKenzie FA, Zankl A, Brown MA, Hartley JL, Maher ER, Li C, Leroux MR, Scambler PJ, Zhan SH, Jones SJ, Kayserili H, Tuysuz B, Moorani KN, Constantinescu A, Krantz ID, Kaplan BS, Shah JV, Hurd TW, Doherty D, Katsanis N, Duncan EL, Otto EA, Beales PL, Mitchison HM, Saunier S, Hildebrandt F, UK10K Consortium : Defects in the IFT-B component IFT172 cause Jeune and Mainzer-Saldino syndromes in humans. Am J Hum Genet 93: 915–925, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D: Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 72: 9873–9880, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahuzier A, Gaudé H-M, Grampa V, Anselme I, Silbermann F, Leroux-Berger M, Delacour D, Ezan J, Montcouquiol M, Saunier S, Schneider-Maunoury S, Vesque C: Dishevelled stabilization by the ciliopathy protein Rpgrip1l is essential for planar cell polarity. J Cell Biol 198: 927–940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, Gubler MC, Antignac C, Esquivel EL: Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol 20: 2181–2189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.