Abstract
Common human experience shows that stress and anxiety may modulate gut function. Such observations have been combined with an increasing evidence base that has culminated in the concept of the brain–gut axis. Nevertheless, it has not been until recently that the gut and its attendant components have been considered to influence higher cerebral function and behaviour per se. Moreover, the proposal that the gut and the bacteria contained therein (collectively referred to as the microbiota) can modulate mood and behaviours, has an increasing body of supporting evidence, albeit largely derived from animal studies. The gut microbiota is a dynamic and diverse ecosystem and forms a symbiotic relationship with the host. Herein we describe the components of the gut microbiota and mechanisms by which it can influence neural development, complex behaviours and nociception. Furthermore, we propose the novel concept of a ‘state of gut’ rather than a state of mind, particularly in relation to functional bowel disorders. Finally, we address the exciting possibility that the gut microbiota may offer a novel area of therapeutic intervention across a diverse array of both affective and gastrointestinal disorders.
 |
Dr Adam Farmer PhD MRCP and Professor Qasim Aziz PhD FRCP work at the Wingate Institute of Neurogastroenterology, Queen Mary University of London. Their main research interests focus around the brain gut axis and visceral nociception and they have published widely in this area. They are also both honorary consultant gastroenterologists at Barts Health and run a busy tertiary referral Neurogastroenterology service with a special interest in patients with functional gastrointestinal disorders.
Introduction
It is almost a universal human experience that stress or anxiety may modulate gut function, often culminating in symptoms such as diarrhoea, nausea and discomfort. Indeed, the influence that anxiety may exert on the gastrointestinal (GI) tract is often reflected in common phrases such as ‘butterflies in my stomach’ and ‘gut feelings’. Nevertheless, it was not until the beginning of the nineteenth century that such observations began to be objectively investigated by luminaries in the field such as William Beaumont. While the methodologies and technologies utilized to evaluate GI function have advanced since these early days, the relationship between emotional state and gut function, both in health and disease, remain prominent in the contemporaneous research agenda. Arguably, the most unequivocal evidence of the brain's influence on human GI function derives from reports of alterations of this function following lesions within the CNS. Perhaps the most frequently encountered clinical instance is that of dysphagia following a cerebrovascular accident (Hamdy et al. 1997). Further examples include gastric emptying delay occurring as a consequence of spinal cord transection or constipation related to Parkinson's disease (Gunterberg et al. 1976; Fealey et al. 1984). Despite these insights, it was not until the advent of a number of neurophysiological techniques, that these interactions have been studied non-invasively in vivo. This increased understanding has led to the development of the concept of the brain–gut axis, a bidirectional intercommunication between the gut and the brain, providing an explanation of both normal activity and acute and chronic perturbations of GI function (Camilleri & Di Lorenzo, 2012). Moreover, this model of circuitous communication underpins the biopsychosocial concept, first explicitly formulated by George Engel in the late 1970s, postulates that all illnesses, but especially in GI disorders, result from a complex reciprocal interaction between biological/genetic, psychological and social factors (Engel, 1977).
Despite the brain–gut axis being by definition bidirectional, it is a recent concept that the gut, and its attendant components, may influence higher cerebral function and behaviour per se (Hughes et al. 2013). Moreover, the postulation that the gut and its microbiota can modulate mood and behaviours has an increasing body of supporting evidence, albeit in its infancy. In this review, we describe the components of the gut microbiota and mechanisms by which it can influence neural development, complex behaviours and nociception. Furthermore, we shall address the intriguing possibility that gut microbiota may offer a novel area of therapeutic intervention across a diverse array of GI disorders. Finally, we propose an innovative concept of a ‘state of gut’ rather than a state of mind.
