Abstract
Background
CD4+CD25hiCD127lo/− regulatory T cells have been suggested to be critical regulators of inflammatory processes in allergic asthma. Recent studies reported a selective decrease in the frequency of regulatory T cells in the bronchoalveolar lavage fluid of allergic asthmatic (AA) subjects, prompting the possibility of defective recruitment of these cells to the airway in response to chemokines produced during asthmatic inflammation.
Objectives
This study aimed to characterize the chemotactic profile of circulating regulatory T cells in AA subjects in response to chemokines abundantly produced in airway inflammation, such as CCL1, CCL17, and CCL22.
Methods
The study was performed in a cohort of 26 AA, 16 healthy control, and 16 non-AA subjects. We used chemotaxis assays to evaluate cell migration, flow cytometry to examine chemokine receptor expression, and phospho-ELISA to study consequent signaling pathways in regulatory T cells.
Results
Regulatory T cells, but not CD4+CD25−T cells, from AA subjects showed decreased chemotactic responses, specifically to CCL1, in comparison with their healthy control and non-AA counterparts. Decreased CCL1-mediated chemotaxis in AA regulatory T cells was associated with decreased phosphorylation of protein kinase B (AKT), a protein involved in chemokine intracellular signaling. Furthermore, the decreased chemotactic response to CCL1 in AA regulatory T cells significantly correlated with asthma severity and decreased pulmonary function in AA subjects.
Conclusions
These results provide the first evidence of dysfunction in the chemokine signaling pathway in AA regulatory T cells.
Keywords: Allergic asthma, regulatory T cells, cell trafficking, cell signaling, CCL1, CCR8, AKT
Allergic asthma is a chronic lung disease characterized by airway hyperreactivity that is generally associated with TH2-biased immune responses. Allergic asthmatic (AA) subjects are distinguished from their nonallergic asthmatic (NA) counterparts based on high IgE levels and positive skin test responses to common allergens. Functional studies of human AA and NA cells also reveal distinct patterns of cellular interactions and intracellular signaling pathways in each disease.1,2 The clinical symptoms of AA involve indicators of systemic inflammation,3 such as increased serum levels of IL-4 and IgE.4,5 Nevertheless, many cardinal inflammatory features of the disease occur at the airway.6 Such localized inflammatory responses have been attributed to the hyperresponsiveness of residential cell subsets and recruited populations of both innate immune cells and TH2-biased CD4+ T cells.7,8 It has been suggested that on allergenic challenge the former produce signaling factors to cause preferential trafficking of the latter to areas of airway inflammation.9
Chemokines are a major class of regulators of immune cell trafficking. Through interaction with G protein–coupled receptors on target cell surfaces, these molecules initiate a signaling cascade with hallmark activation of the phosphoinositide 3-kinase10 and subsequent activation of downstream effectors of the chemotactic signaling pathway, such as protein kinase B (AKT) (a Pleckstrin homology domain protein), extracellular signal-regulated kinase 1/2 (ERK1/2; a mitogen-activated protein kinase), or both.11 Furthermore, a balance between the activities of phosphoinositide kinase-3 and its negative regulator, the lipid phosphatase phosphatase/tensin homolog deleted on chromosome 10 (PTEN), is also critical for the proper transduction of chemotactic signals.12 Previous studies successfully identified a number of chemokines potentially responsible for the recruitment of TH2-biased immune cells to the airways ofAAsubjects. In human subjects eosinophilia is associated with increased levels of IL-5 and eotaxins (CCL11, CCL24, and CCL26) in the bronchoalveolar lavage fluid (BALF) of AA subjects,13–15 whereas neutrophilia is associated with upregulation of BALF CXCL8.16 Airway infiltration of TH2-biased CD4+ T cells is linked to the increased expression of CCL17, CCL22,17,18 and possibly CCL117–20 (conflicting data) in the BALF of AA subjects. These cells also express CCR4 and CCR8, receptors of CCL17/CCL22 and CCL1, respectively,21 implicating the involvement of these chemokines in TH2-biased CD4+ T-cell trafficking. Mechanistic studies of these chemokines in mice confirmed the critical importance of CCL17/CCL22 in the recruitment of TH2-biased CD4+ T cells to the airway.22 Studies of CCL1 resulted in conflicting results. To date, 3 of 5 studies suggested that CCL1 is important for TH2-biased CD4+ T-cell recruitment to the airway,23–25 whereas the other 2 studies did not.26,27 Nevertheless, all studies thus far have focused strictly on chemotactic responses of inflammatory cells instead of potential regulatory cell subsets in asthmatic inflammation.
Emerging evidence suggests that naturally occurring CD4+CD25hiCD127lo/−regulatory T (Treg) cells play a key role in regulating inflammatory responses in asthmatic subjects.28 Treg cells are CD4+CD25hiCD127lo/−T cells chiefly involved in peripheral tolerance.29 In human allergic asthma Treg cells do not suppress the proliferation and cytokine production of allergen-stimulated CD4+ T cells in AA subjects and healthy individuals.30,31 Furthermore, several murine studies have demonstrated that the induction of Treg cell function reverses airway hyperresponsiveness32 and protects against experimentally induced asthma.33 Collectively, these findings suggest a role of Treg cells in controlling TH2 inflammation in patients with allergic asthma.
Immune regulation by Treg cells has been suggested as a multifactorial phenomenon. Several molecules, such as Foxp3, cytotoxic T lymphocyte–associated antigen 4, glucocorticoid-induced TNF receptor, and the lymphocyte activation gene,3 have been associated withTreg cell activity.34,35Treg cells also expressmultiple chemokine receptors, controlling their migration to different organs and tissues.36 Previous reports have shown that like TH2-biased CD4+ T cells, Treg cells express high levels of CCR4 and CCR8 and functionally respond to CCL1 and CCL17/CCL22.37,38 These data suggest that Treg cells are potentially able to migrate toward these chemokines on allergic/airway inflammation to suppress hyperactive immune responses.37,39 Furthermore, the most recent study of Treg cells in allergic asthma revealed that Treg cell numbers decrease in the BALF but not in the peripheral blood of AA subjects,40 suggesting the possibility of defective recruitment of Treg cells to the airway on asthmatic inflammation. Thus this study aims to investigate the migratory potentials of Treg cells in AA subjects toward chemokines implicated in T-cell recruitment during asthmatic inflammation, such as CCL1 and CCL17/CCL22. Our data provide the first line of evidence of a decreased chemotactic response in AA Treg cells, specifically with a dysfunction in CCL1 intracellular signaling pathway.
