Abstract
Young blacks receiving dialysis have an increased risk of death compared with whites in the United States. Factors influencing this disparity among the young adult dialysis population have not been well explored. Our study examined the relation of neighborhood socioeconomic status (SES) and racial differences in mortality in United States young adults receiving dialysis. We merged US Renal Data System patient-level data from 11,027 black and white patients ages 18–30 years old initiating dialysis between 2006 and 2009 with US Census data to obtain neighborhood poverty information for each patient. We defined low SES neighborhoods as those neighborhoods in US Census zip codes with ≥20% of residents living below the federal poverty level and quantified race differences in mortality risk by level of neighborhood SES. Among patients residing in low SES neighborhoods, blacks had greater mortality than whites after adjusting for baseline demographics, clinical characteristics, rurality, and access to care factors. This difference in mortality between blacks and whites was significantly attenuated in higher SES neighborhoods. In the United States, survival between young adult blacks and whites receiving dialysis differs by neighborhood SES. Additional studies are needed to identify modifiable factors contributing to the greater mortality among young adult black dialysis patients residing in low SES neighborhoods.
In the United States, the incidence of ESRD is 3.4 times higher in blacks compared with whites.1 The greater incidence of ESRD among blacks has been attributed to prevalent CKD risk factors (including hypertension,2,3 diabetes,4,5 and obesity6), genetic predisposition,7–9 low socioeconomic status (SES),10–12 and inequalities in the access and quality of kidney disease care.13,14 Despite the greater incidence of ESRD, numerous studies have shown that blacks experience paradoxically better survival on dialysis compared with whites.15–35 Although the reasons for this survival paradox are not well understood, proposed mechanisms include more favorable nutritional and/or inflammatory profiles,29 greater resilience to inflammation,36 and tolerance of lower dialysis dose.15 Others postulate that improved access to health care afforded by the US Centers for Medicare and Medicaid Services (CMS) ESRD insurance coverage program may confer a survival benefit, especially to poor black patients likely to have been uninsured before dialysis initiation.35
A recent study by Kucirka et al.37 challenged the robustness of this survival paradox by showing that the risk of death varied across age strata, with 18- to 30-year-old black dialysis patients having nearly a 2-fold increased risk of death compared with similarly aged whites.37 Reasons for disparate findings among younger (versus older) dialysis patients have been poorly explored. Although older adults with progressive CKD often have access to private and public (e.g., Medicare) forms of health insurance, young adults are more frequently of low SES and uninsured,34 and they may be particularly vulnerable to receiving poor predialysis health care.
Although income is one of the most commonly used metrics to determine an individual’s SES,38 area-based SES factors, such as neighborhood poverty, may capture contextual factors of importance beyond individual measures.39 Area-based SES measures have been shown to be reflective of SES inequalities in health.40 Low area-based SES has been shown to be associated with poor health outcomes41 and linked to poorer dialysis outcomes even in the absence of individual-level SES data.34,35 The association between neighborhood SES and race disparities in mortality among young adult dialysis patients has not been previously examined.
Results
Population Characteristics
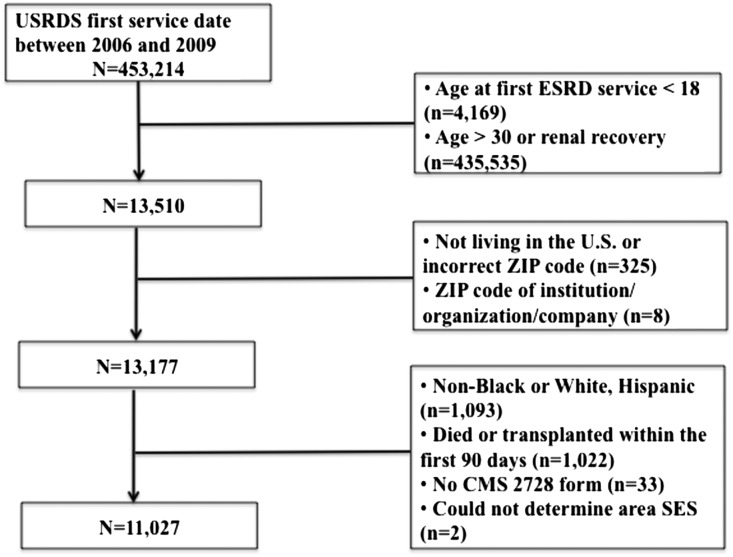
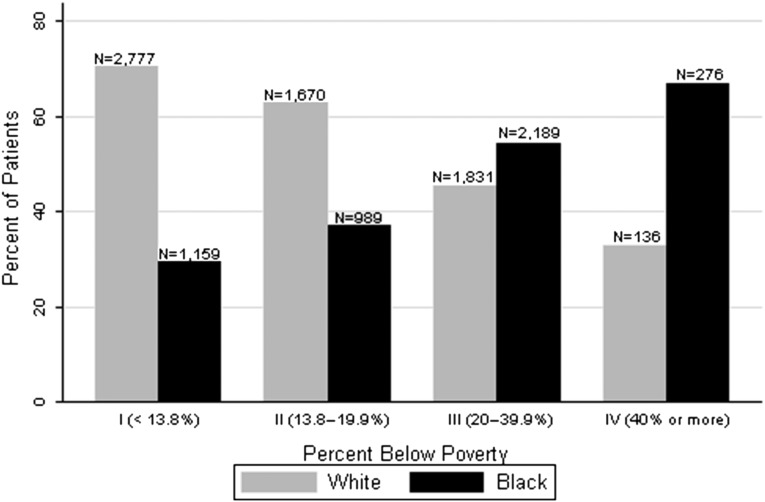
Our cohort of young adult incident ESRD patients was obtained from the US Renal Data System (USRDS). After exclusions (Figure 1), our cohort included 11,027 young adult ESRD patients. Patients’ mean (SD) age was 25.3 (±3.6) years, 42% were black, 56% were men, 22% had diabetes, and 79% had hypertension. Over 90% of young adults were on hemodialysis. Rurality was low, with the majority of patients classified as living in an urban area (82%). More than 50% of these young patients did not see a nephrologist before starting dialysis. Young blacks were nearly two times more likely to live in a category III poverty neighborhood (47% versus 28%, respectively) and three times more likely to live in a category IV severe poverty neighborhood (6% versus 2%, respectively) compared with young whites (Figure 2).
