 |
Jeremy E. Orr (left) is a Fellow in Pulmonary and Critical Care Medicine at the University of California, San Diego. His current research is focused on the interaction between abnormal ventilatory control and circulatory disorders. Bradley A. Edwards (right) is an Instructor in Medicine in Sleep Medicine at the Brigham and Women's Hospital and Harvard Medical School. His research focuses on understanding the pathogenesis of the common sleep disorder, obstructive sleep apnoea, as well as developing and assessing novel ways to treat the disorder. Atul Malhotra is a Professor of Medicine in Pulmonary and Critical Care Medicine, as well as the director of Sleep Medicine at the University of California, San Diego.
Obstructive sleep apnoea (OSA) is a common disease affecting at least 13% of adult men and 6% of adult women in the United States (Peppard et al. 2013) and is characterized by repetitive collapse (apnoea) or partial collapse (hypopnoea) of the pharyngeal airway during sleep (Sullivan & Issa, 1985; Guilleminault et al. 1986; Young et al. 1993; Hamilton et al. 2004). Recent studies suggest that OSA is a multifactorial condition, and not just an anatomical problem (Wellman et al. 2011; Eckert et al. 2013). Alongside anatomical vulnerability, at least three additional physiological traits interact to contribute to the development of OSA including (1) ineffective upper airway dilator muscles, (2) a low threshold for arousal from sleep, and (3) a hypersensitive ventilatory control system (i.e. high loop gain) (Dempsey et al. 2010). In individual patients, the manifestation of OSA may be the result of one or more combinations of abnormalities, and thus multiple underlying causes may need to be addressed for sleep apnoea to be resolved.
Interestingly, recent evidence has questioned whether some of these traits such as a high loop gain are truly pathogenic (i.e. an intrinsic cause of OSA) or merely reflect a consequence of the disorder. Loop gain characterizes the sensitivity of the negative feedback system controlling ventilation and is defined as the size of a ‘corrective’ ventilatory response divided by the size of the ventilatory disturbance that elicits the correction (see Fig. 1); a large response to a small disturbance represents a system with a high loop gain. In favour of an elevated loop gain being an acquired condition (i.e. a consequence of disease) are two investigations whose findings demonstrate that treatment of OSA leads to major reductions in loop gain. Salloum et al. examined the effect of one month of nasal continuous positive airway pressure (CPAP) therapy on the components of the ventilatory control system – plant and controller gain – in a group of recently diagnosed and untreated severe OSA patients (Salloum et al. 2010). They reported that one month of treatment led to reductions in the ventilatory sensitivity to CO2 (i.e. controller gain), and thus loop gain (as plant gain remained unchanged), back to levels similar to healthy controls. In another study, Loewen et al. measured the dynamic ventilatory response to CO2 in a group of severe OSA patients before and after one month of CPAP therapy (Loewen et al. 2009). Similar to the study by Salloum et al., Loewen et al. observed that ventilatory sensitivity to CO2 was markedly diminished following CPAP therapy; taken together, such findings seem to suggest that a high loop gain is a consequence of OSA.
Figure 1. Loop gain and its manipulation.
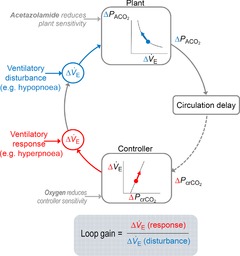
Simplified block diagram of the respiratory control system. A ventilatory disturbance (change in expiratory minute ventilation, 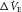 (disturbance), shown in blue) produces a change in alveolar
(disturbance), shown in blue) produces a change in alveolar 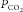 (
(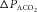 ). The amount of change in alveolar
). The amount of change in alveolar 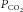 depends on the properties of the plant (which represents the lungs, blood and body tissues where CO2 is stored). After a circulation delay, this
depends on the properties of the plant (which represents the lungs, blood and body tissues where CO2 is stored). After a circulation delay, this 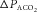 changes the
changes the 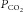 at the chemoreceptors (
at the chemoreceptors (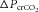 , shown in red). This change in chemoreceptor
, shown in red). This change in chemoreceptor 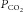 produces a change in ventilation (
produces a change in ventilation (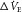 (response)) based on the sensitivity of the controller that acts to correct the initial disturbance. Loop gain, which takes into account the plant, circulation delay and the controller gain, is defined as the magnitude of the ventilatory response divided by the magnitude of the ventilatory disturbance. A large loop gain ratio indicates an unstable system prone to oscillations, and a low loop gain indicates a stable system. Note that oxygen reduces controller gain whereas acetazolamide reduces plant gain.