Human gut microbiota
The human microbiota is a diverse and dynamic ecosystem, which has evolved to form a symbiotic relationship with the host. Cell for cell, the microbiota outnumbers host cells by a factor of 10. An estimated 1014 microorganisms populate the adult gut (Ley et al. 2006). Before birth, the human perinatal gut is a sterile environment and is vertically inoculated from the mother during birth. The ecosystem, established during the first year and refined over the course of an individual's life, is determined in part by genetics, ethnicity, diet and the environment (Dominguez-Bello et al. 2011). The microbiota helps safeguard the host from external pathogens, aids in the metabolism of polysaccharides and lipids, modulates intestinal motility, in addition to modulating visceral perception (Montiel-Castro et al. 2013). The microbiota also acts as a source of neurochemicals used to regulate a vast array of physiological and psychological processes. For example, the microbiota is the source of approximately 95% of the body's 5-hydroxytryptamine, a critical neurotransmitter in the modulation of GI motility and mood (Sommer & Backhed, 2013). The human GI microbiota commences in the oral cavity, containing approximately 102–3 colony forming units of bacteria per gram of saliva. This rises to 1010–12 colony forming units per gram of faeces in the colon comprising of between 400 and 1000 different species (Bercik, 2011). Firmicutes (60–80%) and Bacteroides (20–40%) are the two predominant bacterial phylotypes (Eckburg et al. 2005). The majority of the constituents of these phyla do not grow outside the host (Ley et al. 2006). These bacteria are classified from phylum to species level (see Fig. 1).
Figure 1.
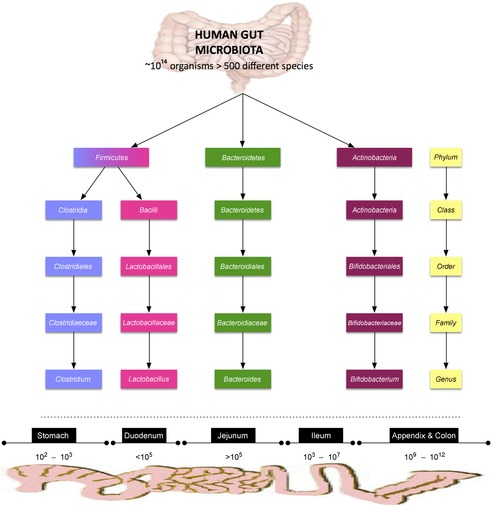
Top panel, the major constituents of the gut microbiota in humans classified according to phylum, class, order, family and genus. Bottom panel, the relative concentrations of microorganisms in the gut from the foregut to the hindgut (Cryan & Dinan, 2012).
Microbiome brain–gut axis: A circuitous relationship
The bidirectional communication between the brain and the gut is facilitated through a number of pathways, including the autonomic and enteric nervous systems, the neuroendocrine system and the immune system (Rhee et al. 2009). The gut has its own dense independent neural network, which is capable of functioning even after connections to the CNS have been severed. The primary neural conduit for this communication between the gut and the CNS is the parasympathetic nervous system and its main substrate, the vagus nerve comprising of both afferent and efferent limbs. Recently, there has been a considerable reappraisal of the manner in which the periphery and CNS communicate as a result of the growing body of experimental data from animal studies focused on the microbiota (De Vadder et al. 2014). This evidence has provided a rational basis for the importance of the ‘bottom-up’ influence of microbes themselves, with several studies showing that commensal bacteria are important to CNS function.
Central nervous system influences on the microbiota
The CNS can influence composition of the GI microbiota directly or indirectly. Direct alterations are exerted by the intraluminal action of neurotransmitters, such as 5-hydroxytrapamine released by enterochromaffin cells, neurones and immune cells located in the lamina propria. Indirectly, changes in the composition of the microbiota may occur through fluctuations in GI motility and secretion (El Aidy et al. 2012). Modification of GI motility can be accompanied by marked changes in blood flow and therefore nutrient delivery and availability to the microbiota (Rhee et al. 2009).
Microbiota to central nervous system communication
There is accumulating evidence that the microbiota can influence behaviour via the CNS; however, the mechanisms by which such communication occurs are incompletely understood. For example, external factors such as stress or depression influence the course of GI diseases such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) (Goodhand & Rampton, 2008). Furthermore, stress can alter the integrity of the GI epithelium, modulate GI motility and induce the release of catecholamines and cortisol that impact on intestinal immunity and cytokine production (see Fig. 2) (Chen et al. 2013). All of these factors can have a direct or indirect influence on gut microbiota composition.
