Abstract
Rickettsia parkeri Luckman (Rickettsiales: Rickettsiaceae), a member of the spotted fever group of Rickettsia, is the tick-borne causative agent of a newly recognized, eschar-associated rickettsiosis. Because of its relatively recent designation as a pathogen, few studies have examined the pathogenesis of transmission of R. parkeri to the vertebrate host. To further elucidate the role of tick feeding in rickettsial infection of vertebrates, nymphal Amblyomma maculatum Koch (Acari: Ixodidae) were fed on C3H/HeJ mice intradermally inoculated with R. parkeri (Portsmouth strain). The ticks were allowed to feed to repletion, at which time samples were taken for histopathology, immunohistochemistry (IHC), quantitative polymerase chain reaction (qPCR) for rickettsial quantification, and reverse transcriptase polymerase chain reaction(RT-PCR)for expression of Itgax, Mcp1, and Il1β. The group of mice that received intradermal inoculation of R. parkeri with tick feeding displayed significant increases in rickettsial load and IHC staining, but not in cytokine expression, when compared with the group of mice that received intradermal inoculation of R. parkeri without tick feeding. Tick feeding alone was associated with histopathologic changes in the skin, but these changes, and particularly vascular pathology, were more pronounced in the skin of mice inoculated previously with R. parkeri and followed by tick feeding. The marked differences in IHC staining and qPCR for the R. parkeri with tick feeding group strongly suggest an important role for tick feeding in the early establishment of rickettsial infection in the skin.
Keywords: Rickettsia, Amblyomma, tick-borne, C3H/HeJ
The dramatic increase in recognition of tick-borne rickettsial diseases (TBRDs) over the past decade is punctuated by the emergence of a rickettsiosis caused by Rickettsia parkeri Luckman (Rickettsiales: Rickettsiaceae) in the southeastern United States and South America. Although originally described >70 yr ago, R. parkeri was first determined to be pathogenic to humans within the past decade (Parker et al. 1939, Paddock et al. 2004). The resulting rickettsiosis has since been diagnosed at least 20 times and demonstrates similarities to Rocky Mountain spotted fever (RMSF); however, R. parkeri rickettsiosis is typically a milder infection (Whitman et al. 2007; Paddock et al. 2008, 2010; Cragun et al. 2010; Romer et al. 2011). Although the geographic distributions of these rickettsiae are vastly different, the range of R. parkeri and its tick vector, Amblyomma maculatum Koch (Acari: Ixodidae), overlap greatly with the range of Rickettsia rickettsii Brumpt (Rickettsiales: Rickettsiaceae), the causative agent of RMSF, in the United States (Sumner et al. 2007, Paddock et al. 2008, Cragun et al. 2010, Trout et al., 2010, Jiang et al. 2012). The paucity of information and sympatry with other spotted fever group (SFG) Rickettsia for this eschar-associated disease necessitate comprehensive exploration of the mechanisms vital to infection establishment.
As a result of a prolonged feeding period, ticks have developed mechanisms to modify the host microenvironment to allow bloodmeal acquisition. Typical mechanisms include modulation of complement activation, natural killer cell function, antibody production, T-lymphocyte proliferative responses, and cytokine elaboration by antigen-presenting cells and T-lymphocytes (Wikel 1996). The influence of tick feeding on bacterial transmission to and infection of vertebrate hosts has been described for other systems. For example, the supplementation of cytokines normally down-regulated by tick feeding resulted in decreased infection rates in mice exposed to ticks infected with Borrelia burgdorferi Johnson (Spirochaetales: Spirochaetaceae) (Zeidner et al. 1996). Animals with acquired resistance to ticks have been shown to be more resistant to infection with pathogens transmitted by those ticks (Bell et al. 1979, Wikel et al. 1997, Nazario et al. 1998, Narasimhan et al. 2007, Dai et al. 2009). Some pathogens also undergo developmental transitions within the tick vector, which result in a form of the pathogen that is more infectious for the vertebrate host (Mastronunzio et al. 2012).
A murine model has recently been proposed for R. parkeri rickettsiosis, in which the C3H/HeJ strain of inbred mouse was determined to be the most susceptible (Grasperge et al. 2012). These mice lack competent TLR4 signaling due to a mutation, which causes an amino acid switch in the cytoplasmic domain of the TLR4 protein (Poltorak et al. 1998, Hoshino et al. 1999, Qureshi et al. 1999). These mice developed eschars upon intradermal inoculation of the tail and transient hypothermia with no other overt clinical signs. Interestingly, the eschars associated with R. parkeri rickettsiosis were inducible by intradermal inoculation of R. parkeri into the tail, but the same did not hold true for the skin over the nape of the neck (Grasperge et al. 2012). The reason for this difference is unclear but may relate to temperature differences at the inoculation sites or differences in immunological response of the tissues. Explanation of the mechanisms preventing infection at the inoculation site at the nape of the neck is central for understanding the pathogenesis of TBRDs, as this is a common site for tick feeding (Teel et al. 2010), and therefore a probable site for introduction of pathogenic rickettsiae. In this context, cutaneous inoculation of SFG Rickettsia represents the best route of infection to understand the pathology of eschar-associated rickettsioses such as those caused by R. parkeri.
Intuitively, the well-recognized effects of tick saliva on the regional immunology of the microenvironment at the feeding site should play a critical role in the inception of infection with tick-borne pathogens, but surprisingly little is known with respect to the influence of tick feeding on rickettsial infection. Here, the recently described model for R. parkeri rickettsiosis was used to evaluate the role of the tick in rickettsial infection of the vertebrate host. It was hypothesized that tick feeding enhances rickettsial infection of the cutaneous feeding site before dissemination of the infection. The results indicate that tick feeding at the site of rickettsial inoculation significantly enhances local rickettsial proliferation.
