Abstract
In 2009, the Scottish government issued a target to reduce Clostridium difficile infection by 30% in 2 years. Consequently, Scottish hospitals changed from cephalosporins to gentamicin for surgical antibiotic prophylaxis. This study examined rates of postoperative AKI before and after this policy change. The study population comprised 12,482 adults undergoing surgery (orthopedic, urology, vascular, gastrointestinal, and gynecology) with antibiotic prophylaxis between October 1, 2006, and September 30, 2010 in the Tayside region of Scotland. Postoperative AKI was defined by the Kidney Disease Improving Global Outcomes criteria. The study design was an interrupted time series with segmented regression analysis. In orthopedic patients, change in policy from cefuroxime to flucloxacillin (two doses of 1 g) and single-dose gentamicin (4 mg/kg) was associated with a 94% increase in AKI (P=0.04; 95% confidence interval, 93.8% to 94.3%). Most patients who developed AKI after prophylactic gentamicin had stage 1 AKI, but some patients developed persistent stage 2 or stage 3 AKI. The antibiotic policy change was not associated with a significant increase in AKI in the other groups. Regardless of antibiotic regimen, however, rates of AKI were high (24%) after vascular surgery, and increased steadily after gastrointestinal surgery. Rates could only be ascertained in 52% of urology patients and 47% of gynecology patients because of a lack of creatinine testing. These results suggest that gentamicin should be avoided in orthopedic patients in the perioperative period. Our findings also raise concerns about the increasing prevalence of postoperative AKI and failures to consistently measure postoperative renal function.
Reported rates of postoperative AKI vary because of the heterogeneity of the populations studied. Uncomplicated AKI is associated with a mortality of 10%, rising to 50% in the context of multiorgan failure and up to 80% if RRT is required.1,2 It was thought that the presence of AKI was a marker of coexisting pathology that increased mortality risk, but recent reports demonstrate AKI as an independent risk factor for mortality.3,4 The increasing incidence of AKI and its long-term consequences have significant socioeconomic and public health effects globally.5
Clostridium difficile infection (CDI) is an important healthcare-associated infection. Antibiotic use increases the risk of CDI for at least 3 months6 and short courses of perioperative antibiotic prophylaxis have also been associated with an increased risk of CDI, particularly in the context of an established outbreak.7
In 2009, the Scottish government issued a new target for all health boards to reduce CDI by at least 30% over 2 years.8 The Scottish Antimicrobial Prescribing Group also produced recommendations for all National Health Service (NHS) boards to restrict the use of antibiotics associated with a high risk of CDI.9 As part of a widespread antibiotic policy change at NHS Tayside, orthopedic antibiotic prophylaxis was changed from cefuroxime to gentamicin and flucloxacillin. After concerns raised by nephrologists and a small uncontrolled study in the Dumfries and Galloway region of Scotland that described an increased rate of AKI in patients after orthopedic surgery after this policy change,10 it was felt that further investigation was required.
This study aimed to use robust methodology, in a larger, population-based study of adult patients undergoing orthopedic implant surgery, to evaluate the effect of the policy change on postoperative AKI. It is noteworthy that patients who underwent repair of a neck of femur (NOF) fracture received coamoxiclav as antibiotic prophylaxis after the policy change because of concerns raised by orthopedic surgeons with regard to administering gentamicin in this particular patient group. This analysis was then extended to evaluate postoperative AKI in other surgical specialties (urology, vascular, gastrointestinal, and gynecology) that had changed to a gentamicin-based regimen.
Results
Descriptive Data
In total, 12,482 patients were included in the analysis from October 1, 2006, to September 30, 2010. Table 1 describes the baseline characteristics of the population. Of note, within the orthopedic patient group, 36% of patients were prescribed a nonsteroidal anti-inflammatory drug (NSAID) in the year before their operation and 38.5% were prescribed a diuretic. A comparison of the patients with and without AKI in the orthopedic group showed that only an increasing Charlson Comorbidity Index (CCI) score was associated with an increased risk of AKI on multivariate analysis (95% confidence interval [95% CI], 1.03 to 1.22; P<0.001). In urology patients, there was no difference between individuals with or without AKI. In vascular patients, an increasing CCI was associated with an increased risk of AKI on multivariate analysis (95% CI, 1.08 to 1.42; P=0.03). In gastrointestinal patients, older age (95% CI, 1.03 to 1.07; P<0.001) and male sex (P=0.02; 95% CI, 0.39 to 0.91) were associated with an increased risk of AKI. In gynecology patients, older age was associated with an increased risk of AKI (95% CI, 1.02 to 1.15; P=0.02).
