Abstract
Endothelial dysfunction begins in early CKD and contributes to cardiovascular mortality. HDL is considered antiatherogenic, but may have adverse vascular effects in cardiovascular disease, diabetes, and inflammatory conditions. The effect of renal failure on HDL properties is unknown. We studied the endothelial effects of HDL isolated from 82 children with CKD stages 2–5 (HDLCKD), who were free of underlying inflammatory diseases, diabetes, or active infections. Compared with HDL from healthy children, HDLCKD strongly inhibited nitric oxide production, promoted superoxide production, and increased vascular cell adhesion molecule-1 expression in human aortic endothelial cells, and reduced cholesterol efflux from macrophages. The effects on endothelial cells correlated with CKD grade, with the most profound changes induced by HDL from patients on dialysis, and partial recovery observed with HDL isolated after kidney transplantation. Furthermore, the in vitro effects on endothelial cells associated with increased aortic pulse wave velocity, carotid intima-media thickness, and circulating markers of endothelial dysfunction in patients. Symmetric dimethylarginine levels were increased in serum and fractions of HDL from children with CKD. In a longitudinal follow-up of eight children undergoing kidney transplantation, HDL-induced production of endothelial nitric oxide, superoxide, and vascular cell adhesion molecule-1 in vitro improved significantly at 3 months after transplantation, but did not reach normal levels. These results suggest that in children with CKD without concomitant disease affecting HDL function, HDL dysfunction begins in early CKD, progressing as renal function declines, and is partially reversed after kidney transplantation.
Patients with CKD no longer die from renal failure but from cardiovascular disease. There is an independent, graded association between a reduced eGFR and the risk of death and cardiovascular events.1 Typically, patients with CKD develop calcification in the tunica media of their arteries,2 but a concomitant process of endothelial damage leading to atherosclerosis is also3 present beginning in predialysis CKD.4,5
LDL is crucially involved in the pathogenesis of atherosclerotic cardiovascular disease in the general population,6 whereas HDL is thought to be antiatherogenic by promoting reverse cholesterol transport and exerting direct protective endothelial effects.7 HDL from healthy participants increases the bioavailability of nitric oxide (NO) by activating endothelial NO synthase inducing vasodilation and decreasing arterial BP. Moreover, HDL diminishes the production of reactive oxygen species such as superoxide (SO) radicals, which have been demonstrated to reduce NO bioavailability leading to endothelial dysfunction and promoting atherogenesis. However, recent evidence suggests that HDL may lose its vasoprotective properties in patients with manifest cardiovascular disease (e.g., coronary artery disease), diabetes, or inflammatory disease states (e.g., antiphospholipid syndrome).8–10 Similarly, in adults on dialysis, HDL has reduced cholesterol efflux capacity and proinflammatory effects on mononuclear cells.11–13 Observational studies have shown a strong association between high HDL levels and reduced risk of cardiovascular disease in the general population14 but not in dialysis patients.15
Cardiac and vascular damage has also been documented in children on dialysis,2,16,17 and cardiovascular disease accounts for the majority of deaths in pediatric dialysis patients.17 In contrast with adult patients with CKD, in whom cardiovascular risk factors such as diabetes dyslipidemia, hypertension, and smoking are highly prevalent,18 CKD in children is mainly caused by inherited disorders such as malformations of the kidney or urinary tract.18 Accordingly, examining HDL function in children who are free of “traditional” cardiovascular risk factors and underlying inflammatory diseases and who are nonsmokers gives us an unique opportunity to study the effects of renal failure on the vascular functions of HDL.
We studied the endothelial properties of HDL in a cohort of children at different stages of CKD on dialysis and after transplantation and compared them with healthy children. Furthermore, to determine the clinical relevance of in vitro effects of HDL, we examined its relationship with clinical measures of the vascular phenotype as well as circulating markers of endothelial dysfunction. Finally, to show a causal link between renal function and HDL properties, we examined children on dialysis and 3 months after kidney transplantation. This study allowed us to examine when HDL dysfunction develops during the natural history of renal decline, its effects on vascular function, and the potential for recovery after kidney transplantation.
Results
Patient Characteristics
We studied 82 children at different stages of CKD who were free of underlying inflammatory diseases or diabetes and were nonsmokers. Patients were compared with 12 healthy age- and sex-matched controls. Table 1 describes clinical characteristics of the study population. There was no difference in age, sex, or race between patients and controls, but the patients had a higher systolic BP SD score and higher serum phosphate levels. Within patient groups, renal transplant recipients had a higher body mass index SD score, systolic BP SD score, and triglyceride levels compared with CKD and dialysis groups (P=0.03, P=0.02, and P=0.01, respectively). To confirm that the vascular involvement in children with CKD is a noninflammatory process, arterial biopsy samples obtained from children in predialysis CKD stage 5 and on dialysis2,19 confirmed an absence of macrophages in both the tunica intima and media (Supplemental Figure 1). IL-6 levels were within the normal range in predialysis patients with stages 4 and 5 CKD (P=0.80) but were increased in children on dialysis (P=0.01; Table 1).
Table 1.
