Abstract
The enteric nervous system (ENS) integrates numerous sensory signals in order to control and maintain normal gut functions. Nutrients are one of the prominent factors which determine the chemical milieu in the lumen and, after absorption, also within the gut wall. This review summarizes current knowledge on the impact of key nutrients on ENS functions and phenotype, covering their acute and long-term effects. Enteric neurones contain the molecular machinery to respond specifically to nutrients. These transporters and receptors are not expressed exclusively in the ENS but are also present in other cells such as enteroendocrine cells (EECs) and extrinsic sensory nerves, signalling satiety or hunger. Glucose, amino acids and fatty acids all activate enteric neurones, as suggested by enhanced c-Fos expression or spike discharge. These excitatory effects are the result of a direct neuronal activation but also involve the activation of EECs which, upon activation by luminal nutrients, release mediators such as ghrelin, cholecystokinin or serotonin. The presence or absence of nutrients in the intestinal lumen induces long-term changes in neurotransmitter expression, excitability, neuronal survival and ultimately impact upon gut motility, secretion or intestinal permeability. Together with EECs and vagal nerves, the ENS must be recognized as an important player initiating concerted responses to nutrients. It remains to be studied how, for instance, nutrient-induced changes in the ENS may influence additional gut functions such as intestinal barrier repair, intestinal epithelial stem cell proliferation/differentiation and also the signalling of extrinsic nerves to brain regions which control food intake.
 |
Michel Neunlist is an INSERM Research Director and head of the Translational Neurogastroenterology Unit Inserm U913 of the Digestive Diseases Institute in Nantes. Our group is studying the role of the digestive neuro-glial-epithelial unit in gut health and during chronic diseases. Michael Schemann is Professor for Human Biology at the Technische Universität Münchenwith a research focus on translational neurogastroenterology to study the neural control of gut functions under physiological and pathological conditions.
Introduction
The enteric nervous system (ENS) autonomously regulates gastrointestinal functions, such as motility, mucosal secretion and absorption, blood flow, the epithelial barrier and epithelial proliferation and differentiation (Furness, 2012). The ENS undergoes profound phenotypic and functional changes during life, ultimately impacting upon gastrointestinal functions. Although some of these changes are genetically programmed, changes in the milieu of the gut wall and lumen directly influence the neurochemistry and neurobiology of the ENS. Among those factors are dietary nutrients. It is therefore likely that the ENS must be able to sense nutrients and that nutrients impact on ENS behaviour and ultimately on ENS-controlled gut functions. In principle, both of the ENS plexi – the myenteric plexus located between the muscle layers and the submucosal plexus located close to the mucosal layer – may be able to integrate nutrient signalling. The submucosal plexus may be strategically better suited to respond directly to nutrients, as the ganglia are closer to the site of absorption and to the dense network of blood vessels in the submucosal layer. This is suggested by the finding that mucosal functions, in particular secretory reflexes, are mainly controlled by the submucosal rather than the myenteric plexus (Raybould et al. 2004). In particular, the presence of food in the gut lumen affects ENS-mediated secretory capacity. For instance, in the jejunum, short circuit currents induced by electrical stimulation of submucosal neurones were greater in hibernating ground squirrels as compared to those exposed to feed (Carey & Cooke, 1992). The feeding state also influences muscle reflexes. Peristaltic reflex activity was increased in ileal segments of animals that were refed after an overnight fasting period (Roosen et al. 2012). This effect was probably driven by the ENS, as myenteric neurones of refed animals were hyperresponsive to high K+ solutions and Cholecystokinin-8 (CCK-8), but less responsive to ghrelin (Roosen et al. 2012). We also showed recently that ENS sensitization can occur after a prolonged nutritional challenge with a high-fat diet (Baudry et al. 2012; Reichardt et al. 2013). Gastric myenteric neurones showed increased responses to electrical stimulation and colonic myenteric neurones exhibited a stronger response to acetylcholine and serotonin. This increased excitability in the colon depended on the feeding status, as it only occurred in obese mice fed for 12, but not for 4, weeks (Reichardt et al. 2013). Interestingly, the degree of neuronal sensitization, upregulation of adipocyte markers and tissue levels of serotonin and acetylcholine were positively correlated with body weight (Reichardt et al. 2013).