Materials and Methods
Mice
C3H/HeJ mice were selected based on previous susceptibility studies (Grasperge et al. 2012). Seven-week-old male mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice appeared healthy with no overt abnormalities and were ear punched for identification purposes. The animals were monitored daily during the course of the experiment for overt clinical signs of disease (i.e., unkempt fur, decreased activity, subcutaneous edema, erythema) and body temperature fluctuations. The research was performed under the approval of the IACUC at Louisiana State University.
Tick Preparation
A colony of A. maculatum was developed from wild-caught adults and maintained as previously described (Troughton and Levin 2007). Briefly, nymphal and adult ticks were fed on adult Sprague-Dawley rats (Division of Laboratory Animal Medicine, Louisiana State University) within capsules fashioned from 50-ml plastic conical tubes and attached with a 3:1 rosin to beeswax mixture. Engorged females were kept in vials at 27°C and ≈90% RH. Larvae were fed on adult BALB/c mice (Louisiana State University Division of Laboratory Animal Medicine) housed on wire grates over fresh water, and engorged larvae were collected twice daily as the water was changed. For this experiment, 120 nymphs, which originated from the same egg clutch were used. This colony of ticks is constitutively infected with rickettsiae, and sequence analysis using standard PCR for rompA (Rickettsia outer membrane protein A) identified this organism as the nonpathogenic Candidatus “Rickettsia andeanae” (Paddock et al. 2010).
Rickettsia Preparation
Semipurified rickettsiae were recovered from R. parkeri Portsmouth strain (Paddock et al. 2004), passage 4 infected Vero cells (5 d postinoculation; dpi) via needle (27 gauge) lysis of host cells and low- and high-speed centrifugation (Simser et al. 2001, Sunyakumthorn et al. 2008). Absolute concentration of rickettsiae was determined using the LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen, Carlsbad, CA), a bacterial counting chamber, and a fluorescent microscope (Kurtti et al. 2005). The rickettsiae were resuspended in sucrose-phosphate-glutamic acid buffer (Feng et al. 2004) to a desired inoculation dose of 5.5 × 106 rickettsiae/200 µl. Uninfected Vero cell culture was prepared using the same techniques as above with the exception of the bacterial counting. The final lysed Vero cell suspension was diluted with the same volume of buffer as the rickettsial suspension.
Inoculation and Tick Infestation
Mice were divided into five groups containing six animals each: age-matched controls, buffer injected control, R. parkeri injected, buffer injected + tick feeding, and R. parkeri injected + tick feeding. The hair over the nape of the neck was clipped for all mice except the age-matched controls. For each injection group, 200 µl of the appropriate inoculum was injected intradermally in the clipped area of skin over the nape of the neck. The bleb formed by the injection was marked with felt-tip pen immediately after inoculation, and the mark was retraced daily in the groups without tick feeding to overcome fading due to grooming and hair regrowth. The tick feeding groups were fitted with capsules fashioned from plastic 15-ml conical tubes directly over the inoculation site within 20 min of the injection. After allowing ≈30 min for the rosin/wax mixture to harden, 10 nymphal A. maculatum were added to each capsule. Ticks were allowed to feed to repletion and removed from the capsules at the time of detachment. The study was concluded as the final tick(s) detached at 8 d postinfestation.
Sample Collection
Mice were sacrificed at 8 dpi. Samples of skin from the inoculation site were snap frozen in liquid nitrogen for nucleic acid extraction, while additional samples of the skin were placed in RNAlater (Ambion, Austin, TX) until RNA extraction could be performed. Samples of the skin were fixed for histopathology.
Histopathology and Immunohistochemistry (IHC)
Tissues for histopathology were fixed overnight in 10% neutral buffered formalin. All sampled tissues were routinely processed and embedded in paraffin, and 3–4-µm sections were cut for hematoxylin and eosin (H&E) staining. The sections were examined in a randomized manner by a veterinary pathologist. Inflammation, edema, and necrosis were scored as follows: 0 = absence of the specified parameter, 1 = mild histologic change (rare to infrequent presence as observed by high-powered magnification), 2 = moderate histologic change (change is commonly observed in multiple high-powered fields or larger foci are present in selected areas), 3 = marked histologic change (changes frequently observed in multiple high-powered fields or severe change in focal areas). Disruption of vessel continuity, fibrinoid change, myodegeneration/regeneration, endothelial cell necrosis, and endothelial cell swelling were scored as either present (+) or absent (−). Tissues were examined by IHC for evidence of infection with R. parkeri using an immunoalkaline phosphate technique with a polyclonal anti-R. rickettsii antibody, diluted at 1/500, as described previously (Paddock et al. 2008).
Real-time PCR Quantitation of Rickettsial Load
Frozen tissue samples for genomic DNA extraction were processed using the DNeasy Blood and Tissue Kit (Qiagen, Limburg, The Netherlands)with some modifications as previously described (Grasperge et al. 2012). Approximately 10 mg of tissue was placed in a 2-ml Safe-Lock microcentrifuge tube (Eppendorf, Hamburg, Germany) to which two sterile 5-mm stainless steel beads (Qiagen) were added. Twenty microliters of proteinase K (Qiagen) and 180 µl of buffer ATL (Qiagen) were then added to each tube, and samples were then placed in a TissueLyser (Qiagen) for two cycles of 30 s at 30 cycles per second. Tubes were centrifuged at 7,500 × g for 5 min and then incubated for ≈16 h in a 56°C water bath. After incubation, extraction was completed according to the manufacturerÕs instructions. Extracted DNA was stored at −20°C until used for qPCR. Rickettsia primers and probe for the 17 kDa antigen gene and mouse primers and probe for mouse cfd were used as previously described (Grasperge et al. 2012). The 17 kDa antigen gene encodes a common rickettsial surface antigen protein, while the mouse cfd encodes the complement factor D protein common to most mammals. To quantify a portion of the R. parkeri 17 kDa gene in mouse tissues, serial dilutions of a plasmid containing single-copy portions of the R. parkeri 17 kDa and mouse cfd genes were amplified along with the sample unknowns. Briefly, qPCR components and the template that included 2 × LightCycler 480 Probe Master (Roche, Basel, Switzerland); 75 nM of each primer; 200 nM of each probe; DNase/RNase-free water; and 5 µl of gDNA template (samples), water (negative control), or serial 10-fold dilutions (3.5 × 108 to 3.5 × 103 copies) of pCR4-TOPO- Rp17 kDa + MmCfd were premixed in 35-µl volumes in 96-well plates and aliquoted in triplicate 10-µl reactions on 384-well plates (Reif et al. 2011). Quantitative PCR was then performed using a LightCycler 480 system II (Roche). Analysis of amplification was conducted with LightCycler 480 software. To ensure that our qPCR assay did not identify C. “Rickettsia andeanae,” we attempted to perform the assay on genomic DNA from C. “Rickettsia andeanae.” While the primers do amplify a similarly sized portion of the 17 kDa gene, the probe fails to label this amplicon.