Figure 2. Bidirectional brain–gut–microbiota pathways.
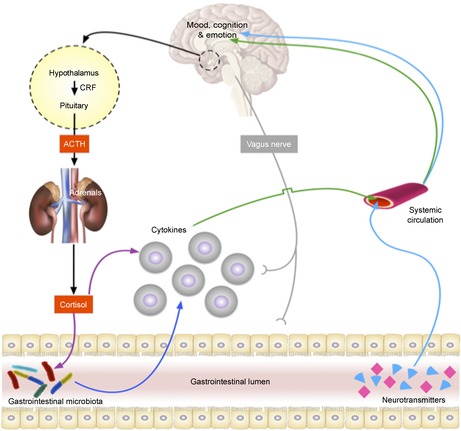
Multiple pathways, including but not limited to neural, endocrine and immune, exist in which the gastrointestinal microbiota may modulate the brain (Cryan & Dinan, 2012). ACTH, adrenocorticotrophic hormone; CRF, corticotrophin releasing hormone.
The use of germ-free animals has facilitated the evaluation of the role of the microbiota on many aspects of GI physiology. This approach takes advantage of the fact that the uterine environment is sterile and that colonization of the gut occurs in the direct postnatal period. Germ-free animals, most commonly rodents, are maintained in a sterile environment thereby eliminating postnatal colonization. Such a stratagem allows the direct comparison with conventionally reared animals although the sterile uterine premise has recently been questioned in humans (Funkhouser & Bordenstein, 2013). Nevertheless, in a series of elegant experiments, to address the question of whether the microbiome can influence the CNS both at the neuronal and behavioural levels (Cryan & O'Mahony, 2011). Using such techniques, Bercik et al. (2011) demonstrated that the microbiota influence the CNS by observing that behavioural traits of donor mice can be adoptively transferred into adult germ-free mice of a different strain via the gut microbiota. This study exploited well-documented differences in behavioural traits and the microbiota profiles of strains of laboratory mice. BALB/c mice exhibit anxiety-like behaviour, but when germ-free BALB/c mice are colonized with the microbiota from affable Swiss mice, they exhibit more exploratory behaviour than their un-colonized counterparts. Conversely, germ-free Swiss mice colonized with the microbiota from BALB/c mice exhibit a reduction in exploratory behaviour. Moreover, these changes in behavioural traits were associated with alterations in brain-derived neurotrophic factor (BDNF) in the hippocampal region, a critical neurotrophic protein involved in neuronal growth and survival. In an influential study published in the Journal of Physiology, Sudo et al. (2004) demonstrated that at the neuronal level, germ-free animals had a relative paucity of BDNF and reduced expression of the N-methyl-d-aspartate receptor subunit 2A in the cortex and hippocampus compared with controls. Interestingly, alterations of BDNF have been implicated in anxiety states, although further work is needed to clarify the absolute molecular contribution to behavioural change (Cryan & Sweeney, 2011). This evidence highlighting ‘bottom-up’ is counterbalanced by studies investigating the ‘top-down’ influence, i.e. the effect of mood manipulation on the microbiota. For example, in a mouse model, the induction of chronic depression has been shown to alter both the microbial profile and colonic motor activity, through a mechanism dependent on the hypothalamic–pituitary–adrenal axis (Park et al. 2013). The maintenance of the intraluminal environment for healthy microbiota requires nucleotide-binding oligomerization domain protein-like receptors, pyrin-domain containing (NLRP)-6 inflammasomes. Sun et al. (2013) demonstrated that stress inhibits NLRP6 thereby influencing the composition of the gut microbiota, which culminates in inflammation of the GI tract. Such evidence offers the enticing translational prospect that modulation of the GI microbiota may present a potential therapeutic strategy for stress-related disorders and for modulating the affective disorders that are frequently comorbid with functional bowel diseases. Although these studies, and others, provide evidence that the microbiota influences brain function on a real-time basis, to date there is a paucity of information regarding the mechanisms underlying this link. While germ-free mice have provided insights, their use is limited by the fact that their immune systems fail to have the opportunity to mature. Furthermore, these studies entrust DNA sequencing per se to identify the gut microbiota. In future such sequencing approaches will need to be combined with metabolomic and transcriptomic techniques to develop a mechanistic appreciation of the processes involved (Collins et al. 2012). Whether such murine models are concordant with human disease states remains to be elucidated.