It is therefore increasingly recognized that the ENS is able to sense nutrients and that nutrients impact on ENS behaviour and ultimately on ENS-controlled gut functions. This review focuses on two aspects: the short-term effects of key nutrients after acute application to enteric neurones, and the ability of nutrients or diet to induce long-term changes in the ENS (Fig. 1). A focused review cannot do justice to the wealth of studies investigating chemosensitivity in the gut. The reader is referred to some excellent recent reviews which cover aspects beyond the ones discussed in our review (Bertrand, 2009; Raybould, 2010; Page et al. 2012; Furness et al. 2013).
Figure 1. The cellular and molecular bases for direct and indirect effects of nutrients on enteric neurones.
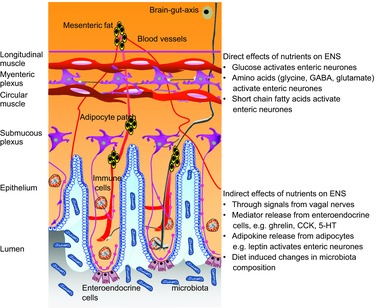
See text for details.
Neurally mediated effects of nutrients on gastrointestinal functions
It has been known since the 1960s that nutrients activate vagal afferents. Receptors, ligands and mediators involved in carbohydrate, amino acid and fatty acid effects on extrinsic sensory nerves, and their possible roles in obesity, have been comprehensively reviewed recently (Page et al. 2012). Besides chemosensitive extrinsic afferents, the ENS is also a peripheral neural target for nutrients or mediators released upon the presence of nutrients in the lumen. Nutrient sensing by extrinsic afferents primarily serves to monitor the metabolic state and to relay hunger and satiety signals (Page et al. 2012). The functional impact of changes in ENS phenotype and activity induced by nutrients is not as clear, but probably serves to adjust motility, secretion and blood flow to the presence of food in the lumen. Although it is an intriguing thought, it is not clear if the ENS responses to nutrients reflect its ability to monitor and regulate nutrient absorption. There is some circumstantial evidence that vagal–ENS signalling may be involved in food intake. It has been shown that the trypsin inhibitor, camostat, reduced food intake and increased c-Fos expression in the dorsal vagal complex as well as in the submucosal plexus (Raboin et al. 2008). These effects were mediated by cholecystokinin (CCK) acting on CCK1 receptors. The c-Fos expression was, as expected, abolished in the dorsal motor complex after subdiaphragmatic vagotomy, but, interestingly, also significantly reduced in submucosal neurones.
Application of the short-chain fatty acids (SCFAs) acetate, proprionate or butyrate onto the colonic mucosa induced peristaltic activity or increased transit time via activation of ENS pathways (Grider & Piland, 2007; Soret et al. 2010). Strikingly, luminal perfusion of the small intestine with the fatty acid decanoic acid triggered segmental activity, a motility pattern responsible for slowing transit (Ellis et al. 2013). Both peristaltic and segmental motility were critically dependent on 5-HT release from enteroendocrine cells (EECs) and hence were not a result of direct effects of the fatty acids on the ENS. The reason for the discrepant results remain unknown but may be related to the use of SCFA versus fatty acid, small versus large intestine, or the possibility that the SCFA-induced peristaltic reflex is a rather local phenomenon which does not propagate to cause peristalsis.
Nutrients also regulate mucosal secretion via ENS pathways, as illustrated by the ability of glucose to increase the rate of secretion of both sodium and chloride ions (See & Bass, 1993). SCFAs can also increase chloride secretion in the colon and small intestine, in part via neurally mediated enteric pathways (Yajima, 1988; Diener et al. 1996).