RNA Extraction and Reverse Transcriptase PCR Quantitation
RNA was purified from the samples stored in RNAlater using the Quick-RNA MiniPrep kit (Zymo Research, Irvine, CA) according to manufacturerÕs instructions. Extracted samples were stored at −80°C. RNA was treated with DNaseI for 30 min and repurified using an RNA clean-up kit (Zymo Research). cDNA was generated from RNA samples using an iScript reverse transcription kit (Bio-Rad, Hercules, CA) following manufacturerÕs instructions. cDNAwas diluted fivefold in RNase-free water before use in real-time PCR. All primers used for real-time PCR analysis were designed using Primer3 software (Butchi et al. 2011). Primer sequences were blasted against the National Center for Biotechnology Information (NCBI) database to confirm that all primer pairs were specific for the gene of interest and that no homology to other genes was present. PCR reactions were prepared using SYBR green mix with Rox (Bio-Rad) in a 10-µl volume with ≈10 ng of cDNA and 1.8 µM forward and reverse primers. Samples were run in triplicate on an ABI PRISM 7900 Sequence Detection System (Applied Biosystems). Analysis of dissociation curves was used to confirm the amplification of a single product for each primer pair per sample. Confirmation of a lack of DNA contamination was achieved by analyzing samples that had not undergone reverse transcription. Untranscribed controls had at least a 1,000-fold lower expression level than analyzed samples or were negative for all genes after 40 cycles. Gene expression was quantified by the cycle number at which each sample reached a fixed fluorescence threshold (CT). To control for variations in RNA amounts among samples, data were calculated as the difference in CT values (log2) between the housekeeping gene, Gapdh, and the gene of interest for each sample (ΔCT = CT Gapdh − CT gene of interest). Data were calculated as a percentage of Gapdh expression for each gene of interest per sample. These data were then calculated as fold expression relative to the average of mock samples for each gene and each group.
PCR of Ticks Fed on Infected and Uninfected Mice
All of the engorged nymphal ticks from both tick feeding groups were maintained in separate vials at 27°C and 90% RH. After molt, the adult ticks were processed for DNA extraction and standard PCR. Briefly, ticks were halved and pooled into groups of two ticks, which were always from the same mouse. The samples were processed for DNA extraction using the Qiagen DNeasy Blood and Tissue Kit as previously described with a few modifications. Initially, 180 µl of buffer ATL and 20 µl of proteinase K were added to each sample. The samples were then incubated overnight in a 56°C water bath. Extractions were then completed according to manufacturerÕs instructions with a final elution volume of 100 µl. Standard PCR was performed using 190.70p and 190.602n and 190.70p and 190.701 primer pairs for rompA as previously described (Regnery et al. 1991, Fournier et al. 1998).
Statistics
Mouse temperature data were evaluated using the mixed procedure in SAS. Real-time quantitative PCR and RT-PCR data were evaluated by paired t-tests using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). P values of 0.05 were considered significant.
Results
Tick Feeding Results in Enhanced Rickettsial Proliferation at the Site of Intradermal Inoculation
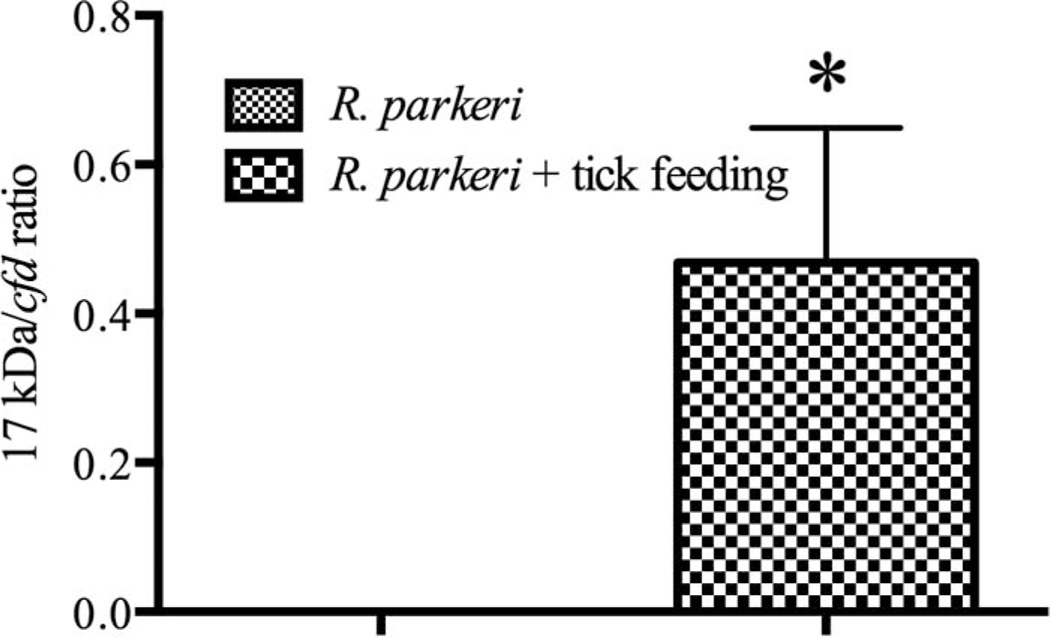
Quantification of the genus-specific 17 kDa antigen gene revealed a significant increase in rickettsial proliferation when nymphal A. maculatum were allowed to feed at the site of R. parkeri inoculation. While no amplification was observed in the Rickettsia-inoculated group that did not include tick feeding, the skin of the mice from the Rickettsia inoculated with tick feeding all had detectable levels of rickettsial DNA, totaling larger numbers of rickettsiae than were originally inoculated into the skin (average of 640,500 copies per 5 µl of extracted DNA), indicating enhancement of rickettsial infection (Fig. 1). Rickettsial DNA was not recovered from the skin of any of the mice from the remaining groups, buffer inoculated control; R. parkeri inoculated without tick feeding; and buffer inoculated with tick feeding.
