Abstract
Polyploidy plays a crucial role in plant evolution. Brassica napus (2n = 38, AACC), the most important oil crop in the Brassica genus, is an allotetraploid that originated through natural doubling of chromosomes after the hybridization of its progenitor species, B. rapa (2n = 20, AA) and B. oleracea (2n = 18, CC). A better understanding of the evolutionary relationship between B. napus and B. rapa, B. oleracea, as well as Arabidopsis, which has a common ancestor with these three species, will provide valuable information about the generation and evolution of allopolyploidy. Based on a high-density genetic map with single nucleotide polymorphism (SNP) and simple sequence repeat (SSR) markers, we performed a comparative genomic analysis of B. napus with Arabidopsis and its progenitor species B. rapa and B. oleracea. Based on the collinear relationship of B. rapa and B. oleracea in the B. napus genetic map, the B. napus genome was found to consist of 70.1% of the skeleton components of the chromosomes of B. rapa and B. oleracea, with 17.7% of sequences derived from reciprocal translocation between homoeologous chromosomes between the A- and C-genome and 3.6% of sequences derived from reciprocal translocation between non-homologous chromosomes at both intra- and inter-genomic levels. The current study thus provides insights into the formation and evolution of the allotetraploid B. napus genome, which will allow for more accurate transfer of genomic information from B. rapa, B. oleracea and Arabidopsis to B. napus.
Introduction
Polyploidy plays a crucial role in plant evolution [1], [2], [3]. Most flowering plants, including the majority of agricultural crops, are polyploid [2], [4]. Polyploidization can result in chromosomal rearrangements, changes in gene expression, reductions in chromosome numbers and evolution of the centromere [5], [6], [7], [8], [9]. However, the genetic, genomic and cytological factors determining nascent polyploid formation remain to be elucidated.
The Brassica genus consists of three elementary diploid species, Brassica rapa (AA, 2n = 2x = 20), B. nigra (BB, 2n = 2x = 16), and B. oleracea (CC, 2n = 2x = 18), and three amphidiploid species derived from the three diploids, B. napus (AACC, 2n = 4x = 38), B. juncea (AABB, 2n = 4x = 36), and B. carinata (BBCC, 2n = 2x = 34) [10]. B. napus, as the most important oil crop among the six species in the U-triangle, is estimated to have been generated 5,000 to 10,000 years ago by the natural hybridization of its two progenitor diploids, B. rapa and B. oleracea [11], [12]. B. rapa and B. oleracea were produced by extensive triploidization of their ancestral species at the genomic level [13]. The three species are believed to share a common ancestor with Arabidopsis thaliana (2n = 2x = 10) [9], [14]. B. napus is a relatively young species in terms of its evolutionary age and has a short history of artificial domestication (about 400–500 years) [12], [15]. Therefore, B. napus is an ideal model species to study the evolution of allopolyploidy [6], [16].
Compared to diploidy, the study of genomic structure in polyploidy is much more difficult and complex. With the vast amount of information gained from the genome sequences of an ever-increasing number of plant species, comparative genomics has proved to be a useful tool for understanding the complicated polyploid genome through the transfer of information and resources of related species [17], [18]. Therefore, it is especially important to conduct efficient comparative genomic studies of Brassica crops with Arabidopsis, which is a model plant for the whole plant kingdom [19], although complete genome sequences are currently still lacking for B. napus.
The conserved blocks of the Arabidopsis genome or ancestral karyotype (AK, 2n = 2x = 16) have been demonstrated for most of the Brassica species by comparative genomic analysis [9], [12]. Such comparative mapping with Arabidopsis normally uses markers with known sequences, such as restriction fragment length polymorphisms (RFLPs) [18], [20], [21], [22], [23], [24], [25], [26], intron polymorphisms (IPs) [27], EST-SSR markers [28], [29], EST-based SNP markers [30], [31] and gene-specific markers [32]. Recently, we developed a method for comparative mapping with Arabidopsis with SSR markers in B. napus [33]. Delourme et al. used a SNP Infinium array to construct a high-density integrated genetic map for comparative analysis with Arabidopsis [34].
Another important aspect of comparative genomic studies in Brassicaceae is the comparison within the agronomically important species of the Brassica genera, especially between the three elementary species and their respective aggregated species, for example, the collinear relationship between the A and C genomes of B. napus [18], [27], [35]. Wang et al. mapped the sequence-tagged markers from an integrated linkage map of B. napus onto the B. rapa A genome and identified discrepancies and inconsistent regions (maybe deletion, inversion and translocation) between the B. napus A genome and the B. rapa A genome [26]. Similarly, Chen et al. aligned 3,116 SNPs that were on a B. napus ultrahigh-density SNP bin map to the B. rapa reference genome and also identified inversions and insertion/deletion fragments [36]. Jiang et al. identified several genomic rearrangement events covering totally at least 5% of the A genome between B. napus and B. rapa [37]. Xiong et al. used cytological methods to study homoeolog pairing and chromosome rearrangements, aneuploidy, and homoeologous chromosome compensation in resynthesized B. napus [16]. The completion of the genome sequences of B. rapa [14] and B. oleracea [38], and the availability of 6K [39] and 60K [40], [41] SNP arrays for B. napus offer new opportunities for comparative genomic research on B. napus and its ancestral species, B. rapa and B. oleracea, as well as Arabidopsis using whole-genome high-throughput data.
In this study, a 6K SNP array (Illumina Infinium HD Assay) for B. napus [39] was applied to genotyping of a doubled haploid (DH) population and its parental lines [42]. A high-density genetic map with SNP and SSR markers was constructed and used for comparative genomic analysis with the B. rapa, B. oleracea and Arabidopsis genomes. The conserved blocks of Arabidopsis, as well as the homoeologous collinear fragments of B. rapa and B. oleracea were identified in the B. napus genetic map by screening the homoeologous loci of the markers in the genetic map. With this information, we were able to dissect the genetic composition in the A and C genomes of B. napus and uncover their evolutionary relationships with the ancestral species at the genomic level.
Results and Discussion
Construction of a high-density genetic map
SSR markers and a 6K SNP array containing 5,306 probes for B. napus [39] were used to genotype the HJ DH population and its parental lines. From 2,400 SSR primer pairs, 406 (16.9%) exhibited high quality polymorphism between the two parental lines, generating 473 SSR loci in the population. The loci were subsequently used for constructing the framework of the genetic map.
The call rate of the 6K SNP array was >0.7 for all 192 samples (190 DH lines and 2 parents), with an average of 0.86. There were 578 probes (10.9%) that were detected in less than 80% of samples and thus not included in further analysis. The remaining 4728 SNPs were used for cluster analysis using the GenomeStudio software. A total of 1850 SNPs from the array showed polymorphisms between the parental lines Hua 5 and J7005.
Linkage analysis was conducted with the 2,323 polymorphic loci (1850 SNPs and 473 SSR loci), and 2,115 markers (1667 SNPs and 448 SSR loci) were mapped onto 19 linkage groups (LGs) of B. napus (Table 1, Figure 1, Table S1). The total length of the genetic map was 2,477.4 cM, with an average distance of 1.27 cM between the markers (Table 1). The marker density (1.07 cM/marker) on the A genome of B. napus (designated as BnA-genome thereafter) was higher than that (1.49 cM/marker) on the C genome of B. napus (designated as BnC-genome thereafter).