METHODS
Human subjects
The study was approved by the Stanford Administrative Panel on Human Subjects in Medical Research. The study population included 22 AA subjects, 16 healthy control (HC) subjects, and 16 NA subjects. All subjects signed informed consent forms before participating in the study. Comprehensive clinical data were collected at each patient visit, including history, disease severity, medication status, common allergens, IgE level, and FEV1 (see Tables E1 and E2 in this article’s Online Repository at www.jacionline.org). A detailed description of human subjects is available in the Methods section in this article’s Online Repository at www.jacionline.org.
Cell isolation
Buffy coats were derived from up to 450 mL of whole blood. CD4+ T cells were purified with CD4+ Rosette Kits (Stemcell Technologies, Vancouver, British Columbia, Canada). From these CD4+ T cells, Treg and CD4+CD25−T (non-Treg) cells were pre-enriched by means of magnetic isolation and subsequently purified through flow cytometric sorting. A detailed description of the cell isolation protocol is available in this article’s Online Repository.
Chemotaxis assays
The chemotaxis protocol was performed as previously described.41 Specific migration of purified Treg and non-Treg cells was evaluated with a 5-µm Transwell system (Corning Costar, Corning, NY). Cells (5 × 105) were suspended in 100 µL of RPMI 0.5% BSA and added to the top compartment. Five hundred microliters of chemokine solutions (PeproTech, Inc, Rocky Hill, NJ) at various concentrations was added to the lower compartment. Migration was assayed for 2.5 hours at 37°C, and then the inserts were removed. Migrated Treg and non-Treg cells were enumerated with an automatic cell counter (Beckman Coulter, Fullerton, Calif) in a similar method used to quantify the starting cell populations. The number of cells migrated to specific chemokines was divided by the number of spontaneously migrated cells to calculate chemotactic indices. Each assay was performed in triplicate.
Flow cytometry
Surface staining for CCR4 and CCR8 was performed with an R&D Systems (Minneapolis, Minn) protocol. Data were acquired on a FACSCalibur (BD Biosciences, San Jose, Calif). The data acquisition threshold was set on a forward-scatter channel to exclude dead cells and debris with very low size. Compensation of flow cytometric data was performed electronically with FlowJo software (Treestar, Inc, Ashland, Ore) for standardization.
ELISA and Western blotting
Quantitation of phosphorylated AKT (phospho-AKT; also known as protein kinase B), phosphorylated ERK1/2, total AKT, and total PTEN protein levels was performed with ELISA kits from R&D Systems. Treg cells (106) were stimulated with optimal chemokine concentrations and incubation times (derived from chemotaxis assays) and lysed with lysis buffer (R&D Systems). Each sample was assayed in triplicate. Western blot reconfirmation experiments were performed with phospho-AKT antibodies (Cell Signaling Technology, Danvers, Mass). After stimulation, 106 Treg cells were lysed in sample buffer and subjected to downstream assays according to the manufacturer’s protocol.
Statistical analysis
All statistical procedures were performed with Prism software (GraphPad, Inc, San Diego, Calif). Data were tested for normality (Koromonov-Smirnov test) and variance equality (Bartlett test) before being subjected to appropriate statistical tests. Differences with a P value of less than .05 were considered statistically significant. Correction for multiple comparisons was performed by using the Bon-ferroni method. In dot-plot graphs horizontal bars represent mean or median values for normally distributed or nonnormally distributed data, respectively. A detailed description of each statistical test is available in the figure legends.
RESULTS