Fig. 1.
qPCR for R. parkeri 17 kDa antigen gene relative to mouse cfd in the skin at 8 dpi. Relative quantification was used to account for variation in weight of tissues at the time of nucleic acid extraction. The mean R. parkeri numbers ± SEM as detected in mice that either (A) had no nymphal A. maculatum infestation or (B) had nymphal A. maculatum feeding at the inoculation site (* denotes significance between groups of P ≤ 0.05).
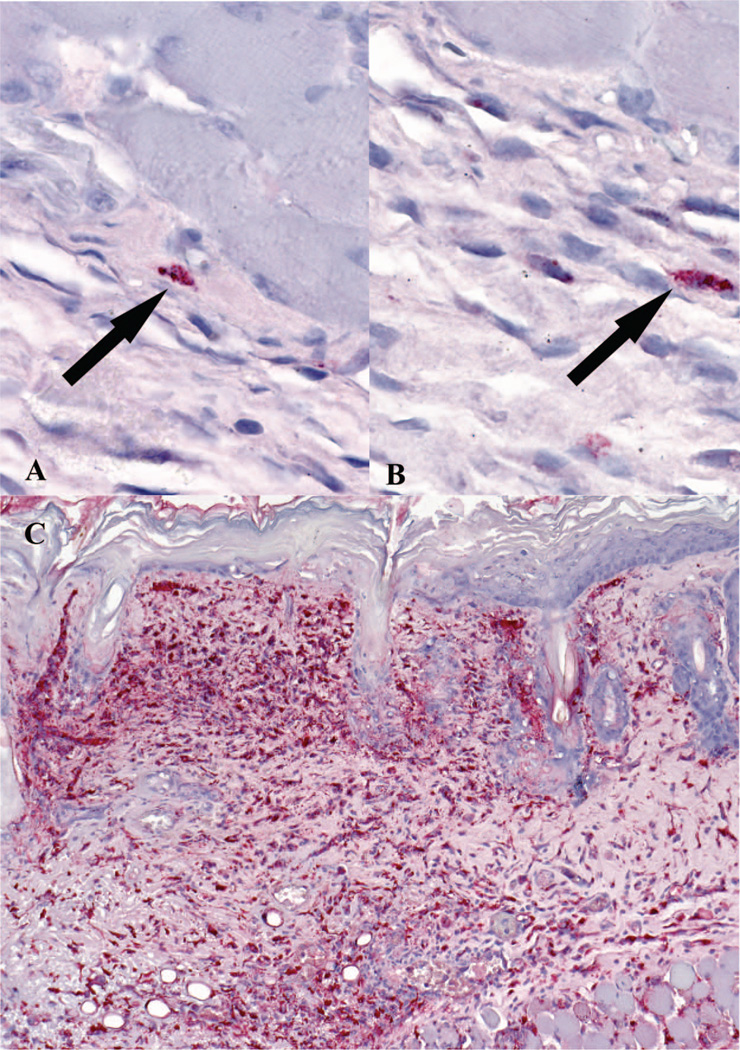
IHC revealed a marked increase in cells staining positive for SFG Rickettsia in the R. parkeri inoculated with tick feeding group when compared with the R. parkeri inoculated without tick feeding group (Fig. 2). Rare SFG Rickettsia-positive cells could also be found in the R. parkeri inoculated without tick feeding group and the buffer inoculated with tick feeding group, but not in the buffer inoculated without tick feeding group.
Fig. 2.
Immunohistochemical detection of SFG Rickettsia at tick feeding sites. A and B represent cutaneous tissues of the buffer-inoculated mice with tick feeding group. Arrows indicate rare positive cells (red) for SFG Rickettsia. Frame C displays florid staining of SFG Rickettsia in the cytoplasm of histiocytic cells and endothelial cells in the skin of R. parkeri inoculated mice with tick feeding at 8 dpi. Immunoalkaline phosphatase technique with naphthol-fast red and hematoxylin counterstain.
Tick Feeding Leads to Inflammation and Necrosis at the Feeding Site
Histological examination of both tick feeding groups displayed mixed lymphoplasmacytic/histiocytic inflammation and necrosis; however, the R. parkeri inoculated with tick feeding group presented with more extensive necrosis and more abundant inflammatory cell infiltrates (Table 1). In contrast, only one mouse displayed mild inflammation from the R. parkeri without tick feeding group, while the remaining mice failed to present any signs of inflammation or tissue damage, paralleling the minimal rickettsial proliferation observed by qPCR and IHC.