Table 1. Comparative genomic analysis of B. napus with B. rapa, B. oleracea and Arabidopsis (the source of the data come from Table S1).
| B. napus | Homoeologous collinear locus | Block | Island | |||||||
| LG | SSR marker | SNP marker | Total marker | Length (cM) | Average distance (cM/marker) | Arabidopsis | B. rapa | B. oleracea | ||
| A01 | 19 | 110 | 129 | 105.1 | 0.81 | 74 | 104 | 0 | 5 | 0 |
| A02 | 15 | 175 | 190 | 144.8 | 0.76 | 106 | 43 | 44 | 14 | 18 |
| A03 | 47 | 149 | 196 | 233.2 | 1.19 | 99 | 95 | 45 | 11 | 4 |
| A04 | 11 | 40 | 51 | 69.5 | 1.36 | 14 | 33 | 0 | 1 | 1 |
| A05 | 16 | 58 | 74 | 83.5 | 1.13 | 35 | 44 | 4 | 3 | 0 |
| A06 | 16 | 62 | 78 | 87.4 | 1.12 | 50 | 56 | 0 | 7 | 3 |
| A07 | 33 | 154 | 187 | 155.9 | 0.83 | 89 | 112 | 14 | 12 | 5 |
| A08 | 17 | 64 | 81 | 79.9 | 0.99 | 49 | 62 | 0 | 7 | 7 |
| A09 | 36 | 61 | 97 | 143.9 | 1.48 | 40 | 67 | 0 | 7 | 2 |
| A10 | 20 | 73 | 93 | 90.3 | 0.97 | 50 | 50 | 0 | 4 | 2 |
| Subtotal | 230 | 946 | 1176 | 1193.5 | 1.07 | 606 | 666 | 107 | 71 | 42 |
| C01 | 18 | 99 | 117 | 190.1 | 1.62 | 50 | 9 | 37 | 7 | 6 |
| C02 | 21 | 81 | 102 | 127.1 | 1.25 | 42 | 25 | 17 | 8 | 1 |
| C03 | 22 | 37 | 59 | 110.4 | 1.87 | 20 | 0 | 33 | 3 | 1 |
| C04 | 40 | 102 | 142 | 200.2 | 1.41 | 68 | 34 | 58 | 5 | 0 |
| C05 | 27 | 113 | 140 | 186.9 | 1.34 | 77 | 50 | 27 | 13 | 3 |
| C06 | 27 | 72 | 99 | 100.7 | 1.02 | 52 | 29 | 35 | 7 | 1 |
| C07 | 15 | 97 | 112 | 93.7 | 0.84 | 41 | 0 | 73 | 8 | 2 |
| C08 | 32 | 99 | 131 | 172.3 | 1.32 | 63 | 13 | 76 | 8 | 7 |
| C09 | 16 | 21 | 37 | 102.5 | 2.77 | 14 | 0 | 20 | 2 | 3 |
| Subtotal | 218 | 721 | 939 | 1283.9 | 1.49 | 427 | 160 | 376 | 61 | 24 |
| Total | 448 | 1667 | 2115 | 2477.4 | 1.27 | 1033 | 826 | 483 | 132 | 66 |
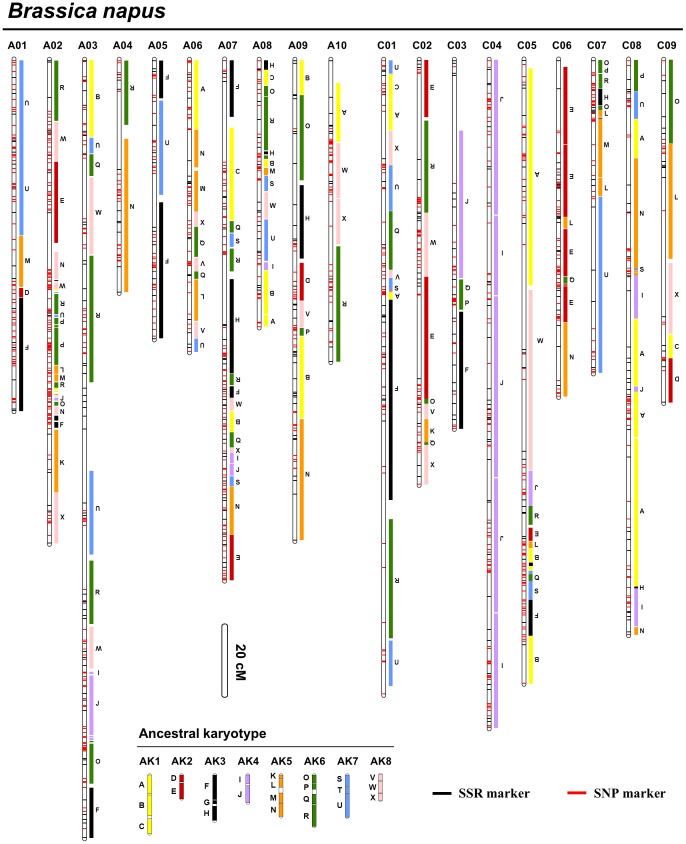
Figure 1. Conserved blocks of Brassicaceae Ancestral Karyotype on Brassica napus genetic map.
For each linkage group (LG), the left vertical bar represents the LG with mapped markers (red dashes for single nucleotide polymorphisms (SNPs) and black for simple sequence repeat (SSR)). The conserved Arabidopsis blocks are listed on the right of each LG. The length of LG bars is proportional to their genetic distances. The conserved blocks are identified according to their positions in the Arabidopsis genome (see Materials and Methods) and depicted with colors based on the Ancestral Karyotype chromosome positions as described by Schranz et al. [9]. Inverted letters for respective conserved blocks indicate inversions in the LGs relative to Arabidopsis chromosomes. The length of each vertical bar for Ancestral karyotype chromosome is proportional to its physical length.
Comparative genomic analysis of B. napus with Arabidopsis
To identify the conservation and variation of the B. napus chromosomes compared with Arabidopsis or Ancestral Karyotype genome, the conserved blocks of Arabidopsis [9] in B. napus were identified through comparative genomic analysis of B. napus with Arabidopsis. For SSR markers, the locus sequences were inferred with the aid of homoeologous collinear loci in the B. rapa/B. oleracea genomes and then subjected to a BLASTn analysis with the Arabidopsis genome as described by Cai et al. [33]. For the SNP markers, the sequences of individual probes (300 bp on average) were directly used in the BLASTn analysis with Arabidopsis (E-value ≤ 1E-10). We identified 2,115 loci on the B. napus genetic map that could be matched to 1,930 loci in the Arabidopsis genome (Table S1). Conserved blocks and islands of Arabidopsis in B. napus were then detected using these loci. A conserved block on the map refers to a region harboring at least three molecular markers that includes at least two homoeologous loci in a 2 Mb fragment of one of the 24 defined blocks in the Arabidopsis genome for every 10 cM of the B. napus genetic map. If an Arabidopsis conserved block in B. napus had only two corresponding homoeologous loci, the block was classified as an island and named according to the block to which it belonged. In total, there were 132 conserved blocks and 66 islands that were deduced from 1033 Arabidopsis homoeologous loci (Figure 1, Table 1, 2 and S1). Together, these conserved blocks and islands covered 2,252 cM of the genetic linkage map of B. napus, accounting for approximately 90.9% of the total length (Figure 1, Table S1). The block T was not detected and the block G was only detected once (Table 2) in the map. The other 23 blocks were detected with an average frequency of 5.7 (Table 2).
Table 2. The copy number of the 24 identified conserved blocks and islands in the B. napus genetic map.
| Conserved Block a | Copy | |
| Block b | Island | |
| A | 8 | 4 |
| B | 6 | 4 |
| C | 4 | 2 |
| D | 2 | 1 |
| E | 10 | 0 |
| F | 11 | 1 |
| G | 1 | 0 |
| H | 6 | 2 |
| I | 5 | 3 |
| J | 7 | 4 |
| K | 1 | 1 |
| L | 7 | 1 |
| M | 3 | 4 |
| N | 6 | 4 |
| O | 4 | 5 |
| P | 5 | 2 |
| Q | 7 | 5 |
| R | 9 | 7 |
| S | 4 | 3 |
| T | 0 | 0 |
| U | 12 | 2 |
| V | 3 | 3 |
| W | 7 | 3 |
| X | 4 | 5 |
| Total | 132 | 66 |
The conserved blocks are defined by Schranz et al. [9].