(response)) based on the sensitivity of the controller that acts to correct the initial disturbance. Loop gain, which takes into account the plant, circulation delay and the controller gain, is defined as the magnitude of the ventilatory response divided by the magnitude of the ventilatory disturbance. A large loop gain ratio indicates an unstable system prone to oscillations, and a low loop gain indicates a stable system. Note that oxygen reduces controller gain whereas acetazolamide reduces plant gain.
However, we would argue that the findings of these two investigations do not provide conclusive evidence that an elevated loop gain is solely a consequence of OSA. An important implication of the aforementioned studies is that one month of effective treatment was sufficient to reverse the consequences of disease and allowed an individual's ‘intrinsic’ physiology to be assessed. However, studies that have manipulated loop gain in CPAP-treated OSA patients have consistently shown that lowering the ‘intrinsic’ loop gain is associated with an improvement in OSA severity, highlighting the importance of loop gain as a cause of OSA. For instance, administration of oxygen, which is known to lower loop gain via reductions in controller gain, led to marked improvement in OSA among those patients with elevated loop gain at baseline (Wellman et al. 2008; Chowdhuri et al. 2010). No such improvement was observed in patients with low loop gain, highlighting that the intrinsic elevation in loop gain (at baseline) was pathophysiologically important in some OSA patients. In addition to oxygen therapy, the administration of acetazolamide has also been shown to lower loop gain and OSA severity (Edwards et al. 2012, 2013). Furthermore, the use of cardiac resynchronization therapy as a treatment for congestive heart failure additionally improves OSA (Stanchina et al. 2007). In this study, the observed improvement in OSA was strongly correlated with the improvement in circulatory delay, the effect of which is expected to decrease loop gain. Elevated loop gain may be critical to OSA pathogenesis in some patients, and will likely be dependent on the interaction with other pathophysiological traits that predispose towards apnoea. Depending on the underlying anatomy, loop gain can explain a large proportion of the variance in OSA severity (Wellman et al. 2004; Eckert et al. 2013). Patients with extreme pharyngeal closing pressures (Pcrit) were either protected (negative Pcrit) or predisposed (positive Pcrit) to apnoea based on intrinsic anatomy, whereas those with intermediate values were most susceptible to OSA if their loop gain was elevated.
In order to reconcile the apparent disconnect between cause vs. consequence, we would offer our opinion that an elevated loop gain is an important cause of OSA, rather than simply a consequence. Loop gain is intrinsically elevated in some OSA patients and lowering loop gain leads to improvement in OSA (Younes et al. 2001; Eckert et al. 2013). On the other hand, treating OSA also lowers loop gain, suggesting that the presence of OSA is also responsible for elevating loop gain. OSA-induced loop gain elevation could in fact be perpetuating further apnoea, in part causing the disease progression that is observed in some patients. The data regarding the progressive nature of OSA are mixed after controlling for changes in body weight, but clinical experience certainly suggests that occasional OSA patients do worsen over time (Fisher et al. 2002). Moreover, disease progression from onset to established severe OSA clearly happens gradually, a process that may be a function of loop gain elevation. Thus, efforts to lower loop gain would be predicted to reduce the severity of OSA, as is observed by several interventional studies.
