Abstract
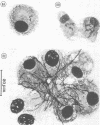
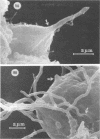
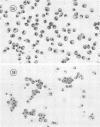
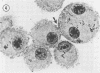
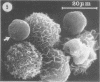
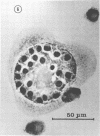
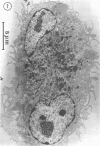
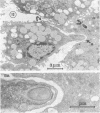
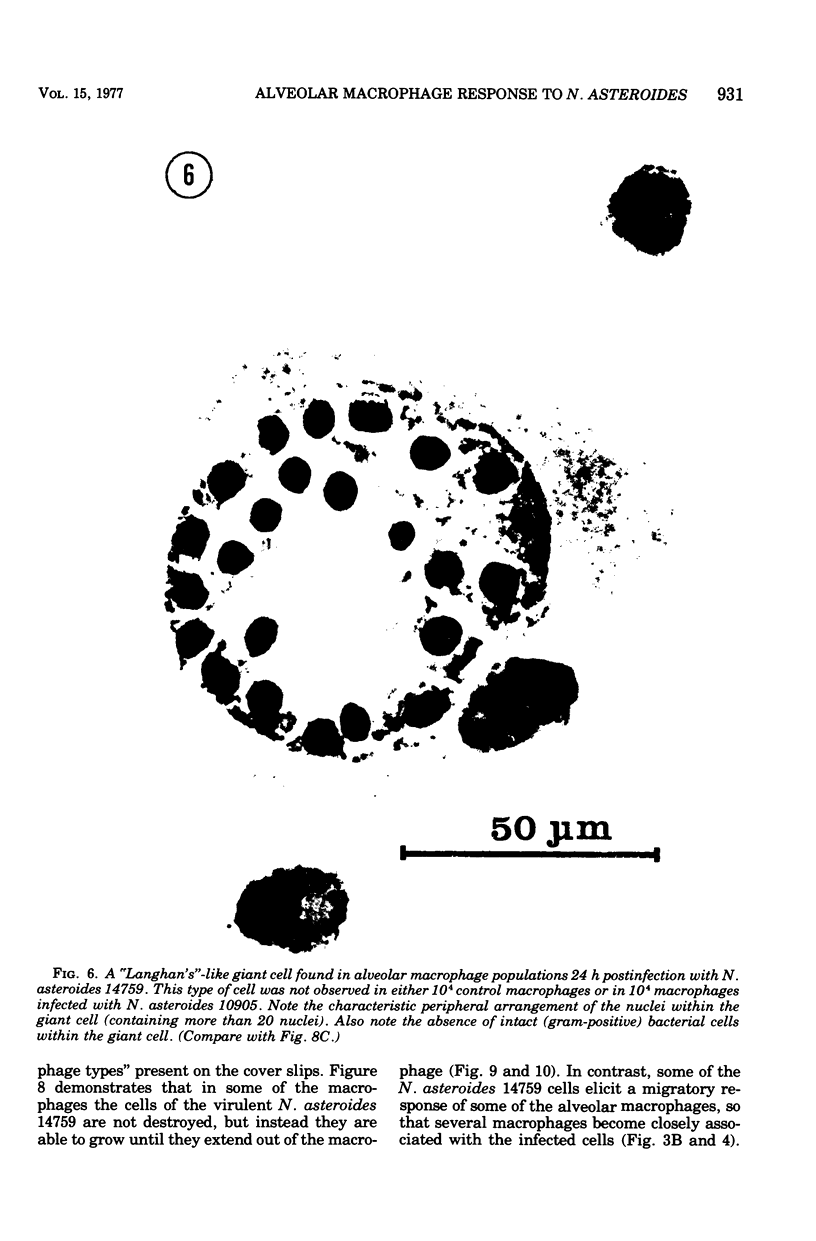
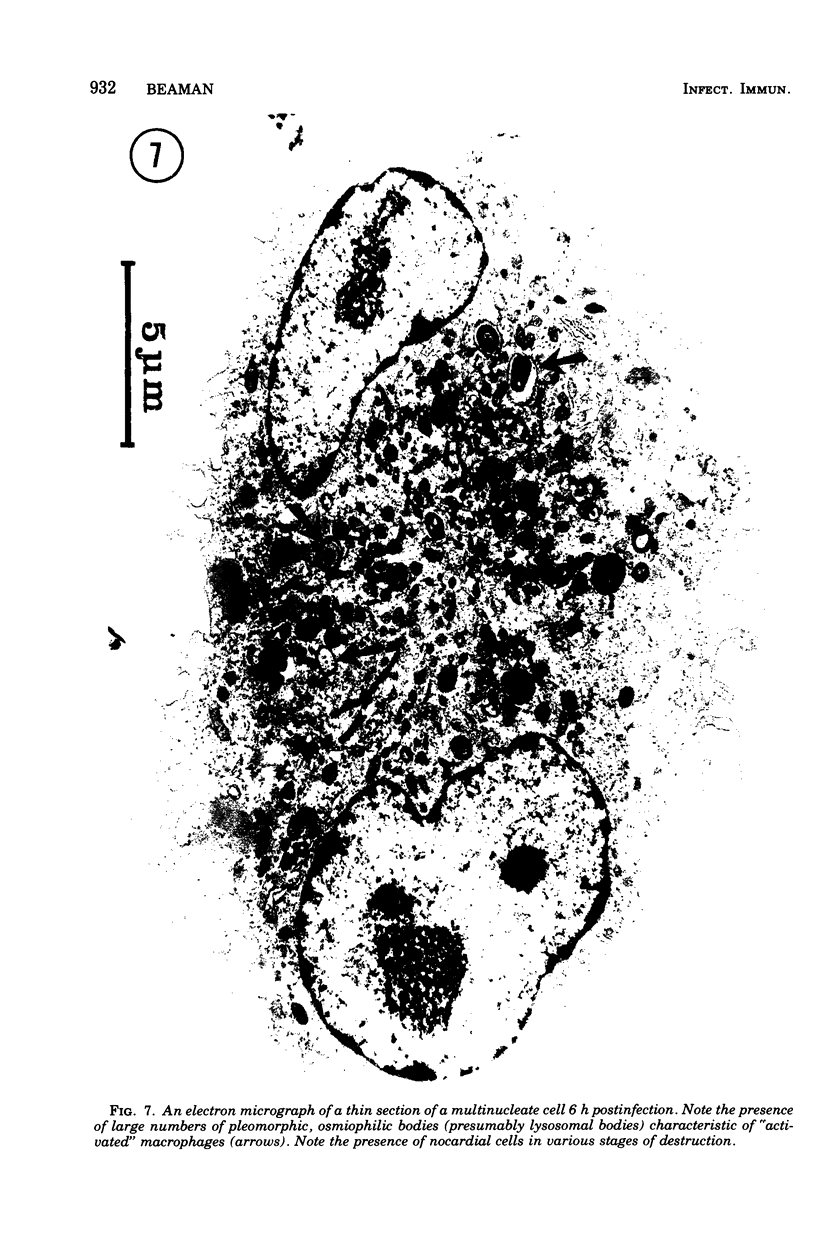
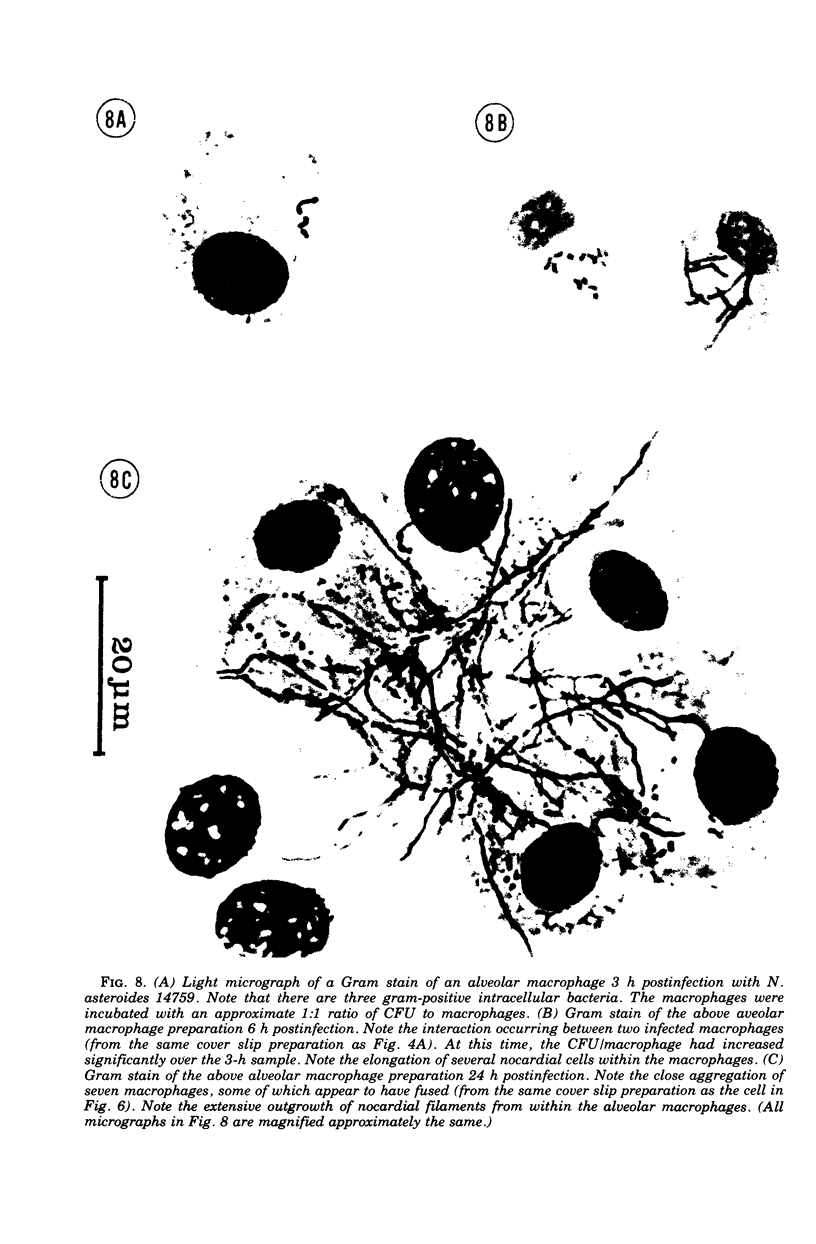
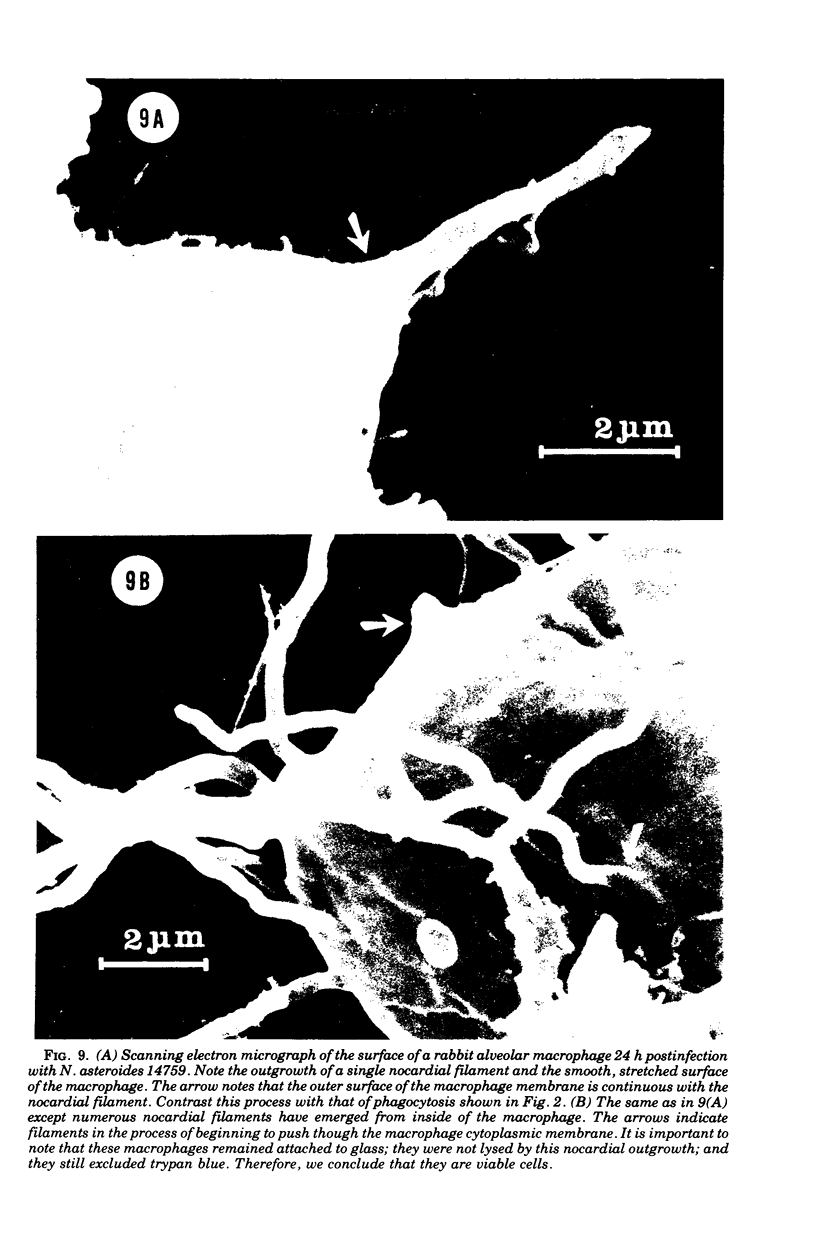
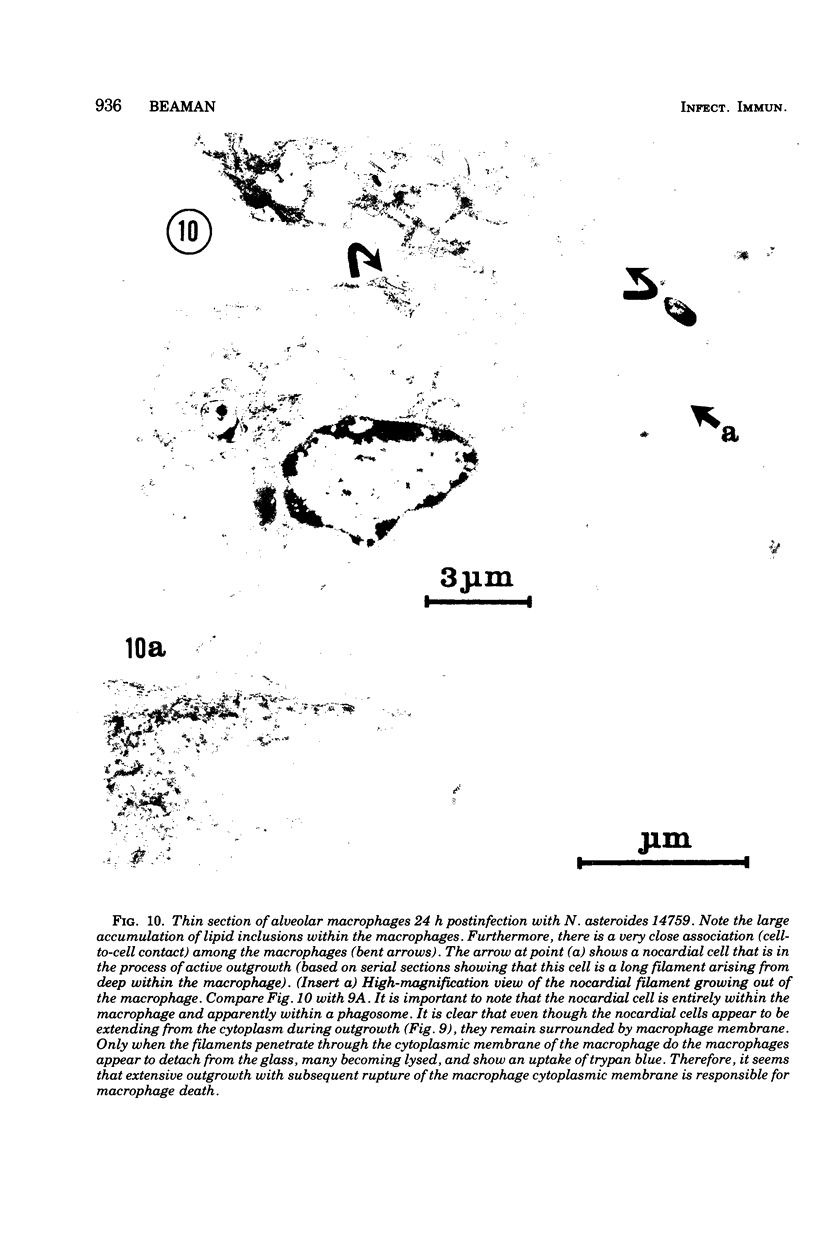
The interaction of Nocardia asteroides with cultured "normal" nonimmune rabbit alveolar macrophages was studied by light and electron microscopy. It was shown that the alveolar macrophage response to the more virulent strain (N. asteroides 14759) was quite different from the response to the less virulent