Table 1.
Descriptive data for other surgical specialties
| Characteristic | Orthopedics | Urology | Vascular Surgery | Gastrointestinal Surgery | Gynecology | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Intervention | After Intervention | Before Intervention | After Intervention | Before Intervention | After Intervention | Before Intervention | After Intervention | Before Intervention | After Intervention | |
| Recommended antibiotics (dose) | Cefuroxime (1.5 g) | Flucloxacillin (1 g)×2 plus gentamicin (4 mg/kg) | Coamoxiclav (1.2 g) | Gentamicin (4 mg/kg) | Coamoxiclav (1.2 g) | Flucloxacillin (1 g)×2 plus metronidazole (500 mg)±gentamicin (4 mg/kg) | Coamoxiclav (1.2 g) | Metronidazole (500 mg) plus gentamicin (4 mg/kg) | Coamoxiclav (1.2 g) | Metronidazole (500 mg) plus gentamicin (4 mg/kg) |
| Patients (n) | 3674 | 3992 | 421 | 402 | 358 | 362 | 1599 | 1672 | 176 | 227 |
| Age (yr) | 71.2 (13.5) | 70.7 (13.9) | 71.4 (12.5) | 70.0 (13.1) | 70.0 (11.3) | 69.0 (12.9) | 62.1 (15.9) | 61.8 (16.3) | 54.9 (13.7) | 53.5 (13.7) |
| Baseline SCr (µmol/L) | 79.0 (66.0, 94.0) | 75.0 (62.0, 90.0) | 92.0 (77.0, 114.0) | 90.0 (74.0, 114.0) | 89.5 (75.0, 113.0) | 84.0 (67.0, 108.0) | 75.0 (62.0, 89.0) | 71.0 (60.0, 85.0) | 71.0 (61.0, 83.0) | 65.0 (58.0, 75.0) |
| Sex (%) | ||||||||||
| Women | 2359 (64.2) | 2451 (61.4) | 101 (24.0) | 87 (21.6) | 117 (32.7) | 241 (67.3) | 953 (57.0) | 902 (56.4) | 176 (100) | 227 (100) |
| Men | 1315 (35.8) | 1541 (38.6) | 320 (76.0) | 315 (78.4) | 241 (67.3) | 234 (64.6) | 719 (43.0) | 697 (43.6) | 0 | 0 |
| SIMD (%) | ||||||||||
| 1–3 | 706 (19.4) | 788 (20.0) | 88 (21.2) | 108 (27.2) | 111 (31.2) | 104 (29.0) | 402 (24.4) | 352 (22.4) | 40 (23.0) | 59 (26.0) |
| 4–7 | 1616 (44.5) | 1716 (43.6) | 176 (42.4) | 161 (40.6) | 143 (40.2) | 151 (42.1) | 682 (41.4) | 676 (43.1) | 70 (40.2) | 100 (44.1) |
| 8–10 | 1311 (36.1) | 1429 (36.3) | 151 (36.4) | 128 (32.2) | 102 (28.7) | 104 (29.0) | 565 (34.3) | 541 (34.5) | 64 (36.8) | 68 (30.0) |
| CCI (%) | ||||||||||
| Low (0 or 1) | 3494 (95.1) | 3774 (94.5) | 206 (48.9) | 230 (57.2) | 244 (68.2) | 236 (65.2) | 951 (59.5) | 1040 (62.2) | 122 (69.3) | 152 (67.0) |
| Medium (2) | 121 (3.3) | 145 (3.6) | 185 (43.9) | 128 (31.8) | 68 (19.0) | 73 (20.2) | 432 (27.0) | 425 (25.4) | 45 (25.6) | 65 (28.6) |
| High (≥3) | 59 (1.6) | 73 (1.8) | 30 (7.1) | 44 (10.9) | 46 (12.8) | 53 (14.6) | 216 (13.5) | 207 (12.4) | 9 (5.1) | 10 (4.4) |
Data are presented as the mean (SD) or median (IQR). SCr, serum creatinine; SIMD, Scottish Index of Multiple Deprivation.
