Abstract
The gut–brain axis is the bidirectional communication between the gut and the brain, which occurs through multiple pathways that include hormonal, neural and immune mediators. The signals along this axis can originate in the gut, the brain or both, with the objective of maintaining normal gut function and appropriate behaviour. In recent years, the study of gut microbiota has become one of the most important areas in biomedical research. Attention has focused on the role of gut microbiota in determining normal gut physiology and immunity and, more recently, on its role as modulator of host behaviour (‘microbiota–gut–brain axis’). We therefore review the literature on the role of gut microbiota in gut homeostasis and link it with mechanisms that could influence behaviour. We discuss the association of dysbiosis with disease, with particular focus on functional bowel disorders and their relationship to psychological stress. This is of particular interest because exposure to stressors has long been known to increase susceptibility to and severity of gastrointestinal diseases.
 |
Dr. Verdu, MD PhD, is associate professor and director of the Axenic Gnotobiotic Unit at McMaster University. Dr. Verdu is interested in the pathogenesis of chronic inflammatory disorders such as celiac disease, and is also investigating host-bacterial interactions in particular in the context of probiotics and functional gastrointestinal diseases. Dr. De Palma, PhD, is a post-doctoral fellow atMcMaster University working on themicrobiota–gut–brain axis in health and disease.
Introduction
The central nervous system and the gastrointestinal (GI) tract are in constant bidirectional communication through neural pathways, such as the vagus nerve, and by humoral and cellular mediators that include the immune system and the hypothalamic–pituitary–adrenal (HPA) axis.
The gut is colonized with a complex community of bacteria (microbiota), which helps to shape the immune system, metabolic function and behaviour in health and disease throughout life. The microbiota is a relatively new player in the gut–brain axis, fulfilling key roles in its communication (Bailey & Coe, 1999; Bercik et al. 2011a; Heijtz et al. 2011; Neufeld et al. 2011; Matsumoto et al. 2013), which has led to the term ‘microbiota–gut–brain axis’ (Rhee et al. 2009; Collins et al. 2012). Alterations in gut microbiota (dysbiosis) can arise as a consequence of gastrointestinal disease or of its treatment. All major chronic disorders of the gut, namely inflammatory bowel disease, irritable bowel syndrome and coeliac disease, are associated with dysbiosis (Nadal et al. 2007; Collado et al. 2009; De Palma et al. 2010). Although an overall decrease in diversity and richness of the microbiota seems to be a common finding across studies, no specific dysbiotic signature has emerged between studies. This may be due, in part, to differences in sampling (small intestinal, colonic, faecal), as well as analytical techniques employed (culture, Denaturing Gradient Gel Electrophoresis, DGGE, Illumina, 454 sequencing, Matrix-assisted laser desorption ionization time-of-flight mass spectrometer, MALDI-TOF) (Lagier et al. 2012). However, there is now increasing evidence that dysbiosis modulates peripheral and central nervous system function, leading to alterations in brain signalling and behaviour (Bercik et al. 2011a; Collins et al. 2013; Mulle et al. 2013). This observation is important in view of the fact that stress and depression, common co-morbidities in GI disorders, in turn influence the natural course of these illnesses (Collins, 2001; Wu, 2012).
The microbiota–gut–brain axis has been the subject of numerous reviews in recent years (Rhee et al. 2009; Mayer, 2011, 2014; Bercik et al. 2012; Collins et al. 2012, 2013). The influence of psychosocial and environmental stressors on the pathogenesis of gastrointestinal diseases has long been recognized. Recently, the mechanisms through which stress may affect various physiological functions of the GI tract have been reviewed (Konturek et al. 2011). We will discuss the recent progress on specific mechanisms of interaction between gut microbiota and brain, with focus on the effect of psychological stress.
Gut microbiota and its host: a mutualistic relationship
A unique combination of different populations of organisms inhabits our gut, mainly bacteria but also archaea, viruses and protozoa, roughly approximating 1014 cells, outnumbering the human cells in our bodies by a factor of 10 (Sekirov et al. 2010). While bacterial profiling and its understanding has become easier during the last decade, the analysis of the mycobiome and the virome is still in its infancy (Minot et al. 2011, 2013; Cui et al. 2013).
The human intestinal tract is essentially sterile at birth, when it is immediately colonized. The gut microbiota evolves during early life until a unique, subject-specific (fingerprint) adult-like community arises, which is relatively stable throughout life (Rajilić-Stojanović et al. 2013). Out of the more than 50 phyla described in the literature, only few are found in the human GI tract, dominated by two phyla in particular (Firmicutes, Bacteroidetes), together with members of Actinobacteria, Verrucomicrobia, Proteobacteria, Fusobacteria and Cyanobacteria phyla (Sommer & Bäckhed, 2013). These autochthonous phyla colonize the GI tract and are present in a majority of individuals. The concept of ‘enterotypes’ has recently been proposed and, according to this, humans can be subdivided into Bacteroides, Prevotella or Ruminococcus types (Zoetendal et al. 2008; Arumugam et al. 2011). However, this categorization has recently become a matter of debate, and the term ‘enterogradients’ has been proposed instead, to describe bacterial communities with prevalence of Bacteroides or Prevotella (Jeffery et al. 2012). Microbes in the human gut undergo selective pressure from the host as well as from microbial competitors, and once the ecosystems reaches homeostasis, some species will occur in high and many in low abundance (Bäckhed, 2011; Nicholson et al. 2012).