Baseline characteristics of the study cohort
| Characteristic | Controls (n=12) | Stage 2 CKD (n=10) | Stage 3 CKD (n=13) | Stages 4 and 5 CKD (n=16) | Dialysis (n=20) | Transplant (n=23) | P Value |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Age (yr) | 13.3±3.7 | 12.7±2.1 | 11.3±3.3 | 12.4±0.9 | 14.2±3.9 | 14.4±4.0 | 0.72 |
| Men | 6 (50) | 7 (70) | 7 (54) | 9 (56) | 13 (65) | 15 (65) | 0.88 |
| Ethnic origin | — | ||||||
| White | 7 | 6 | 8 | 11 | 12 | 15 | |
| Asian | 4 | 3 | 4 | 4 | 6 | 6 | |
| Black | 1 | 0 | 1 | 0 | 1 | 1 | |
| Other | 0 | 1 | 0 | 1 | 1 | 1 | |
| Underlying renal disease | — | 0.82 | |||||
| Hypodysplasia | 7 | 8 | 10 | 13 | 14 | ||
| Glomerulopathies | 2 | 5 | 4 | 6 | 6 | ||
| Other | 1 | 0 | 2 | 1 | 3 | ||
| Clinical | |||||||
| eGFR (ml/min per 1.73 m2) | 103±4.8 | 68±6.1 | 41±10.7 | 14±8.7 | — | 47±14.2 (34–65) | <0.001 |
| Duration of CKD, yr (eGFR<60 ml/min/1.73 m2) | — | — | 6.8±4.1 | 6.2±2.2 | 7.9±5.1 | 6.2±4.6 | 0.74 |
| Type of dialysis | — | — | — | — | PD, 11; HD, 9 | — | — |
| BMI SD scorea | 1.2±0.3 | 0.9±0.2 | 1.1±0.1 | 0.9±0.4 | 0.23±0.6 (−0.2 to 0.8) | 1.3±0.6 | 0.06b |
| Systolic BP SD scorea | 0.7±0.3 | 0.8±0.1 | 0.8±0.4 | 1.1±0.2 | 1.2±0.5 | 1.6±0.2 | 0.02b |
| Diastolic BP SD scorea | 0.4±0.1 | 0.8±0.2 | 1.3±0.1 | 0.8±0.4 | 1.0±0.4 | 1.4±0.4 | 0.07 |
| Children taking antihypertensive agents | 0 | 0 | 1 | 2 | 1 | 9 (median 2 drugs per patient) | <0.001 |
| Antihypertensive agents | — | — | — | ||||
| ACE inhibitor | 1 | 2 | — | 2 | |||
| Calcium channel blocker | — | — | 1 | 7 | |||
| Diuretic | — | — | — | 6 | |||
| Biochemical | |||||||
| Total cholesterol (mg/dl) | 187±13 | 182±21 | 188±22 | 202±42 | 190±29 | 211±34 | 0.51 |
| HDL cholesterol (mg/dl) | 44±7.1 | 45±5.9 | 41±9.2 | 43±8.2 | 41±11.2 (37.8–46) | 44.0±9.5 (39–47) | 0.29 |
| LDL cholesterol (mg/dl) | 131±13 | 127±18 | 145±24 | 151±39 | 151±50 | 144±23 | 0.12 |
| Triglycerides (mg/dl) | 91±11 | 93±17 | 90±19 | 127±32 | 119±40 | 140±65 | 0.08b |
| Phosphate (mmol/L) | 1.5±0.1 | 1.6±0.4 | 1.4±0.6 | 1.6±0.3 | 1.4±0.7 | 1.1±0.3 | 0.04 |
| High-sensitivity CRP (mg/L) | 2.4±0.4 | 1.3±0.2 | 1.6±0.8 | 2.2±1.0 (0.3–2.7) | 2.9±6.8 (0.7–22.8) | 1.7±1.3 | 0.07 |
| IL-6 (pg/ml) | 6.2±3.9 (median 4.4; 0–9.3) | — | — | 7.1±3.1 (median 4.7; 0–9.1) | 11.9±8.4 (median 8.9; 3–31.5) | — | 0.01c |
All data are presented as mean±SD, n, n (%), or median (interquartile range). P values are for comparison across all groups and are obtained from ANOVA or Kruskal–Wallis tests. PD, peritoneal dialysis; HD, hemodialysis; BMI, body mass index; ACE, angiotensin-converting enzyme; CRP, C-reactive protein.
Statistical analyses have not been performed on BMI, systolic BP, and diastolic BP values because there is very high variability in normal levels across the pediatric age range. SD score values provide a more accurate estimate.
P values between transplant patients and the CKD plus dialysis groups: for BMI SD score, P=0.03; for systolic BP SD score, P=0.002; and for triglyceride levels SD score, P=0.01.
P values between stages 4 and 5 CKD and dialysis groups.
Importantly, total cholesterol, HDL cholesterol, and LDL cholesterol levels were not significantly different between the patients and controls or between patient groups. Only one child with stage 5 CKD and one child on dialysis were receiving statin therapy (pravastatin for both); their clinical, biochemical, in vitro studies and vascular measures did not differ from the overall cohort.
