Abstract
Background:
Harms associated with prescription opioids are a major and increasing public health concern. Prescribing of opioids for inpatients may contribute to the problem, especially if primary care practitioners continue opioid therapy that is initiated in hospital.
Objectives:
To describe the extent and nature of opioid prescribing for opioid-naive patients (i.e., no use of opioids within 2 weeks before admission) on an internal medicine unit.
Methods:
This single-centre study involved chart review for opioid-naive patients admitted to the internal medicine unit of a large academic health sciences centre in Toronto, Ontario. Over 12 weeks, patients were prospectively identified for the study, and charts were later reviewed to characterize opioid use during the hospital stay and upon discharge. The primary outcomes were the proportions of opioid-naive patients for whom opioids were prescribed in hospital and upon discharge. Data on serious adverse events related to opioid use (e.g., need for naloxone or occurrence of falls) were also collected through chart review.
Results:
From July 4 to September 22, 2011, a total of 721 patients were admitted to the study unit, of whom 381 (53%) were classified as opioid-naive. Opioids were prescribed for 82 (22%) of these opioid-naive patients while they were in hospital. Among the opioid-naive patients, there were a total of 247 opioid prescriptions, with hydromorphone (110 prescriptions) and morphine (92 prescriptions) being the drugs most commonly prescribed. For 23 (28%) of the patients with a prescription for opioids in hospital (6% of all opioid-naive patients), an opioid was also prescribed upon discharge. The indication for opioids was documented in 16 (70%) of the 23 discharge prescriptions. No adverse events or deaths related to opioid use were identified during the hospital stays.
Conclusions:
Among opioid-naive patients admitted to the internal medicine unit, opioids were prescribed for about 1 in 5 patients, and less than one-third of these patients were continued on opioids at the time of discharge. These results, if replicated elsewhere, suggest that efforts to improve opioid prescribing and reduce attendant harm should be focused primarily on the outpatient setting.
Keywords: opioid abuse, opioid prescribing, internal medicine, discharge, medication safety, opioids
RÉSUMÉ
Contexte :
Les préjudices liés aux opioïdes d’ordonnance représentent un enjeu important et croissant en santé publique. Le fait de prescrire des opioïdes aux patients hospitalisés pourrait aggraver le problème, particulièrement si les praticiens de premier recours poursuivent le traitement opioïde amorcé à l’hôpital.
Objectifs :
Décrire dans quelle mesure l’on prescrit quels opioïdes dans un service de médecine interne à des patients n’ayant pas reçu ces analgésiques au cours des deux semaines précédant leur hospitalisation.
Méthodes :
Cette étude menée dans un seul centre comportait une analyse des dossiers médicaux de patients ayant été admis au service de médecine interne d’un important centre de santé universitaire à Toronto en Ontario et n’ayant pas reçu d’opioïdes au cours des deux semaines précédant l’hospitalisation. Sur une période de douze semaines, ces patients ont été recrutés de façon prospective pour l’étude et leurs dossiers ont été examinés ultérieurement afin de décrire l’utilisation d’opioïdes au cours de leur séjour à l’hôpital et au moment du congé. Les principaux paramètres d’évaluation étaient les proportions de patients n’ayant pas reçu d’opioïdes au cours des deux semaines précédant leur hospitalisation qui s’en sont vu prescrire au cours du séjour et au moment du congé. Des données sur les événements indésirables graves liés à la prise d’opioïdes (p. ex., le recours à la naloxone ou les cas de chutes) ont aussi été recueillies à l’aide de l’analyse des dossiers médicaux.