Figure 1.
Study flow chart. There were 453,214 incident dialysis patients from 2006–2009 in the USRDS. The study population included incident dialysis patients aged 18-30 years without renal recovery (or dialysis requiring AKI). Patients whose neighborhood poverty status could not be determined, race/ethnicity other than black or white, and those who died or received a transplant (censored) within the first 3 months after starting dialysis were excluded. After these exclusions, 11,027 patients were included in our analyses.
Figure 2.
Neighborhood poverty category and race. Proportion of young adult incident dialysis patients by race in each US Census Bureau poverty category: I includes neighborhoods with fewer than 13.8% residents living below poverty; II includes neighborhoods with 13.8% to 19.9% residents living below poverty; III includes neighborhoods with 20% to 39.9% residents living below poverty; and IV includes neighborhoods with 40% or more residents living below poverty. Category III and IV poverty neighborhoods were combined and categorized as low SES neighborhoods for the analyses.
Among patients classified as residing in low SES neighborhoods, young blacks were more likely to be women, reside in urban areas, and have higher body mass indexes (BMIs) compared with young whites. AIDS nephropathy was seen almost exclusively in young black patients, and young blacks were also more likely to have systemic lupus erythematosus or FSGS listed as a cause of ESRD compared with young whites. The prevalence of potential indicators of AKI (i.e., acute interstitial nephritis and tubular necrosis) as a cause of ESRD was low (1%) and similar between whites and blacks. Young blacks were more likely to have comorbidities, such as diabetes, hypertension, and congestive heart failure. The prevalence of tobacco use as well as drug and alcohol dependence was low and similar among the groups. More than 25% of young adults were uninsured at the time of dialysis initiation. Low SES young blacks were more likely than young whites to have Medicaid insurance and less likely to have private insurance. Most young adult patients in the low SES neighborhoods were not seen or it was unknown if they were seen by a nephrologist before dialysis initiation, and there was no difference by race. We observed similar trends among young blacks and young whites in the higher neighborhood SES group; however, the proportion of young blacks not seen (or unknown if seen) by a nephrologist was significantly greater compared with young whites (Table 1).
Table 1.
Baseline characteristics by neighborhood SES and race
| Characteristics | Low SESa (n=4432) | P Value | Higher SESa (n=6595) | P Value | ||
|---|---|---|---|---|---|---|
| Black (n=2465)b | White (n=1967) | Black (n=2148) | White (n=4447) | |||
| Mean age, yr±SD | 25.7±3.4 | 25.0±3.7 | <0.001 | 25.6±3.4 | 24.9±3.6 | <0.001 |
| Mean BMI±SD | 29.1±9.0 | 27.6±7.7 | <0.001 | 28.8±8.7 | 26.4±5 | <0.001 |
| Men | 1194 (48.4) | 1109 (56.4) | <0.001 | 1159 (54.0) | 2671 (60.1) | <0.001 |
| Hemodialysis | 2324 (94.3) | 1759 (89.4) | <0.001 | 2011 (93.2) | 3910 (88.0) | <0.001 |
| Urbanc | 2045 (83.0) | 1579 (80.5) | 0.03 | 1912 (89.6) | 3575 (80.8) | <0.001 |
| Primary cause of ESRD | <0.001 | <0.001 | ||||
| Diabetes mellitus | 549 (22.3) | 386 (19.6) | 435 (20.3) | 783 (17.6) | ||
| GNd | 767 (31.1) | 740 (37.6) | 782 (36.4) | 1817 (40.9) | ||
| AIDS-associated nephropathy | 169 (6.9) | 4 (0.2) | 95 (4.4) | 4 (0.1) | ||
| Other | 980 (39.7) | 837 (42.6) | 836 (38.9) | 1843 (41.4) | ||
| Comorbidities | ||||||
| Hypertension | 2050 (83.2) | 1495 (76.0) | <0.001 | 1801 (83.9) | 3314 (74.5) | <0.001 |
| Diabetes mellitus | 649 (26.3) | 414 (21.1) | <0.001 | 491 (22.9) | 853 (19.2) | 0.001 |