Specific reduction in CCL1-mediated chemotaxis in AA Treg cells
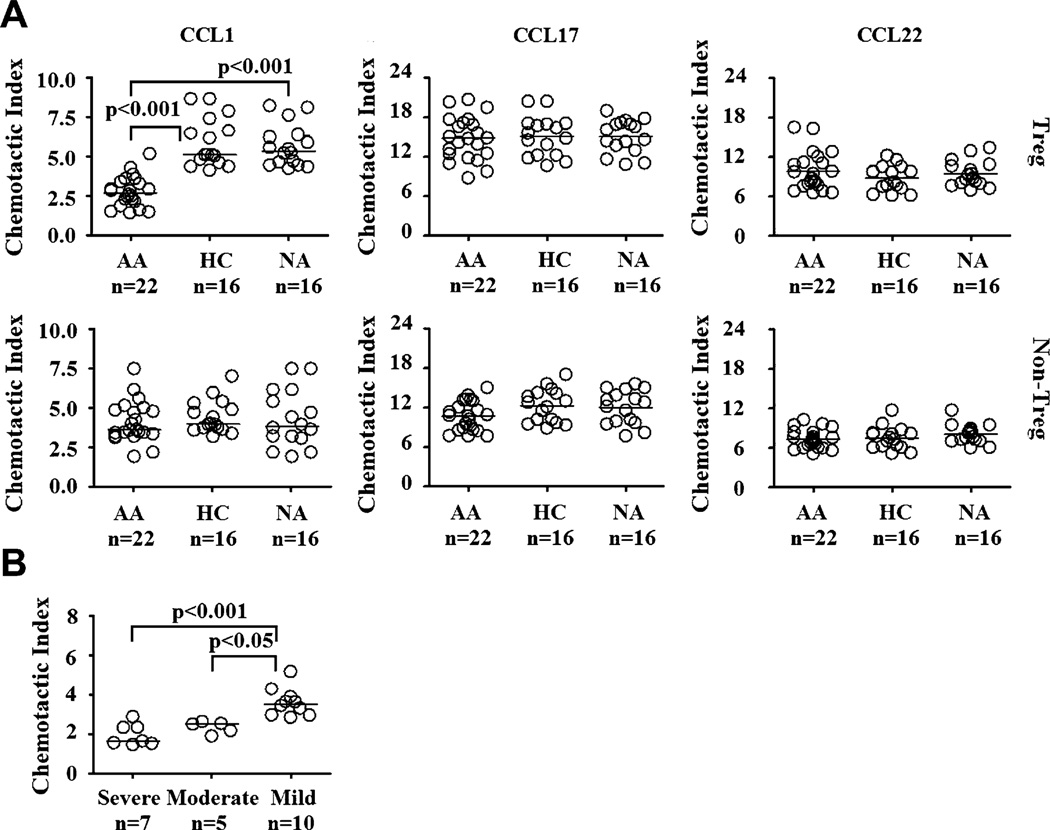
Treg and non-Treg cells were isolated by using a combined method of magnetic and flow cytometric sorting to greater than 95%purity on reanalysis (see Fig E1 in this article’s Online Repository at www.jacionline.org). In vitro chemotaxis assays were used to evaluate the migratory potentials of each cell type toward several chemokines known to be associated with AA pathophysiology, as well as migration of Treg and non-Treg cells, such as CCL1, CCL17, and CCL22. The chemotactic response of each cell subset to a particular chemokine was analyzed by using chemotactic indices. Because there might be variations in sensitivity to chemokines by cells obtained from different clinical groups, we performed titration experiments for all AA, HC, and NA groups. Titration experiments showed that optimal chemotaxis in Treg and non-Treg cells was induced at specific concentrations of each chemokine (Treg cells: 200 nmol/L CCL1, 100 nmol/L CCL17, and 200 nmol/L CCL22; non-Treg cells: 200 nmol/L for all chemokines in all subject groups; see Fig E2, A, in this article’s Online Repository at www.jacionline.org). These specific conditions were then used to evaluate the migration of Treg and non-Treg cells in each clinical group. At the optimal chemokine concentration, chemotactic response to CCL1 in AA Treg cells was significantly lower than that seen in HC and NA subjects (Fig 1, A). In contrast, CCL17 and CCL22 induced chemotaxis of AA Treg cells as potently as those from HC and NA subjects. We did not observe differential chemotactic patterns to CCL1, CCL17, and CCL22 in non-Treg cells among AA, HC, and NA subjects. A possible explanation for this differential responsiveness to CCL1 in Treg and non-Treg cells from AA subjects is that Treg cells, but not non-Treg cells, were exposed to magnetic beads during the isolation process and consequentially exhibited lower chemotactic response to CCL1. To investigate whether magnetic beads affect Treg cell migration, we compared chemotactic responses to CCL1, CCL17, and CCL22 of Treg cells that were only sorted by means of flow cytometry with those of Treg cells obtained from our combined sorting scheme. We found no significant differences in chemotactic responses in Treg cells obtained from 2 different sorting methods (see Fig E2, B). Therefore it is unlikely that magnetic beads significantly influenced Treg cell motility. Altogether, these results suggest that the hyporesponsiveness to CCL1-mediated chemotaxis is specific to the Treg cell population in AA subjects.
FIG 1.
Chemotactic patterns of Treg and non-Treg cells. A, Specific migration of Treg and non-Treg cells toward CCL1, CCL17, and CCL22. Optimal dosages of each chemokine were used in these experiments. B, Chemotactic responses of Treg cells from AA clinical subsets with respect to disease severity. The Kruskal-Wallis test was used for analyses of CCL1 response by Treg and non-Treg cells and severity analysis because the data were not normally distributed. ANOVA was used for the rest of the analyses because the data had normal distributions and equal variances.
Surface receptor of CCL1 is not decreased in AA Treg cells
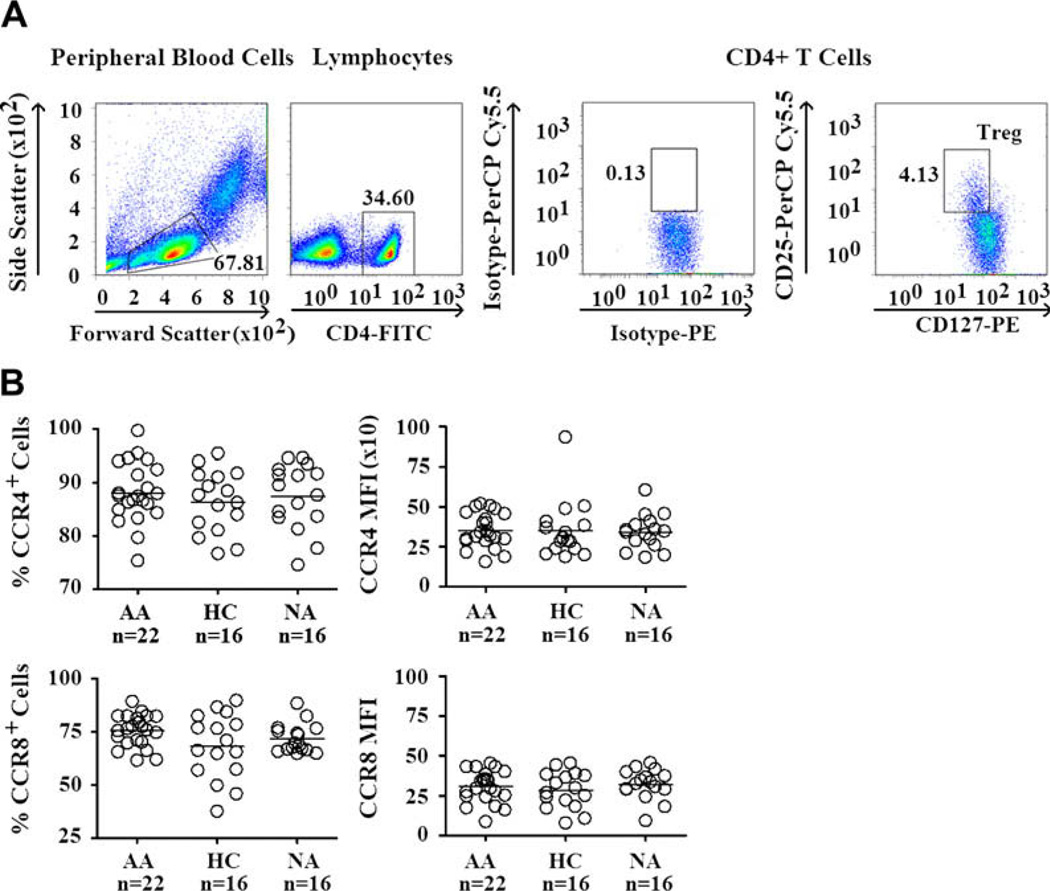