Nutrient signalling in the ENS
Role of enteroendocrine cells in mediating nutrient effects on the ENS
As previously reviewed, EECs are important intermediary cells to relay information on the nutritive state to extrinsic sensory neurones (Raybould, 2010; Furness et al. 2013). The same principle seems to apply to the ENS. Chemical stimulation of the mucosa by SCFAs evoked action potential discharge in enteric neurones via mediators released from EECs (Kunze et al. 1995; Bertrand et al. 1997). There is also differential activation of enteric neurones along the small intestine and between the submucosal and myenteric plexi, which also depends on nutrient type. Intra-intestinal perfusion of oleate increased c-Fos expression in rat myenteric and submucosal neurones of the duodenum and jejunum, but not the ileum. The activation in the duodenum was the most pronounced (Sayegh et al. 2004). In contrast, glucose infusion increased c-Fos expression in myenteric neurones of the jejunum but not the duodenum or ileum, while c-Fos expression in submucosal neurones occurred in all three regions (Sayegh et al. 2004). Both oleate and glucose appeared primarily to activate c-Fos expression in myenteric nitric oxide-synthesizing neurones, which function as inhibitory muscle motor neurones, and in calretinin-expressing submucosal neurones which, in the rat, may function as sensory neurones (Sayegh et al. 2004). The finding that nutrients activated motor neurones in the myenteric plexus, but putative sensory neurones in the submucous plexus, suggests that nutrient signalling, whether direct or indirect, occurred in submucosal rather than myenteric neurones. Oleate-mediated effects on enteric neurones were indirect, involving CCK1-dependent pathways. Indeed, the CCK1 antagonist, lorglumide, attenuated oleate-induced c-Fos expression in myenteric and submucosal neurones. In contrast, lorglumide failed to attenuate glucose-induced c-Fos expression in enteric neurones (Gulley et al. 2005).
Direct effects of nutrients on the enteric nervous system
The pattern of mucosal innervation by the ENS, in particular the fact that enteric nerve endings terminate at the basal membrane of the epithelium and never extend into the lumen, precludes any direct detection of the luminal content by the ENS. However, direct effects of nutrients on the ENS become relevant once nutrients have been absorbed or produced by metabolic activity.
Enteric neurones express similar transporters and receptors which are involved in nutrient sensing by EECs (Furness et al. 2013), thereby allowing them to directly sense and respond to nutrients. For instance, enteric neurones express the Na+–d-glucose transporter SGLT1 (Balen et al. 2008), monocarboxylate transporters (MCT), in particular MCT-2 (Soret et al. 2010), the SCFA receptor GPR41 (Nohr et al. 2013), the dipeptide transporter Pept2 (Ruhl et al. 2005), or amino acid receptors activated by glutamate, glycine or GABA (Neunlist et al. 2001; Galligan, 2002).
The expression of these transporters/receptors sets the basis for the modulation of the activity of enteric neurones by specific nutrients. About 60% of guinea pig myenteric neurones behaved as glucoresponsive neurones, with hyperpolarization upon removal of extracellular glucose (Liu et al. 1999). Glucose-induced excitation of myenteric neurones was mediated by inhibition of KATP channels, which was similar to the properties and behaviour of glucoresponsive neurones in the ventromedial hypothalamus and glucose-sensing pancreatic β cells (Liu et al. 1999).
Direct application of the amino acids GABA, glutamate or glycine increased excitability in enteric neurones (Cherubini & North, 1984; Liu et al. 1997; Neunlist et al. 2001). Concerning glutamate, data remain controversial, as a recent study showed no effect of glutamate or receptor agonists on enteric neurone excitability (Wang et al. 2014). While glutamate and GABA, but probably not glycine, are synthesized by enteric neurones, it is not known to what degree their actions may reflect a response to dietary amino acids.