| Congestive heart failure | 267 (10.8) | 144 (7.3) | <0.001 | 205 (9.5) | 257 (5.8) | <0.001 |
| Atherosclerotic heart disease | 26 (1.6) | 39 (1.3) | 0.47 | 25 (1.3) | 59 (1.2) | 0.58 |
| Other cardiac disease | 130 (5.3) | 70 (3.6) | <0.01 | 106 (4.9) | 160 (3.6) | 0.01 |
| Cerebrovascular disease | 41 (1.7) | 25 (1.3) | 0.28 | 41 (1.9) | 52 (1.2) | 0.02 |
| Chronic obstructive pulmonary disease | 26 (1.0) | 14 (0.7) | 0.23 | 12 (0.6) | 29 (0.7) | 0.65 |
| Peripheral vascular disease | 52 (2.1) | 33 (1.7) | 0.30 | 41 (1.9) | 65 (1.5) | 0.18 |
| Malignancy | 19 (0.8) | 18 (0.9) | 0.60 | 13 (0.6) | 49 (1.1) | 0.05 |
| Alcohol dependence | 20 (0.9) | 17 (0.8) | 0.84 | 11 (0.5) | 21 (0.5) | 0.83 |
| Drug dependence | 78 (3.2) | 51 (2.6) | 0.26 | 54 (2.5) | 100 (2.3) | 0.50 |
| Tobacco use | 190 (7.7) | 129 (6.6) | 0.14 | 128 (6.0) | 314 (7.0) | 0.09 |
| Needs assistance for ADL | 115 (4.6) | 88 (4.5) | 0.76 | 74 (3.5) | 160 (3.6) | 0.75 |
| Insurance | ||||||
| Private | 406 (16.5) | 389 (19.8) | 0.004 | 555 (25.8) | 1381 (31.1) | <0.001 |
| Medicaid | 1162 (47.1) | 803 (40.8) | <0.001 | 808 (37.6) | 1494 (33.6) | <0.001 |
| No medical insurance | 723 (29.3) | 583 (29.6) | 0.82 | 599 (27.9) | 1090 (24.5) | 0.003 |
| Seen by a nephrologist pre-ESRD | 0.45 | <0.001 | ||||
| No | 1092 (44.3) | 903 (45.9) | 914 (42.5) | 1777 (39.9) | ||
| Unknown | 295 (12.0) | 241 (12.3) | 251 (11.7) | 406 (9.1) | ||
| Type of access (hemodialysis patients) | 0.04 | 0.001 | ||||
| Arteriovenous fistula | 151 (6.5) | 133 (7.6) | 137 (6.8) | 392 (10.0) | ||
| Arteriovenous graft | 57 (2.4) | 37 (1.3) | 37 (1.9) | 58 (1.5) | ||
| Catheter | 2096 (90.2) | 1585 (90.1) | 1811 (90.5) | 3443 (87.8) | ||
| Unknown | 20 (0.9) | 18 (1.0) | 16 (0.8) | 27 (0.7) | ||
ADL, activities of daily living.
Low neighborhood SES is a neighborhood with ≥20% below poverty. Higher neighborhood SES is a neighborhood with <20% living below poverty.
Data are shown as number (%) unless otherwise specified.
Patients with rural-urban communicating area information (n=10,986).
Systemic lupus erythematosus: low SES: blacks, 300 (39); whites, 143 (19); higher SES: blacks, 290 (37); whites, 298 (16); FSGS: low SES: blacks, 248 (32); whites, 165 (22); higher SES: blacks, 258 (33); whites, 326 (18).
Outcomes in Young Blacks and Young Whites by Level of Neighborhood SES
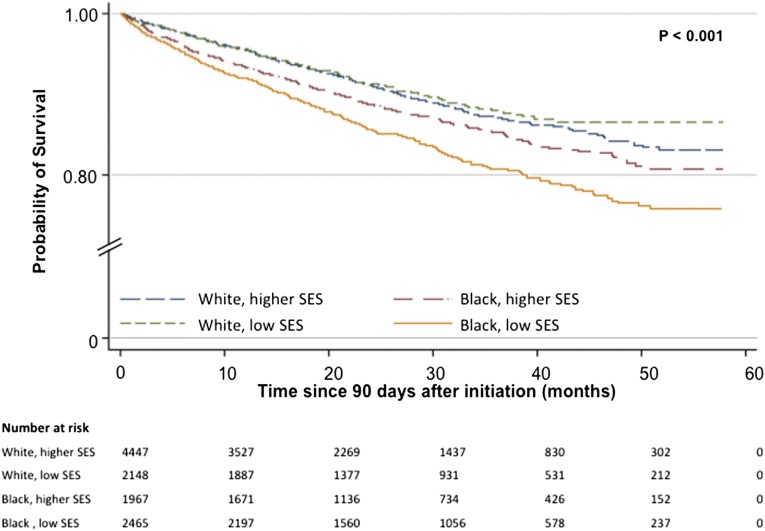
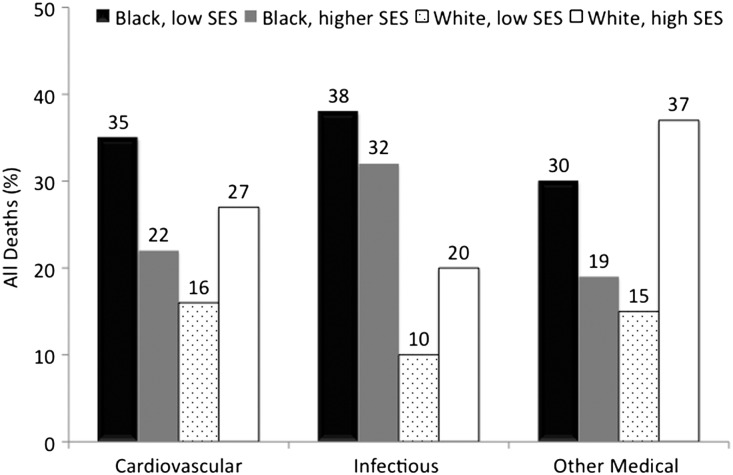
During a median follow-up of 23 months, 1242 young adult patients died (11%), and 2383 (22%) patients received a kidney transplant. Mortality was highest in low SES young blacks (16%) followed by higher SES young blacks (12%) and similar (9%) in the low and higher SES young whites (P<0.001) (Figure 3). Cardiovascular (29%) and infectious (12%) causes accounted for 41% of all deaths. Respiratory failure, diabetes complications, and chronic renal failure complications accounted for the majority of other medical deaths. There were no deaths attributable to accidents, suicides, homicides, or trauma among those patients with a known cause of death. Among patients who died from cardiovascular causes, blacks residing in low SES neighborhoods had the highest proportion of deaths (35%). Within deaths attributable to infectious causes, young adult black patients accounted for 70% of deaths (38% low SES blacks and 32% higher SES blacks). Whites living in higher SES neighborhoods had the largest proportion of deaths (37%) from other medical causes. Low SES whites were equally likely to die from cardiovascular (16%) and other medical causes (15%) (Figure 4). Of 28% of deaths with an unknown cause, 26% occurred in young blacks, and 30% occurred in young whites. Characteristics of those patients with a known cause of death were similar to the characteristics of patients with an unknown cause of death (Supplemental Table 1).