HDL Functionality Is Substantially Impaired in Children with CKD
Human aortic endothelial cells (HAECs) were incubated with HDL isolated from children with CKD (HDLCKD) and from healthy children (HDLHealthy) to measure endothelial properties of HDL. HDLHealthy increased endothelial NO production, but HDLCKD substantially inhibited endothelial NO production (Figure 1A). HDLCKD promoted basal endothelial production of SO radicals (Figure 1B), which are known to reduce NO bioavailability and to promote vascular damage. To analyze the anti-inflammatory properties of HDL, we examined the expression of the vascular cell adhesion molecule-1 (VCAM-1) on the surface of TNF-α–treated HAECs (Figure 1C). We found that HDLCKD increased endothelial VCAM-1 expression, whereas HDLHealthy could reduce TNF-α–mediated VCAM-1 expression. Furthermore, we assessed the cholesterol efflux from macrophages to apoB-depleted serum (i.e., serum without LDL) as a measure for the reverse cholesterol transport capacity of HDL. Notably, compared with healthy children, cholesterol efflux capacity was significantly reduced when apoB-depleted serum from children with CKD was used (Figure 1D).
Figure 1.
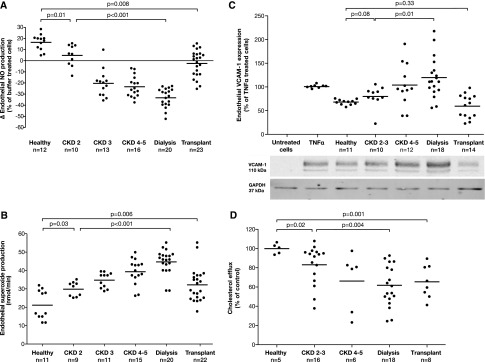
Endothelial effects of HDL in healthy controls and children with CKD stages 2–5, on dialysis and after transplantation. (A) Endothelial NO production (measured by ESR spectroscopy) in HAECs incubated with HDL (50 µg/ml, 60 minutes). (B) Endothelial SO production (measured by ESR spectroscopy) in HAECs treated with HDL (50 µg/ml, 60 minutes). (C) Endothelial VCAM-1 expression (determined by Western blot analysis and normalized to expression of GAPDH) in HAECs preincubated with HDL (50 µg/ml, 60 minutes) and stimulated with TNF-α (0.1 ng/ml, 4 hours). (D) Cholesterol efflux from cholesterol-loaded J774 macrophages to apoB-depleted serum. Bar shows mean level in each group. Some data have similar values and therefore the dots may not equal the sample size. ESR, electron spin resonance; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
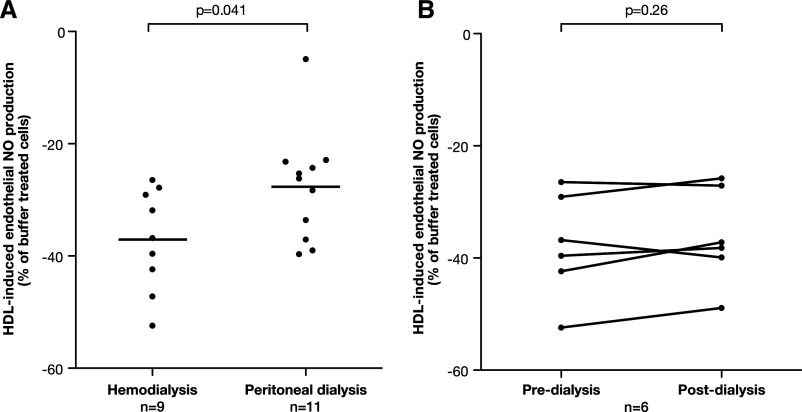
There was a graded change in HDL-induced endothelial NO and SO production from CKD stages 2–5, with the most profound changes in dialysis patients and only partial recovery after transplantation (Figure 1, A and B). For each 10-ml/min per 1.73 m2 reduction in eGFR, HDLCKD reduced NO by 13.2% and increased SO by 8.2 nmol/min (Supplemental Figure 2). HDL from children with even mildly reduced kidney function (stage 2 CKD) substantially inhibited endothelial NO release (P=0.01) and increased SO production (P=0.03) compared with healthy controls (Figure 1, A and B). The number of years that a patient spent on dialysis was not associated with any aspect of his or her HDL function. HDL-induced NO production was lower in children on hemodialysis compared with children receiving peritoneal dialysis (P=0.04; Figure 2A). However, when paired samples were taken before and immediately after a hemodialysis session, there was no change in NO production (P=0.25; Figure 2B). In renal transplant recipients, HDLCKD function was improved compared with patients with CKD stages 2–5 and patients on dialysis but remained lower than levels seen in healthy controls (Figure 1). There was no association between the eGFR after transplantation and HDLCKD-induced NO or SO production (P=0.41 and P=0.55, respectively).
Figure 2.
Association between HDL functionality, eGFR, and different treatment regimens. (A) Endothelial NO production in HAECs treated with HDL (50 µg/ml, 1 hour) from children on hemodialysis and peritoneal dialysis. (B) Endothelial NO production in HAECs incubated with HDL (50 µg/ml, 1 hour) obtained from children before and immediately after hemodialysis. Bar shows mean level in each group.