Application of the SCFA butyrate onto guinea pig myenteric neurones with identified mucosal projections evoked a biphasic response consisting initially of an enhanced spike discharge followed by a transient hyperpolarization (Neunlist et al. 1999). Butyrate evoked only the hyperpolarization in dissociated, cultured rat myenteric neurones via activation of charybdotoxin-sensitive, Ca2+-dependent K+ channels (Hamodeh et al. 2004). It needs to be studied whether the lack of butyrate-induced excitation is due to downregulation of receptors in the culture or to species differences. Although not yet studied, the excitatory effect of SCFAs on enteric neurones may, specifically, involve the G-protein-coupled free fatty acid receptor GPR41/FFAR3, but not GPR43/FFAR2, as only GPR41/FFAR3 is expressed in some myenteric and submucosal neurones in mice (Nohr et al. 2013).
Some nutrients, in particular amino acids, may indirectly influence ENS activity, as they affect the synthesis and release of transmitters. l-Cysteine and l-arginine, for example, are substrates for enzymes involved in the synthesis of the enteric neurotransmitters, nitric oxide and hydrogen sulfide, respectively. The functional consequences are difficult to predict, as their effects depend on whether they are released from interneurones or motor neurones and whether they act pre- or postsynaptically. Similar effects may occur with other amino acids, for example d-serine, which enhanced contractility of the lower oesophageal sphincter by modulating glutamatergic transmission (Ghasemi-Kasman et al. 2012).
We would like to highlight two aspects common to the neural actions of amino acids, glucose and fatty acids. Firstly, all three activate myenteric and/or submucosal neurones. Secondly, all nutrients act preferentially on enteric neurones which possess mechano- and/or chemosensitivity and hence must be considered as neurones directly encoding sensory stimuli.
Long-term effects of nutrients on the ENS
Besides the ability of nutrients to stimulate the ENS acutely, long-term exposure to food or individual nutrients induces major neuroplastic changes in the ENS or alters the ENS response to nutrients. For instance, the sensitivity of myenteric and submucosal neurones to intraluminal infusion of oleate (as assessed by the proportion of c-Fos immunoreactive neurones) was reduced in rats maintained on a high-fat diet but not in animals on a normal diet (Covasa et al. 2000). These differential responses may be due to an altered response of EECs and/or neurones to nutrients. Similar tuning of enteric neurone sensitivity involving CCK, ghrelin or 5-HT was reported in animals with different feeding statuses (Roosen et al. 2012). The hunger-related ghrelin evoked stronger activation of enteric neurones in animals that had been fasted than those that had been refed, while the effects of the satiety-related CCK or 5-HT were not different. Besides modulating neuronal excitability, nutrients also induce neuroplastic changes, as suggested by the altered expression of neurotransmitters (Soret et al. 2010). Thus, chronic administration of a resistant starch-supplemented diet increased butyrate production in the colon, leading to neuroplastic changes in myenteric neurones characterized by an increase in the proportion of cholinergic neurones with no change in the nitrergic population. Butyrate- but not acetate- or proprionate-induced neuroplastic changes were mediated in part via neuronal MCT-2 and involved epigenetic regulatory mechanisms (Soret et al. 2010). As a functional consequence, this study found an increased cholinergic contractility, together with a slower distal colonic transit time. Daily butyrate enemas in rat pups between postnatal days 7 and 17 increased the proportion of cholinergic neurones and, in contrast to adult animals, also the proportion of nitrergic neurones (Suply et al. 2012), which suggested age-dependent neuroplastic changes induced by butyrate. This was associated with an enhanced amplitude of cholinergic- and nitrergic-mediated muscle responses and again a slower distal colonic transit time. Besides SCFAs, other nutrients also induce neuroplastic changes. In particular, administration of ω–3 polyunsaturated fatty acids (PUFAs) to pregnant pigs and to piglets increased the proportion of cholinergic neurones, involving PUFA metabolites such as eicosapentaenoic acid (De Quelen et al. 2011), and increased the cholinergically mediated paracellular mucosal permeability.