Reduced chemotactic response to a chemokine could result from a decreased expression of the receptor specific to that chemokine, abnormal intracellular signaling pathway downstream of the chemokine receptor, or both. Thus we first investigated the expression of CCR8, the specific receptor of CCL1 on Treg cells. In addition, we also analyzed the expression of CCR4, the receptor of CCL17 and CCL22 on Treg cells. Treg cells in PBMCs were identified in a similar manner to that of sorted Treg cells, with triple staining for CD4, CD25, and CD127 (Fig 2, A). Negative thresholds for CCR4 and CCR8 expression were derived by staining of Treg cells with an isotype control antibody (mouse anti-human IgG1–allophycocyanin; see Fig E3, A, in this article’s Online Repository at www.jacionline.org). Similar to previous reports, we found that a majority of Treg cells express CCR4 and CCR8.37,38 We found no significant differences in the expression of CCR4 and CCR8 on Treg cells among AA, HC, and NA subjects (Fig 2, B). Similar analysis of CCR4 and CCR8 expression was performed on non-Treg cells, which showed no significant differences among the 3 subject groups (see Fig E3, B). Thus these results suggest that the selective decrease in CCL1-mediated chemotaxis in AA Treg cells is unlikely to result from a decreased expression of CCR8.
FIG 2.
CCR4 and CCR8 expression by Treg cells. A, Gating of Treg cells from PBMCs. Lymphocytes were gated by means of characteristic side scatter and forward scatter. CD4+ T cells were gated from these lymphocytes. From CD4+ T cells, Treg cells were identified by using CD127 and CD25 markers. CD25 and CD127 gates were defined by using isotype controls. PerCP, Peridinin-chlorophyll-protein complex; FITC, fluorescein isothiocyanate; PE, phycoerythrin. B, CCR4 and CCR8 expression by Treg cells. CCR4 and CCR8 expression by Treg cells was measured based on mean fluorescence intensity (MFI), as well as percentages of cells that express these chemokine receptors. ANOVA was used for all analyses because all data sets have normal distribution and equal variances.
Decreased CCL1-induced phosphorylation of AKT in AA Treg cells
We next attempted to investigate the signaling cascade downstream of the CCL1-CCR8 axis in Treg cells. Although the precise signaling mechanism of the CCL1-CCR8 axis has not been fully characterized, the phosphoinositide 3-kinase signaling pathway has been implicated as a pivotal regulator of signaling cascades of several chemokines, including CCL1.10 Downstream effectors of this phosphoinositide 3-kinase signaling pathway involve several molecules, such as AKT and ERK1/2.11 On receiving chemokine stimulus, the phosphorylation of these proteins occurs and triggers further downstream activation of transcription factors required for cell migration. Thus we used phospho-ELISA to characterize the phosphorylation status of these signaling molecules in response to CCL1, CCL17, and CCL22 in vitro.
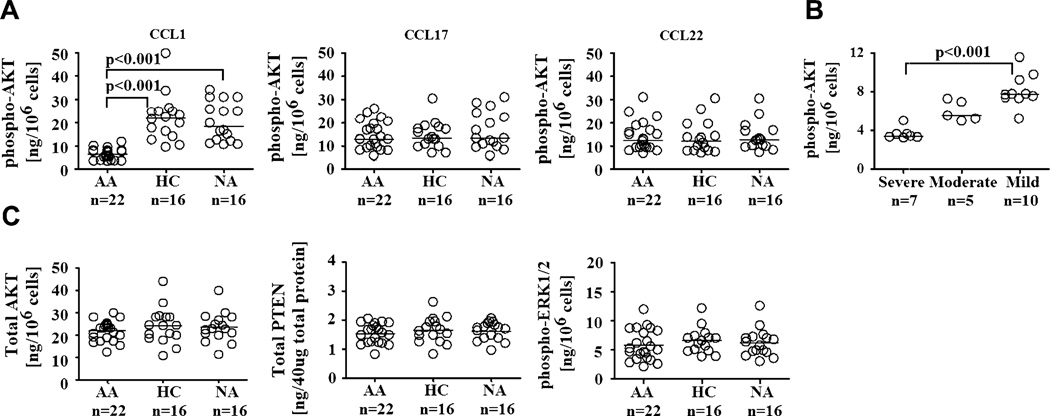
We first performed a kinetic study of the phosphorylation of AKT and ERK1/2 in Treg cells from each clinical group. Phosphorylated forms of AKT and ERK1/2 were detected by means of analysis of their expressions in cell lysates collected from purified Treg cells that were stimulated with CCL1, CCL17, or CCL22. Stimulation of Treg cells with CCR4 ligand (CCL17/CCL22) and CCR8 ligand (CCL1) phosphorylated ERK1/2 and AKT, confirming the involvement of these proteins in the signaling pathways of CCR4 and CCR8 ligands (see Fig E4, A, in this article’s Online Repository at www.jacionline.org).42–45 Kinetic study showed that optimal phosphorylation of both AKT and ERK1/2 in Treg cells occurred after 5 minutes (see Fig E4, A). Thus this time point was used to evaluate the expression of phospho-AKT and ERK1/2 in Treg cells from all clinical groups. ELISA results showed a significant decrease in phospho-AKT induction in AA Treg cells compared with that seen in their HC and NA counterparts in response to CCL1 (Fig 3, A). In contrast, phospho-AKT induction in AA Treg cells in response to CCL17 and CCL22 was comparable with that of HC and NA Treg cells. These ELISA results were subsequently confirmed by means of Western blotting, which indeed showed a specific reduction in CCL1-induced AKT phosphorylation in AA Treg cells compared with HC and NA Treg cells (see Fig E4, D).