Importantly, there was no association between the serum levels of total cholesterol, HDL cholesterol, LDL cholesterol, or triglycerides and any of the endothelial effects of HDL.
HDL Functionality Is Associated with Circulating Markers of Vascular Dysfunction
In patients with stages 4 and 5 CKD and patients on dialysis, the in vitro measures of HDL functionality were compared with circulating markers of vascular dysfunction including serum urate, angiopoietin-2, and the proinflammatory cytokine IL-6, which were previously associated with cardiovascular disease.5 Increasing serum urate and angiopoietin-2 were significantly associated with reduced NO production by HDLCKD (Figure 3, A and B). IL-6 levels were within the normal range in patients with stages 4 and 5 CKD (P=0.80) but increased in patients on dialysis (P=0.01; Table 1). IL-6 significantly correlated with HDL-induced SO production (Figure 3C). There was no correlation with endothelial VCAM-1 expression (P=0.60).
Figure 3.
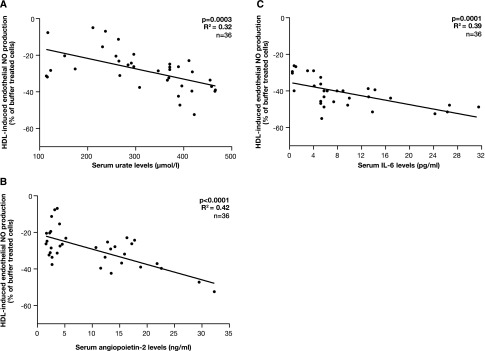
Association between HDL function and circulating markers of vascular dysfunction in children with CKD. Association between HDLCKD-induced endothelial NO production and serum urate levels (A), serum angiopoietin-2 levels (B), and serum IL-6 levels (C) (n=36). Some data have similar values and therefore the dots may not equal the sample size.
HDL Functionality Is Associated with Clinical Measures of Vascular Dysfunction
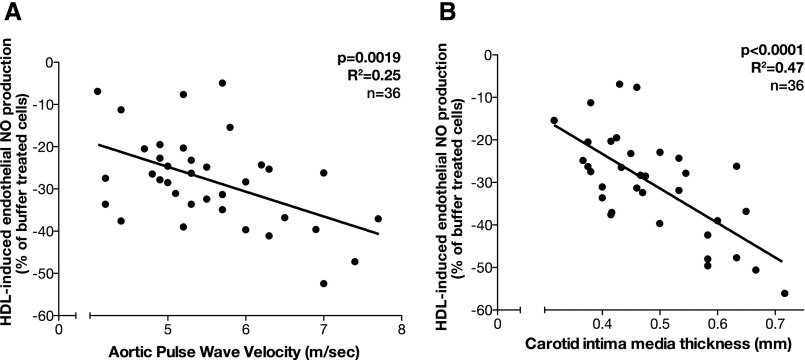
The in vitro effects of HDL on endothelial NO release showed a strong association with clinical measures of the vascular phenotype. HDLCKD-induced endothelial NO release significantly correlated with aortic pulse wave velocity (aPWV; P=0.002; Figure 4A) and carotid intima-media thickness (cIMT; P<0.001; Figure 4B).
Figure 4.
Association between HDLCKD-induced NO production and aPWV and cIMT. (A) aPWV measured by applanation tonometry. (B) cIMT measured by an ultrasonography scan of the common carotid artery. Some data have similar values and therefore the dots may not equal the sample size.
There was no association between cIMT or aPWV and circulating levels of HDL cholesterol or with endothelial SO or VCAM-1 production or cholesterol efflux. There was no difference in the BP SD score between patients with stages 4 and 5 CKD and patients on dialysis (P=0.07) to account for these differences. The calcium and phosphate levels were not significantly different across patient groups, suggesting that factors other than dysregulated mineral metabolism may account for the increased cIMT and vessel stiffness.
Symmetric Dimethylarginine Is Increased in HDLCKD
Our group recently showed that the accumulation of symmetric dimethylarginine (SDMA) in the HDL particle from patients with CKD at least partially mediated the adverse endothelial effects of HDL.20 Therefore, we measured SDMA in the serum as well as directly in the HDL fractions of our study cohort. Children with CKD showed significantly elevated serum SDMA levels compared with healthy children (P<0.001), with the highest levels in children on dialysis (P<0.001 compared with patients with stage 2 CKD; Figure 5A). Levels of SDMA in the HDL fraction showed a similar pattern with increased levels in the CKD cohort compared with controls (P<0.01), with the highest levels in dialysis patients, and only partial recovery after transplantation (Figure 5B). Levels of SDMA in the HDL fractions of children with CKD significantly correlated with both endothelial NO production (P<0.001) and endothelial SO production (P=0.003; Figure 5, C and D).
Figure 5.