A state of gut (microbiota)?
Mood disorders are common across a diverse array of GI disorders, including IBD, coeliac disease, non-coeliac gluten sensitivity, lactose intolerance and IBS (Cho et al. 2011; Goodhand et al. 2012; Smith & Gerdes, 2012). IBS is a prevalent disorder, characterized by abdominal pain and change in bowel habit, whose pathogenesis is incompletely understood. Conventionally, IBS is subtyped according to the predominant stool pattern with diarrhoea and constipation being the most prevalent. The development of culture independent techniques has facilitated considerable advances to be made in ascertaining the role of the microbiota in these subtypes (Simren et al. 2013). A recent study by Jeffery et al. (2012) performed pyrosequencing analysis of the composition of faecal microbiota and demonstrated two species-specific subtypes of IBS, which were independent of symptom-based classification. The first of these showed a microbial composition similar to normal, while the second was characterized by an increase in Firmicutes-associated taxa with a relative depletion of Bacteroides-related taxa. The implication of these data is that in the future, GI microbial enterotyping may facilitate stratifications of IBS subpopulations. However, currently, such methods have limited practicality as a routine clinical biomarker as they are resource and labour intensive (Arumugam et al. 2011). Pimentel et al. (2011) have reported the combined results of two phase III trials evaluating the utility of the non-absorbable antibiotic rifaximin in non-constipated IBS, demonstrating a small but significant improvement of global symptoms, bloating, abdominal pain, and loose or watery stools. In IBD, preliminary data also suggest that rifaximin may offer a therapeutic benefit in inducing and maintaining remission (Guslandi, 2011). The potential therapeutic activity of rifaximin in IBD and IBS warrants further investigation and replication in larger, controlled studies.
IBS can also occur after an enteric infection, where it is termed postinfectious IBS (PI-IBS), or if as a sequelae of amoebiasis, post-amoebiasis IBS (Neal et al. 2002). While the overwhelming majority of individuals who develop bacterial gastroenteritis have acute self-limiting symptoms, between 4 and 32% of patients develop symptoms consistent with IBS that outlast the initial infection (Ghoshal & Ranjan, 2011). IBS symptoms have been documented after a variety of enteric pathogens, including Campylobacter, Salmonella, Shigella strains and Escherichia coli. Indeed, public health disasters such as the E. coli outbreak in Walkerton (Ontario, Canada) have afforded researchers the opportunity to prospectively study the natural history, pathophysiology and genetic susceptibility of PI-IBS at the population level (Garg et al. 2005). Although there is an absence of universally applicable pathophysiological features, intestinal inflammation, alterations in GI motility and mucosal permeability have all been implicated in PI-IBS. The increased recognition and appreciation of PI-IBS as a clinical entity has facilitated the prospective examination of the role of psychological factors. Early data, subsequently confirmed by others, has shown that at the time of the initial infectious illness those who had higher scores for anxiety, depression, somatization, and neuroticism were more likely to develop symptom chronicity (Gwee et al. 1996). Although these studies have resulted in pathophysiological insights being made, it is entirely possible that the initial infectious pathogen causes an alteration in mood, culminating in the modulation of behavioural traits and symptom chronicity. Taking evidence from both human and animal studies, we propose that an acute insult to the gut microbiota can cause central changes culminating in alterations in both mood and behaviour in susceptible individuals (see Fig. 3. Clearly, this proposition warrants further exploration and objective assessment in stringently designed population-based longitudinal studies.