Characteristics of Patients with Missing Data
Table 2 shows the percentage of available data for each specialty. Biochemistry data were available in only 35% of gynecology patients and 58% of urology patients before the intervention and in 47% of gynecology patients and 52% of urology patients after the intervention. The majority of missing data were missing postoperative serum creatinine measurements, rather than preoperatively. Supplemental Table 3 shows multivariate analyses of the characteristics of patients included in the study versus patients excluded because of missing data. An examination of the characteristics of included patients compared with patients who were excluded due to missing data showed that the included orthopedic patients were older men with higher CCI comorbidity scores, as were the gastrointestinal patients.
Table 2.
Completeness of creatinine data
| Operation | Before Policy Change | After Policy Change | Chi Square | P Value | Preoperative | Postoperative | Preoperative and Postoperative |
|---|---|---|---|---|---|---|---|
| All orthopedic NOF | 3674 (81) | 4009 (82) | 3.68 | 0.05 | 8883 (94.5) | 7917 (84.2) | 7698 (81.9) |
| Urology | 421 (58) | 402 (52) | 4.38 | 0.04 | 1396 (92.9) | 828 (55.1) | 823 (54.8) |
| Vascular | 358 (89) | 362 (86) | 2.00 | 0.16 | 798 (96.8) | 727 (88.2) | 720 (87.4) |
| Gastrointestinal | 1599 (78) | 1672 (78) | 0.50 | 0.48 | 4089 (97.6) | 3315 (79.2) | 3271 (78.1) |
| Gynecology | 176 (35) | 227 (47) | 12.76 | <0.001 | 927 (94.2) | 423 (43.0) | 403 (41.0) |
Data are presented as n (%) of patients with creatinine data.
Results of Interrupted Time Series Analysis
Orthopedic Patients
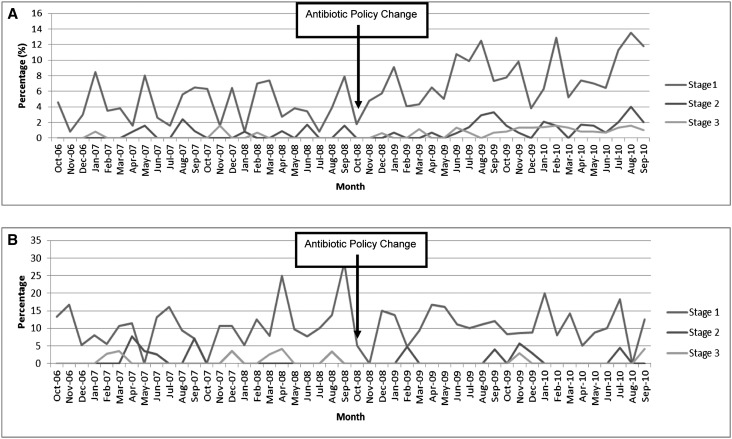
Figure 1A shows the percentage of AKI stages 1, 2, and 3 for each study month. Interrupted time series (ITS) analyses showed that there was a significant increase in AKI after the change in prophylactic regimen. After adjustment for age, sex, and use of other nephrotoxic drugs, only the change in policy was significantly associated with an increase in AKI, with a significant change in all levels of severity of AKI after the policy change (β=0.30; 95% CI, 0.01 to 0.59; P=0.04) for the highest serum creatinine postoperatively (Table 3).
Figure 1.
(A) Increase in percentage of AKI (adjusted) stages 1, 2, and 3 for each month (excluding NOF) following policy change. (B) No increase in percentage of AKI stage 1, 2, and 3 for each month in patients with a NOF fracture.
Table 3.