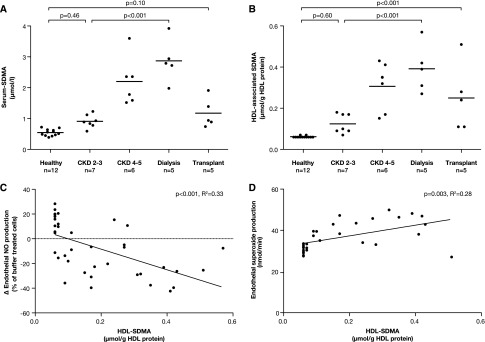
Levels of SDMA measured by HPLC-ESI-MS/MS in children with CKD stages 2–5, on dialysis and after transplantation and in healthy controls. (A) Serum SDMA levels. (B) SDMA in the HDL fraction. (C) Association between HDL-associated SDMA and HDL-induced endothelial NO production. (D) Association between HDL-associated SDMA and HDL-induced endothelial SO production. Bar shows mean level in each group. HPLC-ESI-MS/MS, HPLC with electrospray ionization coupled to tandem mass spectrometry.
HDL Functionality Improves after Kidney Transplantation
Finally, to show a causal link between renal function and HDL properties, we examined children on dialysis and 3 months after kidney transplantation. Compared with HDL properties while on dialysis, there was an increase in HDL-induced endothelial NO production (P=0.001; R2=0.80) and a significant reduction in endothelial SO (P=0.01; R2=0.66) and VCAM-1 (P=0.002; R2=0.88) expression after transplantation (Figure 6). There was no change in cholesterol efflux (P=0.80).
Figure 6.
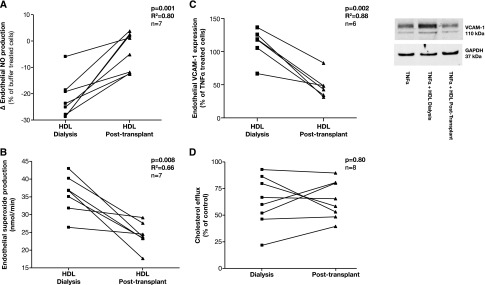
Longitudinal changes in the vascular effects of HDL in children on dialysis and 3 months after a kidney transplant. Endothelial NO production (A) and endothelial SO production (B) in HAECs incubated with HDL (50 µg/ml, 1 hour) determined by ESR spectroscopy. (C) Endothelial VCAM-1 expression in HAECs preincubated with HDL (50 µg/ml, 1 hour) and stimulated with TNF-α (0.1 ng/ml, 4 hours) determined by Western blot analysis and normalized to expression of GAPDH. (D) Cholesterol efflux from cholesterol-loaded J774 macrophages to apoB-depleted serum. Bar shows mean level in each group. ESR, electron spin resonance; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
We have shown that the endothelial properties of HDL as well as its cholesterol efflux capacity are potently impaired in children with CKD. These changes were seen even in children with early CKD without any underlying inflammatory disease, diabetes, or coronary artery disease, suggesting that CKD itself transforms HDL into a noxious proatherogenic particle that mediates endothelial dysfunction. HDL properties were altered even in early CKD and show a graded association with eGFR. Renal transplantation produced a partial recovery in HDL-induced endothelial function, suggesting a causal role of CKD in converting HDL into a proatherogenic particle. Importantly, there was a strong association between dysfunctional HDL and clinical measures of abnormal vascular function. This study also demonstrates the benefit from transplantation, as opposed to dialysis, in protecting the vasculature and potential opportunities for therapies targeting restoration of HDL function.
HDL from healthy participants is known to be an important regulator of vascular integrity mainly by modulating endothelial properties such as NO and SO production, preventing proinflammatory endothelial activation, and promoting cholesterol transport from lipid-laden macrophages in atheromata to the liver for excretion.21 By contrast, our group recently showed that, in adults, the presence of CKD not only abolishes the vasoprotective properties of HDL, but rather transforms HDL into a noxious particle, inhibiting endothelial NO production and increasing arterial BP.20 Other studies have also reported that HDL from adults with advanced CKD has a reduced cholesterol efflux capacity and proinflammatory effects on mononuclear cells.12,13,22 However, adults with CKD often have concomitant diseases such as diabetes and coronary artery disease and may be smokers, all of which may independently impair HDL function.8,10,23 Therefore, the impaired vasoprotective properties of HDL in adult patients cannot be definitely attributed to the presence of CKD alone. In this study, abnormal endothelial properties of HDL were seen even in children in predialysis CKD who had normal levels of IL-6 and C-reactive protein and no evidence of macrophage infiltration of their vessel wall. Dialysis per se has been shown to induce inflammation even in children,24,25 and the significantly worse HDL functional properties in dialysis patients may, in part, be a result of the proinflammatory milieu on dialysis.
