Abstract
Podocytes are terminally differentiated cells with an elaborate cytoskeleton and are critical components of the glomerular barrier. We identified a bHLH transcription factor, Tcf21, that is highly expressed in developing and mature podocytes. Because conventional Tcf21 knockout mice die in the perinatal period with major cardiopulmonary defects, we generated a conditional Tcf21 knockout mouse to explore the role of this transcription factor in podocytes in vivo. Tcf21 was deleted from podocytes and podocyte progenitors using podocin-cre (podTcf21) and wnt4-cre (wnt4creTcf21) driver strains, respectively. Loss of Tcf21 from capillary-loop stage podocytes (podTcf21) results in simplified glomeruli with a decreased number of endothelial and mesangial cells. By 5 weeks of age, 40% of podTcf21 mice develop massive proteinuria and lesions similar to FSGS. Notably, the remaining 60% of mice do not develop proteinuria even when aged to 8 months. By contrast, earlier deletion of Tcf21 from podocyte precursors (wnt4creTcf21) results in a profound developmental arrest of podocyte differentiation and renal failure in 100% of mice during the perinatal period. Taken together, our results demonstrate a critical role for Tcf21 in the differentiation and maintenance of podocytes. Identification of direct targets of this transcription factor may provide new therapeutic avenues for proteinuric renal disease, including FSGS.
Keywords: podocyte, transcription factors, diabetic nephropathy, focal segmental glomerulosclerosis
Over the past decade, many landmark studies have demonstrated a central role for podocytes in renal health and disease. Mature podocytes extend elaborate foot processes that interdigitate with each other and are connected by a specialized intercellular junction called the slit diaphragm. The slit diaphragm is composed of several proteins, including nephrin, podocin, and Cd2-associated protein, that interact with actin-based cytoskeletal proteins, such as α-actinin 4. Mutations in each of these genes can cause neonatal and adult human nephrotic syndromes.1 Furthermore, podocytes communicate with other cell types in the glomerulus, and are able to monitor their extracellular environment and modify their shape and function accordingly.2 As a result, dysregulation in a number of signaling pathways and factors produced by podocytes, such as vascular endothelial growth factor (VEGF), can also cause glomerular injury.3,4
During development, podocyte precursors appear at the end of the S-shaped body farthest from the ureteric bud and differentiate through a well defined sequence of morphologic events from a columnar-shaped epithelium facing the vascular cleft to mature podocytes with their elaborate foot processes. Several transcription factors have been identified that are expressed by podocyte precursors, including Wilms’ tumor suppressor 1 (Wt1), Lmx1b, Foxc2, and MafB. Wt1 is a zinc finger–containing transcription factor, and genetic deletion of Wt1 leads to loss of nephrogenic induction and renal agenesis.5 Glomeruli in knockout mice for Lmx1b or Foxc2 are abnormal, with arrested podocyte differentiation, simplified capillary loop structure, and defects in mesangial cell ingrowth.6,7 Global deletion of MafB also results in abnormal foot process formation, with persistence of “flat feet” in podocytes.8
Tcf21 (Pod1/capsulin/epicardin) is a basic helix-loop-helix (bHLH) transcription factor whose expression is highest in podocyte precursors and is maintained in mature podocytes. However, at earlier stages of metanephric development, Tcf21 is expressed in both Six2-expressing nephron progenitors and Foxd1-expressing stromal mesenchyme.9–11 Global deletion studies of Tcf21 have shown that it is required for the development of lung, heart, kidney, gonad, spleen, and facial muscles.9,12–14 The kidneys of Tcf21 knockout mice are severely hypoplastic because of a delay of nephrogenesis and abnormal branching morphogenesis of the ureteric bud.9 The few glomeruli found in kidneys of Tcf21 knockout mice appear to arrest at the capillary loop stage of development. However, because Tcf21 is also involved in the induction of nephrogenesis from the Six2-positive progenitor population, it has not been possible to determine the roles of Tcf21 in developing and mature podocytes. Indeed, the glomerular defects might reflect roles of Tcf21 in the progenitor or adjacent cell populations, rather than in the podocyte itself.
Here we report the phenotype in mice following podocyte-selective knockout of Tcf21. Deletion of Tcf21 in relatively mature podocytes (podocin-cre) allows podocyte differentiation to occur but results in a simplified glomerular structure. However, 40% of podocyte-specific Tcf21 knockout mice develop massive proteinuria at 3–5 weeks of age with prominent glomerular lesions similar to human FSGS. By contrast, earlier deletion of Tcf21 at the renal vesicle stage of glomerular development using a wnt4-cre driver line results in major defects in podocyte differentiation. Finally, we show rapid development of kidney failure in a diabetic model in the “protected” podTcf21 knockout cohort. Together, our data demonstrate key roles of Tcf21 in podocytes in developing and mature animals, especially under disease conditions.
Results
Generation of a Floxed Tcf21 Founder Mouse Line
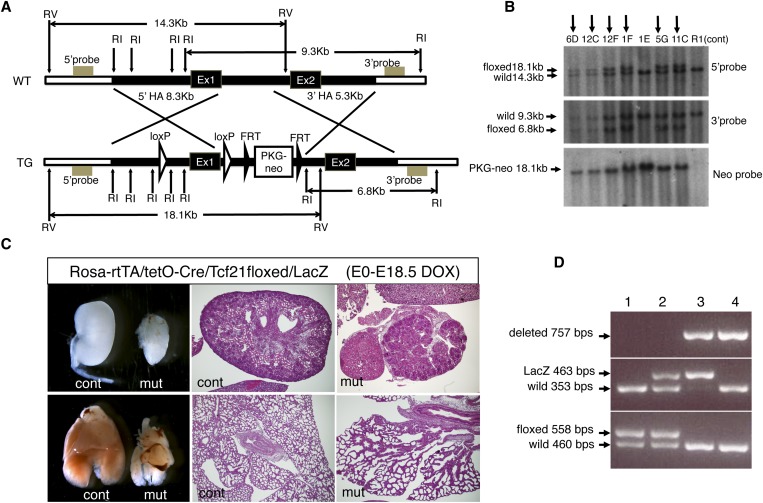
The Tcf21 conditional allele was designed with LoxP sites inserted around the first exon, which contains the bHLH domain (Figure 1A). After electroporation, embryonic stem (ES) cell clones were screened by Southern blot analysis with probes outside the homology regions. One positive clone (5G clone in Figure 1B) was used to generate chimeric mice.
Figure 1.