R. parkeri Infection During Tick Feeding Fails to Induce a Demonstrable Systemic Response as Monitored by Body Temperature
As measured by rectal temperature changes, no difference was demonstrated among groups at any time point during the study, indicating minimal systemic response to the early localized rickettsial infection. Mean rectal temperatures in degrees Fahrenheit were 97.6 (SEM = 0.327), 96.3 (SEM = 0.674), 95.6 (SEM = 0.158), 96.9 (SEM = 0.252), and 97 (SEM = 0.397) at day 0 for the control, buffer inoculated, R. parkeri inoculated, buffer with tick feeding, and R. parkeri with tick feeding, respectively, while day 8 rectal temperatures were 100.2 (SEM = 0.174), 99.9 (SEM = 0.101), 99.2 (SEM = 0.204), 97.1 (SEM = 0.980), and 96.2 (SEM = 0.578). No significant differences in rectal temperature were found between treatments and day of the experiment (data not shown).
Ticks Acquire R. parkeri From Feeding on Skin Intradermally Inoculated With R. parkeri
All pooled tick samples from the R. parkeri inoculated with tick feeding group showed appropriately sized amplicons using the rompA 190.70p and 190.602n primers, while all ticks from the buffer inoculated with tick feeding group failed to amplify with these primers. This demonstrates that the nymphal ticks acquired R. parkeri during the blood feeding over the R. parkeri inoculation site. Because the A. maculatum ticks used in this study are persistently infected with C. “Rickettsia andeanae,” these findings also indicate that the 190.70p and 190.602n primer pair fails to amplify C. “Rickettsia andeanae.” The 190.70p and 190.701 primer pair successfully amplified rickettsial DNA from all ticks, indicating this primer pair can be used to amplify C. “Rickettsia andeanae.”
Interestingly, only 11 of 60 ticks from the R. parkeri inoculated with tick feeding group successfully molted, and all of these were dead by the time of collection for DNA extraction. In contrast, all of the nymphs from the buffer inoculated with tick feeding group successfully molted and were still alive and mobile at the time of collection for DNA extraction. This suggests a possible fitness loss associated with R. parkeri infection in A. maculatum in the current model, which may be due to the much higher number of rickettsiae at the feeding site than a naïve tick might be expected to encounter feeding on a systemically infected host.
C. “Rickettsia Andeanae” Is Not Efficiently Transmitted to the Skin During Nymphal Tick Feeding
PCR for rompA using the 190.70p and 190.701 primer pair amplified rickettsial DNA in the R. parkeri inoculated with tick feeding group of mice but failed to amplify in the buffer inoculated with tick feeding group. The 190.70p and 190.602n primer pair also successfully amplified rickettsial DNA within the skin samples from the R. parkeri inoculated with tick feeding group but again failed to amplify from the buffer inoculated with tick feeding group. The failure of the more general primer pair to amplify rickettsial DNA from the buffer inoculated with tick feeding group suggests that C. “Rickettsia andeanae” is poorly, if at all, transmitted to the mouse during feeding.
No Obvious Immunological Alterations Presented as Differential RNA Expression
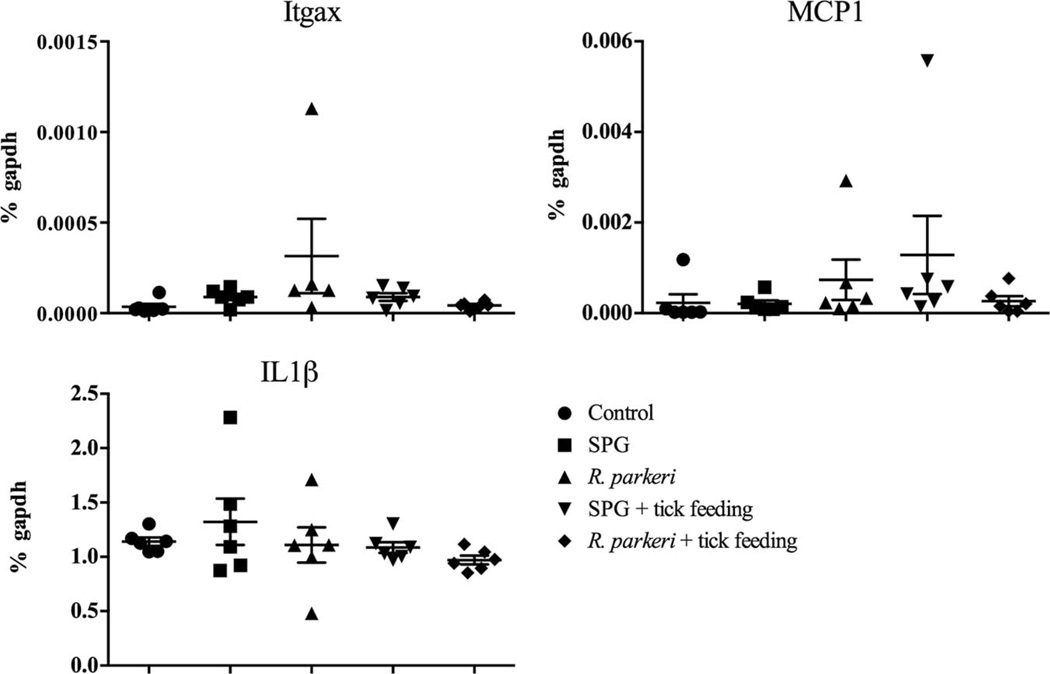
RT-PCR for Itgax (CD11c), Il1β, and Mcp1 (monocyte chemotactic protein-1 or CCL2) showed no significant difference among the groups (Fig. 3). CD11c is a marker for dendritic cells, while IL1β is a cytokine produced by activated macrophages. MCP1 is a chemokine secreted by monocytes, macrophages, and dendritic cells, and it primarily recruits monocytes, memory T-cells, and dendritic cells to sites of tissue injury. CD207, which is a marker for Langerhans cells in the skin, also failed to show differential expression among the groups (data not shown).