The method for identification of the conserved block and island is described in the Materials and Methods section.
Interestingly, the LG C04 of B. napus (BnC04) was composed of only blocks I and J, indicating that it may entirely originate from the AK4 (ancestral karyotype) chromosome of the ancestral species (Figure 1, Table S1). The BnA04 and BnA05 were composed of the blocks and islands from two AK chromosome fragments, the BnC03 and BnC06 from three, the BnA01, BnA10, BnC02 and BnC07 from four, the BnA06 and BnC09 from five, the BnA03, BnA09, BnC01 and BnC08 from six, and the BnA08 was from seven. The BnA02, BnA07, and BnC05 were the most complex LGs/chromosomes and each of them contained all eight AK chromosomes (Table 1, Figure 1 and Table S1). These results suggest that B. napus chromosomes may vary greatly in terms of their origins of the ancestral genetic components. Understanding the mechanisms underlying the phenomenon will provide insights on how natural and artificial selection could shape the genetic variations in B. napus [43], [44].
Dissection of the genomic composition of B. napus through comparative mapping with B. rapa and B. oleracea
B. napus is an allotetraploid species that is believed to have originated 5,000–10,000 years ago by natural doubling of chromosomes after the hybridization of its progenitor species, B. rapa and B. oleracea [10], [14]. With genetic maps constructed using RFLP and SSR markers, a large number of homoeologous collinear loci were identified in the BnA-genome and BnC-genome in addition to replacement, duplication, inversion, and translocation events [17], [27], [37], [45], [46],[47]. It was also proposed that there is a close collinear relationship of the BnA-genome and BnC-genome with the genomes of B. rapa and B. oleracea [9], [26], [36], [48], [49]. Although several studies have been conducted based on such an assumption [39], [50], [51], [52], [53], [54], the relationship between the BnA- and BnC- genomes and their relationships to their counterparts in the progenitor species are still elusive.
To conduct comparative genomic analyses with B. rapa and B. oleracea, the sequences of SNP markers on the map were aligned with B. rapa and B. oleracea genome sequences by means of the BLASTn tool. A locus with an E-value ≤ 1E-20 (best hit) in the B. rapa or B. oleracea genomes was considered to be a homoeologue to the query sequence of the SNP locus on the map. The loci in the B. rapa and B. oleracea genomes that were homoeologous to SSR markers were identified with the method described by Cai et al. [33]. In total 1,923 of the 2,115 markers (90.9%) on the map were matched to their homoeologous loci in the B. rapa and B. oleracea genomes (Table S1).
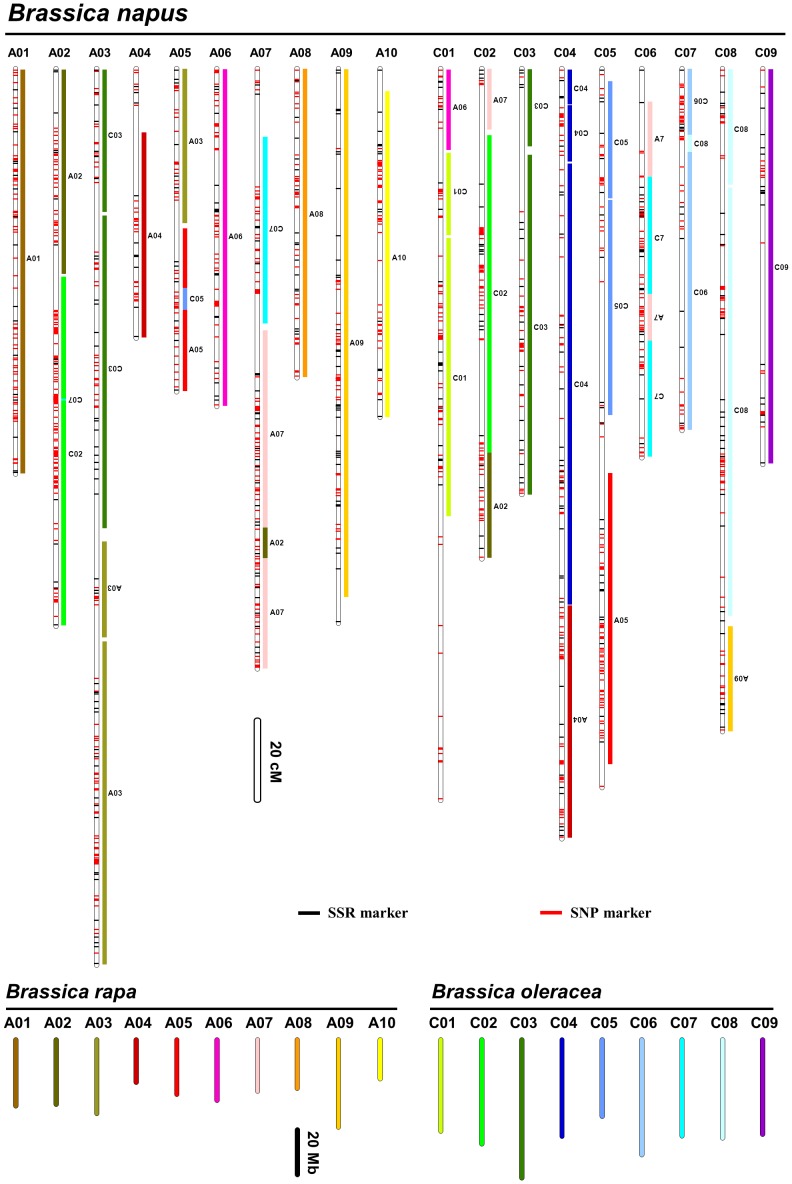
Based on the above analysis, we further identified homoeologous collinear fragments of B. rapa and B. oleracea in the B. napus genome using a similar method described by Parkin et al. [18]. Homoeologous collinear fragments of B. rapa/B. oleracea in B. napus were defined as DNA sequences that included at least four molecular markers in every 5 cM of the map, and simultaneously contained at least one homoeologous locus in a 2.5 Mb region of the corresponding B. rapa/B. oleracea genomes. Using this criterion, 22 homoeologous collinear fragments of B. rapa and 24 of B. oleracea were identified in B. napus (Figure 2, Table 3 and Table S1), which corresponded to 1,309 loci (Table 1, Table S1) and covered 2,237.1 cM (90.3%) of the whole B. napus genome (Table 3, Figure 2). Except for a 2.74 Mb fragment in chromosome A07 of B. rapa (BrA07) that had one duplicated copy located on BnC06 (yellow ribbon in Figure 3, Table 3), the rest of the 21 B. rapa fragments and 24 B. oleracea fragments appeared only once on each of the 19 LGs/chromosomes of B. napus (Table 3). These homoeologous collinear fragments of B. rapa and B. oleracea identified in B. napus genome accounted for 90.3% and 71.4% of the total length of the B. rapa and B. oleracea genomes, respectively, based on the known physical lengths of the two species (Figure 2, Table 3 and Table S1).
Figure 2. The relationship of the Brassica napus genetic map to the B. rapa and B. oleracea genomes.
For each B. napus linkage group (LG), the left vertical bar represents the LG with mapped markers (red dashes for single nucleotide polymorphisms (SNPs) and black for simple sequence repeat (SSR)). The length of LG bars is proportional to their genetic distances. The homoeologous collinear fragments of B. rapa and B. oleracea identified in the B. napus genetic map are listed on the right, and colored based on the positions on B. rapa or B. oleracea chromosomes. Inverted letters for respective homoeologous collinear fragments indicate inversions in the LGs relative to B. rapa or B. oleracea chromosomes. The length of each vertical bar for B. rapa and B. oleracea chromosome is proportional to its physical length.