Besides the above-described neuroplasticity in the ENS, recent studies suggest that nutrients also affect neuronal survival and proliferation. During early life, neurotrophic factors in the milk favour neuronal survival and neurite outgrowth (Fichter et al. 2011). However, diet may also negatively affect the survival of enteric neurones. Adult mice and rats fed with high-fat diets experienced loss of ileal and colonic myenteric neurones, which was associated with delayed intestinal transit (Voss et al. 2013; Nezami et al. 2014). Others report contrary findings, in that a high-fat diet had no effect on the survival rate of colonic or small intestinal myenteric neurones, and even increased neuronal survival in the gastric myenteric plexus involving leptin and glial cell-derived neurotrophic factor (GDNF)-dependent pathways (Baudry et al. 2012). Such discrepant results may be attributed to the composition of the diet (Voss et al. 2013), the age of the animal at the onset of high-fat feeding, how long the animals received the diet, whether animals developed diabetes or not, and which gut region was studied. Furthermore, the study of long-term effects of high-fat diet upon the ENS and gut dysfunctions, including disturbed motility and altered intestinal permeability (Teixeira et al. 2012; Mushref & Srinivasan, 2013), also identified adipocytes as putative major actors involved in the effects of nutrients upon the ENS. Indeed, enteric ganglia, especially in the submucosal plexus, and patches of adipocytes are closely spaced, in particular in vascularized regions in human and guinea pig intestine (Fig. 2). This anatomical association has functional relevance, as activation of the submucosal adipocytes by β3-adrenoreceptors causes inhibition of enteric neurones through the release of somatostatin (Schemann et al. 2010). The adipokine leptin, however, activates enteric neurones (Reichardt et al. 2011). It is therefore reasonable that the adipocyte–enteric neurone interactions are involved in altered neurophysiology or neurochemistry of enteric neurones in animal models of obesity. However, the mouse may not be the ideal animal model to study such interactions because we could not find evidence for an adipocyte–enteric neurone association in the mouse gut (Fig. 2).
Figure 2. Adipocyte–enteric neurone association in the submucosal layer of the gut wall.
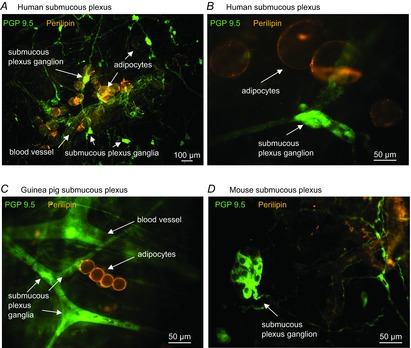
Patches of adipocytes (perilipin-positive) are very close to enteric neurones (PGP9.5 positive, A–C) in the human and guinea pig mucosa, in particular in vascularized regions. Note that the mouse intestine lacks such an adipocyte–enteric neurone axis because there are no patches of adipocytes in the mouse intestinal wall (D).
Summary and outlook
Acute and chronic exposure to nutrients has well-established and validated direct and indirect (via intermediary cells) effects in the ENS (Fig. 1). Enteric neurones express the molecular machinery to respond directly to carbohydrates, amino acids and fatty acids, and share this feature with EECs and extrinsic sensory nerves. Together with EECs and extrinsic sensory nerves, the ENS must be recognized as an important player initiating concerted responses to nutrients. Despite the ability of the ENS to respond directly to nutrients, much remains to be learned about its contribution to nutrient-induced changes in gut functions and the mechanisms involved. In addition, it is not known whether nutrient effects on the ENS play any role beyond the local regulation of muscle and mucosal functions. The anatomical and functional association between extrinsic sensory nerves signalling hunger or satiety and the ENS raises the intriguing possibility that nutrient effects on the ENS may impact on food intake.
Glossary
- CCK
cholecystokinin
- EEC
enteroendocrine cell
- ENS
enteric nervous system
- MCT-2
monocarboxylate transporter 2
- SCFA
short-chain fatty acid
Additional information
Competing interests
None declared.