Fig. 3.
Cytokine profile from the R. parkeri intradermal inoculation site as determined by reverse transcriptase qPCR. Itgax (CD11c, a histiocytic cell marker), Mcp1 (monocyte chemotactic protein 1, a chemokine for histiocytic cells), and Il1β (produced by activated macrophages) were assessed to determine histiocytic cell activation, and results showed no significant difference among groups. Data presented as mean ± SEM.
Discussion
The results of this work suggest that the ability of R. parkeri to establish infection at the intradermal inoculation site is greatly enhanced by the process of tick feeding. The proliferation of R. parkeri at the site of tick feeding provides compelling evidence that the tick vector does more than simply deposit R. parkeri at the bite site. The influence of tick feeding on R. parkeri infection, specifically the cellular architecture in the cutaneous microenvironment, should be examined further.
The localization of R. parkeri primarily to histiocytictype cells within the cutaneous tissues is intriguing. Dendritic cells serve as the initial line of defense against rickettsial infection and produce IL-12p40 upon stimulation of TLR4 by Rickettsia (Fang et al. 2009). A samodel for Rickettsia conorii, the TLR4 deficiency of C3H/HeJ mice failed to cause the normal lag phase of growth observed early in rickettsial infection and, instead, allowed exponential growth during early infection (Jordan et al. 2009). Our findings suggest that defective dendritic cells fail to induce protective immune mechanisms and may also serve as the primary target cell for R. parkeri during the initiation of infection of the vertebrate host, which is similar to studies evaluating infection with Orientia tsutsugamushi (Paris et al. 2012). This is an interesting possibility when considering that rickettsial infection of endothelial cells is often described for the systemic infection, but enhanced resistance to rickettsial infection in TLR4-competent mice is independent of nitric oxide production or rickettsial growth within endothelial cells (Jordan et al. 2009). It must be recognized, however, that the infected cells present in the current work appear morphologically similar to histiocytic cells, but this has not been confirmed by other means.
We believe that the very rare SFG Rickettsia-positive cells identified by IHC of the skin of the buffer with tick feeding group could represent an abortive infection with C. “Rickettsia andeanae,” an SFG Rickettsia. Recent isolation of C. “Rickettsia andeanae” showed tick cells to be permissive while a mammalian cell line was unable to sustain infection, suggesting that replication of this organism is host cell dependent (Ferrari et al. 2013). This inability to sustainably infect mammalian cells provides further support for the limited transmission of C. “Rickettsia andeanae” to the vertebrate host observed in the current study. The observation of IHC-positive cells despite no detectable rickettsial DNA in the R. parkeri inoculated group without tick feeding is unexpected, as it is conventional that the PCR assay would be of greater sensitivity than the IHC technique. It is also possible that the Rickettsia had spread beyond the initial inoculation area and was therefore missed during sampling. Subsequent analysis using this model should examine the dissemination patterns in a temporal manner to better understand rickettsial infection kinetics.
The influence of rickettsial infection on tick fitness has been examined for a number of tick–Rickettsia pairings. For example, R. rickettsii infection of Dermacentor variabilis resulted in decreased fitness as measured by engorgement, survival through molt, and fecundity (Niebylski et al. 1999). Consistent with other rickettsial pathogens of humans, the death of the ticks feeding on R. parkeri inoculation sites may represent pathogenicity toward A. maculatum nymphs. While the inoculum a tick receives during feeding on a rickettsemic host is not known, the inoculum introduced into the skin in this study representsmuchmore rickettsiae than encountered naturally. Because of the nature of the PCR for detection of Rickettsia in the ticks, it is not possible to determine if the ticks were truly infected with R. parkeri versus harboring an infected bloodmeal; however, the probability of R. parkeri being pathogenic within the tick is worthy of further investigation.
Studies with hematophagous arthropods have revealed specific molecules present in the saliva of these blood feeders as promoting local infection of an infectious agent in the skin of a murine model (Volfova et al. 2008). This provides a framework for future studies into the mechanisms involved in the increased proliferation of Rickettsia secondary to tick feeding and emphasizes the importance and redundancy of these processes, as many hematophagous arthropods share similar salivary protein profiles. Specifically in ticks, the role of cystatins is intriguing. Cystatins are a family of cysteine protease inhibitors, and they have been described in tick saliva, including that of A. maculatum (Karim et al. 2012). Interestingly, the saliva of Ixodes ricinus has been shown to alter the immune response to lipotechoic acid, an activator of toll-like receptor 2, and Borrelia afzelii. The saliva suppressed the downstream signaling of toll-like receptors present on dendritic cells while enhancing the production of the immunosuppressive cytokine interleukin 10 (Lieskovska and Kopecky 2012). In regards to SFG rickettsiae, these alterations may result in impairment of the influx and efflux of inflammatory cells at the site of tick feeding, thereby limiting rickettsial recognition and clearance by the vertebrate host.
The lack of differential transcript of skin-associated immune factors was not entirely unexpected because the mice lack functional TLR4, and TLR4 is known to be an important component of the mouse innate immune response to Rickettsia (Jordan et al. 2009). However, other pattern recognition receptors may recognize Rickettsia and induce a level of immune cell activation that was undetected in this study. Tick salivary components can suppress inflammatory responses (Zeidner et al. 1996) and may disrupt the immune stimulation associated with rickettsial infection. Further studies are required to determine if tick immunomodulation of the host immune response at the inoculation site facilitates the changes in rickettsial infection observed in the current study. Specifically, the kinetics of antibody response to rickettsial infection with and without tick feeding requires further investigation.