Table 3. The detailed information of 46 homoeologous collinear fragments of B. rapa and B. oleracea genomes identified in the B. napus genetic map (the source of the data come from Table S1).
| B. napus | Homoeologous collinear fragment | ||||||||
| LG a | Length b | B. rapa | B. oleracea | ||||||
| Chr c | Span (bp) | % d | Length b | Chr c | Span (bp) | % d | Length b | ||
| BnA01 | 105.1 | BrA01 | 284,970–28,424,481 | 98.4 | 105.1 | ||||
| BnA02 | 144.8 | BrA02 | 1,510,618–14,212,023 | 45.6 | 54.3 | BoC02 | 25,050,175–41,757,926 | 37.9 | 90.1 |
| BoC07 | 36,597,360–37,488,062 | 2.2 | 0.5 | ||||||
| BnA03 | 233.2 | BrA03 | 501,255–16,229,444 | 49.6 | 84.1 | BoC03 | 24,221–7,268,049 | 12.5 | 47.9 |
| BrA03 | 26,608,007–28,648,208 | 6.4 | 27.4 | BoC03 | 32,383,728–47,645,315 | 26.4 | 38.4 | ||
| BnA04 | 69.5 | BrA04 | 276,812–7,920,797 | 40.3 | 57.5 | ||||
| BnA05 | 83.5 | BrA05 | 22,210,227–23,831,506 | 6.8 | 40.4 | BoC05 | 32,350,587–32,701,848 | 1.1 | 5.4 |
| BrA03 | 18,834,614–26,272,710 | 23.5 | 38.7 | ||||||
| BnA06 | 87.4 | BrA06 | 2,993,650–26,241,942 | 88.5 | 87.4 | ||||
| BnA07 | 155.9 | BrA07 | 277,845–11,178,806 | 48.3 | 50.2 | BoC07 | 15,896,409–26,012,560 | 24.9 | 50.4 |
| BrA07 | 11,472,403–18,261,993 | 30.1 | 28.9 | ||||||
| BrA02 | 14,309–810,600 | 2.9 | 8.0 | ||||||
| BnA08 | 79.9 | BrA08 | 759,127–17,858,014 | 79.2 | 79.9 | ||||
| BnA09 | 143.9 | BrA09 | 936,877–29,820,627 | 77.8 | 137.0 | ||||
| BnA10 | 90.3 | BrA10 | 135,760–17,501,817 | 98.7 | 90.3 | ||||
| Subtotal | 1193.5 | 889.2 | 232.6 | ||||||
| BnC01 | 190.1 | BrA06 | 1,168,297–2,902,867 | 6.6 | 22.4 | BoC01 | 10,671,090–11,302,996 | 1.6 | 34.6 |
| BoC01 | 13,821,490–38,372,637 | 63.3 | 57.2 | ||||||
| BnC02 | 127.1 | BrA02 | 15,879,592–27,246,329 | 40.8 | 26.6 | BoC02 | 6,520,290–24,759,788 | 41.4 | 83.7 |
| BrA07 | 20,511,170–21,918,751 | 6.2 | 16.8 | ||||||
| BnC03 | 110.4 | BoC03 | 8,598,996–29,818,084 | 36.7 | 86.9 | ||||
| BoC03 | 55,727,445–56,984,210 | 2.2 | 23.5 | ||||||
| BnC04 | 200.2 | BrA04 | 8,431,313–18,248,518 | 51.8 | 60.2 | BoC04 | 28,531–2,985,740 | 7.2 | 10.5 |
| BoC04 | 4,503,191–7,283,093 | 6.8 | 16.5 | ||||||
| BoC04 | 19,590,343–40,800,903 | 51.9 | 113.0 | ||||||
| BnC05 | 186.9 | BrA05 | 1,492,477–21,646,834 | 84.2 | 75.0 | BoC05 | 283,542–3,205,578 | 8.9 | 37.3 |
| BoC05 | 7,007,360–26,021,073 | 57.9 | 34.0 | ||||||
| BnC06 | 100.7 | BrA07 | 14,970,124–17,705,637 | 12.1 | 13.0 | BoC07 | 302,532–8,913,646 | 21.2 | 30.4 |
| BrA07 | 21,961,835–22,193,291 | 1.0 | 17.1 | BoC07 | 27,513,541–35,677,613 | 20.1 | 29.7 | ||
| BnC07 | 93.7 | BoC06 | 12,451,594–15,878,853 | 7.1 | 17.0 | ||||
| BoC06 | 24,097,329–44,570,444 | 42.3 | 72.7 | ||||||
| BoC08 | 23,087,748–26,244,524 | 7.6 | 4.0 | ||||||
| BnC08 | 172.3 | BrA09 | 30,571,654–36,860,095 | 16.9 | 27.3 | BoC08 | 1,501,208–18,653,316 | 41.3 | 30.2 |
| BoC08 | 27,420,490–41,433,239 | 33.8 | 114.8 | ||||||
| BnC09 | 102.5 | BoC09 | 171,077–34,857,697 | 86.4 | 102.5 | ||||
| Subtotal | 1283.9 | 258.4 | 898.5 | ||||||
| Total | 2477.4 | 1147.5 | 1131.0 | ||||||
Linkage group.
The unit of the length is cM.
Chromosome.
Percentage. The percentage refers that the proportion of the physical length of the homoeologous collinear fragment accounts for the whole physical length of the corresponding B. rapa (chromosome_v1.5) or B. oleracea (chromosome_v1.0) chromosome.
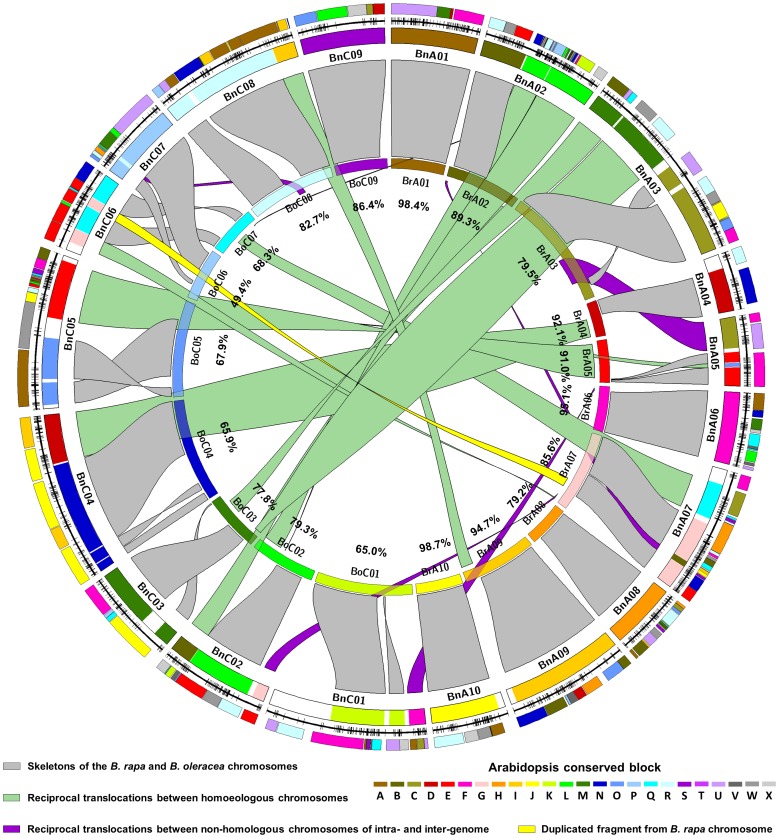
Figure 3. Evolutionary relationship between Brassica napus and its progenitor species B. rapa and B. oleracea.