Résultats :
Entre le 4 juillet et le 22 septembre 2011, un total de 721 patients ont été admis dans le service à l’étude et, parmi eux, 381 (53 %) n’avaient pas reçu d’opioïdes au cours des deux semaines précédant leur hospitalisation. Des opioïdes ont été prescrits à 82 de ces derniers (22 %) alors qu’ils séjournaient à l’hôpital. Parmi les patients admissibles à l’étude, on a relevé 247 ordonnances d’opioïdes, dont 110 ordonnances d’hydro-morphone et 92 ordonnances de morphine, les opioïdes les plus couramment prescrits. Vingt-trois (28 %) des patients à qui l’on a prescrit des opioïdes à l’hôpital (6 % de l’ensemble des patients n’ayant pas reçu d’opioïdes au cours des deux semaines précédant leur hospitalisation) ont aussi obtenu une ordonnance pour des opioïdes au moment du congé. Les indications pour les opioïdes ont été consignées dans 16 (70 %) des 23 cas d’ordonnances données au moment du congé. Aucun événement indésirable ou décès lié à l’utilisation d’opioïdes n’a été noté pendant les séjours à l’hôpital.
Conclusions :
Parmi les patients n’ayant pas reçu d’opioïdes au cours des deux semaines précédant leur hospitalisation dans le service de médecine interne, un patient sur cinq s’est vu prescrire des opioïdes et moins d’un tiers de ces patients ont vu se poursuivre ce traitement au moment du congé. Les résultats, si cette étude est reproduite ailleurs, suggèrent que les efforts visant à améliorer les pratiques de prescription d’opioïdes et à réduire les risques inhérents pour le patient doivent être axés principalement sur les soins externes.
[Traduction par l’éditeur]
Keywords: abus d’opioïdes, prescription d’opioïdes, médecine interne, congé, sécurité des médicaments, opioïdes
INTRODUCTION
Over the past decade, there has been increasing recognition that the more liberal use of opioids for chronic noncancer pain has been associated with a substantial increase in opioid addiction and fatal overdoses.1–5 In the United States, the Centers for Disease Control and Prevention reported that the number of overdose deaths involving opioid analgesics rose from 14 800 in 2008 to more than 16 000 in 2010.6,7 The increase in deaths from opioid overdose has occurred concurrently with a 4-fold increase in sales of opioid analgesics between 1999 and 2010.7 In Ontario, the number of deaths associated with oxycodone use increased 5-fold from 1999 to 2004.5 Popova and others8 examined the number of individuals in Canada’s general population who were using prescription opioids nonmedically, specifically the population of street drug users. They found that street drug users (except those in Montréal and Vancouver) used prescription opioids more than heroin in 2003, and from 2002 to 2005, they documented a 24% relative increase in the nonmedicinal use of prescription opioids by street drug users.
The rate of prescription opioid use in certain populations is of particular concern. For example, a state of emergency was declared in the Nishnawbe Aski Nation of Northern Ontario after a survey found that about 9000 to 10 000 of the 45 000 First Nations and Aboriginal people represented by the organization were addicted to prescription opioids.9,10 Harms associated with prescription opioids are a major public health concern in both Canada5,8–11 and the United States.7,12,13
Most studies of opioid prescribing practices have involved patients in outpatient and emergency department settings.4,14,15 To date, opioid prescribing patterns among opioid-naive hospital inpatients has not been well characterized. In the hospital setting, opioids are used frequently for the treatment of both acute and chronic pain.
Given the well-described trend of increasing opioid-related harm in the outpatient setting,1,3–10 and the lack of research attention directed toward inpatient opioid prescribing, this study examined patterns of opioid prescribing for inpatients admitted to a medical inpatient unit. The study also described the number and types of opioid-related adverse events occurring in hospital among patients for whom opioids had been newly prescribed.
METHODS
This study was conducted on the internal medicine clinical teaching unit at St Michael’s Hospital, a large academic health sciences centre in Toronto, Ontario. In a typical year, this internal medicine service admits about 3300 patients, almost all of them from the emergency department.
The study included all opioid-naive patients admitted over a 12-week period (July 4 to September 22, 2011), where “opioid-naive” was defined as absence of opioid medication use within the 2 weeks before admission to hospital. Patients were excluded if they had been transferred from an intensive care unit, if they had been transferred to internal medicine from another service, or if they were transferred from internal medicine to another medical service before discharge.