FIG 3.
Expression of molecules involved in chemotactic signaling pathways by Treg cells. A, Phosphorylation of AKT by Treg cells in response to CCL1, CCL17, and CCL22 stimulation. B, Phospho-AKT expression of Treg cells from AA clinical subsets with respect to disease severity. C, Expression of total AKT and PTEN and phosphorylation of ERK1/2 by Treg cells in response to CCL1 stimulation. Optimal chemokine dosages from chemotaxis assays and time points for phospho-AKT and phosphorylated extracellular signal-regulated kinase 1/2 detection (5 minutes) from kinetics experiments were used in these experiments. The Kruskal-Wallis test was used for the analyses of phospho-AKT expression by Treg cells and severity analysis because the data were not normally distributed. ANOVA was used for the rest of the analyses because the data had equal variances and normal distributions.
We next investigated the expression of total AKT protein and PTEN, the negative regulator of AKT phosphorylation,12 as potential contributors to the decreased phosphorylation of AKT in AA Treg cells. However, no significant differences in the expression of total AKTand PTEN were found in Treg cells among AA, HC, and NA subjects (Fig 3, D). Analysis of the phosphorylation of ERK1/2, a signaling molecule involved (but not exclusively) in phosphoinositide 3-kinase signaling response to CCL1 in Treg cells, also showed no significant differences among the 3 subject groups (Fig 3, C).
Defective CCL1 signaling pathway in allergic asthmatic Treg cells correlated with disease severity and clinical assessment of pulmonary function (FEV1)
Correlation analysis of in vitro cellular function and clinical parameters of allergic asthma was performed. We found a significant positive correlation between phospho-AKT induction in AA Treg cells and their chemotactic indices in response to CCL1 (Table I and see Fig E5 in this article’s Online Repository at www.jacionline.org), suggesting that decreased AKT phosphorylation might contribute to the overall reduction in the migratory potential of Treg cells in response to CCL1. Furthermore, Treg cells from subjects with severe and moderate allergic asthma had significantly lower chemotactic indices in response to CCL1 compared with their counterparts with mild allergic asthma (Fig 1, B). Phospho-AKT expression in Treg cells from subjects with severe allergic asthma was also significantly less than that of Treg cells from subjects with mild allergic asthma (Fig 3, B). Both chemotactic indices and phospho-AKT induction by Treg cells in response to CCL1 positively correlated with FEV1, a parameter of pulmonary function (Table I and see Fig E5). Other clinical parameters, such as subject age, asthma duration, and IgE level, were not significantly correlated with any parameters of in vitro cellular function (Table I and see Fig E5).
TABLE I.
Correlation of in vitro cell function and clinical parameters of AA
| Age | Asthma duration | IgE | FEV1 | Phospho-AKT | |
|---|---|---|---|---|---|
| Phospho-AKT | |||||
| N value | 22 | 22 | 22 | 22 | — |
| R value | −0.3192 | −0.3737 | −0.2249 | 0.9005 | — |
| P value | .1476 | .0866 | .3144 | <.0001 | — |
| Chemotactic index | |||||
| N value | 22 | 22 | 22 | 22 | 22 |
| R value | −0.3208 | −0.2929 | −0.2146 | .8197 | 0.8478 |
| P value | .1454 | .1859 | .3375 | <.0001 | <.0001 |
Data were presented with N, P, and R values. The Spearman test was used for all analyses because all data sets were not normally distributed.
Modulation of CCL1 responsiveness in Treg cells by corticosteroids
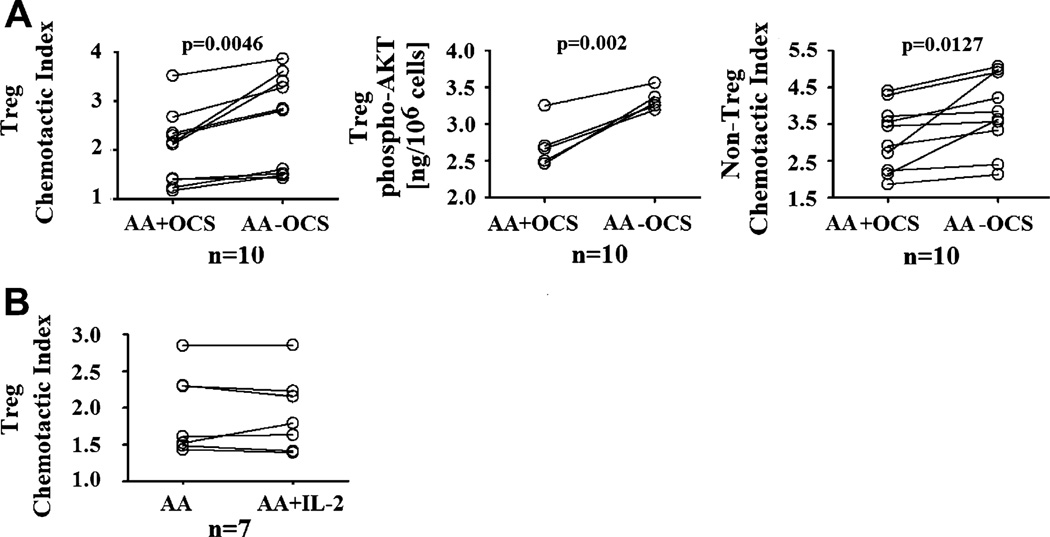
We next investigated the possible influences of corticosteroids on chemotactic responses of Treg cells to CCL1. Because corticosteroids have been reported to impede lymphocyte chemotaxis, a 12-hour steroid withdrawal on blood donation was enforced in all AA subjects used in our analysis of Treg cell function. For selected subjects with different disease severity and steroid dosages, we collected blood samples twice: the first time without steroid withdrawal and the second time with steroid withdrawal. These samples were then used to evaluate the effects of steroids on chemotaxis of Treg cells. Paired analysis of Treg cells from these subjects showed that steroids indeed exerted a negative influence on the chemotactic response of Treg cells toward CCL1, as shown by significantly lower chemotactic indices and phospho-AKT levels of Treg cells from blood samples without steroid withdrawal (Fig 4, A). Steroid withdrawal experiments of Treg cells from NA subjects, as well as studies of Treg cells from HC subjects, incubated with high-dose prednisone ex vivo (1000 ng/mL for 24 hours) showed similar inhibitory effects of steroids on chemotactic response of Treg cells to CCL1 (see Fig E6 in this article’s Online Repository at www.jacionline.org). Studies