Generation of conditional allele for Tcf21. (A) Targeting construct for floxed Tcf21 allele. LoxP sites were inserted around exon1 (Ex1). FRT, FRT sequence; HA, homology arm; RI, EcoRI site; RV, EcoRV site; TG, targeted; WT, wild type. (B) Targeted clones were identified by Southern blot analysis using probes outside the 5′ and 3′ homology arms. Arrows indicate positively targeted clones. Clones 12F and 5G were used for ES cell aggregation; only the 5G clone resulted in germline transmission. (C) Validation of the floxed allele using the Rosa-rtTA/tetO-Cre system. Doxycycline induction to excise the floxed alleles in embryos from E0 (conception) resulted in a phenotype in newborn pups identical to that observed in conventional Tcf21 knockout pups. Note the severely hypoplastic kidneys (upper panels) and lungs (lower panels) in floxed mutants. Histology, hematoxylin and eosin; original magnification, ×40. (D) PCR genotyping shows floxed Tcf21 allele (558 bp), Tcf21-LacZ allele (463 bp; conventional knockout), and floxed allele after Cre-mediated deletion (757 bp). Lane 3 shows the genotype from a mouse carrying one deleted floxed allele and a null LacZ allele. cont, control littermate; DOX, doxycycline; mut, mutant, Rosa-rtTA/tetO-Cre/Tcf21floxed/LacZ.
To confirm that the Cre-mediated gene deletion results in a null Tcf21 allele, we generated inducible whole-body Tcf21 knockout mice using the ROSA-rtTA/tetO-Cre system. Administration of doxycycline to the dam from the time of conception resulted in mutant embryos that show pericardial bleeding and severely hypoplastic lungs and kidneys at embryonic day 18.5, which are identical to the defects caused by phenotype observed in conventional Tcf21 knockout mice (Figure 1C). This finding confirmed that the deleted conditional allele is indeed a null allele. Subsequent genotyping was performed by PCR (Figure 1D).
Normal Differentiation of Podocytes and Delay of Glomerular Maturation in Podocyte-Specific Tcf21 Knockout Mice
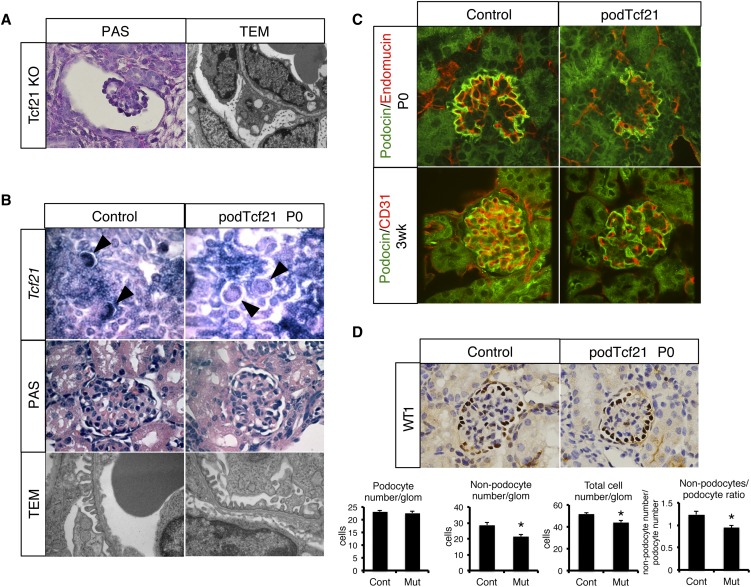
Previously we showed that conventional Tcf21 knockout mice demonstrate an arrest of glomerular maturation (Figure 2A).9 To examine the role of Tcf21 exclusively in podocytes, floxed Tcf21 mice were bred to transgenic podocin-cre mice to create podocyte-specific knockout (podTcf21) mice. Using a Z/EG reporter mouse,15 we confirmed that podocin-cre results in gene excision from the capillary loop stage onward and not at the S-shape stage (Supplemental Figure 1A). At postnatal day 0, although Tcf21 expression was properly decreased in podocytes, podTcf21 glomeruli showed almost normal histologic features and ultrastructure (Figure 2B). Mice were born in the expected Mendelian ratio, and podTcf21 pups appeared healthy for 2 weeks (data not shown). These results indicate that Tcf21 is not required for terminal differentiation of podocytes after the capillary loop stage.
Figure 2.
Delay of glomerular maturation in podTcf21 mice. (A) Periodic acid-Schiff (PAS)–staining (×1000) of a glomerulus and a TEM (×12,400) of the glomerular barrier in the kidney from conventional Tcf21 knockout at E18.5. (B) Upper panel: in situ hybridization for Tcf21 (×200) confirms loss of Tcf21 message in capillary loop stage podocytes in podTcf21 mice (arrowheads). Middle panel: periodic acid-Schiff–staining of glomeruli (×1000). Lower panel: TEM that shows relatively normal glomerular filtration barrier of podTcf21 at postnatal day 0 (P0) (×12,400). (C) Immunostainings for endothelial (CD31 or endomucin) markers and podocin demonstrate simplified structure of the glomerulus at P0 (upper) and 3 weeks (lower) in podTcf21 mice compared with control (×400). (D) Reduced nonpodocyte cell number in podTcf21 glomeruli. Upper panel: immunostainings for a podocyte marker, Wt1 (×1000). Lower panels: numbers of podocytes, non-podocytes, total cell number in each glomerulus, and non-podocyte/podocyte ratio. Total cell number and non-podocyte cell number in mutant glomeruli were reduced by 15% (51.8 cells in control versus 44.1 cells in podTcf21; P<0.05) and 25% (28.6 cells in control versus 21.4 cells in podTcf21; P<0.05), respectively. *P<0.05.
However, immunostainings for podocin and endothelial markers (Cd31 and endomucin) revealed a surprisingly simplified glomerular structure at postnatal day 0 and 3 weeks of age (Figure 2C). Immunostaining for a mesangial marker, desmin, showed a similar finding (Supplemental Figure 1B). Interestingly, at P0, although the Wt1-positive podocyte number did not differ, total cell number and nonpodocyte cell number in mutant glomeruli were reduced by 15% and 25%, respectively (Figure 2D). Taken together, these findings suggest a delay in glomerular maturation with reduced influx and/or in situ proliferation of endothelial and mesangial cells.