Medication histories were obtained by the admitting physician or staff pharmacist and were documented according to routine practice. For each eligible patient, a research assistant used a structured data collection form to collect the following information from the health record: age and sex; serum creatinine level on admission; initial opioid and nonopioid regimens and subsequent changes prescribed in hospital, including changes to dose, route, frequency, and routine or as needed (PRN) use; time of initial opioid order in relation to admission; number of doses administered; adverse events related to opioids; and opioid regimen (or regimens) prescribed upon discharge.
Adverse events potentially related to opioids were defined in terms of need for naloxone during the admission (based on review of the medication administration record) or diagnosis of respiratory depression, aspiration pneumonia, falls, or death (as recorded in the discharge summary). Renal function was determined at the time opioids were prescribed, as it was theorized that hydromorphone might be preferentially prescribed for patients with impaired renal function. Hydromorphone is often preferred over morphine and codeine for patients with renal failure and those undergoing dialysis.16 Renal function was calculated using the Cockcroft–Gault formula. If a patient’s weight had not been recorded, an estimated weight of 70 kg was used, irrespective of the patient’s sex. Serum creatinine at the time of admission was used for this calculation. Renal dysfunction was subsequently classified as mild, moderate, or severe according to the following cut-off values: ≥ 50 mL/min, 10–49 mL/min, or < 10 mL/min, respectively.
The primary outcomes were the number of opioid-naive patients for whom standing or PRN orders for opioids were prescribed in hospital and upon discharge. The secondary outcome was occurrence of an adverse event related to opioids. The study also examined the specific opioids that were prescribed, as well as the time between initial prescription of an immediate-release product and subsequent prescription of a controlled-release opioid.
Descriptive statistics were used to summarize prescribing patterns, and χ2 tests and t tests (Microsoft Excel 2007) were used for comparisons between patients for whom opioids were and were not prescribed.
The protocol was approved by the St Michael’s Hospital Research Ethics Board.
RESULTS
Prescribing of Opioids in Hospital
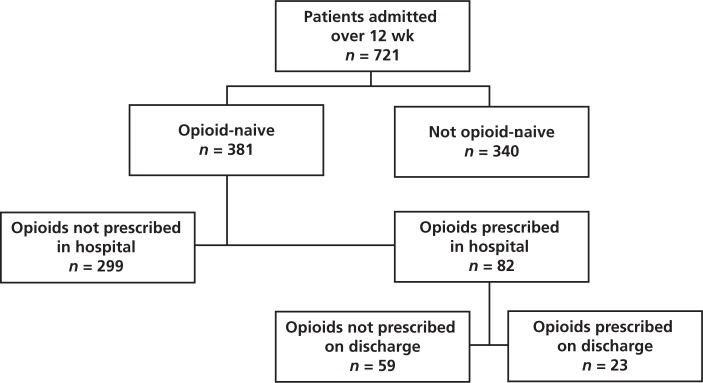
A total of 746 patients were admitted to the internal medicine service during the study period. Of these, 721 (97%) patients were screened for opioid-naivety, and 381 (53%) of those screened were identified as being opioid-naive (Figure 1). Demographic and clinical data for opioid-naive patients are summarized in Table 1. Of the 381 opioid-naive patients, 82 (22%) had a prescription (standing or PRN) for opioids in hospital. The total number of opioid orders (for IV, subcutaneous, or oral administration) was 247, of which 110 (45%) were for hydromorphone. The second most commonly prescribed opioid was morphine (92 prescriptions) (Table 2).
Figure 1.
Flow chart for patients included in the study.
Table 1.