ITS of monthly percentage of AKI in orthopedic surgery patients using preoperative versus highest postoperative creatinine measurement, excluding patients with a NOF fracture
| Variable | β (95% Confidence Interval) | P Value |
|---|---|---|
| Time | 0.08 (−0.12 to 0.28) | 0.40 |
| Intervention (0, before policy change; 1, after policy change) | −1.65 (−5.45 to 2.16) | 0.39 |
| Time after intervention | 0.30 (0.01 to 0.59) | 0.04 |
| Percentage of men (+1%) | −0.07 (−0.30 to 0.16) | 0.52 |
| Mean age (+1 year) | 0.09 (−0.69 to 0.88) | 0.81 |
| Percentage of β-blockers (+1%) | −3.85 (−8.24 to 0.54) | 0.08 |
Patients who underwent repair of the NOF received coamoxiclav throughout the study period. An analysis of these patients alone showed that the changes in slope for before and after the policy change were virtually zero (β=−0.106; 95% CI, −0.69 to 0.48, P=0.77) (Figure 1B).
Other Surgical Specialties
Results of ITS analyses are shown in Tables 4 and 5 for urology, vascular, gastrointestinal, and gynecologic surgery patients. There was no significant increase in the rates of AKI in these surgical specialties related to the policy change. However, the baseline rates of AKI in vascular surgery were high at 23.2% but did not increase significantly after the policy change. In gastrointestinal surgery, there was a 1.2% monthly increase in AKI (P=0.29) before the policy change, with a 0.6% monthly increase after the change in policy, indicating rates that were already increasing and slowing after the policy changed.
Table 4.
Summary of results in all surgical specialties
| Variable | Fracture NOF | Other Orthopedic Implant Surgery | Urological Surgery | Vascular Surgery | Gastrointestinal | Gynecology |
|---|---|---|---|---|---|---|
| Postoperative AKI before the policy change (%)a | 104 (15.0) | 186 (6.2) | 49 (11.6) | 83 (23.2) | 118 (7.4) | 8 (4.5) |
| Postoperative AKI after the policy change (%)a | 94 (14.8) | 361 (10.8) | 63 (15.7) | 91 (25.1) | 204 (12.2) | 9 (4.0) |
| Percent change in AKI (after versus before the policy change) (95% CI) | −9.6 (−10.2 to −9.1) | +94 (93.8 to 94.3) | +28.6 (26.8 to 30.4) | +9.6 (8.9 to 10.3) | +72.9 (72.2 to 73.6) | +12.5 (4.4 to 20.6) |
| IRR after versus before the policy changeb | 0.99 (0.75 to 1.30) | 1.72 (1.43 to 2.05) | 1.35 (0.93 to 1.95) | 1.08 (0.80 to 1.45) | 1.65 (1.32 to 2.07) | 0.87 (0.34 to 2.26) |
Data are presented as n (%), percent change (95% CI), or IRR (95% CI). IRR, incidence rate ratio.
Percentage of patients with Acute Kidney Injury Network stages 1–3 (at least 150% increase in serum creatinine).
The incidence rate ratio is calculated as follows: C-post/T-post or C-pre/T-pre, where C-post is number of patients in the postoperative period, T-post is number of person-years at risk in the postoperative period, C-pre is the number of patients in the preoperative period, and T-pre is the number of person-years at risk in the preoperative period
Table 5.
Segmented regression analysis, point estimates (β), and P values for all surgical specialties
| Variable | Fracture NOF | Other Orthopedic Implant Surgery | Urological Surgery | Vascular Surgery | Gastrointestinal | Gynecology | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | β | P Value | β | P Value | β | P Value | |
| Preintervention slope | 0.17 (−0.24 to 0.58) | 0.41 | 0.08 (−0.12 to 0.28) | 0.40 | −0.00 (−0.01 to 0.02) | 0.99 | 0.02 (−0.01 to 0.04) | 0.18 | 0.01 (−0.01 to 0.04) | 0.29 | −0.04 (−0.13 to 0.05) | 0.35 |
| Change in level | −3.68 (−11.74 to 4.37) | 0.36 | −1.65 (−5.45 to 2.16) | 0.39 | −0.01 (−0.24 to 0.22) | 0.91 | −0.03 (−0.50 to 0.45) | 0.92 | −0.14 (−0.52 to 0.25) | 0.49 | −0.48 (−2.42 to 1.46) | 0.63 |
| Change in slope | −0.11 (−0.69 to 0.48) | 0.77 | 0.30 (0.01 to 0.59) | 0.04 | 0.01 (−0.01 to 0.03) | 0.16 | −0.03 (−0.07 to 0.01) | 0.11 | 0.01 (−0.02 to 0.03) | 0.68 | 0.04 (−0.09 to 0.16) | 0.55 |
Data are presented with 95% CIs.