A Subset of Podocyte-Specific Tcf21 Knockout Mice Develop Proteinuria and FSGS
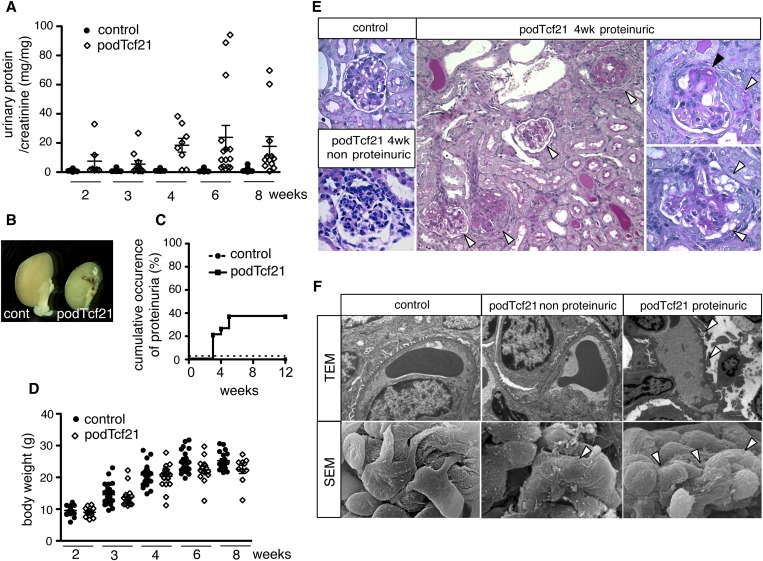
By 5 weeks of age, 40% of the podTcf21 mice developed massive proteinuria (Figure 3, A and C). Although there was large variation between mutants, podTcf21 mice showed significant increase of the mean urinary protein-to-creatinine ratios (P<0.05 for 3 and 8 weeks; P<0.01 for 4 and 6 weeks-of-age). Kidneys from the proteinuric podTcf21 mice were atrophic and pale, and the surface was irregular (Figure 3B). Some mutants quickly reached ESRD and died at 3–6 weeks of age. Interestingly, 60% of podTcf21 mice never developed proteinuria even up until 8 months of age, suggesting there are two populations of podTcf21 mutants: protected and susceptible (Figure 3C). Susceptible PodTcf21 mice showed significant growth retardation at 6 and 8 weeks-of-age (Figure 3D, Supplemental Figure 2A). Proteinuria occurred in both male and female mutants to the same degree (41.7% and 38.5%, respectively), suggesting no sex differences.
Figure 3.
Forty percent of podTcf21 mice develop proteinuria and FSGS. (A) Urinary protein-to-creatinine ratio in control and podTcf21 mice. Bars indicate mean±SEM. Forty percent of podTcf21 mutants developed massive proteinuria at 3 weeks onward. (B) Macroscopic picture of control and podTcf21 kidneys from mutant with massive proteinuria at 4 weeks of age. (C) Cumulative occurrence of proteinuria that is >3+ by dipstick or 20 mg/mg protein-to-creatinine ratio. Only 40% of the mutants develop proteinuria, identifying both a protected and a susceptible cohort. (D) Body weight of control versus podTcf21 mice. Bars indicate mean±SEM (24.5 g control versus 22.4 g podTcf21 at 6 weeks, 25.2 g control versus 22.5 g podTcf21 at 8 weeks; for both P<0.05). (E) Periodic acid-Schiff–staining of podTcf21 kidneys shows FSGS lesions. Various degrees of glomerulosclerosis and protein casts in tubules (arrowheads, middle panel), partial glomerulosclerosis (black arrowhead) with crescent formation (white arrowhead, right upper panel), and vacuolations in podocytes (white arrowheads, right lower panel) are shown. Middle panel, ×200; others, ×1000. (F) Ultrastructural analysis of podTcf21 glomeruli. In non-proteinuric podTcf21 mice (middle panels), no abnormality was detectable by TEM, but scanning electron micrograph (SEM) shows partial flattening and disorganization of podocyte foot processes (arrowhead). These findings are more prominent and global in proteinuric podTcf21 glomeruli (arrowheads, right panels). TEM, ×9600; scanning electron micrograph, ×12,400.
Glomeruli from the proteinuric podTcf21 show dramatic pathologic changes with focal and segmental sclerosis similar to human FSGS, with occasional crescent formation and podocyte vacuolation (Figure 3E). Transmission electron micrographs (TEM) of kidneys from nonproteinuric podTcf21 mice at 3 weeks of age were similar to those of control kidneys. However, scanning electron microscopy revealed subtle defects with focal flattening and disorganization of foot processes (Figure 3F). In glomeruli from proteinuric podTcf21 mice, podocyte foot processes were extensively effaced (Figure 3F). At 6 weeks of age, glomeruli of proteinuric podTcf21 mice showed foot process effacement with large bumpy subepithelial protrusion of the glomerular basement membrane (Supplemental Figure 2B). Taken together, these results indicate that Tcf21 is required for the maintenance of podocyte structure and the function of the glomerular filtration barrier, but the extent of the effect varies among individual mice.
PodTcf21 Mutants Show Podocyte Injury
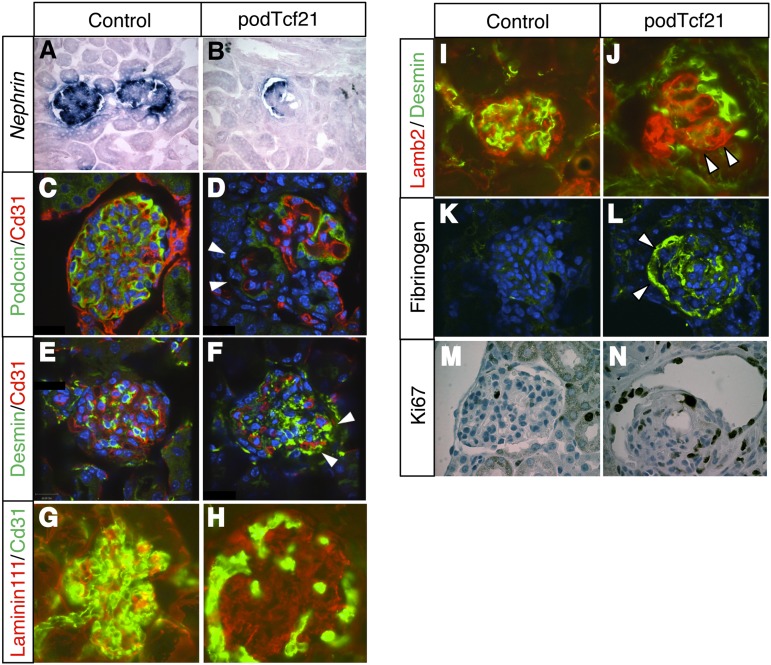
To characterize the glomerular injury in kidneys of podTcf21 mice, we performed in situ hybridization and immunostainings for glomerular markers. In injured glomeruli, Nephrin mRNA expression and Podocin protein were partially lost (Figure 4, A–D). Desmin expression was increased with a punctate pattern at the edge of the glomeruli (Figure 4, E and F), presumably in podocytes. These observations suggested that podocytes are injured. Laminin 111–positive area, normally seen in mesangium and immature glomerular basement memrane,16 was expanded in podTcf21 glomeruli with reduced capillary loops (Figure 4, G and H). Staining for Laminin β2, a specific laminin in the glomerular basement membrane, shows an aberrantly tangled and clustered pattern (Figure 4, I and J). The mutant glomeruli showed prominent fibrinogen deposition, indicating the leakage of plasma components (Figure 4, K and L). In addition, the mutant glomeruli demonstrated dynamic proliferation of cells in Bowman's capsule (Figure 4, M and N).