Demographic and Clinical Characteristics of Opioid-Naive Inpatients with and without a Prescription for Opioids
| Characteristic | No. (%) of Patients* | p Value | |
|---|---|---|---|
| Opioids Prescribed (n = 82) | Opioids Not Prescribed (n = 299) | ||
| Age (years), mean | 59 | 68 | 0.001 |
| Sex, male | 45 (55) | 177 (59) | 0.48 |
| Length of stay (days), mean | 9 | 7 | 0.009 |
| Creatinine clearance (mL/min) | 0.18 | ||
| ≥ 50 (mild impairment) | 59 (72) | 188 (63) | |
| 10–49 (moderate impairment) | 20 (24) | 104 (35) | |
| < 10 (Severe impairment) | 3 (4) | 7 (2) | |
Except where indicated otherwise.
Table 2.
Number of Opioid Prescriptions for Opioid-Naive Inpatients, According to Renal Function
| Opioid | Renal Impairment*; No. of Prescriptions | |||
|---|---|---|---|---|
|
| ||||
| Mild | Moderate | Severe | Total | |
| Hydromorphone (PO, IV, SC) | 74 | 33 | 3 | 110 |
| Morphine (PO, IV, SC) | 79 | 12 | 1 | 92 |
| Percocet (acetaminophen 325 mg, oxycodone 5 mg) | 8 | 6 | 0 | 14 |
| Oxycodone | 5 | 1 | 0 | 6 |
| Codeine (PO only) | 0 | 1 | 0 | 1 |
| Tylenol | ||||
| No. 3 (acetaminophen 300 mg, caffeine 15 mg, codeine 30 mg) | 16 | 2 | 0 | 18 |
| No. 2 (acetaminophen 300 mg, caffeine 15 mg, codeine 15 mg) | 2 | 3 | 0 | 5 |
| No. 1 (acetaminophen 300 mg, caffeine 8 mg, codeine 8 mg) | 1 | 0 | 0 | 1 |
| Total | 185 | 58 | 4 | 247 |
IV = intravenous, PO = by mouth, SC = subcutaneous.
Renal impairment was defined in terms of creatinine clearance, as follows: mild = creatinine clearance ≥ 50 mL/min; moderate = creatinine clearance 10–49 mL/min; severe = creatinine clearance < 10 mL/min.
All but one of the patients with a standing prescription for an opioid also had a PRN opioid prescription. Of the 82 opioid-naive patients with a prescription for an opioid, 68 (83%) also had a prescription for a nonopioid analgesic (Table 3). For 8 of the patients, a controlled-release opioid such as morphine, oxycodone, or hydromorphone was prescribed. The interval from initial prescription of short-acting products to prescription of a controlled-release product ranged from 0 to 7 days.
Table 3.
Nonopioid Medications Prescribed for Inpatients with Opioid Prescriptions
| Nonopioid Medication | No. of Prescriptions* |
|---|---|
| Acetaminophen, PRN | 56 |
| Acetaminophen, standing | 29 |
| Tricyclic antidepressants | 3 |
| Nonsteroidal anti-inflammatory drugs | 9 |
| COX-2 inhibitors | 4 |
| Gabapentin | 4 |
| Cyclobenzaprine | 1 |
| Calcitonin spray | 2 |
| Total | 108 |
COX = cyclo-oxygenase, PRN = as needed.
A total of 68 patients had a prescription for a nonopioid analgesic. Some patients had prescriptions for more than one such medication.
Patients for whom opioids were prescribed were younger than those for whom opioids were not prescribed (59 versus 68 years, p = 0.001) (Table 1). Patients for whom opioids were prescribed also had a longer stay in hospital than those for whom opioids were not prescribed (9 versus 7 days, p = 0.009). There was no difference in opioid prescribing between men and women (p = 0.48).
Of the 82 patients for whom opioids were prescribed in hospital, one died from cardiac arrest, but this death was due to sepsis and multiorgan failure (i.e., not related to opioid therapy). There were no documented adverse events related to opioid use in hospital.