References
- Balen D, Ljubojević M, Breljak D, Brzica H, Žlender V, Koepsell H, Sabolić I. Revised immunolocalization of the Na+d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol. 2008;295:C475–C489. doi: 10.1152/ajpcell.00180.2008. [DOI] [PubMed] [Google Scholar]
- Baudry C, Reichardt F, Marchix J, Bado A, Schemann M, des Varannes SB, Neunlist M, Moriez R. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J Physiol. 2012;590:533–544. doi: 10.1113/jphysiol.2011.219717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009;3:48. doi: 10.3389/neuro.21.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol Gastrointest Liver Physiol. 1997;273:G422–G435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Carey HV, Cooke HJ. Intestinal secretion after jejunal bypass in the ground squirrel. Am J Physiol Regul Integr Comp Physiol. 1992;263:R1209–R1214. doi: 10.1152/ajpregu.1992.263.6.R1209. [DOI] [PubMed] [Google Scholar]
- Cherubini E, North RA. Actions of gamma-aminobutyric acid on neurones of guinea-pig myenteric plexus. Br J Pharmacol. 1984;82:93–100. doi: 10.1111/j.1476-5381.1984.tb16445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covasa M, Grahn J, Ritter RC. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton Neurosci. 2000;84:8–18. doi: 10.1016/S1566-0702(00)00176-4. [DOI] [PubMed] [Google Scholar]
- De Quelen F, Chevalier J, Rolli-Derkinderen M, Mourot J, Neunlist M, Boudry G. n–3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol. 2011;589:4341–4352. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener M, Vujicic Z, Scharrer E. Neuronally mediated anion secretion induced by short-chain fatty acids in the rat distal small intestine. Acta Physiol Scand. 1996;157:33–40. doi: 10.1046/j.1365-201X.1996.470184000.x. [DOI] [PubMed] [Google Scholar]
- Ellis M, Chambers JD, Gwynne RM, Bornstein JC. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304:749–761. doi: 10.1152/ajpgi.00358.2012. [DOI] [PubMed] [Google Scholar]
- Fichter M, Klotz M, Hirschberg DL, Waldura B, Schofer O, Ehnert S, Schwarz LK, Ginneken CV, Schaefer KH. Breast milk contains relevant neurotrophic factors and cytokines for enteric nervous system development. Mol Nutr Food Res. 2011;55:1592–1596. doi: 10.1002/mnfr.201100124. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil. 2002;14:611–623. doi: 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Ghasemi-Kasman M, Dehpour AR, Mani AR. d-serine modulates non-adrenergic non-cholinergic contraction of lower esophageal sphincter in rats. Eur J Pharmacol. 2012;696:155–160. doi: 10.1016/j.ejphar.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:G429–G437. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- Gulley S, Covasa M, Ritter RC, Sayegh AI. Cholecystokinin1 receptors mediate the increase in Fos-like immunoreactivity in the rat myenteric plexus following intestinal oleate infusion. Physiol Behav. 2005;86:128–135. doi: 10.1016/j.physbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Hamodeh SA, Rehn M, Haschke G, Diener M. Mechanism of butyrate-induced hyperpolarization of cultured rat myenteric neurones. Neurogastroenterol Motil. 2004;16:597–604. doi: 10.1111/j.1365-2982.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci. 1997;17:4764–4784. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Seino S, Kirchgessner AL. Identification and characterization of glucoresponsive neurons in the enteric nervous system. J Neurosci. 1999;19:10305–10317. doi: 10.1523/JNEUROSCI.19-23-10305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med. 2013;1:14. doi: 10.3978/j.issn.2305-5839.2012.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol. 1999;517:533–546. doi: 10.1111/j.1469-7793.1999.0533t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Michel K, Reiche D, Dobreva G, Huber K, Schemann M. Glycine activates myenteric neurones in adult guinea-pigs. J Physiol. 2001;536:727–739. doi: 10.1111/j.1469-7793.2001.