Figure 3.
Unadjusted Kaplan–Meier survival curves by race and neighborhood SES. Patients were censored at time of death or transplantation. Low SES, neighborhoods with ≥20% of residents living below poverty; higher SES, neighborhoods with <20% residents living below poverty.
Figure 4.
Cause of death by race and neighborhood SES. Absolute (not relative) proportions are shown. P value <0.05 for each death category. Missing cause of death: 30% whites and 26% blacks.
In the Cox model adjusted for age and sex, blacks had worse survival compared with whites, and this difference in survival differed by neighborhood SES (P<0.01 for interaction). Among patients residing in low SES neighborhoods, blacks had a 65% higher risk of death compared with whites (95% confidence interval [95% CI], 1.38 to 1.97) (Table 2, model 1). This risk was attenuated in the higher SES group, in which blacks had an 18% greater risk of death compared with similarly aged whites (95% CI, 1.01 to 1.38). Additional adjustments for BMI, baseline comorbidities, cause of ESRD, dialysis modality, rurality, and access to care factors showed that young adult blacks in low SES neighborhoods still had increased risk of death compared with low SES young adult whites (adjusted hazard ratio [aHR], 1.46; 95% CI, 1.21 to 1.74) (Table 2, model 4). Conversely, the relative hazard of death (aHR, 1.11; 95% CI, 0.94 to 1.30) was not statistically significant between blacks and whites in higher SES neighborhoods. The rates of transplantation differed by race and neighborhood SES: 10% in low SES young blacks, 15% in higher SES young blacks, 20% in low SES young whites, and 32% in higher SES young whites. The median time to transplantation was 26 and 21 months for young blacks and whites, respectively. Competing risk analyses accounting for these differential rates of transplantation showed similar findings among patients residing in low SES neighborhoods, with young adult blacks experiencing greater risk of death compared with similarly aged whites after adjusting for BMI, baseline comorbidities, rurality, and access to care factors (adjusted subhazard ratio [aSHR], 1.53; 95% CI, 1.28 to 1.85). However, among those patients living in higher SES neighborhoods, young blacks still had a modestly higher risk of death compared with whites (aSHR, 1.26; 95% CI, 1.07 to 1.48; P=0.10 for interaction) (Table 2, model 4).
Table 2.
Adjusted relative risk of death for blacks versus whites by level of neighborhood SES
| Model | Cox Models, HR (95% CI) | Competing Risk, SHR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Low SES (n=4432) | Higher SES (n=6595) | P Interaction | Low SES (n=4432) | Higher SES (n=6595) | P Interaction | |
| 1 | 1.65 (1.38 to 1.97) | 1.18 (1.01 to 1.38) | <0.001 | 1.79 (1.50 to 2.14) | 1.40 (1.17 to 1.60) | 0.03 |
| 2 | 1.44 (1.19 to 1.73) | 1.09 (0.93 to 1.28) | 0.02 | 1.51 (1.26 to 1.83) | 1.24 (1.06 to 1.45) | 0.10 |
| 3 | 1.45 (1.21 to 1.74) | 1.09 (0.93 to 1.28) | 0.02 | 1.53 (1.27 to 1.85) | 1.24 (1.05 to 1.46) | 0.09 |
| 4a | 1.46 (1.21 to 1.74) | 1.11 (0.94 to 1.30) | 0.03 | 1.53 (1.28 to 1.85) | 1.26 (1.07 to 1.48) | 0.10 |
Model 1, age+sex; model 2, model 1+BMI+primary cause of ESRD and baseline comorbidities; model 3, model 2+access to care factors.
Model 4, model 3+rurality (n=10,986).
Sensitivity Analyses
AIDS nephropathy primarily occurred in blacks, but excluding these patients did not significantly change our risk estimates. Young blacks still had a greater risk of death compared with their white counterparts in the low SES group (aHR, 1.42; 95% CI, 1.18 to 1.71). Among patients residing in higher SES neighborhoods, young blacks had similar survival to young whites (aHR, 1.13; 95% CI, 0.96 to 1.33). In the competing risk model, the mortality disparity between young adult blacks and whites persisted among patients residing in low SES neighborhoods (aSHR, 1.51; 95% CI, 1.24 to 1.82). This difference was attenuated but still statistically significant in the higher SES group (aSHR, 1.28; 95% CI, 1.08 to 1.51 for young blacks compared with young whites). The P for interaction was >0.05 in both models. Analyses excluding patients receiving peritoneal dialysis and adjusting for access type in the fully adjusted Cox proportional and competing risk models yielded similar findings.