Prescribing of Opioids at Time of Discharge
Twenty-three (28%) of the 82 opioid-naive patients for whom opioids were prescribed in hospital also had a prescription for opioids upon discharge; this group represented 6% (23 of 381) of all opioid-naive patients admitted during the 12-week study period. The most commonly prescribed opioid on discharge was hydromorphone (Table 4). For 4 of these 23 patients, controlled-release opioids were prescribed on discharge. The duration of hospital stay for patients with a prescription for controlled-release opioids upon discharge ranged from 1 to 16 days. For the remaining 19 patients, immediate-release opioids, to be given on an as-needed basis, were prescribed at the time of discharge. Of note, for 5 patients a different opioid was prescribed at discharge than had been most recently prescribed in hospital. The discharge summaries for these patients did not indicate the reason for the change in therapy upon discharge. Four of these 5 patients were given a less potent opioid upon discharge.
Table 4.
Opioids Prescribed at Discharge for Opioid-Naive Patients
| Opioid Prescribed at Discharge | Renal Impairment*; No. of Patients | ||
|---|---|---|---|
|
| |||
| Mild | Moderate | Total | |
| Hydromorphone | |||
| Immediate release | 5 | 2 | 7 |
| Controlled release | 1 | 1 | 2 |
|
| |||
| Morphine | |||
| Immediate release | 2 | 0 | 2 |
| Controlled release | 1 | 0 | 1 |
|
| |||
| Oxycodone, controlled release | 1 | 0 | 1 |
|
| |||
| Percocet (acetaminophen 325 mg, oxycodone 5 mg) | 3 | 1 | 4 |
|
| |||
| Tylenol | |||
| No. 3 (acetaminophen 300 mg, caffeine 15 mg, codeine 30 mg) | 4 | 1 | 5 |
| No. 2 (acetaminophen 300 mg, caffeine 15 mg, codeine 15 mg) | 1 | 0 | 1 |
|
| |||
| Total | 18 | 5 | 23 |
Renal impairment was defined in terms of creatinine clearance, as follows: mild = creatinine clearance ≥ 50 mL/min; moderate = creatinine clearance 10–49 mL/min.
The indication for prescribing opioids was clearly indicated on the discharge summary or the prescription for 16 (70%) of the 23 patients. For the remaining 7 prescriptions, the indication was not clearly stated.
The prescribing of nonopioid analgesia was less common upon discharge than during the hospital stay. For 3 patients, acetaminophen prescribed at discharge was clearly intended for management of pain. For 2 patients, tricyclic antidepressants appeared on the discharge prescription, possibly as part of the pain regimen. For a majority of the patients (16 of 23), non-opioid analgesia was not prescribed on discharge.
DISCUSSION
In this study, approximately 1 of every 5 opioid-naive patients admitted to an internal medicine service at a large academic health science centre was given a prescription for opioid therapy. Upon discharge, 28% of opioid-naive patients for whom opioids had been prescribed in hospital (6% of all opioid-naive patients) also received an outpatient prescription for an opioid (Table 4). The indication for opioids prescribed in hospital could not be determined because of the study design; however, for 70% of discharge opioid prescriptions, the indication was clearly documented. There were no adverse events due to opioids. Although this finding probably indicates that prescribing and administration practices at this centre were reasonably safe, it is also possible that some adverse events, particularly any that were mild, were not documented on the discharge summary.
The most commonly prescribed opioid was hydromorphone, a potent opioid that is often preferred for patients with renal failure and those undergoing dialysis.16 This typical use for hydromorphone may explain why it was preferred in this study for patients with moderate and severe renal dysfunction, although it was also prescribed for patients without renal impairment. In contrast to the current study, a 3-month evaluation of all opioids prescribed in study wards at a tertiary referral teaching hospital in Australia showed that oxycodone was the most commonly prescribed opioid.17
In the current study, 83% of the inpatients receiving opioids also received nonopioid analgesia, which is in line with the 2012 Guidelines for the Pharmacological Management of Non-Cancer Pain in Adults of the East Lancashire Health Economy Medicines Management Board (part of the National Health Service).18 These guidelines recommend consideration of nonopioid analgesics at each step of the pain ladder.