Schematic diagram of the B. napus genome as revealed by a genetic linkage map comprised of simple sequence repeat (SSR) and single nucleotide polymorphisms (SNP) markers and comparative analyses with the B. rapa, B. oleracea and Arabidopsis genomes. The colored blocks at the outermost circle represent the Arabidopsis conserved blocks in the B. napus genome identified with the genetic linkage groups of B. napus, which is represented in the second outer circle (all circles were orientated clockwise). The third circle (from the outermost one) represents the B. napus genome that is reconstructed with 46 homoeologous collinear fragments of B. rapa and B. oleracea. Each homoeologous collinear fragment of B. napus (the third circle) is the same color as the corresponding chromosome of B. rapa and B. oleracea in the inner circle. The ribbons between the third and inner circle depict the origins of the homoeologous collinear fragments from B. rapa and B. oleracea. The inverted homoeologous collinear fragments are indicated with twisted ribbons. The gray ribbons represent the skeletons from the B. rapa and B. oleracea genomes retained in B. napus genome; The green ribbons represent the reciprocal translocations between homoeologous chromosomes from the A and C genomes; The purple ribbons represent the reciprocal translocations between non-homologous chromosomes from the A and C genomes; The yellow ribbon represents the repeat fragment from B. rapa/B. oleracea chromosome. The numbers in the inner circle (under each B. rapa or B. oleracea chromosome) are the percentages of all homoeologous collinear fragments of B. rapa or B. oleracea retained in the B. napus genome relative to the physical length of the corresponding B. rapa (chromosome_v1.5) or B. oleracea (chromosome_v1.0) chromosome.
Evolutionary relationship between the genomes of B. napus and its progenitor species B. rapa and B. oleracea
Further analysis was conducted to determine the relationships of the identified homoeologous collinear fragments of B. napus with the genomes of their two progenitor species (Table 3). Based on the comparison of the homoeologous collinear fragments with the genomes of B. rapa and B. oleracea, a total of 393.5 Mb of genomic components from 10 chromosomes of B. rapa and 9 of B. oleracea were identified to be conserved (Table 3 and Table S1), which formed a basic skeleton for each of 19 B. napus LGs/chromosomes (grey ribbons in Figure 3), corresponding to a total length of 1,736.9 cM of the B. napus map and 29 homoeologous collinear fragments (Table 3, grey ribbons in Figure 3, and Table S1). The remainder of the B. napus genome could result from chromosome re-arrangements (exchanges), including fragment duplication (yellow ribbon in Figure 3), inversions within a chromosome (twisted ribbons in Figure 3, Table S1) and translocations among different chromosomes (green and purple ribbons in Figure 3, Table S1).
Since B. napus is an allotetraploid containing the A-genome and C-genome from its progenitor species of B. rapa and B. oleracea, it would be expected that chromosome translocations could happen at both intra- and inter-genome levels. To distinguish the origins of translocation events in B. napus genome, we considered the reciprocal translocations occurred between the homoeologous chromosomes of the A-genome and C-genome [18] as homoeologous recombination. On the other hand, all other translocations between non-homologous chromosomes within the A-genome or C-genome (intra-genome) as well as between the A-genome and C-genome (inter-genome) were regarded as non-homologous recombination. Under such definitions, the reciprocal translocations between homoeologous chromosomes in the B. napus genome covered 438.3 cM of the genetic distance on the B. napus map, and could be linked to 11 corresponding homoeologous collinear fragments of B. rapa and B. oleracea that were equal to 97.5 Mb of the genomic components from B. rapa and B. oleracea (green ribbons in Figure 3, Table 3). On the other hand, the reciprocal translocations through non-homologous recombination of intra- and inter-genome covered 90.4 cM of the genetic distance on the B. napus map, and could be linked to 6 corresponding homoeologous collinear fragments of B. rapa and B. oleracea that were equal to 15.4 Mb of genomic components (purple ribbons in Figure 3, Table 3).
Based on the origins of the chromosome fragments, eight LGs/chromosomes of B. napus (BnA01, BnA04, BnA06, BnA08, BnA09, BnA10, BnC03 and BnC09) were found to contain only the skeletons from the corresponding chromosomes of B. rapa or B. oleracea (Figure 3, Table 3). These eight chromosomes of B. napus all contained intact genetic components of their progenitor species' chromosomes, except BnC03 on which one inversion and one translocation occurred (Figure 3). The rest of the 11 LGs/chromosomes of B. napus were composed of various chromosome fragments along with a skeleton of the progenitor species' chromosome (Figure 3, Figure 2 and Table 3). Interestingly, the DNA sequences in BrA01, BrA08 and BrA10, and BoC01, BoC04, BoC06 and BoC9 were only identified in their corresponding B. napus LGs/chromosomes of BnA01, BnA08 and BnA10, and BnC01, BnC04, BnC06 and BnC09 (Figure 3, Table 3).
Three types of the chromosome re-arrangements among the 11 LGs/chromosomes that consisted of a skeleton and various chromosome fragments from different origins were found. The first type could be defined as rearrangement from the reciprocal translocations between the homoeologous chromosomes of the A and C genomes, as well as intra-chromosome recombination (inversion and translocation), which were identified in BnA03, BnC04, BnC05, BnC06, and BnC08 (Figure 3 and Table 3). For example, BnC06 was composed of two different chromosomal fragments from BoC07 and BrA07. The chromosome contained a skeleton from BoC07, but the skeleton was divided into two reversed fragments with a BrA07 insertion that was duplicated once in a reversed orientation (Figure 2 and 3). BnC06 has previously been found to be aligned with BoC07, as well as BnC07 with BoC06 [27], [33]. Such reversed nomenclatures are based on the nomenclatures from Parkin et al. [17], [18]. It is possible now the obviously reversed nomenclatures of BnC06-BoC07 and BnC07-BoC06 could be corrected with the aids of sequencing and cytology.
With the genome sequences of B. rapa [14] and B. oleracea [38] publically available, we were able to examine the consistency of comparative mapping of B. napus-Arabidopsis (Figure 1) and B. napus-two progenitor species (Figure 2 and 3), To that end, we took BnC04 as an example. BnC04 was composed of a BoC04 skeleton and a large section of BrA04 at the distal end (Figure 2 and 3). The BoC04 skeleton on BnC04 consisted of two close but separated fragments at its upper end from the upper part of BoC04 and one large fragment at its middle part from the lower part of BoC04 (all circles were orientated clockwise in Figure 3). At the same time, there was a BrA04 fragment at the distal end of BnC04, which was originated from the distal end of BrA04 (Figure 3). Such an origin and distribution could explain why BnC04 only contained I and J blocks from AK4 in the comparison with Arabidopsis (Figure 1). The most recent study of de novo sequencing of B. oleracea and collinearity analysis of B. rapa and B. oleracea with Arabidopsis has showed that the upper and lower parts of the BoC04 (Supplementary Figure 22 of Liu et al. [38]) only contained I and J blocks, and the lower half of BrA04 was only composed of I and J blocks (Supplementary Figure 23 of Liu et al. [38]). Our results are consistent with these new sequencing data.
The second type of chromosome rearrangements in the B. napus genome was derived from reciprocal translocations between non-homologous chromosomes of the intra- and inter-genome, for example BnC01 and BnC07. These were formed by two reciprocal translocations between the non-homologous chromosomes in the A and C genomes and one intra-chromosomal inversion (Figure 2 and 3).
The third type of chromosome rearrangements included reciprocal translocations between the homoeologous chromosomes and between the non-homologous chromosomes, including BnA02, BnA05, BnA07 and BnC02. For example, BnA07 consisted of three different chromosomal sources, i.e. the skeleton of BrA07, a homologous fragment of BoC07, and a non-homologous fragment of BrA02 through intra-genomic translocation (Figure 2 and 3).
