Abstract
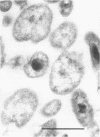
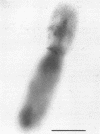
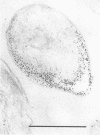
By using the cytochemical staining procedure with concanavalin A, horseradish peroxidase, and diaminobenzidine, no surface carbohydrates with terminal alpha-glucosyl or sterically closely related residues could be detected on the cell walls of Coxiella burnetii phases I and II. Using a polycationized ferritin derivative as a cytochemical probe, anionic binding sites were visualized in the electron microscope on cell membranes of C. burnetii phase II, but not on phase I organisms. The sites appeared to be masked in phase I particles. Anionic sites could be demonstrated on phase I organisms after treatment with NaIO4 or dimethyl sulfoxide. A number of different biological properties of C. burnetii phases I and II may depend on the presence or absence of a net negative charge on the surface of the cell walls of these organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREZINA R., KAZAR J. PHAGOCYTOSIS OF COXIELLA BURNETI AND THE PHASE VARIATION PHENOMENON. Acta Virol. 1963 Sep;7:476–476. [PubMed] [Google Scholar]
- BREZINA R., KAZAR J. STUDY OF THE ANTIGENIC STRUCTURE OF COXIELLA BURNETI. IV. PHAGOCYTOSIS AND OPSONIZATION IN RELATION TO THE PHASES OF C. BURNETI. Acta Virol. 1965 May;9:268–274. [PubMed] [Google Scholar]
- Bernhard W., Avrameas S. Ultrastructural visualization of cellular carbohydrate components by means of concanavalin A. Exp Cell Res. 1971 Jan;64(1):232–236. doi: 10.1016/0014-4827(71)90217-5. [DOI] [PubMed] [Google Scholar]
- Bretton R., Wicker R., Bernhard W. Ultrastructural localization of concanavalin A receptors in normal and SV 40 -transformed hamster and rat cells. Int J Cancer. 1972 Sep 15;10(2):397–410. doi: 10.1002/ijc.2910100222. [DOI] [PubMed] [Google Scholar]
- Brezina R. New advances in the study of rickettsial antigens. Zentralbl Bakteriol Orig. 1968 Apr;206(3):313–321. [PubMed] [Google Scholar]
- Brezina R., Pospísil V., Schramek S. Study of the antigenic structure of Coxiella burneti. VII. Properties of phenol-extracted phase I antigenic component. Acta Virol. 1970 Jul;14(4):295–301. [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Downs C. M. Phagocytosis of coxiella burneti, phase I and phase II by peritoneal monocytes from normal and immune guinea pigs and mice. Zentralbl Bakteriol Orig. 1968 Apr;206(3):329–343. [PubMed] [Google Scholar]
- FISET P. Phase variation of Rickettsia (Coxiella) burneti; study of the antibody response in guinea pigs and rabbits. Can J Microbiol. 1957 Apr;3(3):435–445. doi: 10.1139/m57-046. [DOI] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A. The antibody response to antigens of Coxiella burneti. Zentralbl Bakteriol Orig. 1968 Apr;206(3):321–329. [PubMed] [Google Scholar]
- HOYER B. H., ORMSBEE R. A., FISET P., LACKMAN D. B. Differentiation of Phase I and Phase II Coxiella burnetti by equilibrium density gradient sedimentation. Nature. 1963 Feb 9;197:573–574. doi: 10.1038/197573a0. [DOI] [PubMed] [Google Scholar]
- ORMSBEE R. A. AN AGGLUTINATION-RESUSPENSION TEST FOR Q FEVER ANTIBODIES. J Immunol. 1964 Jan;92:159–166. [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B. Antigens of Coxiella burnetii. I. Extraction of antigens with non-aqueous organic solvents. J Immunol. 1962 Jun;88:741–749. [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- Schiefer H. G., Krauss H., Brunner H., Gerhardt U. Ultrastructural visualization of anionic sites on mycoplasma membranes by polycationic ferritin. J Bacteriol. 1976 Jul;127(1):461–468. doi: 10.1128/jb.127.1.461-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer H. G., Krauss H., Brunner H., Gerhardt U. Ultrastructural visualization of surface carbohydrate structures on mycoplasma membranes by concanavalin A. J Bacteriol. 1975 Dec;124(3):1598–1600. doi: 10.1128/jb.124.3.1598-1600.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz H. D., Schmatz S., Krauss H. Der Einfluss der Inokulationsdosis auf die Gewinnung vonCoxiella burnetii-Antigen aus dem Dottersack embryonierteer Hühnereier. Berl Munch Tierarztl Wochenschr. 1976 Mar 15;89(6):120–122. [PubMed] [Google Scholar]
- Schramek S., Brezina R. Characterization of an endotoxic lipopolysaccharide from Coxiella burnetii. Acta Virol. 1976 Apr;20(2):152–158. [PubMed] [Google Scholar]
- Schramek S., Brezina R., Pospísil V. Improved method of preparing phase II Coxiella burneti antigen for the microagglutination test. Acta Virol. 1970 Sep;14(5):415–415. [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Fiset P., Ormsbee R. A. Interaction of rickettsiae and phagocytic host cells. V. Phagocytic and opsonic interactions of phase 1 and phase 2 Coxiella burneti with normal and immune human leukocytes and antibodies. J Immunol. 1967 Oct;99(4):669–674. [PubMed] [Google Scholar]