Discussion
In this national study of 18- to 30-year-old incident United States ESRD patients, we found that mortality was greatest among low SES young blacks. Young blacks in both low and higher SES neighborhoods had greater mortality than young whites, regardless of their neighborhood SES. Young whites had similar mortality in higher and low SES neighborhoods. In the adjusted traditional Cox models accounting for patients’ baseline comorbidities, access to care, and rurality, young blacks living in low SES neighborhoods had approximately a 50% greater hazard of death compared with young whites living in low SES neighborhoods. In contrast, the relative hazard of death between young blacks and young whites living in higher SES neighborhoods was not statistically significant. Competing risk analyses accounting for racial disparities in transplantation showed an even greater risk of death among young blacks compared with young whites living in low SES neighborhoods as well as worse mortality among young blacks compared with young whites living in higher SES neighborhoods. The overall mortality rate for this young adult dialysis population was high, with 1 in 10 patients dying over a median follow-up of 2 years. Notably, the deaths attributable to infection were much greater among young blacks compared with young whites and also two times greater among young blacks compared with deaths from infection in a general dialysis cohort.42
Few studies have examined the association between race, SES, and mortality in ESRD. Earlier United States studies found an association between low SES and higher mortality and indicated that this relationship may be limited to blacks.43,44 However, recent large population-based studies have not shown a consistent association. Eisenstein et al.21 found no difference in survival by income level, and blacks maintained their survival advantage across all income groups.21 Rodriguez et al.34 also found no difference in survival for blacks living in zip codes where 75% or more of residents were black compared with zip codes where less than 10% of the residents were black. Kimmel et al.35 found that having a very low income was associated with worse survival for both blacks and whites; however, blacks maintained a survival advantage over whites. None of these studies evaluated race survival differences among younger adults. Our findings confirm the results by Kucirka et al.,37 showing that the race survival paradox is not present among young adults initiating dialysis; we extend them by showing the substantial and differential relation of neighborhood SES with race disparities in survival among young adults.
In the setting of dialysis, social and environmental determinants may be particularly influential on the health of young blacks. In our study, baseline access to care and quality of care measures (i.e., the provision of health insurance, initiation of dialysis with appropriate vascular access, and receipt of pre-ESRD care by a nephrologist) were generally poor and similar between blacks and whites of low and higher SES. Nonetheless, these pre-ESRD measures may still contribute to the disparate findings in mortality that we observed by race and level of neighborhood SES. Although the access to care may not differ among young adult patients residing in low SES neighborhoods, challenging psychosocial circumstances for low SES young blacks (such as experiences of social stigmatization and discrimination or poor health literacy) may lead to greater distrust in medical institutions,45 potentially resulting in their underuse of available medical resources and lower medical adherence.46 These factors may contribute to uncontrolled diabetes47 and HIV48 as well as greater rates of dialysis initiations with a catheter49 and fewer conversions to arteriovenous access50 among young blacks with low SES. These factors may underlie the greater risk of deaths attributable to infections compared with young whites. Our findings of better survival in higher SES young blacks compared with low SES young blacks would suggest that improved access to care, likely better health literacy, and potentially less social stigmatization and discrimination could at least partially attenuate some of the postulated effects of low SES on the health for young blacks.
Our findings also suggested that the effects of low SES on mortality were only present among young blacks, which is counter to some studies in the general population that show that low SES may result in worse health outcomes in both blacks and whites.51,52 However, our finding of a differential association of low SES in blacks compared with whites has been observed in other kidney disease studies.10,11,53 Our study, however, did not measure certain factors, including residential segregation,35 density of neighborhood poverty,54 minority-serving status of the dialysis facility,55 and individual-level factors, such as health literacy56 and adherence to dialysis treatment,57 which may influence mortality differentially among young blacks and whites. Furthermore, social stigmatization and discrimination may be more frequently experienced among low SES blacks compared with low SES whites and could also influence outcomes.
Our study has limitations. First, both low individual SES and low neighborhood SES may contribute to poor outcomes among young blacks compared with young whites. The USRDS registry does not include individual-level SES data, and we were, therefore, not able to fully explore the potential interactions between these SES indicators. However, prior epidemiologic studies provide rationale for the use of area-based measures to reflect SES in the absence of other socioeconomic data.11,35,58 Second, the lack of data available on AKI limited our ability to fully assess it as a contributor to our findings. The prevalence of potential indicators of AKI, specifically acute interstitial nephritis and tubular necrosis as primary causes of ESRD, however, was very low and therefore, unlikely to be a major contributor to our overall results. Furthermore, to avoid misclassification of dialysis-requiring AKI cases as ESRD cases, we excluded patients with recovery at any point during follow-up. Third, although we made a distinction between non-Hispanic and Hispanic individuals (with the recognition that Hispanics also have better survival on dialysis),29 there were participants in our study with unspecified ethnicity, especially among whites. We also could not account for unmeasured confounders that could impact racial differences in ESRD risk and survival (e.g., genetic susceptibility, patients’ attitudes and beliefs, ecological toxins, medication adherence, and medical treatment). As with all observational cohort studies, there is possibility of residual confounding, and causality is difficult to establish. Notwithstanding these limitations, our study is the first national study to examine the influence of neighborhood SES on race differences in mortality among young dialysis patients. We examined a contemporary cohort of incident patients over a fairly short timeframe, which likely limited the influence of secular trends in ESRD care. We were able to adjust for many important confounders in our analyses and accounted for racial disparities in renal transplantation in this young cohort of individuals with ESRD. Finally, our data linkage to the National Death Index (NDI) facilitated a robust descriptive analysis of cause-specific mortality that has not previously been possible.
In summary, the survival differences among young adult blacks compared with young adult whites are most striking in low SES neighborhoods. In addition to practices that improve transplantation rates, predialysis access to care, and predialysis health, additional studies are needed to identify modifiable factors contributing to the higher mortality, especially among young blacks on dialysis residing in low SES neighborhoods.