Overall, in the B. napus genome studied, there were 21 fragments from 10 B. rapa chromosomes and 24 fragments from 9 B. oleracea chromosomes. During the formation of the 19 B. napus chromosomes from the 45 progenitor fragments, 1 duplication (Figure 3, yellow ribbon), 17 inter-chromosomal reciprocal translocations (Figure 3, green and purple ribbons), 13 inversions (Figure 2, the fragments with inverted letters) and 3 intra-chromosomal translocations (Figure 3; BnA03, BnC03, and BnC05) were identified (Figure 2, Figure 3, Table 3 and Table S1). These results advanced our understanding of the formation and evolution of the B. napus genome, and allowed for a more effective utilization of B. rapa and B. oleracea genomic information in future B. napus genetic and genomic research, especially for fine mapping and the identification of genes for economically valuable traits in B. napus. However, the breakpoints of chromosomal rearrangements in B. rapa and B. oleracea, the evolution of the centromere, and the process of the formation of the new chromosomes await further in-depth study.
Based on the genetic distances, approximately 70.1% (1,736.9 cM) of the genetic components in the newly formed genome of B. napus was derived from the corresponding skeletons of the chromosomes of B. rapa and B. oleracea, 17.7% (438.3 cM) from homoeologous chromosome reciprocal translocations between the A and C genomes, and only 3.6% (90.4 cM) from non-homologous chromosome of intra- and inter- genomic translocations (Table 3 and Table S1). We found a higher number of genomic rearrangement events in the A genome (10%) than a previous report (5%) by Jiang et al. [37]. This is likely to be because of the lower density genetic map and absence of the C genome (B. oleracea) reference sequence at that time. The proportion of reciprocal translocations between homoeologous and non-homologous chromosomes in B. napus in this study may be overestimated. Firstly, the density of the HJ DH population genetic map was not saturated, which may result in an incomplete resolution on variations. Secondly, only two B. napus lines were analyzed. Thirdly, the current comparative analysis is only based on one diploid progenitor species sequences. Both B. rapa and B. oleracea are known to be two of the most genetically diverse diploid Brassica species. Fourth, B. napus in China has been modified by the frequent interspecific crossing with B. rapa in recent times. It remains to be determined how the mapping population in this study is similar or different compared with other germplasm of B. napus in terms of chromosome re-arrangements. Therefore, it would be difficult to establish if the variation observed in the B. napus genome was produced by the variation of the progenitor species, B. rapa and B. oleracea, or was derived from the modifications and selections during the demonstration of the B. napus genome.
Based on our analysis, approximately 90.3% and 71.4% of the genomic components of the B. rapa and B. oleracea genomes were conserved, respectively (Figure 3, Table 3). These differences in the genetic conservation of the A and C genomes may result from the partial loss of the C genome sequences during evolution. The stabilities of the A, B, and C genomes (or nucleolar dominance) in the Brassica genus were different, and the B genome was generally considered to be the most stable, whereas the C genome was considered to be the least stable (B>A>C) [55], [56], [57]. Therefore, a partial loss of the C genome sequences during the formation and demonstration of B. napus may not be surprising. Another possible explanation is that there are fewer differences in the C genome between the two parental lines used in this study. B. rapa, as one of the ancestors of B. napus, has been extensively planted in China with a great deal of variability. Chinese breeders introduced various components of the B. rapa genome for the improvement of various traits of B. napus [58], [59], resulting in a rich variation in the B. napus A genome. On the other hand, chromosome re-arrangement in B. napus has been reported not only in the accessions originated from China, but also in the materials originated from other regions such as European countries [17], [26], [27], [36], [37], [45], [46], [47], [59]. However, it is still not clear how the chromosome rearrangements among different B. napus accessions could vary. Further studies are needed to reveal how extensive the changes are in B. napus globally and to elucidate the evolutionary divergence between the A and C genomes of B. napus.
Conclusions
With the high-resolution genetic map constructed with SSR and SNP markers, we were able to conduct a comparative genomic analysis of B. napus and its ancestral species, B. rapa and B. oleracea, as well as Arabidopsis. Compared to the other LGs/chromosomes of B. napus, LG C04 (BnC04) varied the least during the process of evolution; all of the genetic information of BnC04 came from chromosome 4 of the Ancestral Karyotype. Furthermore, the BnA02, BnA07, and BnC05 were the most complicated. According to the analysis of the homoeologous collinear fragments of B. rapa and B. oleracea identified in the B. napus genetic map, approximately 2/3 of the B. napus genome consists of the skeleton components of the chromosomes of B. rapa and B. oleracea, while approximately 1/5 consists of sequences reciprocal translocated between homoeologous chromosomes, and 1/20 consists of sequences reciprocal translocated between non-homologous chromosomes of the intra- and inter-genome. Our study advances the understanding of the complex pattern of the evolution of the B. napus genome, and allows for a more effective utilization of B. rapa and B. oleracea genomic information in B. napus genetic and genomic research.
Materials and Methods
Plant materials
The HJ DH population was produced from microspore culture of F1 buds of the cross between Huashuang 5 (Hua 5), a semi-winter type B. napus variety, and J7005, a winter-type B. napus pure line. The two parents were purified by microspore culture before hybridization. A total of 254 DH lines were obtained, and a random subset of 190 DH lines was sampled for the subsequent experiments. Detailed information about this population was described in Wu et al. [41]. The plant materials used in this study will be available to interested researchers according to PLoS ONE's requirements.
Molecular markers and SNP array genotyping
Primer sequences for the SSR markers used for genetic map construction were described by Cai et al. [33] and the sequence information of all SSR markers is provided in Table S2.
The genotyping of SNPs was performed using 6K Illumina Infinium HD Assay SNP arrays of B. napus (Illumina Inc., San Diego, CA) developed by the University of Queensland. The high-quality DNA was extracted from young leaf tissues as described by Porebski et al. [60]. Each DNA sample was diluted to a final concentration of 50 ng/ µl using ddH2O. The SNP genotyping was carried out in accordance with the Illumina protocol (Infinium HD Assay Ultra Protocol Guide, http://www.illumina.com/).
All the SNP array data were analyzed using the Illumina GenomeStudio software (Illumina Inc., San Diego, CA), which were clustered and visualized for further analysis. Each SNP was re-checked manually to determine if any error was observed during the clustering analysis. The data processing is described by Raman et al. [39].
Construction of genetic linkage map
Linkage analysis with all markers was performed using MAPMAKER/EXP 3.0 [61] and MSTmap [62] softwares. The MSTmap software can process more than 10,000 markers at one-time, while the MAPMAKER/EXP 3.0 can only process no more than 101 markers for one group. We firstly used MSTmap to process all the loci, and group the loci at 5.0 of log likelihood of the odds (LOD) score. Marker orders of each group were then calculated by finding the minimum spanning tree of a graph based on pairwise recombination frequencies [62]. At the same time, each group through MSTmap was calculated again through MAPMAKER/EXP 3.0 with a minimum LOD score of 11.0 and a maximum distance of 25 cM. The marker orders of each group obtained by MSTmap and MAPMAKER/EXP 3.0 were compared and the consistent regions of marker orders were retained. For inconsistent regions of marker orders, adjustments were made through re-calculating with more rigorous parameters (a minimum LOD score greater than 15, and a maximum distance less than 20 cM) by MAPMAKER/EXP 3.0. Genetic distances between markers were calculated using the Kosambi mapping function [63]. The nomenclature of LGs follows the rules proposed by The Multinational Brassica Genome Project (http://www.brassica.info/index.php). The linkage groups and corresponding graphs were drawn using the softwares MapDraw [64] and circos-0.62 (http://www.circos.ca/).
Identification of Arabidopsis conserved blocks and homoeologous collinear fragments of B. rapa and B. oleracea genomes in the B. napus genetic map
The method of identifying the homoeologous locus in Arabidopsis with the SSR markers in the B. napus genetic map was as described in Cai et al. [33]. The SNP probe sequences were used as queries in searching for homoeologues using the BLASTn program [65] against TAIR10 (http://www.arabidopsis.org/) with an E-value threshold of 1E-10. The best-hit locus of BLASTn results was the homoeologous locus with Arabidopsis of each SNP locus in B. napus genetic map. A conserved block in the B. napus genome was defined as a region that, for every 10 cM of the B. napus genetic map with at least three molecular marker loci, at least two of the loci are homoeologous with a 2 Mb fragment of one of the 24 Arabidopsis conserved blocks [9]. If the region had only 2 homoeologous loci related to an Arabidopsis conserved block, then the block was classified as an island. Each island was named according to the block to which it belonged [66].