organism (N. asteroides 10905). N. asteroides 14759 elicited a dramatic in vitro response of the macrophages toward the nocardial infection. Within a few hours postinfection, there was a migration of macrophages toward other cells actively infected with viable nocardia, so that at 6 h considerable macrophage aggregation on the cover slips had occurred. Many of the macrophages within these aggregates exhibited tight cell-to-cell contact, whereas others were observed to fuse, forming multinucleate giant cells, with many containing more than 10 nuclei. Upon continued incubation, these giant cells appeared to destroy the intracellular nocardia, so that, at 24 h postinfection, gram-positive, ultrastructurally intact bacteria could not be observed. At the same time, some of the macrophage aggregates that did not fuse appeared to be unable to stop the intracellular growth of nocardia. At 12 to 24 h large numbers of gram-positive, acid-fast filaments were observed growing out from within these macrophage aggregates. The macrophage response seemed dependent upon the strain of Nocardia infecting them, since N. asteroides 10905 did not induce a similar response within the macrophage population.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L. An ultrastructural analysis of Nocardia during experimental infections in mice. Infect Immun. 1973 Nov;8(5):828–840. doi: 10.1128/iai.8.5.828-840.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois L., Beaman B. L. Probable L-forms of Nocardia asteroides induced in cultured mouse peritoneal macrophages. Infect Immun. 1974 Mar;9(3):576–590. doi: 10.1128/iai.9.3.576-590.