Concise Methods
Study Population and Design
We obtained our data from the USRDS, a national registry of ESRD patients funded by the National Institute of Diabetes and Digestive and Kidney Diseases in conjunction with the CMS. Patients who develop ESRD are included in this registry and assigned a unique identifier that is linked to Medicare and a Social Security Master Death file. Information on patients’ demographics, cause of ESRD, comorbidities, Medicare Part A institutional claims, Medicare Part B physician/supplier claims, and outcomes, such as hospitalizations and mortality, are captured in this database.1 In this study, we also linked USRDS data to NDI data from the Centers for Disease Control and Prevention.59
We conducted a retrospective cohort study that included all young adult non-Hispanic white and black patients ages 18–30 years who initiated dialysis and did not spontaneously recover renal function between January 1, 2006, and December 31, 2009. The patient time at risk was defined as day 91 after ESRD diagnosis until death, renal transplantation, or the end of the study (December 31, 2010). Patients who died or received a renal transplant within the first 90 days were excluded, which typical for most observational analyses of USRDS data on patients <65 years of age, because these patients are not Medicare-eligible within the first 90 days.35,60 We also excluded individuals without a United States residential zip code and individuals for whom socioeconomic information could not be obtained (Figure 1). Our study qualified for an exemption under the Code of Federal Regulations, Protection of Human Subjects (45 CFR 46.101[b]) by the Institutional Review Board at Johns Hopkins School of Medicine.
Data Sources and Study Variables
Patient Level
We derived patient-level characteristics from the CMS 2728 Medical Evidence Form—a federally mandated form that must be completed and signed by the supervising physician within 45 days of dialysis initiation. Our primary exposure was race (non-Hispanic white versus black). We determined covariates a priori based on their association with the primary exposure (race) and their possible association with the primary outcome (all-cause mortality) as well as to allow for comparison with similar population-based studies.37 We collected information on the following covariates at ESRD onset (baseline): age, sex, race and ethnicity, BMI, zip code, dialysis modality, insurance status and type, pre-ESRD nephrology referral, primary cause of ESRD, type of dialysis access, and presence or absence of comorbidities (hypertension, diabetes mellitus, atherosclerotic heart disease, congestive heart failure, other cardiac disease, cerebrovascular disease, chronic obstructive pulmonary disease, peripheral vascular disease, current tobacco use, alcohol dependence, drug dependence, and need for assistance with activities of daily living).
Zip Code Level
We merged patient-level USRDS zip code data with the US Census data to obtain community-level SES for each patient’s zip code of residence. We obtained zip code-level poverty information from the US Census Bureau American Community Survey (ACS) 5-year estimates from 2007 to 2011. The ACS is a nationwide survey with an annual sample size of approximately 3 million addresses across the United States and Puerto Rico, and it includes both housing units and group quarters (e.g., nursing facilities and prisons).61 Poverty status is determined by comparing annual income with a set of dollar values termed poverty thresholds that varied by family size, the number of children, and the age of the head of the household. The poverty thresholds are updated annually to account for changes in the cost of living using the Consumer Price Index. If the total household income is less than the threshold appropriate for the family, then every member of the family is considered to be living below the poverty level. For people not living in families, poverty status is determined by comparing the individual’s income with his or her poverty threshold. For comparison with the US Census Bureau literature, we categorized zip codes into four categories: I (<13.8% living below poverty), II (13.8%–19.9% living below poverty), III (20%–39.9% living below poverty), and IV (40% or more living below poverty).62 We defined low neighborhood SES as a zip code with 20% or more of the residents living below the federal poverty level, consistent with the federal definition.62 To determine rurality, we used the rural–urban commuting area (RUCA) code version 2.0.63
Ascertainment of the Outcomes
The primary outcome was all-cause mortality. We determined the date of death from the USRDS data, and we determined the cause of death through linked NDI International Classification of Disease (ICD-10-CM) codes. We classified causes of death into cardiovascular, infection, or other as follows: (1) cardiovascular: I05–I15, I20–I25.9, I33–I37, I42–I51, I60–I79, I98–I99, K55, and R02; (2) infectious: A00–B99, E06.0, G00–G08, I00–I01, I33, I38, J00–J06, J10–J18, J20–J22, J36, J39.0, J39.1, J44.0, J85–J86, K35, K63.0, K65.0, K65.9, K80.0, K80.3, K80.4, K81, K83.0, L00–L08, M00–M02, M86, N30.0, N30.8, N41.0, N41.2, N41.3, O03.0, O03.5, O04.5, O07.0, O08.0, O75.3, O85–O86, R57.2, T80.2, T81.4, T82.6–T82.7, T83.5–T83.6, T84.5–T84.7, T85.7, T87.4; and T88.0; and (3) other (all other ICD-10 codes).
Statistical Analyses
Descriptive Data Analyses
We described patients’ characteristics at dialysis initiation stratified by neighborhood SES and race. We calculated means and SDs for age and BMI and performed t tests (for continuous variables) and chi-squared tests (for categorical variables) to compare distributions between the groups. We generated Kaplan–Meier curves by race and SES status.
Cox Proportional Hazards and Competing Risk Models
We quantified the racial differences in mortality between patients living in low SES and higher SES neighborhoods using multivariable Cox proportional hazards models (estimating aHRs), which adjusted for patients’ baseline demographics, clinical characteristics, access to care factors, and rurality. We estimated differences in the association of race and mortality according to neighborhood SES using interaction terms. We also performed parallel competing risk regressions (estimating aSHRs) according to the methods of Fine and Gray64 to assess the influence of differential censoring secondary to lower transplantation rates for blacks compared with whites.37 We adjusted for the presence of AIDS nephropathy in all our primary models. Although predominantly observed in blacks, the overall prevalence was low (2.5%), and, therefore, our assumption was that excluding patients with AIDS nephropathy would not significantly change our risk estimates and may decrease the power of our study to detect interaction. To test this assumption, we performed a sensitivity analysis excluding patients with a diagnosis of AIDS nephropathy. We also limited our analyses to hemodialysis patients and adjusted for patients’ hemodialysis access types to explore the influence of dialysis modality and access type (i.e., catheter versus graft or fistula for hemodialysis).