of non-Treg cells also yielded similar effects of steroids on CCL1 chemotactic response (Fig 4, A, and see Fig E6). The down modulatory effects of steroids on Treg and non-Treg cell chemotaxis were not specific to CCL1 because these phenomena were observed in assays with CCL17 and CCL22 (see Fig E7 in this article’s Online Repository at www.jacionline.org). In addition to steroids, we also performed incubation experimentation of Treg cells from AA subjects with IL-2, a critical cytokine for regulatory function and signaling pathways of Treg cells, to investigate whether this cytokine could reverse the defective CCL1 signaling in AA Treg cells. However, IL-2 (at 100 IU/mL for 24 hours) did not significantly upregulate the chemotactic response of Treg cells to CCL1 (Fig 4, B). Increasing (or decreasing) IL-2 concentration, as well as incubation time, also did not enhance the chemotactic response of Treg cells (see Fig E4, C).
FIG 4.
Modulation of CCL1 response in Treg cells by oral corticosteroids (OCS) and other cytokines. A, The effects of OCS on chemotactic response to CCL1 in Treg and non-Treg cells. Comparisons were made between cells from the same AA subjects that were collected with and without steroid withdrawal enforcement. B, The effects of IL-2 on chemotactic response to CCL1 in Treg cells. IL-2 data represented incubation of Treg cells with 100 IU/mL IL-2 for 24 hours (see this article’s Online Repository for other time points and dosages). The paired t test was used for the analyses of chemotactic indices. The Wilcoxon test was used for all other analyses because the data were not normally distributed.
DISCUSSION
Whether CCL1 is involved in the recruitment of inflammatory CD4+ T cells to the airway on asthmatic inflammation has been a controversial subject,23–25 possibly because of different protocols to obtain and examine BALF,17–19 as well as the use of different mouse models of asthma, which recapitulate most, but not all, features of human allergic asthma.46 Nevertheless, the most recent study has suggested that CCL1 is important in human Treg cell motility and possibly involved in their recruitment to areas of allergic inflammation.38 Our study here reveals a dysfunction in CCL1 signaling that is specific to allergic asthmatic Treg cells but not non-Treg cells. Decreased chemotactic response to CCL1 in AA Treg cells is not due to differential CCR8 expression because ex vivo expression of CCR8 by Treg cells was similar among the 3 subject groups. In addition, it has been reported that there was no significant difference in serum levels of CCL1 between AA and HC subjects.47 Thus it is unlikely that this in vitro hyporesponsiveness to CCL1 by AA Treg cells resulted from previous exposure to circulating CCL1 in vivo. In fact, our study of the CCL1/CCR8 signaling pathway in Treg cells reveals that the decreased response to CCL1 by AA Treg cells is associated with a decreased phosphorylation of AKT, an important protein in chemotactic signaling pathway. It is unlikely that a global defect in the phosphoinositide-3 kinase signaling pathway occurred in AA Treg cells because CCL1 stimulation of AA Treg cells leads to phosphorylation of ERK1/2, a downstream effector of phosphoinositide-3 kinase signaling that is also involved in several other signaling pathways at a level similar to that seen in HC Treg cells. Furthermore, decreased phosphorylation of AKT in AA Treg cells is specific to the CCL1-CCR8 signaling pathway but not the CCL17/CCL22-CCR4 pathway. These results suggest that a specific deregulation of signaling events proximally downstream of CCL1-CCR8 ligation and before AKT phosphorylation might be present in AA Treg cells. This hypothesis is currently being investigated in our laboratory.
Another important issue related to CCL1 response by Treg cells is the medication status of study subjects. Even though inhaled corticosteroids have been shown to impede T-cell migration toward TH2-associated chemokines in airway inflammation,48 it is unlikely that the observed reduction in chemotactic response of Treg cells in AA subjects was chiefly mediated by steroids. First, all subjects in our study were undergoing a 12-hour steroid withdrawal procedure before blood was drawn for chemotaxis study. Second, the decreased chemotactic response of Treg cells was specific to CCL1, which is unlikely to only be mediated by nonspecific immunosuppressive reagents, such as steroids. Our data indeed showed a nonspecific inhibition of chemotaxis of both HC Treg and non-Treg cells, which were incubated in vitro with prednisone, toward CCL1, CCL17, and CCL22. Paired comparison of chemotatic responses of T cells from selected NA and AA subjects before and after the steroid withdrawal study also showed a nonspecific decrease in the chemotactic potential of these cells mediated by steroids.