Orthopedic Outcome Data
A higher proportion of orthopedic patients with AKI died within 1 year of surgery compared with patients without AKI (20.8% versus 8.2%, respectively). The median length of hospital stay was 8 days (interquartile range [IQR], 5–13) in patients with AKI compared with 7 days (IQR, 4–10) in patients without AKI. There were 25 patients with persistent stage 2 or stage 3 AKI at 7 days postoperatively. None of these patients underwent a renal biopsy. Five patients died during the admission without receiving RRT and six patients received RRT. All surviving patients recovered their renal function to baseline.
Discussion
In this large population-based study of >12,000 patients, we found that increased rates of AKI were associated with a change in prophylactic antibiotic policy from cefuroxime to flucloxacillin and gentamicin in patients undergoing orthopedic surgery (excluding repair of a NOF fracture) in the Tayside region of Scotland. The majority of patients had transient stage 1 AKI but there were patients with persisting stages 2 and 3. This association with the antibiotic policy change is strengthened because there was no increase in AKI rates in patients with a NOF fracture who received coamoxiclav as prophylaxis after the policy change. CDI rates fell in both of these groups, suggesting that the orthopedic antibiotic prophylaxis policy change was not responsible for this reduction. We did not find any association between the change in prophylactic antibiotic policy to include gentamicin with AKI in urology, vascular, gastrointestinal, and gynecology surgical patients.
In addition, we identified three areas of concern. First, rates of postoperative AKI in vascular surgery were especially high, at 23% before the intervention and 25% after the intervention. Second, rates of postoperative AKI steadily increased in gastrointestinal surgery patients throughout the study period. Finally, rates of postoperative AKI data could only be ascertained in 52% of urology patients and 47% gynecology of patients because of missing postoperative serum creatinine data.
The strengths of our study are that we addressed risks of bias for ITS studies in our analysis plan (Supplemental Table 2), defined the operations using operation procedure codes (OPCs) (Supplemental Table 1), and used the Kidney Disease Improving Global Outcomes (KDIGO) definition of AKI. The limitations of our study are common to all nonrandomized studies, including potential ascertainment and selection biases. Randomized controlled trials are often not appropriate and/or are too expensive to assess the effects of global policy change. The ITS is the strongest quasi-experimental design to assess intervention effects in nonrandomized settings. It controls for trends existing before the intervention by using multiple points before and after the intervention.11,12 A specific limitation of this study was missing preoperative or postoperative serum creatinine measurements in a large proportion of urology and gynecology patients. The patients who were included were older and had greater comorbidity compared with patients who were excluded because of missing creatinine data. This could bias the results in these groups in either direction in estimating the rates of AKI. In addition, we were unable to adjust for potential effects of medication and intraoperative events on AKI rates because these data are not collected electronically.
AKI occurs in 5%–7.5% of acute care hospital inpatients. Of these patients, 30%–40% of AKI incidences occur perioperatively depending on the surgical setting.13,14 The incidence of AKI varies according to the surgical specialty, with the majority of the epidemiologic literature in cardiac and vascular surgery.15
We have shown that rates of AKI vary depending on the surgical setting, ranging from 6% in orthopedic surgery to 25% in vascular surgery. This suggests that patients undergoing vascular surgery are already at risk of developing AKI. Factors that may contribute to this predisposition include age, comorbidity, use of intravenous contrast for vascular imaging and intervention, renovascular disease, and presence of sepsis. Although gentamicin is included in the vascular surgery policy, it is optional and many patients may not receive it because they are deemed to be at high risk for developing AKI, which may account for the results in vascular patients. In gastrointestinal patients, rates of AKI were increasing before the policy change, with a slower increase after the policy change. Therefore, although rates of AKI are higher in the postintervention period, this increase cannot be attributed to the policy change and thus requires investigation into other causes. The large amount of missing data in urology and gynecology patients with a difference in testing before and after intervention threatens the validity of these data. It is, however, important to note that a large number of patients who are attending for major surgical procedures are not having their renal function checked in the preoperative or postoperative period, particularly as the evolving literature indicates an increase in short- and long-term consequences of AKI in this population. The KDIGO AKI guidelines state that major surgery is an exposure for AKI; thus, these patients should have their serum creatinine and urine output measured, according to their clinical status.16
Patients who receive gentamicin prophylaxis are generally undergoing major surgery and we thus suggest that a minimum standard of care should be that they have their blood levels checked preoperatively and at least 24 hours postoperatively.