Figure 4.
Podocyte injury in glomeruli from proteinuric podTcf21 mice. (A and B) In situ hybridization shows decreased expression of Nephrin in proteinuric podTcf21 mice. (C–F) Immunostainings reveal decreased expression of Podocin (arrowheads, D) and Desmin (arrowheads, F) in presumptive podocytes of proteinuric podTcf21 mice compared with controls (C and E). (G and H) Expansion of Laminin111 expression domain was seen in proteinuric podTcf21 mutants. (I and J) Laminin β2 (Lamb2)/Desmin staining shows a collapsed and tangled pattern (arrowhead, J) compared with a beautifully organized structure in controls (I). (K and L) Proteinuric podTcf21 glomeruli show fibrinogen deposits (arrowheads). (M and N) Ki67 staining shows proliferation in the Bowman capsules and in crescents. A–F and K–N, ×400; G–J, ×600.
Microarray Analysis of Isolated Glomeruli
To characterize the molecular response in the glomeruli and to identify putative downstream targets for Tcf21, we performed a comprehensive gene expression analysis using Affymetrix microarray on total glomerular RNAs isolated from podTcf21 and control mice. A total of 3065 genes whose transcript varied significantly (P<0.05) were identified (8.6% of the 35,556 genes). Table 1 shows a partial list of genes associated with glomerular development or function. Some of these changes were confirmed by quantitative realtime PCRs and in situ hybridization (Supplemental Figure 3; primer sequences are shown in Supplemental Table 1). Complete lists of genes that show at least a 1.5-fold difference are provided (Supplemental Tables 2 and 3).
Table 1.
List of genes differentially expressed in glomeruli isolated from Pod1tcf21 versus wild-type mice
| Ref Seq | podTcf21 Glomeruli Microarray Analysis (Categorized) | Fold Change (Mut/Con) | ||
|---|---|---|---|---|
| Gene Assignment | Gene Symbol | P Value | ||
| NM_011545 | Transcription factor 21 | Tcf21 | 4.02E-11 | 0.116851 |
| Podocyte-related genes | ||||
| NM_144783 | Wt1 homolog | Wt1 | 0.96 | 1.00341 |
| AB513652 | Nephrosis 1 homolog, nephrin (human) | Nphs1 | 0.71 | 0.965275 |
| NM_019459 | Nephrosis 1 homolog, nephrin (human) | Nphs1 | 0.82 | 0.967055 |
| NM_130456 | Nephrosis 2 homolog, podocin (human) | Nphs2 | 0.69 | 0.952228 |
| NM_015764 | Gene regulated by estrogen in breast cancer protein | Greb1 | 0.04 | 1.09796 |
| NM_009847 | CD2-associated protein | Cd2ap | 0.04 | 0.913255 |
| NM_001109975 | Synaptopodin | Synpo | 0.01 | 0.834113 |
| NM_001099331 | R3H domain containing-like | R3hdml | <0.001 | 0.195615 |
| Mesangial genes | ||||
| NM_010043 | Desmin | Des | 0.60 | 0.955636 |
| NM_001146268 | Pdgfrb | Pdgfrb | <0.002 | 0.894954 |
| Endothelial genes | ||||
| NM_01061 | Kinase insert domain protein receptor | Kdr | 0.09 | 0.953623 |
| NM_001111059 | CD34 antigen | Cd34 | 0.41 | 1.02267 |
| NM_010228 | FMS-like tyrosine kinase 1 | Flt1 | <0.002 | 0.883751 |
| NM_013690 | Endothelial-specific receptor tyrosine kinase | Tek | 0.01 | 0.890548 |
| NM_008816 | Platelet/endothelial cell adhesion molecule 1 | Pecam1 | 0.22 | 0.953775 |
| VEGF-related genes | ||||
| NM_001025250 | VEGF-A | Vegfa | 0.01 | 0.858069 |
| NM_011697 | VEGF-B | Vegfb | <0.001 | 1.20935 |
| NM_009506 | VEGF-C | Vegfc | <0.006 | 1.46325 |
| NM_008827 | Placental growth factor | Pgf | 1.29E-05 | 0.432535 |
| Notch pathway | ||||
| NM_008714 | Notch gene homolog 1 (Drosophila) | Notch1 | 0.25 | 0.959897 |
| NM_010928 | Notch gene homolog 2 (Drosophila) | Notch2 | 0.54 | 0.975687 |
| NM_019454 | Delta-like 4 (Drosophila) | Dll4 | <0.001 | 0.692084 |
| NM_013904 | Hairy/enhancer-of-split related with YRPW motif 2 | Hey2 | <0.012 | 0.663724 |
| NM_013905 | Hairy/enhancer-of-split related with YRPW motif-like | Heyl | <0.012 | 0.8704 |
| Heparan sulfate proteoglycans | ||||
| NM_178870 | Heparan sulfate (glucosamine) 3-O-sulfotransferase 3A1 | Hs3st3a1 | 2.83E-10 | 0.130029 |
| NM_016696 | Glypican 1 | Gpc1 | 4.04E-06 | 0.614059 |
| NM_009929 | Collagen, type XVIII, α1 | Col18a1 | 6.17E-10 | 0.473699 |
| NM_028072 | Sulfatase 2 | Sulf2 | 1.56E-07 | 1.84207 |
| NM_001198565 | Sulfatase 1 | Sulf1 | <0.001 | 1.57147 |
| NM_016771 | Sulfotransferase family 1D, member 1 | Sult1d1 | <0.001 | 2.43938 |
| Collagens and laminins | ||||
| NM_007743 | Collagen, type I, α2 | Col1a2 | 6.51E-06 | 0.66233 |
| NM_007739 | Collagen, type VIII, α1 | Col8a1 | 2.42E-05 | 2.39189 |
| NM_007730 | Collagen, type XII, α1 | Col12a1 | <0.001 | 1.42416 |
| NM_181277 | Collagen, type XIV, α1 | Col14a1 | <0.001 | 2.93957 |
| NM_009929 | Collagen, type XVIII, α1 | Col18a1 | 6.17E-10 | 0.473699 |
| NM_008480 | Laminin, α1 | Lama1 | <0.001 | 1.37389 |
| NM_008485 | Laminin, γ2 | Lamc2 | 0.02 | 1.3046 |
| Wnt Signaling | ||||
| Cannonical Wnt pathway | ||||
| NM_007614 | Catenin (cadherin associated protein), β1 | Ctnnb1 | 0.04 | 0.957209 |
| NM_010703 | Lymphoid enhancer binding factor 1 | Lef1 | 0.01 | 0.848828 |