With the emergence of new rickettsioses, and the desire to better understand their pathogenesis, the need for adequate animal models is evident. The recent identification of C3H/HeJ mice as a model for R. parkeri rickettsiosis allowed further characterization of this disease process, including the inoculation lesion (eschar) seen in human infections (Grasperge et al. 2012). The current study built upon this model and demonstrates a role for the tick vector as more than just an inert vessel for the transmission of the pathogen. Our study provides preliminary evidence that the initiation of rickettsial infection of the vertebrate host is largely dependent on the alteration of the vertebrate host microenvironment by tick feeding. The results of the current study emphasize the necessary components for acceptable animal models of rickettsioses, as most models typically exclude the role of the tick vector even though the cutaneous route of inoculation is often included (Eisemann et al. 1984). The aspects of tick feeding that facilitate rickettsial infection are under way; this model is an excellent tool for the investigation of the pathogenesis and ecology of R. parkeri rickettsiosis.
Table 1.
Histopathology associated with R. parkeri infection at 8 dpi
| Group | Vessel continuity disrupted |
Inflammation | Edema | Necrosis | Fibrinoid change |
Myodegeneration/ regeneration |
Endothelial necrosis |
Endothelial swelling |
|---|---|---|---|---|---|---|---|---|
| Control | − | 0 | 0 | 0 | − | − | − | − |
| Buffer | − | 0 | 0 | 0 | − | − | − | − |
| R. parkeri | − | 0 | 0 | 0 | − | − | − | − |
| Buffer + tick feeding | − | 2 | 1 | 1 | + | + | − | + |
| R. parkeri + tick feeding | + | 3 | 2 | 2 | + | + | + | + |
0, absence of the specified parameter; 1, mild histologic change (rare to infrequent presence as observed by high-powered magnification); 2, moderate histologic change (change is commonly observed in multiple high-powered fields or larger foci are present in selected areas); 3, marked histologic change (changes frequently observed in multiple high-powered fields or severe change in focal areas).
Acknowledgments
We thank Michael T. Kearney for his assistance with statistical analysis, also, the members of the Macaluso laboratory for their technical assistance. This work was supported by the National Institutes of Health (AI077784 and OD011124). This work was also part of B. GraspergeÕs doctoral dissertation.
Footnotes
The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References Cited
- Bell JF, Stewart SJ, Wikel SK. Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity. Am. J. Trop. Med. Hyg. 1979;28:876–880. [PubMed] [Google Scholar]
- Butchi NB, Woods T, Du M, Morgan TW, Peterson KE. TLR7 and TLR9 trigger distinct neuroin-flammatory responses in the CNS. Am. J. Pathol. 2011;179:783–794. doi: 10.1016/j.ajpath.2011.04.011. doi: http://dx.doi.org/10.1016/j.ajpath.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragun WC, Bartlett BL, Ellis MW, Hoover AZ, Tyring SK, Mendoza N, Vento TJ, Nicholson WL, Eremeeva ME, Olano JP, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch. Dermatol. 2010;146:641–648. doi: 10.1001/archdermatol.2010.48. doi: http://dx.doi.org/2010.48 [pii] 10.1001/archdermatol.2010.48. [DOI] [PubMed] [Google Scholar]
- Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, Fikrig E. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe. 2009;6:482–492. doi: 10.1016/j.chom.2009.10.006. doi: http://dx.doi.org/10.1016/j.chom.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisemann CS, Nypaver MJ, Osterman JV. Susceptibility of inbred mice to rickettsiae of the spotted fever group. Infect. Immun. 1984;43:143–148. doi: 10.1128/iai.43.1.143-148.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Ismail N, Shelite T, Walker DH. CD4+ CD25+ Foxp3- T-regulatory cells produce both gamma interferon and interleukin-10 during acute severe murine spotted fever rickettsiosis. Infect. Immun. 2009;77:3838–3849. doi: 10.1128/IAI.00349-09. doi: IAI.00349-09 [pii] 10.1128/IAI.00349-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HM, Whitworth T, Olano JP, Popov VL, Walker DH. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 2004;72:2222–2228. doi: 10.1128/IAI.72.4.2222-2228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FAG, Goddard J, Moraru GM, Smith WEC, Varela-Stokes AS. Isolation of “Candidatus Rickettsia andeanae” (Rickettsiales: Rickettsiaceae) in embryonic cells of naturally infected Amblyomma maculatum (Ixodida: Ixodidae) J. Med. Entomol. 2013;50:1118–1125. doi: 10.1603/me13010. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Roux V, Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- Grasperge BJ, Reif KE, Morgan TD, Sunyakumthorn P, Bynog J, Paddock CD, Macaluso KR. Susceptibility of Inbred Mice to Rickettsia parkeri. Infect. Immun. 2012;80:1846–1852. doi: 10.1128/IAI.00109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Jiang J, Stromdahl EY, Richards AL. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast Ticks Collected from Humans in the United States. Vector Borne Zoonotic Dis. 2012;12:175–182. doi: 10.1089/vbz.2011.0614. doi: http://dx.doi.org/10.1089/vbz.2011.0614. [DOI] [PubMed] [Google Scholar]
- Jordan JM, Woods ME, Soong L, Walker DH. Rickettsiae stimulate dendritic cells through toll-like receptor 4, leading to enhanced NK cell activation in vivo. J. Infect. Dis. 2009;199:236–242. doi: 10.1086/595833. doi: http://dx.doi.org/10.1086/595833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS ONE. 2012;6:e28525. doi: 10.1371/journal.pone.0028525. doi: http://dx.doi.org/ 10.1371/journal.pone.0028525 PONE-D-11-19009 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti TJ, Simser JA, Baldridge GD, Palmer AT, Munderloh UG. Factors influencing in vitro infectivity and growth of Rickettsia peacockii (Rickettsiales: Rickettsiaceae), an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni (Acari, Ixodidae) J. Invertebr. Pathol. 2005;90:177–186. doi: 10.1016/j.jip.2005.09.001. doi: S0022-2011(05)00190-4 [pii] 10.1016/j.jip.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieskovska J, Kopecky J. Effect of tick saliva on signalling pathways activated by TLR-2 ligand and Borrelia afzelii in dendritic cells. Parasite Immunol. 2012;34:421–429. doi: 10.1111/j.1365-3024.2012.01375.x. doi: http://dx.doi.org/10.1111/j.1365-3024.2012.01375.x. [DOI] [PubMed] [Google Scholar]