The time from prescription of short-acting opioid products to prescription of controlled-release opioids ranged from 0 to 7 days. Notably, the Ontario Workplace and Safety Insurance Board will not provide reimbursement for controlled-release opioids until a patient has received at least 12 weeks of treatment with a short-acting opioid.19 Given this restriction, it is reasonable to recommend that initiation of controlled-release opioids in the inpatient setting should usually be avoided, except for patients who were using controlled-release opioids before admission.
No overt explanation could be found to justify hydromorphone as the most commonly prescribed opioid at the time of discharge (Table 4), given that 6 of the 9 discharge prescriptions for hydromorphone were for patients with creatinine clearance of at least 50 mL/min (i.e., mild renal impairment). The East Lancashire Health Economy Medicines Management Board guidelines state that there is no evidence for superior analgesic effect of any opioid over morphine.18 In 2012, the Institute for Safe Medication Practices Canada published the results of a hydromorphone knowledge assessment survey, which showed that the greatest knowledge deficit among nurses, pharmacists, and physicians occurred in the area of pharmacology.20 The authors of this survey report noted that, on the basis of incidents resulting in harm, it appears that many health care providers do not have a good understanding of the difference in potency between morphine and hydromorphone. The 2010 Canadian Guideline for the Safe and Effective Use of Opioids for Chronic Non-cancer Pain recommended caution when prescribing oxycodone, hydromorphone, or hydrocodone, because of the greater risk of abuse relative to morphine.21
In the current study, most of the patients had a prescription for nonopioid analgesia while in hospital, but a smaller proportion of patients had prescriptions for nonopioid analgesia upon discharge (83% versus 39%). The reason for prescribing opioids upon discharge was not always clearly documented in the discharge summary or discharge prescription. Improving the documentation of the indication for opioids may help to guide therapy when the patient returns to the community. Conversely, if the indication for opioids is not clearly stated, a patient’s family doctor may inappropriately prescribe these medications simply to continue treatment that was started in hospital.
The study methodology allowed for 97% of patients admitted during the study period to be included in the study, which eliminated selection bias. The rate of opioid prescribing within this internal medicine unit may provide a benchmark for future studies of opioid prescribing in the inpatient population.
This study had several limitations that merit emphasis. First, because this was a single-centre study at a teaching hospital, the results may not be generalizable to other inpatient care settings. Second, the indication for opioid prescribing was difficult to ascertain because of the brevity of chart notes. Third, although adverse events such as death and use of naloxone could be identified through medication administration records, other adverse events related to opioids, including respiratory depression, aspiration pneumonia, falls, or delirium, were more difficult to identify because doing so relied on the physician clearly documenting these events in the discharge summary. A complete chart review, including interviews with patients and health care providers, would likely yield a greater number of adverse events due to opioids. Finally, the number of prescriptions for opioids did not necessarily reflect the actual number of doses administered to patients. Use of this variable could overestimate the prescribing of potent opioids if some of the prescribed medications were never administered. However, the intent of this study was to identify physicians’ prescribing decisions, so the selected variable was appropriate.
CONCLUSION
About 20% of opioid-naive patients admitted to an internal medicine service at a large teaching hospital had a prescription for an opioid during their admission. For the majority of these patients, opioids were not prescribed at discharge, and adverse events related to opioids appeared to be uncommon. These results, if generalizable, may imply that efforts to improve opioid prescribing and reduce attendant harm should be focused primarily in the outpatient setting.
Acknowledgments
TThe authors would like to thank Dr. Heather Kertland and Dr. Clarence Chant for their support and guidance, as well as Vivian Li for her organization and dedication.
This study was partially funded by a grant from the Medbuy Research, Education and Development Fund. Dr. Irfan Dhalla was supported by a New Investigator Award from the Canadian Institutes of Health Research. The funding agencies had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The opinions, results and conclusions reported in this paper are those of the authors, and are independent from the funding sources.
Footnotes
Competing interests: None declared.