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezami BG, Mwangi SM, Lee JE, Jeppsson S, Anitha M, Yarandi SS, Farris AB, 3rd, Srinivasan S. MicroRNA 375 mediates palmitate-induced enteric neuronal damage and high-fat diet-induced delayed intestinal transit in mice. Gastroenterology. 2014;146:473–483. doi: 10.1053/j.gastro.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs. FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- Page AJ, Symonds E, Peiris M, Blackshaw LA, Young RL. Peripheral neural targets in obesity. Br J Pharmacol. 2012;166:1537–1558. doi: 10.1111/j.1476-5381.2012.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboin SJ, Reeve JR, Jr, Cooper MS, Green GM, Sayegh AI. Activation of submucosal but not myenteric plexus of the gastrointestinal tract accompanies reduction of food intake by camostat. Regul Pept. 2008;150:73–80. doi: 10.1016/j.regpep.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil. 2004;16(Suppl. 1):60–63. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- Reichardt F, Baudry C, Gruber L, Mazzuoli G, Moriez R, Scherling C, Kollmann P, Daniel H, Kisling S, Haller D, Neunlist M, Schemann M. Properties of myenteric neurones and mucosal functions in the distal colon of diet-induced obese mice. J Physiol. 2013;591:5125–5139. doi: 10.1113/jphysiol.2013.262733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt F, Krueger D, Schemann M. Leptin excites enteric neurons of guinea-pig submucous and myenteric plexus. Neurogastroenterol Motil. 2011;23:165–170. doi: 10.1111/j.1365-2982.2010.01665.x. [DOI] [PubMed] [Google Scholar]
- Roosen L, Boesmans W, Dondeyne M, Depoortere I, Tack J, Vanden Berghe P. Specific hunger- and satiety-induced tuning of guinea pig enteric nerve activity. J Physiol. 2012;590:4321–4333. doi: 10.1113/jphysiol.2012.231134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl A, Hoppe S, Frey I, Daniel H, Schemann M. Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J Comp Neurol. 2005;490:1–11. doi: 10.1002/cne.20617. [DOI] [PubMed] [Google Scholar]
- Sayegh AI, Covasa M, Ritter RC. Intestinal infusions of oleate and glucose activate distinct enteric neurons in the rat. Auton Neurosci. 2004;115:54–63. doi: 10.1016/j.autneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schemann M, Hafsi N, Michel K, Kober OI, Wollmann J, Li Q, Zeller F, Langer R, Lee K, Cellek S. The β3-adrenoceptor agonist GW427353 (Solabegron) decreases excitability of human enteric neurons via release of somatostatin. Gastroenterology. 2010;138:266–274. doi: 10.1053/j.gastro.2009.09.046. [DOI] [PubMed] [Google Scholar]
- See NA, Bass P. Glucose-induced ion secretion in rat jejunum: a mucosal reflex that requires integration by the myenteric plexus. J Auton Nerv Syst. 1993;42:33–40. doi: 10.1016/0165-1838(93)90339-v. [DOI] [PubMed] [Google Scholar]
- Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- Suply E, de Vries P, Soret R, Cossais F, Neunlist M. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. Am J Physiol Gastrointest Liver Physiol. 2012;302:1373–1380. doi: 10.1152/ajpgi.00338.2011. [DOI] [PubMed] [Google Scholar]
- Teixeira TFS, Souza NCS, Chiarello PG, Franceschini SCC, Bressan J, Ferreira CLLF, Peluzio MdoCG. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735–740. doi: 10.1016/j.clnu.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Voss U, Sand E, Olde B, Ekblad E. Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PLoS One. 2013;8:81413. doi: 10.1371/journal.pone.0081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Wang XY, Xia Y, Wood JD. Dietary glutamate: interactions with the enteric nervous system. J Neurogastroenterol Motil. 2014;20:41–53. doi: 10.5056/jnm.2014.20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T. Luminal propionate-induced secretory response in the rat distal colon in vitro. J Physiol. 1988;403:559–575. doi: 10.1113/jphysiol.1988.sp017264. [DOI] [PMC free article] [PubMed] [Google Scholar]