| NM_013685 | Transcription factor 4 | Tcf4 | 0.73 | 0.990435 |
| NM_015732 | Axin2 | Axin2 | 0.61 | 0.951149 |
| Wnt ligands | ||||
| NM_009523 | Wingless-related MMTV integration site 4 | Wnt4 | 0.03 | 0.894278 |
| ENSMUST00000045747 | Wingless-related MMTV integration site 4 | Wnt4 | 0.16 | 0.913155 |
| NM_009524 | Wingless-related MMTV integration site 5A | Wnt5a | 1.30E-05 | 0.646918 |
| NM_009528 | Wingless-related MMTV integration site 7B | Wnt7b | 0.67 | 1.03785 |
| NM_011720 | Wingless related MMTV integration site 8b | Wnt8b | 2.26E-05 | 1.45817 |
| NM_011719 | Wingless-type MMTV integration site 9B | Wnt9b | 0.39 | 1.08941 |
| NM_009519 | Wingless-related MMTV integration site 11 | Wnt11 | 0.05 | 0.882975 |
| Wnt inhibitors and related | ||||
| NM_013834 | Secreted frizzled-related protein 1 | Sfrp1 | >0.001 | 1.65044 |
| NM_011915 | Wnt inhibitory factor 1 | Wif1 | 2.68E-11 | 3.57431 |
| NM_011356 | Frizzled-related protein | Frzb | <0.001 | 2.78872 |
| NM_021339 | Cell adhesion molecule–related/downregulated by oncogenes | Cdon | 6.57E-08 | 2.6764 |
| Wnt receptors | ||||
| NM_021458 | Frizzled homolog 3 (Drosophila) | Fzd3 | <0.001 | 1.29471 |
| NM_008055 | Frizzled homolog 4 (Drosophila) | Fzd4 | >0.007 | 1.15362 |
| NM_008055 | Frizzled homolog 7 (Drosophila) | Fzd7 | >0.001 | 1.34737 |
| NM_001008231 | Dishevelled associated activator of morphogenesis 2 | Daam2 | <0.012 | 0.861158 |
| Tgfs | ||||
| NM_031199 | TGF-α | Tgfa | 5.28E-05 | 0.737485 |
| NM_011577 | TGF-β1 | Tgfb1 | 0.78 | 1.01081 |
| NM_009368 | TGF-β3 | Tgfb3 | 6.43E-05 | 1.52369 |
| NM_009370 | TGF-β receptor I | Tgfbr1 | 0.72 | 1.01594 |
| NM_009371 | TGF-β receptor II | Tgfbr2 | 0.02 | 0.875323 |
| NM_011578 | TGF-β receptor III | Tgfbr3 | <0.003 | 0.853125 |
| NM_008542 | MAD homolog 6 (Drosophila) | Smad6 | <0.001 | 1.20471 |
| NM_001042660 | MAD homolog 7 (Drosophila) | Smad7 | <0.001 | 1.22369 |
| Fgfs | ||||
| NM_010206 | Fibroblast growth factor receptor 1 | Fgfr1 | 4.12E-05 | 0.740399 |
| NM_010207 | Fibroblast growth factor receptor 2 | Fgfr2 | <0.001 | 1.35853 |
| NM_008010 | Fibroblast growth factor receptor 3 | Fgfr3 | <0.078 | 1.09647 |
| NM_008011 | Fibroblast growth factor receptor 4 | Fgfr4 | 8.70E-08 | 0.559123 |
| NM_054071 | Fibroblast growth factor receptor-like 1 | Fgfrl1 | >0.004 | 1.13961 |
| NM_011896 | Sprouty homolog 1 (Drosophila) | Spry1 | 5.09E-06 | 0.756577 |
| Bmps | ||||
| NM_028472 | Bone morphogenetic protein–binding endothelial regulator | Bmper | >0.002 | 1.24081 |
| NM_007554 | Bone morphogenetic protein 4 | Bmp4 | >0.001 | 1.35746 |
| NM_007555 | Bone morphogenetic protein 5 | Bmp5 | <0.007 | 1.24253 |
| NM_007557 | Bone morphogenetic protein 7 | Bmp7 | 0.01 | 0.867236 |
The affymetrix gene microarray platform was used to identify differences. Genes are categorized according to cell type expression or cell signaling pathways. Complete lists of genes that show at least 1.5-fold difference are shown in Supplemental Tables 2 and 3.
Podocyte Differentiation Is Abnormal in Wnt4creTcf21 Mice
We next hypothesized that Tcf21 may be most important when differentiation is actively occurring in podocyte precursors. Therefore, we crossed the Tcf21 floxed mice to a wnt4-cre driver mouse line (wnt4creTcf21).17 Wnt4 is an inducer of mesenchymal-to-epithelial transformation and is expressed in pretubular aggregates, renal vesicles, and the proximal part of the S-shaped body of the developing nephron. Because Tcf21 is expressed in condensing mesenchyme but not in the renal vesicle and reappears in podocyte precursors, wnt4-cre mice should provide a selective knockout of Tcf21 from podocyte precursors without affecting its expression in the condensing mesenchyme.
In situ hybridization confirmed robust excision of Tcf21 in immature and mature podocytes from the S-shape stage onward, which is earlier than excision of Tcf21 using the podocin-cre line (Figure 5, A and B [compare with Figure 2B] Supplemental Figure 1A). Glomeruli of wnt4creTcf21 mice were primitive and reduced in size at embryonic stage E18.5. Podocytes remained columnar, and the complexity of the glomerulus was reduced (Figure 5, C and D). The expression of podocin was largely reduced and showed granular and patchy distribution at the lateral and apical sides (Figure 5, E–H). Desmin staining also revealed a simplification of the glomerulus (Figure 5, I and J). Expression of synaptopodin, aPKCλ, and Par3 (Figure 5, K–P) was decreased. Expression of Nephrin, Wt1, and Zo1 (Figure 5, Q–V) remained unchanged. Finally, a profound defect of podocyte foot process formation was observed on TEM (Figure 5, W and X). Taken together, these data suggest a major defect in podocyte differentiation if Tcf21 is absent from the precursor stage onward. Notably, wnt4creTcf21 kidneys are slightly smaller but do not show any overt abnormality in tubular formation, suggesting that mesenchyme-to-epithelial transformation is not impaired (Supplementary Figure 4).