The method of identifying the loci in the B. rapa and B. oleracea genomes that are homoeologous and collinear with the SSR markers in the B. napus genetic map was described by Cai et al. [33]. The SNP probe sequences (the length of the sequence corresponding to each SNP probe was 300 bp on average) were used as queries in searching for homoeologues using the BLASTn program [65] against the B. rapa (http://brassicadb.org/brad/index.php, chromosome_v1.5) [14] and B. oleracea (http://www.ocri-genomics.org/bolbase/, chromosome_v1.0) [38] genomes with an E-value threshold of 1E-20. The best-hit locus of BLASTn results was the potential homoeologous collinear locus of each SNP locus in the B. napus genetic map. A homoeologous collinear fragment of the B. rapa/B. oleracea genome in B. napus was defined as a region that, in every 5 cM of the B. napus genetic map, has at least 4 molecular markers that simultaneously contained at least one homoeologous locus in the 2.5 Mb of the corresponding B. rapa/B. oleracea genomes.
Supporting Information
The detailed information of the genetic linkage map of the DH population constructed with SNP and SSR markers, the homoeologous loci and homoeologous collinear loci identified in B. rapa, B. oleracea and Arabidopsis, the homoeologous collinear fragments, and the conserved blocks and islands.
(PDF)
The primer sequences of the SSR markers used in the HJ DH population genetic linkage map construction.
(PDF)
Acknowledgments
We thank Drs. Lingling Chen and Weibo Xie at College of Life Science and Technology, Huazhong Agricultural University, China for critical reading of the manuscript.
Funding Statement
The work is financially supported by funding from the Ministry of Agriculture of China (nycytx-00503 and 948 project (2011-G23)), National Natural Science Foundation of China (31371659, 31171188), and Huazhong Agricultural University (STSIF 2010YB05). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8: 135–141. [DOI] [PubMed] [Google Scholar]
- 2. Otto SP (2007) The evolutionary consequences of polyploidy. Cell 131: 452–462. [DOI] [PubMed] [Google Scholar]
- 3. Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, et al. (2011) Ancestral polyploidy in seed plants and angiosperms. Nature 473: 97–100. [DOI] [PubMed] [Google Scholar]
- 4. Meyers LA, Levin DA (2006) On the abundance of polyploids in flowering plants. Evolution 60: 1198–1206. [PubMed] [Google Scholar]
- 5. Tate JA, Ni Z, Scheen A-C, Koh J, Gilbert CA, et al. (2006) Evolution and expression of homeologous loci in Tragopogon miscellus (Asteraceae), a recent and reciprocally formed allopolyploid. Genetics 173: 1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC (2007) Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 19: 3403–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ozkan H, Levy AA, Feldman M (2001) Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops-Triticum) group. Plant Cell 13: 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osborn TC, Pires JC, Birchler JA, Auger DL, Chen ZJ, et al. (2003) Understanding mechanisms of novel gene expression in polyploids. Trends Genet 19: 141–147. [DOI] [PubMed] [Google Scholar]
- 9. Schranz ME, Lysak MA, Mitchell-Olds T (2006) The ABC's of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci 11: 535–542. [DOI] [PubMed] [Google Scholar]
- 10. Nagaharu U (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jap J Bot 7: 389–452. [Google Scholar]
- 11. Palmer JD, Shields C, Cohen D, Orton T (1983) Chloroplast DNA evolution and the origin of amphidiploid Brassica species. Theor Appl Genet 65: 181–189. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt R, Bancroft I (2011) Genetics and Genomics of the Brassicaceae. New York: Springer. 677 p. [Google Scholar]
- 13. Lysak MA, Koch MA, Pecinka A, Schubert I (2005) Chromosome triplication found across the tribe Brassiceae. Genome Res 15: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X, Wang H, Wang J, Sun R, Wu J, et al. (2011) The genome of the mesopolyploid crop species Brassica rapa . Nat Genet 43: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Campo C (1999) Biology of Brassica coenospecies. Netherlands: Elsevier. 489 p. [Google Scholar]
- 16. Xiong Z, Gaeta RT, Pires JC (2011) Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus . Proc Natl Acad Sci USA 108: 7908–7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parkin I, Sharpe A, Keith D, Lydiate D (1995) Identification of the A and C genomes of amphidiploid Brassica napus (oilseed rape). Genome 38: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 18. Parkin IAP, Gulden SM, Sharpe AG, Lukens L, Trick M, et al. (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana . Genetics 171: 765–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana . Nature 408: 796–815. [DOI] [PubMed] [Google Scholar]
- 20. Kowalski SP, Lan T-H, Feldmann KA, Paterson AH (1994) Comparative mapping of Arabidopsis thaliana and Brassica oleracea chromosomes reveals islands of conserved organization. Genetics 138: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lagercrantz U (1998) Comparative mapping between Arabidopsis thaliana and Brassica nigra indicates that Brassica genomes have evolved through extensive genome replication accompanied by chromosome fusions and frequent rearrangements. Genetics 150: 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lan T-H, DelMonte TA, Reischmann KP, Hyman J, Kowalski SP, et al. (2000) An EST-enriched comparative map of Brassica oleracea and Arabidopsis thaliana . Genome Res 10: 776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryder C, Smith L, Teakle G, King GJ (2001) Contrasting genome organisation: two regions of the Brassica oleracea genome compared with collinear regions of the Arabidopsis thaliana genome. Genome 44: 808–817. [PubMed] [Google Scholar]
- 24. Lukens L, Zou F, Lydiate D, Parkin I, Osborn T (2003) Comparison of a Brassica oleracea genetic map with the genome of Arabidopsis thaliana . Genetics 164: 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gulick PGP, Kaczmarek MKM, Koczyk GKG, Ziolkowski PAZPA, Babula-Skowronska DBSD, et al. (2009) Comparative analysis of the Brassica oleracea genetic map and the Arabidopsis thaliana genome. Genome 52: 620–633. [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Lydiate DJ, Parkin IA, Falentin C, Delourme R, et al. (2011) Integration of linkage maps for the Amphidiploid Brassica napus and comparative mapping with Arabidopsis and Brassica rapa . BMC Genomics 12: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panjabi P, Jagannath A, Bisht NC, Padmaja KL, Sharma S, et al. (2008) Comparative mapping of Brassica juncea and Arabidopsis thaliana using Intron Polymorphism (IP) markers: homoeologous relationships, diversification and evolution of the A, B and C Brassica genomes. BMC Genomics 9: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramchiary N, Li X, Hong CP, Dhandapani V, Choi SR, et al. (2011) Genic microsatellite markers in Brassica rapa: development, characterization, mapping, and their utility in other cultivated and wild Brassica relatives. DNA Res 18: 305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shirasawa K, Oyama M, Hirakawa H, Sato S, Tabata S, et al. (2011) An EST-SSR linkage map of Raphanus sativus and comparative genomics of the Brassicaceae. DNA Res 18: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li F, Kitashiba H, Inaba K, Nishio T (2009) A Brassica rapa linkage map of EST-based SNP markers for identification of candidate genes controlling flowering time and leaf morphological traits. DNA Res 16: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li F, Hasegawa Y, Saito M, Shirasawa S, Fukushima A, et al. (2011) Extensive chromosome homoeology among Brassiceae species were revealed by comparative genetic mapping with high-density EST-based SNP markers in radish (Raphanus sativus L.). DNA Res 18: 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao J, Huang J, Chen F, Xu F, Ni X, et al. (2012) Molecular mapping of Arabidopsis thaliana lipid-related orthologous genes in Brassica napus . Theor Appl Genet 124: 407–421. [DOI] [PubMed] [Google Scholar]