Observational studies have shown an association between low HDL cholesterol levels and an increased risk of cardiovascular disease in the general population,14 but not in patients with impaired kidney function (Zewinger et al. JASN accepted).26 HDL is a complex particle and blood levels of HDL cholesterol do not reflect its diverse vascular protective functions. In CKD, HDL carries a distinct protein cargo with acute phase protein serum amyloid A1, apoCIII, and lipoprotein-associated phospholipase A2.22 In our study, there was no difference in the HDL levels between patients and controls or between patient groups, implying that serum levels of HDL are not a reliable measure of its vascular functions. Novel assays directly examining the vascular function of HDL, instead of its serum levels, may thus be necessary to predict cardiovascular risk in CKD. It also follows that raising HDL-C levels in serum may be harmful in patients with impaired kidney function, as noted in a recent study of cholesterol ester transfer protein inhibition.27
Previous studies have examined HDL function in adults on dialysis.12,13,22 We now show that HDL loses its endothelial protective properties even in early CKD with a graded change with the degree of renal impairment. These findings underscore results of epidemiologic studies that have documented an increased risk of cardiovascular morbidity and mortality even at early stages of CKD.1,18 The most profound impairment in all aspects of HDL function was seen in dialysis patients and there was a partial recovery after transplantation, but not to baseline levels. Interestingly, HDL-induced endothelial NO production significantly inversely correlated with circulating markers of vascular dysfunction and inflammation such as urate, angiopoietin-2, and IL-6 in our CKD cohort.5 These findings underscore the important role of NO, which mediates a broad range of HDL’s vasoprotective properties in addition to regulating the vascular tone. Importantly, HDL function was also significantly associated with increased arterial stiffness as well as increased cIMT, which are well established surrogate markers of cardiovascular disease in CKD28–30 and linked to worse outcomes in adult patients with CKD.31,32 These findings underscore the relevance of HDL in promoting vascular damage and dysfunction in patients with CKD.
Through a longitudinal follow-up of children on dialysis and after kidney transplantation, we have documented that improving the renal function by kidney transplantation improved but did not fully restore the vasoprotective properties of HDL. Although kidney transplantation leads to a significant improvement, but not a normalization, of the GFR, it may also introduce a number of traditional risk factors, including hypertension, diabetes, and increased body mass index, that can adversely affect HDL function. These findings are in line with previous reports indicating the persistence of an elevated risk for cardiovascular events after kidney transplantation, suggesting that uremia is only one of several adverse metabolic and inflammatory states that influence HDL functionality.30,33 In addition, these data show that CKD does not lead to a static irreversible remodeling of the HDL particle, but is a dynamic process that is amenable to improvement when the uremic milieu is reversed after transplantation or with appropriate medications.
The mechanisms leading to reduced vasoprotective effects of HDL in patients with CKD are complex and only partially understood.7 Qualitative and quantitative changes in the protein composition of the HDL particle have been well documented.12,22 We recently showed that SDMA, which has been linked to increased cardiovascular risk,34,35 accumulates in the HDL particle in adult patients with CKD.20 The incorporation of SDMA into HDL from healthy participants reduced endothelial NO bioavailability and increased endothelial SO production, acting via Toll-like receptor-2 on the surface of endothelial cells. In vivo, SDMA-modified HDL reduced re-endothelialization after carotid injury and increased the arterial BP in mice. It is not clear whether SDMA accumulation in the HDL particle is the result of renal failure per se or other vascular insults such as diabetes or inflammation, which are known to alter the HDL particle. In this study, we have shown that substantial amounts of SDMA are present in the HDL fraction from children with CKD without concomitant diseases that affect the endothelium. Accumulation of SDMA in the HDL particle may represent a mechanism for HDL dysfunction in CKD.
Although we have described a large cohort of well characterized children with CKD who were managed with single-center protocols for their renal failure, the effect of different treatment regimens for renal failure cannot be deduced from these data. Only two children were taking statins, so we were unable to examine their effect on HDL function. In vitro assays were performed by a single blinded operator to provide pathophysiologic insights into HDL-driven vascular dysfunction, but further studies are required to study changes in HDL composition by proteomic and lipidemic analyses. Serum high-sensitivity C-reactive protein levels were slightly higher in the control group than usually observed in healthy participants, but IL-6 and all other clinical and anthropometric measures were within the normal range. Vascular scans and in vitro studies were performed in separate cohorts of healthy controls. Our study included a longitudinal follow-up component to show the effect of renal transplantation on HDL functionality, but randomized clinical intervention trials are necessary for proof of causality.
In conclusion, we have shown that HDL is a noxious proatherogenic molecule that promotes endothelial dysfunction and reduces cholesterol efflux in children with CKD and without concomitant diseases that are known to affect HDL function. HDL dysfunction begins in early stages of CKD and progresses in a graded manner as renal function declines, with the most profound changes in dialysis patients and partial recovery after transplantation. Functional changes in HDL are associated with circulating and clinical measures of vascular dysfunction. Treatments that preserve renal function and avoidance of dialysis by preemptive renal transplantation may reduce HDL-driven endothelial dysfunction and vascular stiffness. Future therapeutic strategies may need to focus on improving the functionality of HDL rather than raising the HDL concentration in patients with CKD.
Concise Methods
From January 2010 to December 2012, 82 consecutive children (aged 5–18 years) with CKD attending our nephrology outpatient clinics and 12 healthy age- and sex-matched controls were recruited. Children were excluded from the study if they had underlying inflammatory disorders (e.g., GN and vasculitides), had infections in the 3 months preceding the study period, had diabetes, or smoked. The study was approved by the local research ethics committee and written informed consent was obtained from all parents or caregivers and from children when appropriate.