Figure 5.
Deletion of Tcf21 gene in podocyte progenitors results in arrest of podocyte differentiation. (A and B) Tcf21 expression assessed by in situ hybridization. Ring-shaped or crescent-shaped podocyte pattern (arrowheads) is absent in wnt4creTcf21 mutants. (C and D) Histology of wnt4creTcf21 glomeruli shows a severely simplified capillary loop and a defect of mesangial ingrowth. (E–H) Podocin expression is severely decreased in wnt4creTcf21 glomeruli. In controls, Podocin is detected alongside the capillary loops (arrowheads, G) but is also seen at apical and lateral sides in wnt4creTcf21 podocytes (arrowheads, H). (I and J) Desmin/Cd31 staining shows simplified capillary loops and mesangial migration defects in wnt4Tcf21 mutant glomeruli. (K–P) Synaptopodin, aPKCλ, and Par3 expression are decreased, but the distribution doesn’t change. (Q and R) In situ hybridization for Nephrin doesn’t show any change. (S–V) Wt1 and Zo1 stainings are not changed. (W and X) TEM demonstrate a severe defect in podocyte foot process formation. A and B, ×200; C and D, G and H, ×1000; E and F, K–V, ×400; I and J, ×600; TEM, ×9600.
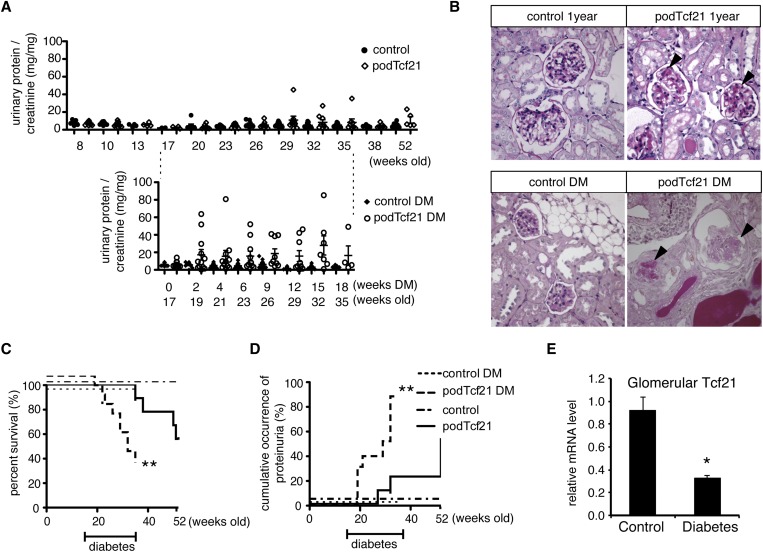
Tcf21 Is Required in Aging Podocytes and Protects against Diabetic Nephropathy
Given the presence of two distinct populations of adult podTcf21 knockout mice (proteinuric versus nonproteinuric), we were keen to examine the protected cohort in more detail. To determine whether podTcf21 mice in the protected cohort might develop proteinuria as they age, we followed nine podTcf21 mice (urinary protein-to-creatinine<10 mg/mg at 8 weeks) until 1 year of age (Figure 6A, upper panel). Outcomes for individual mice are shown in Supplemental Table 4. One developed massive proteinuria at 32 weeks and died at 38 weeks. At 1 year, among five surviving podTcf21, two showed mild proteinuria and focal glomerulosclerosis (Figure 6B, upper panel). These results indicate that podTcf21 mice develop proteinuria either before 5 weeks or after 32 weeks (Figure 6A, upper panel) but are unlikely to develop proteinuria in the interim. Although incomplete or mosaic excision may account for the milder phenotype, we found no difference in the level of Tcf21 expression in glomeruli isolated from kidneys of proteinuric (n=2) and nonproteinuric (n=5) mice, as determined by realtime quantitative PCR (not shown).
Figure 6.
Tcf21 is required in aging podocytes and protects mice from diabetic nephropathy. (A) Upper panel: Long-term follow-up of urinary protein-to-creatinine ratios taken from control and protected podTcf21 mice (defined by protein-to-creatinine ratio<10 mg/mg at 8 weeks of age). PodTcf21 mutants show sporadic mild proteinuria after 32 weeks. Lower panel: Controls and podTcf21 mutants without proteinuria at 17 weeks were rendered diabetic by streptozotocin injection. Diabetic podTcf21 mutants develop massive proteinuria. (B) Representative histologic findings of glomeruli from each group at the end of the study. Aged podTcf21 mutants show mild sclerosis (arrowheads, upper right). Diabetic podTcf21 kidneys show severe glomerulosclerosis and nodular lesions (arrowheads, lower right), while control diabetic mice show mild mesangial expansion only (lower left). Diabetic podTcf21 mice show a significant decrease in survival (C) and increase in occurrence of proteinuria (D). (E) Wild-type diabetic mice show reduced expression of Tcf21 in glomeruli. Bars show mean±SEM. *P<0.05 compared with podTcf21; **P<0.01 compared with podTcf21. DM, diabetes mellitus.
To further examine the role of Tcf21 in podocytes in a clinically relevant disease model, we chose the streptozotocin model of diabetic nephropathy. We selected a cohort of nonproteinuric podTcf21 (urinary protein-to-creatinine ratio< 10 mg/mg) and control mice at 17 weeks of age. Diabetes was induced according to the Diabetic Complications Consortium streptozotocin protocol.18 Both groups were monitored for 18 weeks after injection. Sixty-six percent of the diabetic podTcf21 mice died before the end of the study compared with 11.1% of nondiabetic podTcf21 mice (P<0.01) (Figure 6C). During the course of the disease, the diabetic podTcf21 mice consistently developed massive proteinuria, with significant increase in protein-to-creatinine ratio to a mean±SEM of 18.60±5.36 mg/mg in diabetic podTcf21 mice compared with 5.23±1.18 mg/mg in nondiabetic podTcf21 mice at 9 weeks of diabetes and 26 weeks of age, respectively (P<0.04) (Figure 6A). Diabetic control mice developed only minimal mesangial expansion; however, kidneys from proteinuric diabetic podTcf21 mice showed dramatic glomerulosclerosis with periodic acid-Schiff–positive nodular structures (Figure 6B). In diabetic podTcf21 mutants, cumulative incidence of proteinuria>10 mg/mg protein/creatinine was 87.8% at 18 weeks of diabetes (35 weeks of age) versus 22.2% in nondiabetic podTcf21 mice at the same age (P<0.001) (Figure 6D). Blood glucose did not differ between podTcf21 and control mice in diabetic and nondiabetic conditions, but diabetic podTcf21 mice showed a small decrease in body weight by study end (Supplemental Figure 5). Finally, we examined how diabetes affects expression of Tcf21 in wild-type mice. Real-time PCR showed that glomerular Tcf21 expression is decreased to 36% in wild-type mice with streptozotocin-induced diabetes (Figure 6E). Together, these data clearly demonstrate that podTcf21 mice are susceptible to diabetic nephropathy.