The development of AKI is associated with longer hospital inpatient stays and increased mortality13,14,17 as well as a greater risk of readmission, development and progression of CKD, and poorer long-term survival.18 Epidemiologic data have shown that survivors of AKI have higher long-term mortality rates than those patients who survive hospitalization without AKI. This association becomes stronger as the severity of AKI increases but is present in patients with small reversible rises in serum creatinine.18 There is a lack of therapeutic intervention available once AKI is established. The need for RRT is associated with mortality rates of up to 80%, increased hospital stay, and significantly increased cost. Earlier detection and recognition of AKI to prevent progression and the need for RRT are therefore imperative both for reducing short- and long-term morbidity and mortality as well as economic benefits.
It remains unclear whether gentamicin or flucloxacillin in the doses described, or indeed the combination of both, increased the incidence of AKI in this patient group. None of the patients in our study underwent renal biopsy, so we cannot ascertain the exact mechanism of renal injury. Flucloxacillin is associated with acute interstitial nephritis19 but this is a relatively uncommon adverse effect. Gentamicin is a direct tubular toxin, and toxicity during gentamicin therapy is more commonly observed. After glomerular filtration, some gentamicin remains in the lysosomes of the renal proximal tubular epithelium. With either prolonged dosing or supratherapeutic levels, the accumulation of the drug increases; it leaks from the lysosomes entering and damaging mitochondria. This leads to tubular epithelial cell death and the acute tubular necrosis–like picture that is typical of gentamicin toxicity.20 It has been shown that the nephrotoxic potential of gentamicin varies according to the population studied. Factors potentially relevant to our population include age, dehydration, preexisting renal impairment, and concomitant diuretics and NSAIDs.21 Several recent meta-analyses have shown that the risk of AKI as a result of aminoglycosides is high when used for the empirical treatment of severe Gram-positive or Gram-negative bacterial infections.22–25 Therefore, the KDIGO AKI guideline recommends that aminoglycosides should not be used for the treatment of infection unless no suitable, less nephrotoxic therapeutic alternatives are available.16 However, concerns regarding antimicrobial resistance as well as increasing rates of CDI have led to continued widespread gentamicin use. In Scotland, the relatively low levels of resistance to aminoglycosides among key pathogens in the therapeutic context described make them attractive agents.
The orthopedic population was older, with a mean age of 71 years, but the median baseline serum creatinine was 77 µmol/L (IQR, 64–92), indicating that preoperative renal function was preserved. Our results showed that 36% of the orthopedic patients were prescribed a NSAID in the year before surgery and 39% were prescribed a diuretic. However, we adjusted for these factors in our analysis.
We have demonstrated that a change in prophylactic antibiotic policy to flucloxacillin (two doses of 1 g) and single-dose gentamicin (4 mg/kg) was associated with increased rates of AKI in patients undergoing orthopedic implant surgery. We postulate that this is because they are a high-risk population for developing AKI. Therefore, greater attention to all modifiable risk factors, including prophylactic antibiotic choice, is vital in the perioperative period in order to reduce AKI risk in this vulnerable patient group. Our findings have led to a change in the national antibiotic policy recommendation for orthopedic surgical prophylaxis in Scotland and thus demonstrate the importance of measuring unintended consequences of healthcare interventions. Consequently, it is planned that this study will be repeated across other health boards in Scotland. We have also highlighted that rates of AKI in vascular surgery are high and AKI is rising in gastrointestinal surgery. Furthermore, it is concerning that there was a lack of testing for AKI in urology and gynecology surgery patients. Greater awareness and increased testing for this potentially reversible condition is vital.