Figure 3. A schematic representation of the mood and cognitive effects of the microbiota. In murine models (top) evidence suggests that behaviours can be influenced through microbiota transfer between species.
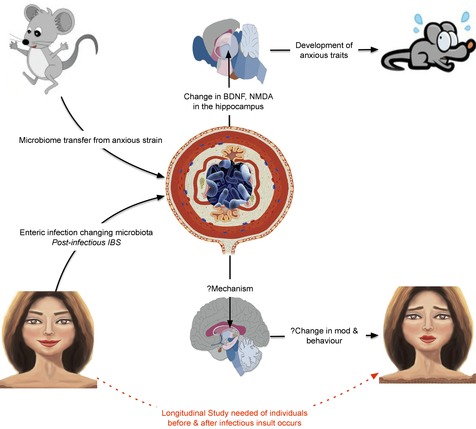
We propose that a similar mechanism may also exert behavioural effects in humans (bottom) after an enteric infection. BDNF, brain-derived neurotrophic factor; IBS, irritable bowel syndrome; NMDA, N-methyl-d-aspartate.
Interest in the microbiota and the metabolome has not been confined to disorders of the GI tract. Notably, animal evidence has implicated these factors in neurodevelopmental disorders, which may have salience the prevalent disorders of autism and ‘pervasive developmental disorder, not otherwise specified’ (Hsiao et al. 2013; Desbonnet et al. 2014). These observations were extended to children with autism, ‘pervasive developmental disorder, not otherwise specified’ in comparison to healthy control, where De Angelis et al. observed significant differences in bacterial phyla between the three groups (De Angelis et al. 2013). Therefore, demonstrable differences in gut microbiota have important implications regarding the development of a disease-specific biomarker(s) as well as treatment and, potentially, prevention.
Therapeutic manipulation of the microbiota: A new hope?
Worldwide, there is considerable commercial interest in the gut microbiota as indexed by the expanding markets for probiotics, some of which have shown significant benefits in the setting of clinical trials of GI disorders (Aziz et al. 2013). Bravo et al. (2011) have elegantly demonstrated that chronic administration of Lactobacillus rhamnosus in mice induces region dependent alterations in GABA receptor expression in the CNS, which has been implicated in the pathogenesis of anxiety and depression. Further evidence suggests that probiotics may prevent the development of changes in brain activity in mice in response to chronic stress (Ait-Belgnaoui et al. 2014). In humans, Tillisch et al. (2013) have shown that consumption of fermented milk product with probiotic affected activity of brain regions that control central processing of emotion and sensation using functional brain imaging. These data suggest that certain organisms may prove to be useful therapeutic adjuncts in stress-related disorders, although well-designed controlled human trials are needed to further evaluate this interesting concept (Saulnier et al. 2013).
A further hypothesis that offers an interesting line of investigation regarding their mechanism of action is that of the psychological/behavioural therapies, such as relaxation therapy, hypnotherapy or cognitive behavioural therapy. Such therapies could potentially modify efferent vagal and/or hypothalamic–pituitary–adrenal axis function thereby possibly modifying the intestinal microbiota.
Conclusions
Considering the gut's multi-faceted capacity to communicate with the CNS, it is implausible that the gut and its components are not playing a crucial role in resultant mood and behaviours. Exciting evidence from animal studies has provided the rationale to warrant further exploration in humans, both in health and disease.
Additional information
Competing interests
None of the authors have any conflict of interests to declare.