Discussion
In recent years, several genetic discoveries have established the podocyte as a key player in glomerular health and disease. Although our understanding of podocyte-expressed cytoskeletal proteins and growth factors has grown extensively, less is known about the transcription factors that control podocyte function. Tcf21 belongs to the bHLH family of transcription factors and is highly expressed in podocyte precursors and mature podocytes.
Previously we reported a severe defect in renal development in Tcf21 conventional knockout mice.9 Here, we show for the first time that loss of Tcf21 from podocyte progenitors results in an arrest of podocyte differentiation. However, somewhat surprisingly, despite persistent high levels of Tcf21 expression throughout podocyte development, deletion at a slightly later developmental time-point in capillary loop glomeruli does not appear to affect differentiation of the complex cytoskeletal architecture of the podocyte. Furthermore, glomerular barrier function remains intact until weaning, when the glomerulus must deal with a substantially increased filtration load. Indeed, in guinea pigs, the GFR increases 7-fold in the first month of life, which is likely similar in mice. In humans, it increases from 30 to 100 ml/min per 1.73 m2 during the first year of life.19–21
Although podTcf21 mice do not demonstrate any obvious defects in podocyte differentiation, they do show retardation in glomerular maturation, with fewer mesangial and endothelial cells per glomerulus. In the developing glomeruli, podocytes express cytokines and growth factors, including VEGF-A, that are necessary to recruit endothelial and mesangial precursor cells into the vascular cleft, where they form the glomerular tuft and capillary loops.4,22 Expression profiling and real-time PCR results from podTcf21 glomeruli reveal the downregulation of several key angiogenic factors, including VEGF-A, Pgf, Dll4, Hey2, Flt1/Vegfr-1, and soluble Flt1. Interestingly, all these factors are increased at 3 weeks of age in glomeruli of control mice compared with day 0, but this upregulation is attenuated in podTcf21 glomeruli, suggesting that Tcf21 is upstream of an angiogenic network.
Another surprising finding in our study was the bimodal penetrance pattern of glomerular injury in podTcf21 mice. Despite equivalent excision of the Tcf21 gene (determined by glomerular mRNA expression), approximately 40% of mice developed massive proteinuria around 3–5 weeks of age, while the remaining 60% appeared protected. Furthermore, most of the protected cohort never developed proteinuria even by 1 year of age. We suspect that this difference relates to major modifying loci that vary because of the mixed background strain (C57Bl6, 129×1/SvJ, and CD1) of mice studied.
The different phenotypes observed in podTcf21 versus wnt4creTcf21 mice supports an important role of Tcf21 in early stages of podocyte differentiation. Tcf21 mRNA was absent from S-shaped body glomeruli in wnt4creTcf21 mice and from the later capillary loop stage in podTcf21 mice, respectively. The excellent podocyte excision (65% at P0 and 97% by 3 weeks) in podTcf21 mice suggests that the earlier timing of excision rather than mosaic excision explains the more severe phenotype observed in wnt4creTcf21 mice.
Diabetic nephropathy is the leading cause of ESRD in North America, and alterations in Tcf21 expression have been reported in biopsy specimens from diabetic patients.23 Therefore, we wondered whether glomeruli in nonproteinuric podTcf21 mice were more susceptible to an injury because of diabetes and induced diabetes in a cohort of protected mutants. PodTcf21 mice showed a dramatic increase in diabetic renal injury with a significantly increased incidence of proteinuria, glomerular injury, and mortality compared with control diabetic mice. Tcf21 levels are decreased in renal mRNA samples isolated from kidneys of patients with diabetic nephropathy.23 In db/db mice, Tcf21 levels are increased during the early phase of diabetic nephropathy but decreased at the late stage.24 We also confirmed its decrease in glomeruli from diabetic control mice after 10 weeks of diabetes. These results suggest that Tcf21 plays a protective role in diabetic nephropathy. It is interesting to speculate that Tcf21 protects the podocyte during times of increased glomerular filtration, which occurs at the time of weaning and also in early phases of diabetic nephropathy and may occur in other renal diseases.
In summary, the current study demonstrates critical roles for Tcf21 in developing and mature podocytes in both physiologic and disease conditions. Tcf21 works to modulate podocyte function and is required when podocytes/glomeruli are undergoing dynamic differentiation, maturation, and aging. In addition, our data reveal protective roles of Tcf21 in diabetic nephropathy. Identification of the direct targets of Tcf21 may provide us new therapeutic approaches for proteinuric renal diseases, including FSGS and diabetic nephropathy.
Concise Methods
Mouse Lines
The floxed Tcf21 targeting vector was created using bacterial artificial chromosome (BAC) recombineering, detailed procedures were described previously.25 Briefly, a first loxP site was inserted at a nonconserved region before the first exon of the Tcf21 gene. A second loxP site and PGK-neo selection cassette were inserted in a nonconserved region of intron 1. ES cell clones were screened using Southern blot analysis with probes located outside the homology arms. One correctly targeted ES cell clone was used for ES cell aggregation that produced chimeras and gave germline transmission.
The Tcf21-LacZ mouse is a conventional knockout mouse for Tcf21 and was described elsewhere.9 Tcf21-lacZ and Tcf21-floxed mice were bred to the podocin-cre driver strain to generate podocyte specific Tcf21 knockout mice (podocin-cre/Tcf21floxed/LacZ, referred to as podTcf21). The Wnt4-cre driver mouse line is described elsewhere, and the gene excision occurs in renal vesicles, pretubular aggregates, and stromal cells.17 The Tcf21 floxed and Tcf21-LacZ mice were bred to wnt4-cre mice, and wnt4-cre/Tcf21floxed/LacZ mice were generated (referred to as wnt4creTcf21). Littermates that lack at least one of the transgenes were used as controls.
Additional information on methods is available in the Supplemental Material.
Phenotypic Analysis
Urine was collected at designated time-points and tested using urine dipsticks (Chemstrip 5L; Roche). Additionally, urinary protein concentration was quantified by Bradford assay. Briefly, the protein assay dye (500–0006; Bio-Rad) was added to urine samples; after 10 minutes’ incubation, absorbance was measured at 595 nm. Urinary creatinine concentration was measured by the Jaffe method.26 The results are reported as urinary protein-to-creatinine ratio (mg/mg).