Blood tests and vascular measures were performed at the same clinical visit: before a mid-week session of hemodialysis or at clinic review for predialysis CKD, peritoneal dialysis, and transplant recipients. All children had their weight, height, and systolic and diastolic BP measured, and body mass index was calculated. SD scores were computed using the least-mean-squares method of Cole and Green36 (Table 1). Routine nonfasting blood samples were collected for renal function tests, measures of mineral dysregulation (calcium, phosphate, parathyroid hormone [Immulite 2500 Intact PTH assay; Siemens Healthcare Diagnostics]), lipid profile (total cholesterol, HDL and LDL cholesterol, and triglycerides [colorimetric enzymatic method; Technicon automatic analyzer RA-1000; Dade Behring, Marburg, Germany]) and inflammatory markers IL-6 (OptEIA; BD Biosciences). Serum was frozen at −80°C and all analyses were performed in a blinded fashion.
HDL Functionality Assays
HDL was isolated by sequential ultracentrifugation (d=1.063–1.21 g/ml) using solid potassium bromide for density adjustment37 and applied to HAECs to assess vascular effects of HDL. All functional assays of HDL were carried out within 2 weeks of isolation by a blinded investigator and all experiments were done in triplicate. Using previously described methods, we studied the effects of HDL on endothelial NO production, endothelial SO production, endothelial VCAM-1 expression, and cholesterol efflux.
Endothelial NO Production
HDL (50 μg/ml; 60 minutes, 37°C) was applied to HAECs. Passages 4–6 (Lonza Bioscience) and endothelial NO production were assessed by electron spin resonance spectroscopy using the spin-probe colloid Fe(DETC)2 (Noxygen).20,38
Endothelial SO Production
The effect of HDL on endothelial cell SO production was compared with controls in unstimulated and TNF-α–stimulated (R&D Systems) HAECs by electron spin resonance spectroscopy.20
Endothelial VCAM-1 Expression
HAECs were incubated with the isolated HDL and TNF-α and VCAM expression was determined by Western blot analysis.20
Cholesterol Efflux
ApoB-depleted serum was used to test cholesterol efflux capacity of HDL. Briefly, whole serum was incubated for 20 minutes with a 20% polyethylene glycol solution (P2139; Sigma-Aldrich) in 200 mM glycine (G8898, pH 10; Sigma-Aldrich). Cholesterol efflux capacity was quantified as previously described.39
These effects were compared with CKD severity, inflammatory and endothelial markers, endothelial and circulating SDMA, and the vascular phenotype. Serum urate, angiopoietin-2, and circulating IL-6 levels were measured in patients with stages 4 and 5 CKD and patients on dialysis only by using the following methods: urate (dry slide urokinase method; Ortho Clinical Diagnostics), angiopoietin-2 (ELISA; R&D Systems), and IL-6 (ELISA; Immulite Systems). SDMA in serum samples as well as in the HDL fraction was measured using HPLC with electrospray ionization coupled to tandem mass spectrometry as previously described.20
All children with stages 4 and 5 CKD and those on dialysis underwent cIMT and aPWV measurements as previously described. Results were compared with 40 healthy age- and sex-matched children who were scanned contemporaneously in our unit.40
For cIMT measurement, B-mode ultrasonography of both common carotid arteries was performed using a 12-MHz linear array transducer (Vivid 7; GE Medical, Horton, Norway). Longitudinal two-dimensional images of the vessel 1–2 cm proximal to the carotid bulb were acquired on the R wave of the electrocardiogram, frozen in diastole, and analyzed off-line. The cIMT was calculated as the distance between the leading edge of the lumen-intima interface and the media-adventitia interface on the far wall of the artery. aPWV was measured using the SphygmoCor system (SphygmoCor version 7.0; Millar Instruments; ScanMed Medical, Gloucestershire, UK). The pressure waveform was recorded consecutively in the carotid and femoral arteries with an electrocardiogram signal that provides an R-timing reference. aPWV is then calculated using the mean time difference in the carotid and femoral arterial length. All measures were performed by a single observer who was blinded to the clinical condition of the child.
Vascular Biopsy Analyses
The inferior epigastric artery was obtained from 10 children with CKD (7 children on dialysis and 3 children with predialysis CKD) at the time of undergoing renal transplantation. The vessel was cut into 1- to 2-mm rings and examined by histology and immunohistochemistry for calcification and changes in the vascular smooth muscle cells using previously described methods.2,19 Macrophages were detected by anti-CD68 antibodies (Dako), using N-Histofine Simple Stain (Nichirei Biosciences Inc.). CD68 was revealed by red staining (NovaRED; Vector Labs).41
Statistical Analyses
Data were presented as mean±SD or median (interquartile range) as appropriate. Comparisons in HDL functionality assays between controls and patients with CKD were performed using ANOVA or the Kruskal–Wallis test with Bonferroni adjustment. Comparisons of HDL functionality assays on children before and after dialysis and before and after transplantation were performed using the paired t test. Correlation analyses between HDL functionality assays and endothelial markers and vascular measures were performed using Pearson’s or Spearman’s test as appropriate. For all analyses, a two-tailed P<0.05 was considered statistically significant. All analyses were performed with GraphPad Prism (version 4.0; GraphPad Software Inc).