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio P. M., Ottino G., Cantino D. Experimental study on development and behaviour of the multinucleated giant cells "in vitro". J Reticuloendothel Soc. 1971 May;9(5):397–408. [PubMed] [Google Scholar]
- Dabrowski W., Bogunowicz A., Stelmaska E., Pietkiewicz D. Fagocytarna aktywnoś makrofagów wobec Nocardia asteroides. Med Dosw Mikrobiol. 1976;28(1):37–42. [PubMed] [Google Scholar]
- GORRILL R. H., HEPTINSTALL R. H. The animal pathogenicity of Nocardia sebivorans nov. spec. J Pathol Bacteriol. 1954 Oct;68(2):387–393. doi: 10.1002/path.1700680211. [DOI] [PubMed] [Google Scholar]
- Galindo B. Antigen-mediated fusion of specifically sensitized rabbit alveolar macrophages. Infect Immun. 1972 Apr;5(4):583–594. doi: 10.1128/iai.5.4.583-594.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Bojalil L. F., Contreras M. F. Delayed hypersensitivity to polysaccharides from nocardia. J Immunol. 1972 May;108(5):1409–1413. [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Bojalil L. F. Delayed skin reactions to cytoplasmic extracts of Nocardia organisms as a means of diagnosis and epidemiological study of Nocardia infection. Clin Exp Immunol. 1972 Oct;12(2):225–229. [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Contreras M. F., Bojalil L. F. The assay of delayed hypersensitivity to ribosomal proteins from Nocardia. Sabouraudia. 1972 Jul;10(2):147–151. doi: 10.1080/00362177285190291. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Contreras M. F., Bujalil L. F. Cytoplasmic antigens from Nocardia elicting a specific delayed hypersensitivity. Infect Immun. 1972 Jun;5(6):879–882. doi: 10.1128/iai.5.6.879-882.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]