References
- 1.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–5. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346–7. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell JC. The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev. 2011;30(3):264–70. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401–35. [PubMed] [Google Scholar]
- 5.Dhalla IA, Mamdani MM, Sivilotti MLA, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181(12):891–6. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opioids drive continued increase in drug overdose deaths Drug overdose deaths increase for 11th consecutive year [news release] Atlanta (GA): US Centers for Disease Control and Prevention; 2013. Feb 20, [cited 2013 Oct 27]. Available from: www.cdc.gov/media/releases/2013/p0220_drug_overdose_deaths.html. [Google Scholar]
- 7.Centers for Disease Control and Prevention (US) Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–92. [PubMed] [Google Scholar]
- 8.Popova S, Patra J, Mohapatra S, Fischer B, Rehm J. How many people in Canada use prescription opioids non-medically in general and street drug using populations? Can J Public Health. 2009;100(2):104–8. doi: 10.1007/BF03405516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Restoring our nation: action plan for community recovery from opioid addiction. Thunder Bay (ON): Nishnawbe Aski Nation Think Tank on Prescription Drug Abuse; 2011. pp. 66–76. in 2010/2011 annual report of Nishnawbe Aski Nation, available from: www.nan.on.ca/upload/documents/finance-2011-annual-report.pdf. [Google Scholar]
- 10.NAN chiefs call for immediate assistance as region braces for major health catastrophe [news release] Thunder Bay (ON): Nishnawbe Aski Nation; 2012. Feb 16, [cited 2014 Sep 11]. Available from: www.nan.on.ca/upload/documents/nr-feb-15-pda-health.pdf. [Google Scholar]
- 11.Fischer B, Rehm J, Goldman B, Popova S. Non-medical use of prescription opioids and public health in Canada: an urgent call for research and interventions development. Can J Public Health. 2008;99(3):182–4. doi: 10.1007/BF03405469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (US) Adult use of prescription opioid pain medications—Utah 2008. MMWR Morb Mortal Wkly Rep. 2010;59(6):153–7. [PubMed] [Google Scholar]
- 13.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 14.Logan J, Liu Y, Paulozzi L, Zhang K, Jones C. Opioid prescribing in emergency departments: the prevalence of potentially inappropriate prescribing and misuse. Med Care. 2013;51(8):646–53. doi: 10.1097/MLR.0b013e318293c2c0. [DOI] [PubMed] [Google Scholar]
- 15.Wisniewski AM, Purdy CH, Blondell RD. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis. 2008;27(1):1–11. doi: 10.1300/J069v27n01_01. [DOI] [PubMed] [Google Scholar]
- 16.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613–24. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murnion BP, Gnjidic D, Hilmer SN. Prescription and administration of opioids to hospital in-patients, and barriers to effective use. Pain Med. 2010;11(1):58–66. doi: 10.1111/j.1526-4637.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- 18.Guidelines for the pharmacological management of non-cancer pain in adults. National Health Service, East Lancashire Health Economy Medicines Management Board; 2012. [cited 2013 Oct 27]. Available from: www.elmmb.nhs.uk/guidelines/disease-specific-guidelines/?assetdetesctl516557=50338. [Google Scholar]
- 19.Enhanced narcotics management for injured workers. Toronto (ON): Workplace Safety and Insurance Board – Ontario; © 1998–2014[cited 2013 Oct 27]. Available from: www.wsib.on.ca/en/community/WSIB/ArticleDetail?vgnextoid=3fc84c23529d7210VgnVCM100000449c710aRCRD. [Google Scholar]
- 20.Report to Health Canada: hydromorphone knowledge assessment survey results. Toronto (ON): Institute for Safe Medication Practices Canada; 2012. [cited 2014 Apr 30]. Available from: www.ismp-canada.org/download/miscpub/ISMPCanada_HYDROmorphoneKnowledgeAssessmentSurveyReport_2012June.pdf. [Google Scholar]
- 21.Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. Hamilton (ON): McMaster University, National Opioid Use Guideline Group; 2010. [cited 2014 Apr 30]. Available from: http://nationalpaincentre.mcmaster.ca/opioid/ [Google Scholar]