Concise Methods
Study Design
This study included all adults aged>18 years who resided in or died in the NHS Tayside region and who underwent surgical procedures in which the revised surgical prophylaxis policy included gentamicin as part of a prophylactic antibiotics regime (Table 2) between October 1, 2006, and September 30, 2010. This study period encompassed 2 years before and after the change in the antibiotic policy. Patients were identified by the OPCs from hospital admissions data (Supplemental Table 1). Table 1 shows the recommended antibiotics and doses before and after the policy change. The primary definition of postoperative renal impairment used was the KDIGO criteria.16 This was applied using baseline serum creatinine as the premeasurement (most recent before surgery) and maximal serum creatinine during the first 7 postoperative days as the postmeasurement. Patients with postoperative AKI were classified according to their most severe degree of AKI. Stage 1 was defined as increase in serum creatinine of ≥26.4 µmol/L or an increase to 150%–200% of baseline. Stage 2 was an increase in serum creatinine to 200%–300% of the baseline value. Stage 3 was an increase in serum creatinine to >300% of baseline or a serum creatinine of ≥354 µmol/L or initiation of RRT.
Data were linked using the Health Informatics Centre (HIC)26 at the University of Dundee. The HIC enables anonymized health record linkage from the population of Tayside, Scotland (n=400,000), using a unique identifying Community Health Index (CHI) number. Data were linked between the following relevant data sets: Scottish Morbidity Record of hospital admissions and OPC-coded procedures, and laboratory results and medicines dispensed by community pharmacies.
Anonymized record linkage was conducted according to HIC standard operating procedure. The Tayside Research Ethics Committee does not require submission of individual studies that follow this standard operating procedure. We obtained permission from NHS Tayside’s Caldicott Guardian to identify patients who had severe postoperative AKI so that their case notes could be reviewed.
Data on age, to the nearest year in the year of surgery, and sex were obtained from the CHI register and social deprivation was obtained from the Scottish Index of Multiple Deprivation, linked to the postal code from the CHI register.
A CCI27 was calculated for each patient from hospital discharge codes and the number of dispensed prescriptions from community pharmacies in the previous year was applied as an additional measure of comorbidity. Exposure to medicines in the previous year that predispose to renal impairment (NSAIDs, cyclooxygenase-2 inhibitors, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, diuretics, and β-blockers) was ascertained from dispensed prescribing data.
Baseline renal function was obtained from the laboratory database. This was the most recent, preoperative serum creatinine measurement and could include preoperative samples taken during the current admission for elective surgery; however, patients undergoing emergency surgery may have AKI on admission to the hospital as a result of trauma. We therefore used the most recent serum creatinine measurement before admission for emergency patients to distinguish CKD from AKI. Only patients with preoperative and postoperative creatinine measurements could be included, so we measured and reported the completeness of data in each group. Patients on RRT were excluded from the analysis.
Statistical Analyses
Data from each surgical specialty (orthopedics, urology, vascular surgery, and obstetrics and gynecology) were analyzed separately. The design was an ITS study with segmented regression analysis28 using 24 monthly time points before and after the intervention in October 2008. The analysis plan included information that addressed the common risks of bias in ITS studies (Supplemental Table 1). The monthly rates of AKI were defined by the number of patients in each AKI stage as a proportion of all patients aged≥18 years undergoing surgery in each month. Rates were plotted over time for descriptive purposes and the functional form of the relationship before and after the intervention was assessed with splines. Rates were analyzed using multiple linear regression if the functional form was linear. Where monthly rates were not normally distributed, log transformation was used in the linear regression models to conform to the statistical criterion of normal distribution of residuals. All models were tested for autocorrelation using the Durbin–Watson statistic.29 Multivariate analyses including age, sex, nephrotoxic drugs, and comorbidity were carried out only when there was a significant change in AKI postintervention. Data for chronic nephrotoxic medication use were adjusted at an aggregate level. Poisson regression analysis was used when there were no or few patients for certain months. In Poisson regression, the outcome variable is in the form of counts or number of patients, and the natural log of the denominator (total number of operations) was included in the model as an offset. Analyses were carried out in IBM SPSS (version 21) and SAS (version 9.2.1) software.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was funded by the Scottish Government Healthcare Associated Infection Task Force.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Seen and the Unseen: Clinical Guidelines and Cost-Effective Care,” on pages 2390–2392.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010035/-/DCSupplemental.