- Mastronunzio JE, Kurscheid S, Fikrig E. Post-genomic analyses reveal development of infectious Anaplasma phagocytophilum during transmission from ticks to mice. J. Bacteriol. 2012;194:2238–2247. doi: 10.1128/JB.06791-11. doi: http://dx.doi.org/ 10.1128/JB.06791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, Nelson KF, Booth CJ, Koski B, Anderson JF, Kantor F, Fikrig E. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS ONE. 2007;2:e451. doi: 10.1371/journal.pone.0000451. doi: http://dx.doi.org/ 10.1371/journal.pone.0000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario S, Das S, de Silva AM, Deponte K, Marcantonio N, Anderson JF, Fish D, Fikrig E, Kantor FS. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am. J. Trop. Med. Hyg. 1998;58:780–785. doi: 10.4269/ajtmh.1998.58.780. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Peacock MG, Schwan TG. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni) Appl. Environ. Microbiol. 1999;65:773–778. doi: 10.1128/aem.65.2.773-778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SLF, Tamminga CL, Ohl CA. Rickettsia parkeri: A Newly Recognized Cause of Spotted Fever Rickettsiosis in the United States. Clin. Infect. Dis. 2004;38:805–811. doi: 10.1086/381894. doi: http://dx.doi.org/10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 2008;47:1188–1196. doi: 10.1086/592254. doi: http://dx.doi.org/10.1086/592254. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, Loftis AD, Varela-Stokes A. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 2010;76:2689–2696. doi: 10.1128/AEM.02737-09. doi: http://dx.doi.org/10.1128/AEM. 02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Phetsouvahn R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M, Ferguson DP, Blacksell SD, Newton PN, Day NP, et al. Orientia tsutsugamushi in human scrub typhus schars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl. Trop. Dis. 2012;6:e1466. doi: 10.1371/journal.pntd.0001466. doi: http://dx.doi.org/10.1371/journal.pntd. 0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Kohls G, Cox G, Davis G. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939;54:1482–1484. [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J. Exp. Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif KE, Kearney MT, Foil LD, Macaluso KR. Acquisition of Rickettsia felis by cat fleas during feeding. Vector Borne Zoonotic Dis. 2011;11:963–968. doi: 10.1089/vbz.2010.0137. doi: http://dx.doi.org/10.1089/vbz. 2010.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer Y, Seijo AC, Crudo F, Nicholson WL, Varela-Stokes A, Lash RR, Paddock CD. Rickettsia parkeri Rickettsiosis, Argentina. Emerg. Infect. Dis. 2011;17:1169–1173. doi: 10.3201/eid1707.101857. doi: http://dx.doi.org/10.3201/eid1707.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simser JA, Palmer AT, Munderloh UG, Kurtti TJ. Isolation of a Spotted FeverGroup Rickettsia, Rickettsia peacockii, in a Rocky Mountain Wood Tick, Dermacentor andersoni, Cell Line. Appl. Environ. Microbiol. 2001;67:546–552. doi: 10.1128/AEM.67.2.546-552.2001. doi: http://dx.doi.org/10.1128/aem. 67.2.546-552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 2007;13:751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyakumthorn P, Bourchookarn A, Pornwiroon W, David C, Barker SA, Macaluso KR. Characterization and Growth of Polymorphic Rickettsia felis in a Tick Cell Line. Appl. Environ. Microbiol. 2008;74:3151–3158. doi: 10.1128/AEM.00025-08. doi: http://dx.doi.org/10.1128/aem. 00025–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, Strey OF. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010;47:707–722. doi: 10.1603/me10029. [DOI] [PubMed] [Google Scholar]
- Troughton DR, Levin ML. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J. Med. Entomol. 2007;44:732–740. doi: 10.1603/0022-2585(2007)44[732:lcosit]2.0.co;2. doi: http://dx.doi.org/10.1603/0022–2585(2007)44[732: lcosit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Trout R, Steelman CD, Szalanski AL, Williamson PC. Rickettsiae in Gulf Coast ticks, Arkansas, USA. Emerg. Infect. Dis. 2010;16:830–832. doi: 10.3201/eid1605.091314. doi: http://dx.doi.org/10.3201/eid1605.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volfova V, Hostomska J, Cerny M, Votypka J, Volf P. Hyaluronidase of bloodsucking insects and its enhancing effect on leishmania infection in mice. PLoS Negl. Trop. Dis. 2008;2:e294. doi: 10.1371/journal.pntd.0000294. doi: http://dx.doi.org/10.1371/journal.pntd. 0000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman TJ, Richards AL, Paddock CD, Tamminga CL, Sniezek PJ, Jiang J, Byers DK, Sanders JW. Rickettsia parkeri infection after tick bite, Virginia. Emerg. Infect. Dis. 2007;13:334–336. doi: 10.3201/eid1302.061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK. The immunology of host-ectoparasitic arthropod relationships, CAB International. Wallingford, Oxon: United Kingdom; 1996. [Google Scholar]
- Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect. Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner N, Dreitz M, Belasco D, Fish D. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor-alpha, interleukin-2, and interferon-gamma. J. Infect. Dis. 1996;173:187–195. doi: 10.1093/infdis/173.1.187. [DOI] [PubMed] [Google Scholar]