There is a significant correlation between the in vitro migratory capacity of Treg cells and asthma severity, as well as pulmonary function, in AA subjects. These results suggest that a decreased chemotactic response to CCL1 in AA Treg cells could possibly lead to decreased accumulation of Treg cells in the airway and subsequent ineffective immune regulation of Treg cells against airway-specific TH2 inflammation. Alternatively, it is possible that non-Treg cell–specific chemoattractants might be produced in abundance, leading to a higher number of non-Treg cells infiltrating the airways. In addition, defective Treg cell function in inflammatory diseases has been suggested to be a reactionary phenomenon to primary immune defects. Thus it could be possible that the predominantly TH2 environment of allergic asthma interfered with the optimal migratory potentials of Treg cells. Further examination of the distribution and function of CCR8+ Treg cells in the BALF of AA subjects, as well as the role of TH2 cytokines in the functional maintenance of Treg cells, is required to confirm these hypotheses.
Our data implicate the potential role of Treg cells and CCL1 in the development of new treatments for allergic asthma. Recently, lovastatin, a hypolipidemic drug, was shown to specifically recruit Treg cells to areas of inflammation through CCL1 in mice.49 However, lovastatin treatment was also associated with a predominant TH2 immune response. This finding not only showed that specifically targeting Treg cells is feasible but also highlighted the specificity issue in current drug delivery systems. The development of Treg cell–specific therapies have been largely hindered because specific surface markers of Treg cells are not known and distinct (chemotactic) signaling pathways in Treg cells in comparison with effector T cells are not fully characterized. It is anticipated that in the near future, advances in Treg cell biology will potentially lead to Treg cell–specific therapies. Our data here presented CCL1 as a promising molecular target for therapeutic development in allergic asthma.
In conclusion, our data show that AA Treg cells might be dysfunctional in the CCL1 chemotactic signaling pathway and could possibly explain the previously reported decreased accumulation of Treg cells in the BALF of AA subjects. CCL1 presents a potential molecular target for therapeutic development in allergic asthma.
Supplementary Material
Clinical implications: The CCL1-specific impaired migration of Treg cells in allergic asthma might contribute to their reduced frequency at sites of airway inflammation.
Acknowledgments
We thank Henriette MacMillan from the Mellins Laboratory for help with Western blot optimization. We also thank the asthmatic and healthy subjects who provided samples for the study.
Supported by grants from the Mary Hewitt Loveless Foundation, the Parker B. Francis Foundation, and the American Academy of Allergy, Asthma & Immunology.
Abbreviations used
- AA
Allergic asthmatic
- AKT
Protein kinase B
- BALF
Bronchoalveolar lavage fluid
- ERK1/2
Extracellular signal-regulated kinase 1/2
- HC
Healthy control
- NA
Nonallergic asthmatic
- Non-Treg
CD4+CD25− T
- Phospho-AKT
Phosphorylated AKT (or protein kinase B)
- PTEN
Phosphatase/tensin homolog deleted on chromosome 10
- Treg
CD4+CD25hiCD127lo/− regulatory T
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Nguyen KD, Vanichsarn C, Nadeau KC. Increased cytotoxicity of CD4 + invariant NKT cells against CD4 + CD25hiCD127lo/− regulatory T cells in allergic asthma. Eur J Immunol. 2008;38:2034–2045. doi: 10.1002/eji.200738082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gernez Y, Tirouvanziam R, Nguyen KD, Herzenberg LA, Krensky AM, Nadeau KC. Altered phosphorylated signal transducer and activator of transcription profile of CD4 + CD161 + T cells in asthma: modulation by allergic status and oral corticosteroids. J Allergy Clin Immunol. 2007;120:1441–1448. doi: 10.1016/j.jaci.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jousilahti P, Salomaa V, Hakala K, Rasi V, Vahtera E, Palosuo T. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol. 2002;89:381–385. doi: 10.1016/S1081-1206(10)62039-X. [DOI] [PubMed] [Google Scholar]
- 4.Johansson SG, Bennich H, Berg T, Högman C. Some factors influencing the serum IgE levels in atopic diseases. Clin Exp Immunol. 1970;6:43–47. [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto T, Mike T, Yamaguchi K, Murakami M, Kawabe T, Yodoi J. Serum levels of soluble IL-2 receptor, IL-4 and IgE-binding factors in childhood allergic diseases. Clin Exp Immunol. 1991;85:288–292. doi: 10.1111/j.1365-2249.1991.tb05720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Byrne PM. Allergen-induced airway hyperresponsiveness. J Allergy Clin Immunol. 1988;81:119–127. doi: 10.1016/0091-6749(88)90230-8. [DOI] [PubMed] [Google Scholar]
- 7.Lee TH. Interactions between alveolar macrophages, monocytes, and granulocytes. Implications for airway inflammation. Am Rev Respir Dis. 1987;135(suppl):S14–S17. doi: 10.1164/arrd.1987.135.6P2.S14. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 9.Averbeck M, Gebhardt C, Emmrich F, Treudler R, Simon JC. Immunologic principles of allergic disease. J Dtsch Dermatol Ges. 2007;5:1015–1028. doi: 10.1111/j.1610-0387.2007.06538.x. [DOI] [PubMed] [Google Scholar]
- 10.Sotsios Y, Ward SG. Phosphoinositide 3-kinase: a key biochemical signal for cell migration in response to chemokines. Immunol Rev. 2000;177:217–235. doi: 10.1034/j.1600-065x.2000.17712.x. [DOI] [PubMed] [Google Scholar]
- 11.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–136. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comer FI, Parent CA. PI 3-kinases and PTEN: how opposites chemoattract. Cell. 2002;109:541–544. doi: 10.1016/s0092-8674(02)00765-1. [DOI] [PubMed] [Google Scholar]