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the British Heart Foundation (London, UK), the Leducq Foundation (Paris, France), the German Research Foundation, the German Hypertension League (Berlin, Germany), and Homburger Forschungsförderungsprogramm. B.S. is a member of the University Institute of France.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111212/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM: Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118: 1748–1757, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Shroff R, Long DA, Shanahan C: Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24: 179–189, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Kari JA, Donald AE, Vallance DT, Bruckdorfer KR, Leone A, Mullen MJ, Bunce T, Dorado B, Deanfield JE, Rees L: Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int 52: 468–472, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Shroff RC, Price KL, Kolatsi-Joannou M, Todd AF, Wells D, Deanfield J, Johnson RJ, Rees L, Woolf AS, Long DA: Circulating angiopoietin-2 is a marker for early cardiovascular disease in children on chronic dialysis. PLoS ONE 8: e56273, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Speer T, Zewinger S, Fliser D: Uraemic dyslipidaemia revisited: Role of high-density lipoprotein. Nephrol Dial Transplant 28: 2456–2463, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Lüscher TF, Landmesser U: Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 121: 2693–2708, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charakida M, Besler C, Batuca JR, Sangle S, Marques S, Sousa M, Wang G, Tousoulis D, Delgado Alves J, Loukogeorgakis SP, Mackworth-Young C, D’Cruz D, Luscher T, Landmesser U, Deanfield JE: Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA 302: 1210–1217, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horváth T, Doerries C, Heinemann M, Flemmer S, Markowski A, Manes C, Bahr MJ, Haller H, von Eckardstein A, Drexler H, Landmesser U: Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 121: 110–122, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S, Konya V, Schuligoi R, Heinemann A, Marsche G: Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: Novel pathways generating dysfunctional high-density lipoprotein. Antioxid Redox Signal 17: 1043–1052, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, Suarna C, Eller P, Tölle M, Gerner C, Zlabinger GJ, van der Giet M, Hörl WH, Stocker R, Säemann MD: Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23: 934–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V: Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60: 2372–2379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR: High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 62: 707–714, 1977 [DOI] [PubMed] [Google Scholar]
- 15.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K: Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol 18: 293–303, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Chavers BM, Li S, Collins AJ, Herzog CA: Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int 62: 648–653, 2002 [DOI] [PubMed] [Google Scholar]
- 17.McDonald SP, Craig JC, Australian and New Zealand Paediatric Nephrology Association : Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 18.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 19.Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM: Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 21: 103–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Müller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Lüscher TF, Fliser D, Bahlmann FH, Landmesser U: Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38: 754–768, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Mineo C, Shaul PW: Novel biological functions of high-density lipoprotein cholesterol. Circ Res 111: 1079–1090, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G: Uremia alters HDL composition and function. J Am Soc Nephrol 22: 1631–1641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Hörkkö S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL: Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 13: 1176–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Bello JA, Gómez-Díaz RA, Contreras-Rodríguez A, Talavera JO, Mondragón-González R, Sanchez-Barbosa L, Diaz-Flores M, Valladares-Salgado A, Gallardo JM, Aguilar-Kitsu A, Lagunas-Munoz J, Wacher NH: Carotid intima media thickness, oxidative stress, and inflammation in children with chronic kidney disease. Pediatr Nephrol 29: 273–281, 20142 [DOI] [PubMed] [Google Scholar]
- 25.Srivaths PR, Silverstein DM, Leung J, Krishnamurthy R, Goldstein SL: Malnutrition-inflammation-coronary calcification in pediatric patients receiving chronic hemodialysis. Hemodial Int 14: 263–269, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, Pfahler K, Seiler S, Heine GH, März W, Silbernagel G, Fliser D: HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol 25: 1073–1082, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, dal-OUTCOMES Investigators : Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367: 2089–2099, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Tröger J, Mehls O, Schaefer F: Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16: 1494–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: Role of calcium-phosphorus metabolism. J Am Soc Nephrol 16: 2796–2803, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F: Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Vlachopoulos C, Aznaouridis K, Stefanadis C: Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Meier-Kriesche HU, Baliga R, Kaplan B: Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 75: 1291–1295, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H: Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol 17: 1128–1134, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D: Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—a meta-analysis. Nephrol Dial Transplant 21: 2446–2451, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Cole TJ, Green PJ: Smoothing reference centile curves: The LMS method and penalized likelihood. Stat Med 11: 1305–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Havel RJ, Eder HA, Bragdon JH: The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 34: 1345–1353, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorrentino SA, Bahlmann FH, Besler C, Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U: Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: Restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 116: 163–173, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J, Rees L: Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18: 2996–3003, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Bories G, Colin S, Vanhoutte J, Derudas B, Copin C, Fanchon M, Daoudi M, Belloy L, Haulon S, Zawadzki C, Jude B, Staels B, Chinetti-Gbaguidi G: Liver X receptor activation stimulates iron export in human alternative macrophages. Circ Res 113: 1196–1205, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.