Missing or Unknown Covariates Information
The number of patients missing a cause of death was similar in both the Social Security Master Death file (30%) and NDI linked file (28%). Information on BMI was missing in 230 (2%) patients and imputed using Rubin’s multiple technique (n=5 iterations).65 For categorical covariates in which information was classified as unknown, we created a separate category for these patients and included them in our model. We excluded patients with indeterminate RUCA information (n=41 or 0.4%) from the statistical analysis.
Model Testing and Statistical Significance
We assessed the proportional hazards assumption both graphically and statistically using Schoenfeld residuals.66 We defined statistical significance as P<0.05 using two-tailed tests. We performed all analyses using multiprocessor Stata version 11.0/MP (StataCorp, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
T.J. was supported by National Institute for Diabetes, Digestive, and Kidney Diseases Grant T32-DK007732. M.M.E. was supported by National Institute for Diabetes, Digestive, and Kidney Diseases Grant K23-DK081317. D.C.C. was supported by the Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation.
T.J. and L.E.B. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111207/-/DCSupplemental.
References
- 1.US Renal Data System: USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, 2012. Available at: http://www.usrds.org/atlas.aspx. Accessed February 10, 2013
- 2.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ: Prevalence of high blood pressure and elevated serum creatinine level in the United States: Findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 161: 1207–1216, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Peralta CA, Lin F, Shlipak MG, Siscovick D, Lewis C, Jacobs DR, Jr., Bibbins-Domingo K: Race differences in prevalence of chronic kidney disease among young adults using creatinine-based glomerular filtration rate-estimating equations. Nephrol Dial Transplant 25: 3934–3939, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whaley-Connell AT, Sowers JR, McFarlane SI, Norris KC, Chen SC, Li S, Qiu Y, Wang C, Stevens LA, Vassalotti JA, Collins AJ, Kidney Early Evaluation Program Investigators : Diabetes mellitus in CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition and Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 51[4 Suppl 2]: S21–S29, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ: The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA 268: 3079–3084, 1992 [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kit BK, Ogden CL: Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, Rajasekaran A, Tzur S, Racusen LC, Skorecki K: APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol 23: 343–350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BI, Murea M: Target organ damage in African American hypertension: Role of APOL1. Curr Hypertens Rep 14: 21–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR: Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis 55: 992–1000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R: Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol 19: 356–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Yan G, Cheung AK, Ma JZ, Yu AJ, Greene T, Oliver MN, Yu W, Norris KC: The associations between race and geographic area and quality-of-care indicators in patients approaching ESRD. Clin J Am Soc Nephrol 8: 610–618, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powe NR, Melamed ML: Racial disparities in the optimal delivery of chronic kidney disease care. Med Clin North Am 89: 475–488, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Owen WF, Jr., Chertow GM, Lazarus JM, Lowrie EG: Dose of hemodialysis and survival: Differences by race and sex. JAMA 280: 1764–1768, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Noori N, Kovesdy CP, Dukkipati R, Feroze U, Molnar MZ, Bross R, Nissenson AR, Kopple JD, Norris KC, Kalantar-Zadeh K: Racial and ethnic differences in mortality of hemodialysis patients: Role of dietary and nutritional status and inflammation. Am J Nephrol 33: 157–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O’Hare AM: White/black racial differences in risk of end-stage renal disease and death. Am J Med 122: 672–678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agodoa L, Eggers P: Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin Dial 20: 577–585, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Bleyer AJ, Tell GS, Evans GW, Ettinger WH, Jr., Burkart JM: Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis 28: 72–81, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Bleyer AJ: Race and dialysis survival. Arch Intern Med 152: 879–880, 1992 [PubMed] [Google Scholar]
- 21.Eisenstein EL, Sun JL, Anstrom KJ, Stafford JA, Szczech LA, Muhlbaier LH, Mark DB: Do income level and race influence survival in patients receiving hemodialysis? Am J Med 122: 170–180, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: Causes of death in dialysis patients: Racial and gender differences. J Am Soc Nephrol 5: 1231–1242, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Buckalew VM, Jr., Freedman BI: Reappraisal of the impact of race on survival in patients on dialysis. Am J Kidney Dis 55: 1102–1110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris D, Samore MH, Pappas LM, Ramkumar N, Beddhu S: Nutrition and racial differences in cardiovascular events and survival in elderly dialysis patients. Am J Med 118: 671–675, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Pugh JA, Tuley MR, Basu S: Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: The emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis 23: 803–807, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Mesler DE, McCarthy EP, Byrne-Logan S, Ash AS, Moskowitz MA: Does the survival advantage of nonwhite dialysis patients persist after case mix adjustment? Am J Med 106: 300–306, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Pei YP, Greenwood CM, Chery AL, Wu GG: Racial differences in survival of patients on dialysis. Kidney Int 58: 1293–1299, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Tanna MM, Vonesh EF, Korbet SM: Patient survival among incident peritoneal dialysis and hemodialysis patients in an urban setting. Am J Kidney Dis 36: 1175–1182, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Streja E, Kovesdy CP, Molnar MZ, Norris KC, Greenland S, Nissenson AR, Kopple JD, Kalantar-Zadeh K: Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis 57: 883–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowie CC, Port FK, Rust KF, Harris MI: Differences in survival between black and white patients with diabetic end-stage renal disease. Diabetes Care 17: 681–687, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Alves