References
- Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, Houdeau E, Theodorou V, Tompkins T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 2014;26:510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J, Meta HITC, Antolin M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Merieux A, Melo Minardi R, M'Rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz Q, Dore J, Emmanuel A, Guarner F, Quigley EM. Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil. 2013;25:4–15. doi: 10.1111/nmo.12046. [DOI] [PubMed] [Google Scholar]
- Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut. 2011;60:288–289. doi: 10.1136/gut.2010.226779. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behaviour in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. 609 e591–593. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behaviour and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Di Lorenzo C. Brain-gut axis: from basic understanding to treatment of IBS and related disorders. J Pediatr Gastroenterol Nutr. 2012;54:446–453. doi: 10.1097/MPG.0b013e31823d34c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, D'Souza R, Hong ST. The role of gut microbiota in the gut-brain axis: current challenges and perspectives. Protein Cell. 2013;4:403–414. doi: 10.1007/s13238-013-3017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Park JM, Lim CH, Cho YK, Lee IS, Kim SW, Choi MG, Chung IS, Chung YK. Anxiety, depression and quality of life in patients with irritable bowel syndrome. Gut Liver. 2011;5:29–36. doi: 10.5009/gnl.2011.5.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behaviour. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140:1713–1719. doi: 10.1053/j.gastro.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aidy S, Kunze W, Bienenstock J, Kleerebezem M. The microbiota and the gut-brain axis: insights from the temporal and spatial mucosal alterations during colonisation of the germfree mouse intestine. Benef Microbes. 2012;3:251–259. doi: 10.3920/BM2012.0042. [DOI] [PubMed] [Google Scholar]
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- Fealey RD, Szurszewski JH, Merritt JL, DiMagno EP. Effect of traumatic spinal cord transection on human upper gastrointestinal motility and gastric emptying. Gastroenterology. 1984;87:69–75. [PubMed] [Google Scholar]
- Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AX, Macnab J, Clark W, Ray JG, Marshall JK, Suri RS, Devereaux PJ, Haynes B Walkerton Health Study I. Long-term health sequelae following E. coli and campylobacter contamination of municipal water. Population sampling and assessing non-participation biases. Can J Public Health. 2005;96:125–130. doi: 10.1007/BF03403675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal UC, Ranjan P. Post-infectious irritable bowel syndrome: the past, the present and the future. J Gastroenterol Hepatol. 2011;26(Suppl 3):94–101. doi: 10.1111/j.1440-1746.2011.06643.x. [DOI] [PubMed] [Google Scholar]
- Goodhand J, Rampton D. Psychological stress and coping in IBD. Gut. 2008;57:1345–1347. doi: 10.1136/gut.2008.154229. [DOI] [PubMed] [Google Scholar]
- Goodhand JR, Wahed M, Mawdsley JE, Farmer AD, Aziz Q, Rampton DS. Mood disorders in inflammatory bowel disease: Relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis. 2012;18:2301–2309. doi: 10.1002/ibd.22916. [DOI] [PubMed] [Google Scholar]
- Gunterberg B, Kewenter J, Petersen I, Stener B. Anorectal function after major resections of the sacrum with bilateral or unilateral sacrifice of sacral nerves. Br J Surg. 1976;63:546–554. doi: 10.1002/bjs.1800630713. [DOI] [PubMed] [Google Scholar]
- Guslandi M. Rifaximin in the treatment of inflammatory bowel disease. World J Gastroenterol. 2011;17:4643–4646. doi: 10.3748/wjg.v17.i42.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, Thompson DG. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet. 1997;350:686–692. doi: 10.1016/S0140-6736(97)02068-0. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–1074. doi: 10.1038/ajg.2013.120. [DOI] [PubMed] [Google Scholar]
- Jeffery IB, O'Toole PW, Ohman L, Claesson MJ, Deane J, Quigley EM, Simren M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Montiel-Castro AJ, Gonzalez-Cervantes RM, Bravo-Ruiseco G, Pacheco-Lopez G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:70. doi: 10.3389/fnint.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410–413. doi: 10.1136/gut.51.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP, Group TS. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, Guarner F. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4:17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simren M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG, Rome Foundation C. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Gerdes LU. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr Scand. 2012;125:189–193. doi: 10.1111/j.1600-0447.2011.01795.x. [DOI] [PubMed] [Google Scholar]
- Sommer F, Backhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang M, Chen CC, Gillilland M, 3rd, Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, Chang YM, Gumucio DL, Owyang C, Kao JY. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144:1478–1487. doi: 10.1053/j.gastro.2013.02.038. 1487 e1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. 1401 e1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]