- 13.Sur S, Gleich GJ, Swanson MC, Bartemes KR, Broide DH. Eosinophilic inflammation is associated with elevation of interleukin-5 in the airways of patients with spontaneous symptomatic asthma. J Allergy Clin Immunol. 1995;96:661–668. doi: 10.1016/s0091-6749(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 14.Miotto D, Christodoulopoulos P, Olivenstein R, Taha R, Cameron L, Tsicopoulos A, et al. Expression of IFN-gamma-inducible protein; monocyte chemotactic proteins 1, 3, and 4; eotaxin in TH1- and TH2-mediated lung diseases. J Allergy Clin Immunol. 2001;107:664–670. doi: 10.1067/mai.2001.113524. [DOI] [PubMed] [Google Scholar]
- 15.Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, et al. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;163:1669–1675. doi: 10.1164/ajrccm.163.7.9812044. [DOI] [PubMed] [Google Scholar]
- 16.Nocker RE, Out TA, Weller FR, Mul EP, Jansen HM, van der Zee JS. Influx of neutrophils into the airway lumen at 4 h after segmental allergen challenge in asthma. Int Arch Allergy Immunol. 1999;119:45–53. doi: 10.1159/000024174. [DOI] [PubMed] [Google Scholar]
- 17.Leung TF, Wong CK, Chan IH, Ip WK, Lam CW, Wong GW. Plasma concentration of thymus and activation-regulated chemokine is elevated in childhood asthma. J Allergy Clin Immunol. 2002;110:404–409. doi: 10.1067/mai.2002.126378. [DOI] [PubMed] [Google Scholar]
- 18.Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J. 2004;23:876–884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- 19.Montes-Vizuet R, Vega-Miranda A, Valencia-Maqueda E, Negrete-García MC, Velásquez JR, Teran LM. CC chemokine ligand 1 is released into the airways of atopic asthmatics. Eur Respir J. 2006;28:59–67. doi: 10.1183/09031936.06.00134304. [DOI] [PubMed] [Google Scholar]
- 20.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 21.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuh JM, Power CA, Proudfoot AE, Kunkel SL, Lukacs NW, Hogaboam CM. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4−/− mice. FASEB J. 2002;16:1313–1315. doi: 10.1096/fj.02-0193fje. [DOI] [PubMed] [Google Scholar]
- 23.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001;193:573–584. doi: 10.1084/jem.193.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalo JA, Qiu Y, Lora JM, Al-Garawi A, Villeval JL, Boyce JA, et al. Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol. 2007;179:1740–1750. doi: 10.4049/jimmunol.179.3.1740. [DOI] [PubMed] [Google Scholar]
- 25.Buckland KF, O’Connor EC, Coleman EM, Lira SA, Lukacs NW, Hogaboam CM. Remission of chronic fungal asthma in the absence of CCR8. J Allergy Clin Immunol. 2007;119:997–1004. doi: 10.1016/j.jaci.2006.12.660. [DOI] [PubMed] [Google Scholar]
- 26.Chung CD, Kuo F, Kumer J, Motani AS, Lawrence CE, Henderson WR, Jr, et al. CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J Immunol. 2003;170:581–587. doi: 10.4049/jimmunol.170.1.581. [DOI] [PubMed] [Google Scholar]
- 27.Goya I, Villares R, Zaballos A, Gutiérrez J, Kremer L, Gonzalo JA, et al. Absence of CCR8 does not impair the response to ovalbumin-induced allergic airway disease. J Immunol. 2003;170:2138–2146. doi: 10.4049/jimmunol.170.4.2138. [DOI] [PubMed] [Google Scholar]
- 28.Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr Allergy Asthma Rep. 2005;5:136–141. doi: 10.1007/s11882-005-0087-8. [DOI] [PubMed] [Google Scholar]
- 29.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4 + CD25 + regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34:1364–1372. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 31.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4 + CD25 + regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 32.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4 + CD25 + regulatory T cells. J Exp Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, et al. CD4 + CD25 + T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shevach EM. CD4 + CD25 + suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 + CD25 + regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 36.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 andCCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soler D, Chapman TR, Poisson LR, Wang L, Cote-Sierra J, Ryan M, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3 + regulatory and Th2 effector lymphocytes. J Immunol. 2006;177:6940–6951. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 39.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, et al. Quantitative and functional impairment of pulmonary CD4 + CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 42.Ward SG. Do phosphoinositide 3-kinases direct lymphocyte navigation? Trends Immunol. 2004;25:67–74. doi: 10.1016/j.it.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki AT, Firtel RA. Regulation of chemotaxis by the orchestrated activation of Ras, PI3 K, TOR. Eur J Cell Biol. 2006;85:873–895. doi: 10.1016/j.ejcb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Spinetti G, Bernardini G, Camarda G, Mangoni A, Santoni A, Capogrossi MC, et al. The chemokine receptor CCR8 mediates rescue from dexamethasone-induced apoptosis via an ERK-dependent pathway. J Leukoc Biol. 2003;73:201–207. doi: 10.1189/jlb.0302105. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura ES, Koizumi K, Kobayashi M, Saitoh Y, Arita Y, Nakayama T, et al. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis. 2006;23:9–18. doi: 10.1007/s10585-006-9006-1. [DOI] [PubMed] [Google Scholar]
- 46.Medoff BD, Thomas SY, Banerji A, Wain JC, Zhang H, Lilly CM, et al. Pathogenic T-cell recruitment into the airway in human disease. Ann N Y Acad Sci. 2005;1062:220–241. doi: 10.1196/annals.1358.026. [DOI] [PubMed] [Google Scholar]
- 47.Lai ST, Hung CH, Hua YM, Hsu SH, Jong YJ, Suen JL. T-helper 1-related chemokines in the exacerbation of childhood asthma. Pediatr Int. 2008;50:99–102. doi: 10.1111/j.1442-200X.2007.02533.x. [DOI] [PubMed] [Google Scholar]
- 48.Hoshino M, Nakagawa T, Sano Y, Hirai K. Effect of inhaled corticosteroid on an immunoreactive thymus and activation-regulated chemokine expression in the bronchial biopsies from asthmatics. Allergy. 2005;60:317–322. doi: 10.1111/j.1398-9995.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 49.Mira E, León B, Barber DF, Jiménez-Baranda S, Goya I, Almonacid L, Márquez G, et al. Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J Immunol. 2008;181:3524–3534. doi: 10.4049/jimmunol.181.5.3524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.