Mice were euthanized at designated time-points as described, and micrographs were obtained using a dissection microscope (Leica MZ6). Organs were fixed in 10% formalin in PBS and subjected to histologic analysis. For ultrastructural analysis using an electron microscope, kidneys were fixed in 0.1 M sodium cacodylate buffer with 2% glutaraldehyde.
Immunostaining
For snap-frozen sections, dissected kidneys were immediately embedded in Tissue-Tek optimal cutting temperature (OCT) 4583 compound (Sakura Finetek, Inc.) after dissection. Some kidney specimens were also fixed in 4% paraformaldehyde overnight at 4°C, cryoprotected in 30% sucrose overnight, and then embedded in optimal cutting temperature compound. For paraffin sections, kidneys were fixed in 10% formalin in PBS and embedded in paraffin. Sections 5 µm thick were cut, rehydrated, and boiled in TEG buffer (10 mM Tris, 0.5 mM ethylene glycol tetraacetic acid; pH, 9.0) for antigen retrieval. After blocking (10% goat serum, 0.3% Triton X100, 3% albumin in PBS), primary antibodies were applied to sections overnight at 4°C. After washing, sections were incubated with secondary antibodies for 1 hour at room temperature.
In Situ Hybridization
For in situ hybridization, the samples were fixed in diethylpyrocarbonate-treated 4% paraformaldehyde overnight at 4°C, cryoprotected in 30% sucrose, embedded in OCT compound, and frozen. Digoxigenin-conjugated RNA probes were made using a DIG RNA labeling kit according to the manufacturer’s instruction (Roche). The detailed procedure of in situ hybridization is described elsewhere.27
Isolation of Glomeruli
The detailed procedure of glomerular isolation was described previously.28 Briefly, mice were anesthetized and perfused with magnetic microbeads (Dynabeads M-450 tosylactivated; Dynal) from the left ventricle. Kidneys were minced, digested with collagenase A (Roche), and filtered through a cell strainer. Glomeruli were then isolated by using magnetic apparatus (Dynal).
RNA Extraction, Microarray Analysis, and Quantitative Real-Time PCR
Total RNA was extracted with an RNeasy mini kit (Qiagen), and the quality was evaluated by a 2100 Bioanalyzer (Agilent Technologies). Affymetrix Mouse GeneChip 1.0 ST was used for RNA microarray analysis, which was done in the Center for Applied Genomics (The Hospital for Sick Children, Toronto, ON, Canada). Microarray data were analyzed using Genomics Suite software (Partek). Reverse transcription was performed using an iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instruction. cDNA samples were amplified by iTaq SYBR Green Supermix (Bio-Rad). Comparative gene expression was calculated using the delta-delta CT method (Applied Biosystems), and described relative to the housekeeping gene, Hprt. Specific primers for the genes are provided in Supplemental Table 1.
Diabetes
Seventeen-week-old control mice (n=12) and nonproteinuric podTcf21 mice (n=12) were made diabetic by 50 mg/kg intraperitoneal injection of streptozotocin (Sigma-Aldrich) for 5 days according to the protocol for animal models from the Diabetic Complications Consortium (www.diacomp.org). Mice with blood glucose>20 mM were used in the experiment.
Statistical Analyses
Statistical analyses were performed using a two-tailed t test using Graphpad Prism. For survival and cumulative occurrence of proteinuria, log-rank analysis was used. P<0.05 was considered to represent a statistically significant difference.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Ken Harpal and Douglas Holmyard for histology and EM analysis. We thank Chao Lu and staff for help with microarray analysis and Dr. Paul Thorner, Mazdak Bagherie, and Antoine Reginensi for helpful discussions. This work is supported by Canadian Institutes of Health Research grant 62931 and a Terry Fox grant.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121307/-/DCSupplemental.
References
- 1.Maezawa Y, Cina D, Quaggin SE: Glomerular Cell Biology. In: Seldin and Giebisch's the Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Moe OW, Caplan M, 5th Ed., San Diego, Academic Press, 2012, pp 721–755 [Google Scholar]
- 2.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R: WT-1 is required for early kidney development. Cell 74: 679–691, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Miner JH, Morello R, Andrews KL, Li C, Antignac C, Shaw AS, Lee B: Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest 109: 1065–1072, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadl V, Jin F, Yu J, Cui S, Holmyard D, Quaggin S, Barsh G, Cordes S: The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol 249: 16–29, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J: The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 126: 5771–5783, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE: Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 131: 4095–4105, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Chang P, Richardson JA, Gan L, Weiler H, Olson EN: The basic helix-loop-helix transcription factor capsulin controls spleen organogenesis. Proc Natl Acad Sci USA 97: 9525–9530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu JR, Bassel-Duby R, Hawkins A, Chang P, Valdez R, Wu H, Gan L, Shelton JM, Richardson JA, Olson EN: Control of facial muscle development by MyoR and capsulin. Science 298: 2378–2381, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Novak A, Guo C, Yang W, Nagy A, Lobe CG: Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28: 147–155, 2000 [PubMed] [Google Scholar]
- 16.Miner JH: Renal basement membrane components. Kidney Int 56: 2016–2024, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Shan J, Jokela T, Skovorodkin I, Vainio S: Mapping of the fate of cell lineages generated from cells that express the Wnt4 gene by time-lapse during kidney development. Differentiation 79: 57–64, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Breyer MD, Böttinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K, AMDCC : Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Dean RF, McCance RA: Inulin, diodone, creatinine and urea clearances in newborn infants. J Physiol 106: 431–439, 1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitzer A, Brandis M: Functional and morphologic maturation of the superficial nephrons. Relationship to total kidney function. J Clin Invest 53: 279–287, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin MI, Bruck E, Rapoport M: Maturation of renal function in childhood; clearance studies. J Clin Invest 28: 1144–1162, 1949 [PubMed] [Google Scholar]
- 22.Maezawa Y, Kreidberg JA, Quaggin SE: CHAPTER 1- Embryology of the Kidney. Brenner and Rector's the Kidney, Philadelphia, PA, W.B. Saunders, 2011 [Google Scholar]
- 23.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K: Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makino H, Miyamoto Y, Sawai K, Mori K, Mukoyama M, Nakao K, Yoshimasa Y, Suga S: Altered gene expression related to glomerulogenesis and podocyte structure in early diabetic nephropathy of db/db mice and its restoration by pioglitazone. Diabetes 55: 2747–2756, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Jenkins NA, Copeland NG: A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13: 476–484, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husdan H, Rapoport A: Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin Chem 14: 222–238, 1968 [PubMed] [Google Scholar]
- 27.Piscione TD, Wu MY, Quaggin SE: Expression of Hairy/Enhancer of Split genes, Hes1 and Hes5, during murine nephron morphogenesis. Gene Expr Patterns 4: 707–711, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.