- 33. Cai G, Yang Q, Yang Q, Zhao Z, Chen H, et al. (2012) Identification of candidate genes of QTLs for seed weight in Brassica napus through comparative mapping among Arabidopsis and Brassica species. BMC Genet 13: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delourme R, Falentin C, Fomeju BF, Boillot M, Lassalle G, et al. (2013) High-density SNP-based genetic map development and linkage disequilibrium assessment in Brassica napus L. BMC Genomics 14: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheung F, Trick M, Drou N, Lim YP, Park J-Y, et al. (2009) Comparative analysis between homoeologous genome segments of Brassica napus and its progenitor species reveals extensive sequence-level divergence. Plant Cell 21: 1912–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen X, Li X, Zhang B, Xu J, Wu Z, et al. (2013) Detection and genotyping of restriction fragment associated polymorphisms in polyploid crops with a pseudo-reference sequence: a case study in allotetraploid Brassica napus . BMC Genomics 14: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang C, Ramchiary N, Ma Y, Jin M, Feng J, et al. (2011) Structural and functional comparative mapping between the Brassica A genomes in allotetraploid Brassica napus and diploid Brassica rapa . Theor Appl Genet 123: 927–941. [DOI] [PubMed] [Google Scholar]
- 38. Liu S, Liu Y, Yang X, Tong C, Edwards D, et al. (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5: 3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raman H, Dalton-Morgan J, Diffey S, Raman R, Alamery S, et al. (2014) SNP markers-based map construction and genome-wide linkage analysis in Brassica napus . Plant Biotechnol J doi: 10.1111/pbi.12186 [DOI] [PubMed] [Google Scholar]
- 40. Ganal MW, Polley A, Graner EM, Plieske J, Wieseke R, et al. (2012) Large SNP arrays for genotyping in crop plants. J Biosci 37: 821–828. [DOI] [PubMed] [Google Scholar]
- 41. Edwards D, Batley J, Snowdon RJ (2013) Accessing complex crop genomes with next-generation sequencing. Theor Appl Genet 126: 1–11. [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Cai G, Tu J, Li L, Liu S, et al. (2013) Identification of QTLs for resistance to Sclerotinia Stem Rot and BnaC.IGMT5.a as a candidate gene of the major resistant QTL SRC6 in Brassica napus . PloS ONE 8: e67740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bennetzen JL (2005) Transposable elements, gene creation and genome rearrangement in flowering plants. Curr Opin Genet Dev 15: 621–627. [DOI] [PubMed] [Google Scholar]
- 44. Sarilar V, Palacios PM, Rousselet A, Ridel C, Falque M, et al. (2013) Allopolyploidy has a moderate impact on restructuring at three contrasting transposable element insertion sites in resynthesized Brassica napus allotetraploids. New Phytol 198: 593–604. [DOI] [PubMed] [Google Scholar]
- 45. Udall JA, Quijada PA, Osborn TC (2005) Detection of chromosomal rearrangements derived from homeologous recombination in four mapping populations of Brassica napus L. Genetics 169: 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Piquemal J, Cinquin E, Couton F, Rondeau C, Seignoret E, et al. (2005) Construction of an oilseed rape (Brassica napus L.) genetic map with SSR markers. Theor Appl Genet 111: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 47. Udall JA, Quijada PA, Lambert B, Osborn TC (2006) Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 2. Identification of alleles from unadapted germplasm. Theor Appl Genet 113: 597–609. [DOI] [PubMed] [Google Scholar]
- 48. Harper AL, Trick M, Higgins J, Fraser F, Clissold L, et al. (2012) Associative transcriptomics of traits in the polyploid crop species Brassica napus . Nat Biotechnol 30: 798–802. [DOI] [PubMed] [Google Scholar]
- 49. Bancroft I, Morgan C, Fraser F, Higgins J, Wells R, et al. (2011) Dissecting the genome of the polyploid crop oilseed rape by transcriptome sequencing. Nat Biotechnol 29: 762–766. [DOI] [PubMed] [Google Scholar]
- 50. Shi T, Li R, Zhao Z, Ding G, Long Y, et al. (2013) QTL for yield traits and their association with functional genes in response to phosphorus deficiency in Brassica napus . PloS ONE 8: e54559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu Z, Hua W, Huang S, Yang H, Zhan G, et al. (2012) Discovery of pod shatter-resistant associated SNPs by deep sequencing of a representative library followed by bulk segregant analysis in rapeseed. PloS ONE 7: e34253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Larkan N, Lydiate D, Parkin I, Nelson M, Epp D, et al. (2013) The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytol 197: 595–605. [DOI] [PubMed] [Google Scholar]
- 53. Liu Z, Liu P, Long F, Hong D, He Q, et al. (2012) Fine mapping and candidate gene analysis of the nuclear restorer gene Rfp for pol CMS in rapeseed (Brassica napus L.). Theor Appl Genet 125: 773–779. [DOI] [PubMed] [Google Scholar]
- 54. Lu W, Liu J, Xin Q, Wan L, Hong D, et al. (2013) A triallelic genetic male sterility locus in Brassica napus: an integrative strategy for its physical mapping and possible local chromosome evolution around it. Ann Bot 111: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen ZJ, Pikaard CS (1997) Transcriptional analysis of nucleolar dominance in polyploid plants: biased expression/silencing of progenitor rRNA genes is developmentally regulated in Brassica . Proc Natl Acad Sci USA 94: 3442–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hua YW, Liu M, Li ZY (2006) Parental genome separation and elimination of cells and chromosomes revealed by AFLP and GISH analyses in a Brassica carinata × Orychophragmus violaceus cross. Ann Bot 97: 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ge XH, Wang J, Li ZY (2009) Different genome-specific chromosome stabilities in synthetic Brassica allohexaploids revealed by wide crosses with Orychophragmus . Ann Bot 104: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu X, Tu J, Chen B, Fu T (2005) Identification and inheritance of a partially dominant gene for yellow seed colour in Brassica napus . Plant Breeding 124: 9–12. [Google Scholar]
- 59. Zou J, Fu D, Gong H, Qian W, Xia W, et al. (2011) De novo genetic variation associated with retrotransposon activation, genomic rearrangements and trait variation in a recombinant inbred line population of Brassica napus derived from interspecific hybridization with Brassica rapa . Plant J 68: 212–224. [DOI] [PubMed] [Google Scholar]
- 60. Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15: 8–15. [Google Scholar]
- 61. Lincoln S, Daly M, Lander E (1993) Constructing genetic maps with MAPMAKER/EXP version 3.0: a tutorial and reference manual. Whitehead Inst Biomed Res Tech Rpt [Google Scholar]
- 62. Wu Y, Bhat PR, Close TJ, Lonardi S (2008) Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet 4: e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kosambi D (1943) The estimation of map distances from recombination values. Ann Eugenic 12: 172–175. [Google Scholar]
- 64. Liu R, Meng J (2003) MapDraw: a microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas (Beijing) 25: 317–321. [PubMed] [Google Scholar]
- 65. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Long Y, Shi J, Qiu D, Li R, Zhang C, et al. (2007) Flowering time quantitative trait loci analysis of oilseed Brassica in multiple environments and genomewide alignment with Arabidopsis . Genetics 177: 2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The detailed information of the genetic linkage map of the DH population constructed with SNP and SSR markers, the homoeologous loci and homoeologous collinear loci identified in B. rapa, B. oleracea and Arabidopsis, the homoeologous collinear fragments, and the conserved blocks and islands.
(PDF)
The primer sequences of the SSR markers used in the HJ DH population genetic linkage map construction.
(PDF)