We suggest that as with many physiological phenomena, a high loop gain is a ‘double edged sword’ for the OSA patient, particularly as it relates to effects on upper airway instability. On one hand, a high loop gain would be expected to increase robustly the output from the central pattern generator to the upper airway dilator muscles, which will in turn act to stiffen the airway, thereby preserving pharyngeal patency. Indeed, intermittent hypoxia can induce long-term facilitation, which may be one mechanism leading to increased upper airway motor tone in OSA patients during wakefulness (Mahamed & Mitchell, 2007; Mateika & Narwani, 2009). On the other hand, a high loop gain will also cause disproportionately large fluctuations in response to small disturbances in ventilation, which can contribute to upper airway compromise when output to these muscles is at its nadir. Of note, dynamic variability of upper airway mechanics itself can contribute to overall instability of ventilatory control. High ventilatory drive may also contribute to worsening inspiratory airflow with increasing driving pressure (i.e. negative effort dependence) (Malhotra et al. 2012; Strohl et al. 2012; Horner et al. 2014; Owens et al. 2014).
In summary, loop gain has clearly been shown to be important in OSA. While it can be said that an elevated loop gain is a consequence of OSA, we believe that it is best stated that loop gain is pathophysiologically important in the development of OSA, depending on its interactions with other individual characteristics. Recognizing that loop gain is a cause of OSA, and not simply a consequence, has important treatment implications. This understanding will hopefully allow clinicians to move beyond the ‘one-size fits all’ treatment approach of CPAP, and begin tailoring therapies that stabilize ventilatory control towards the one-third of OSA individuals with a hypersensitive feedback loop (Eckert et al. 2013; Jordan et al. 2014; Malhotra, 2014).
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;592/14/2903
Additional information
Competing interests
None declared.
References
- Chowdhuri S, Sinha P, Pranathiageswaran S, Badr MS. Sustained hyperoxia stabilizes breathing in healthy individuals during NREM sleep. J Appl Physiol (1985) 2010;109:1378–1383. doi: 10.1152/japplphysiol.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BA, Connolly JG, Campana LM, Sands SA, Trinder JA, White DP, Wellman A, Malhotra A. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36:281–285. doi: 10.5665/sleep.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, Malhotra A, Wellman A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, Pillar G, Malhotra A, Peled N, Lavie P. Long-term follow-up of untreated patients with sleep apnoea syndrome. Respir Med. 2002;96:337–343. doi: 10.1053/rmed.2001.1277. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Motta J, Mihm F, Melvin K. Obstructive sleep apnea and cardiac index. Chest. 1986;89:331–334. doi: 10.1378/chest.89.3.331. [DOI] [PubMed] [Google Scholar]
- Hamilton GS, Solin P, Naughton MT. Obstructive sleep apnoea and cardiovascular disease. Intern Med J. 2004;34:420–426. doi: 10.1111/j.1445-5994.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: basic physiology with clinical implications. J Appl Physiol (1985) 2014;116:325–336. doi: 10.1152/japplphysiol.00531.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen A, Ostrowski M, Laprairie J, Atkar R, Gnitecki J, Hanly P, Younes M. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009;32:1355–1365. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Malhotra A. Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:170–171. doi: 10.1056/NEJMe1314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Butler JP, Wellman A. The pharyngeal airway: is bigger really better? Chest. 2012;141:1372–1375. doi: 10.1378/chest.11-2989. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol. 2009;94:279–296. doi: 10.1113/expphysiol.2008.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RL, Edwards BA, Sands SA, Butler JP, Eckert DJ, White DP, Malhotra A, Wellman A. The classical Starling resistor model often does not predict inspiratory airflow patterns in the human upper airway. J Appl Physiol (1985) 2014;116:1105–1112. doi: 10.1152/japplphysiol.00853.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2010;181:189–193. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, Benser ME, Tosi C, Carlisle C, Millman RP, Buxton A. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest. 2007;132:433–439. doi: 10.1378/chest.06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Butler JP, Malhotra A. Mechanical properties of the upper airway. Compr Physiol. 2012;2:1853–1872. doi: 10.1002/cphy.c110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CE, Issa FG. Obstructive sleep apnea. Clin Chest Med. 1985;6:633–650. [PubMed] [Google Scholar]
- Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Eng J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]