TP, Wang X, Wright JT, Jr., Appel LJ, Greene T, Norris K, Lewis J, AASK Collaborative Research Group : Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol 21: 1361–1369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Kovesdy CP, Norris KC: Racial survival paradox of dialysis patients: Robust and resilient. Am J Kidney Dis 60: 182–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricks J, Molnar MZ, Kovesdy CP, Kopple JD, Norris KC, Mehrotra R, Nissenson AR, Arah OA, Greenland S, Kalantar-Zadeh K: Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis 58: 574–582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM: Geography matters: Relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med 146: 493–501, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Kimmel PL, Fwu CW, Eggers PW: Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol 24: 293–301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crews DC, Sozio SM, Liu Y, Coresh J, Powe NR: Inflammation and the paradox of racial differences in dialysis survival. J Am Soc Nephrol 22: 2279–2286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daly MC, Duncan GJ, McDonough P, Williams DR: Optimal indicators of socioeconomic status for health research. Am J Public Health 92: 1151–1157, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aliprantis D, Zenker M: Concentrated Poverty, 2011. Available at: http://www.clevelandfed.org/research/commentary/2011/2011-26.pdf. Accessed March 15, 2013
- 40.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N: Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: A multilevel analysis of Massachusetts births, 1989–1991. Am J Epidemiol 164: 823–834, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Meijer M, Röhl J, Bloomfield K, Grittner U: Do neighborhoods affect individual mortality? A systematic review and meta-analysis of multilevel studies. Soc Sci Med 74: 1204–1212, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Port FK, Wolfe RA, Levin NW, Guire KE, Ferguson CW: Income and survival in chronic dialysis patients. Trans Am Soc Artif Intern Organs 36: M154–M157, 1990 [PubMed] [Google Scholar]
- 44.Garg PP, Diener-West M, Powe NR: Income-based disparities in outcomes for patients with chronic kidney disease. Semin Nephrol 21: 377–385, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Armstrong K, McMurphy S, Dean LT, Micco E, Putt M, Halbert CH, Schwartz JS, Sankar P, Pyeritz RE, Bernhardt B, Shea JA: Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med 23: 827–833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shoff C, Yang TC: Untangling the associations among distrust, race, and neighborhood social environment: A social disorganization perspective. Soc Sci Med 74: 1342–1352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonds DE, Zaccaro DJ, Karter AJ, Selby JV, Saad M, Goff DC, Jr.: Ethnic and racial differences in diabetes care: The Insulin Resistance Atherosclerosis Study. Diabetes Care 26: 1040–1046, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, Hellinger J, Keiser P, Rubin HR, Crane L, Hellinger FJ, Mathews WC, HIV Research Network : Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr 38: 96–103, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Wasse H, Speckman RA, Frankenfield DL, Rocco MV, McClellan WM: Predictors of central venous catheter use at the initiation of hemodialysis. Semin Dial 21: 346–351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasse H, Speckman RA, Frankenfield DL, Rocco MV, McClellan WM: Predictors of delayed transition from central venous catheter use to permanent vascular access among ESRD patients. Am J Kidney Dis 49: 276–283, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose KM, Foraker RE, Heiss G, Rosamond WD, Suchindran CM, Whitsel EA: Neighborhood socioeconomic and racial disparities in angiography and coronary revascularization: The ARIC surveillance study. Ann Epidemiol 22: 623–629, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saydah S, Lochner K: Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep 125: 377–388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young EW, Mauger EA, Jiang KH, Port FK, Wolfe RA: Socioeconomic status and end-stage renal disease in the United States. Kidney Int 45: 907–911, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Holt JB: The topography of poverty in the United States: A spatial analysis using county-level data from the Community Health Status Indicators project. Prev Chronic Dis 4: A111, 2007 [PMC free article] [PubMed] [Google Scholar]
- 55.Hall YN, Xu P, Chertow GM, Himmelfarb J: Characteristics and performance of minority-serving dialysis facilities [published online ahead of print December 20, 2013]. Health Serv Res 10.1111/1475-6773.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavanaugh KL, Wingard RL, Hakim RM, Eden S, Shintani A, Wallston KA, Huizinga MM, Elasy TA, Rothman RL, Ikizler TA: Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol 21: 1979–1985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saran R, Bragg-Gresham JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, Kurokawa K, Piera L, Saito A, Fukuhara S, Young EW, Held PJ, Port FK: Nonadherence in hemodialysis: Associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int 64: 254–262, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Krieger N: Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health 82: 703–710, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cowper DC, Kubal JD, Maynard C, Hynes DM: A primer and comparative review of major US mortality databases. Ann Epidemiol 12: 462–468, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Wetmore JB, Rigler SK, Mahnken JD, Mukhopadhyay P, Shireman TI: Considering health insurance: How do dialysis initiates with Medicaid coverage differ from persons without Medicaid coverage? Nephrol Dial Transplant 25: 198–205, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.US Census Bureau : American Community Survey and Puerto Rico Community Survey, Suitland, MD, US Census Bureau, 2011 [Google Scholar]
- 62.US Census Bureau: Areas with Concentrated Poverty: 2006–2010, 2011. Available at: http://www.census.gov/prod/2011pubs/acsbr10-17.pdf. Accessed February 10, 2013
- 63.ZIP Code RUCA Approximation Methodology: WWMAI Rural Health Research Center, 2006. Available at: http://depts.washington.edu/uwruca/index.php. Accessed March 15, 2013
- 64.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 494–509, 1999 [Google Scholar]
- 65.Rubin DB: Multiple Imputations for Nonresponse in Surveys, New York, J. Wiley & Sons, 1987 [Google Scholar]
- 66.Schoenfeld D: The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika 68: 316–319, 1981 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.