References
- 1.Liaño F, Junco E, Pascual J, Madero R, Verde E, The Madrid Acute Renal Failure Study Group : The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int Suppl 66: S16–S24, 1998 [PubMed] [Google Scholar]
- 2.Cosentino F, Chaff C, Piedmonte M: Risk factors influencing survival in ICU acute renal failure. Nephrol Dial Transplant 9[Suppl 4]: 179–182, 1994 [PubMed] [Google Scholar]
- 3.Levy EM, Viscoli CM, Horwitz RI: The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996 [PubMed] [Google Scholar]
- 4.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R: Acute kidney injury: An increasing global concern. Lancet 382: 170–179, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ: Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 67: 742–748, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Carignan A, Allard C, Pepin J, Cossette B, Nault V, Valiquette L: Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis 46: 1838–1843, 2008 [DOI] [PubMed] [Google Scholar]
- 8.McGuire M, Keel A, Scott B: A Revised Framework for National Surveillance of Healthcare Associated Infection and the Introduction of a New Health Efficiency and Access to Treatment (HEAT) Target for Clostridium difficile Associated Disease (CDAD) for NHS Scotland. Edinburgh, UK, Scottish Government Health Department, 2009 [Google Scholar]
- 9.Scottish Antimicrobial Prescribing Group : Guidance to Optimise Antimicrobial Use and Reduce Clostridium difficile Associated Disease in Scottish Hospitals. Edinburgh, UK, Scottish Government Health Department, 2008 [Google Scholar]
- 10.Challagundla SR, Knox D, Hawkins A, Hamilton D, W V Flynn R, Robertson S, Isles C: Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 28: 612–619, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Ramsay C, Reeves BC, Deeks JJ, Shea B, Valentine JC, Tugwell P, Wells G: Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Research Synthesis Methods 4: 12–25, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE: Interrupted time series designs in health technology assessment: Lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 19: 613–623, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML: Incidence and outcomes of acute kidney injury in intensive care units: A Veterans Administration study. Crit Care Med 37: 2552–2558, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Thakar CV: Perioperative acute kidney injury. Adv Chronic Kidney Dis 20: 67–75, 2013 [DOI] [PubMed] [Google Scholar]
- 16.KDIGO Clinical Practice Guideline for Acute Kidney Injury Work Group : KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 17.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pusey CD, Saltissi D, Bloodworth L, Rainford DJ, Christie JL: Drug associated acute interstitial nephritis: Clinical and pathological features and the response to high dose steroid therapy. Q J Med 52: 194–211, 1983 [PubMed] [Google Scholar]
- 20.Zahar JR, Rioux C, Girou E, Hulin A, Sauve C, Bernier-Combes A, Brun-Buisson C, Lesprit P: Inappropriate prescribing of aminoglycosides: Risk factors and impact of an antibiotic control team. J Antimicrob Chemother 58: 651–656, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ: New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int 79: 33–45, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Bliziotis IA, Michalopoulos A, Kasiakou SK, Samonis G, Christodoulou C, Chrysanthopoulou S, Falagas ME: Ciprofloxacin vs an aminoglycoside in combination with a beta-lactam for the treatment of febrile neutropenia: A meta-analysis of randomized controlled trials. Mayo Clin Proc 80: 1146–1156, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Falagas ME, Matthaiou DK, Bliziotis IA: The role of aminoglycosides in combination with a beta-lactam for the treatment of bacterial endocarditis: A meta-analysis of comparative trials. J Antimicrob Chemother 57: 639–647, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Falagas ME, Matthaiou DK, Karveli EA, Peppas G: Meta-analysis: Randomized controlled trials of clindamycin/aminoglycoside vs. beta-lactam monotherapy for the treatment of intra-abdominal infections. Aliment Pharmacol Ther 25: 537–556, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L: Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: Systematic review and meta-analysis of randomised trials. BMJ 328: 668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of Dundee: Health Informatics Centre. Available at http://www.medicine.dundee.ac.uk/hic. Accessed March 24, 2014
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D: Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 27: 299–309, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Durbin J, Watson GS: Testing for serial correlation in least squares regression. I. Biometrika 37: 409–428, 1950 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.