Even though the gut microbiota differs greatly between subjects in membership and community structure, it still appears on the whole to be functionally equivalent and necessary for the proper development of the host. Mammals have co-evolved to exist with their gut microbiota largely in a mutualistic relationship; these organisms participate in the conversion of non-digestible carbohydrates (dietary fibre) to short-chain fatty acids, participate in bile acid metabolism, provide a barrier against pathogenic bacteria, and modulate the innate and adaptive immune systems (Nicholson et al. 2012). In turn, the host provides a unique, nutrient-rich niche at constant temperature (Sommer & Bäckhed, 2013).
Studies using germ-free animals have highlighted the importance of the gut microbiota in the maintenance of homeostasis. Germ-free animals have physiological and metabolic abnormalities compared with conventional animals, as well as an imbalanced immune system (Slack et al. 2009; Hapfelmeier et al. 2010; Geuking et al. 2011; Kunii et al. 2011; Hansen et al. 2012; Macpherson et al. 2012; Olszak et al. 2012). In addition, germ-free animals exhibit abnormal gastrointestinal motility (Abrams & Bishop, 1967; Gustafsson et al. 1970; Wostmann, 1981), increased expression of genes encoding transporters throughout the gut (Bäckhed, 2011) and altered perception of inflammatory pain (Amaral et al. 2008). Moreover, germ-free mice have an impaired capacity to harvest energy from the diet (Wostmann, 1981) and are protected against diet-induced obesity (Bäckhed et al. 2007; Rabot et al. 2010).
It is therefore not surprising that alterations in the composition of the normal gut microbiota (dysbiosis) are associated with a variety of GI disorders, such as inflammatory bowel diseases, irritable bowel syndrome and coeliac disease (Nadal et al. 2007; Collado et al. 2009; De Palma et al. 2010). Future work will have to determine whether a microbial signature for dysbiosis is associated with specific disease states. Nevertheless, sufficient data support the concept that changes in the microbiota may arise in adulthood as a consequence of disease, long-term dietary habits, antibiotics and medications. These changes may be short term or long term, depending on the duration of the trigger that induced them and the particular characteristics of the host. In contrast, factors that impact on the normal colonization process during early life, such as psychological stress, may exert long-term effects on the composition of the microbiota that will impact susceptibility to disease.
Microbiota–gut–brain axis
It is well known that the gut and the brain are in bidirectional communication. The concept of the gut–brain axis originated from the field of GI endocrinology and the discovery of hormonal regulation of digestion (Track, 1980). Since then, it has evolved to include the maintenance of homeostasis of several systems, including GI function, appetite and weight control (Collins & Bercik, 2009). Thus, it is only logical to consider and include the gut microbiota as an important modulator of this system and, consequently, the term ‘microbiota–gut–brain axis’ has emerged (Fig. 1; Bercik et al. 2009, 2011a).
Figure 1. The microbiota–gut–brain axis comprises the bidirectional communication, through multiple pathways, between the gut and the brain.
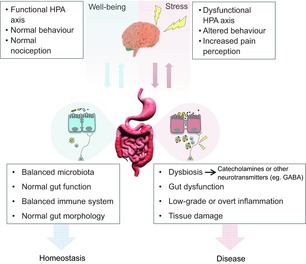
During stress, alterations at the level of the central nervous system can influence gut neuromotor and secretory function, immunity and microbiota composition. In turn, dysbiosis may contribute to perpetuate dysfunction and inflammation, further disrupting gut–brain communication. Some of these effects may be mediated by direct host–microbial interactions at the level of the intestinal epithelium, production of bacterial metabolites (cathecolamines, GABA, etc). The sequence of events can occur in a top-to-bottom or bottom-to-top fashion, but once initiated can perpetuate and exacerbate maladaptive responses that promote a state of disease. We acknowledge dreamdesign and cooldesign (http://FreeDigitalPhotos.net) for the image of the gut and brain, respectively.
The known beneficial effects of laxatives and oral antibiotics in patients with hepatic encephalopathy is perhaps one of the earliest pieces of evidence for a role of gut bacteria in brain function (Victor & Quigley, 2014). Antibiotics have also anecdotally been reported to induce acute psychosis that resolved after withdrawal of the drug (Sternbach & State, 1997; Mehdi, 2010). More recently, an abnormal composition of the microbiota has been associated with autism (Bolte, 1998; Finegold et al. 2010, 2012; Yap et al. 2010; Wang et al. 2011, 2012, 2013; Williams et al. 2011, 2012; De Angelis et al. 2013; Kang et al. 2013); treatment with antibiotics in patients with late-onset autism seems to improve their symptoms (Sandler et al. 2000; Finegold et al. 2012). Bacteroides fragilis, a Gram-negative anaerobic bacterium that inhabits the lower GI tract of most mammals (Ley et al. 2008), has been shown to ameliorate anxiety-like behavior, sensorimotor, communicative and repetitive behavior, but not sociability and social preference in an animal model of autism. The underlying mechanisms may involve modulation of gut microbiota composition and serum metabolomic profile (Hsiao et al. 2013). An association between major depressive disorder and altered gut metabolism has also been proposed (Ledochowski et al. 1998a,b, 2000, 2001; Ochoa-Repáraz et al. 2011).
It is difficult to interpret whether this is a chicken or egg situation, whether brain and behavioural alterations precede gut dysfunction and dysbiosis, or whether gut dysfunction and dysbiosis precede brain and behavioural changes. It has been reported that chronic depression is associated with altered microbial profiles and colonic motility in mice (Park et al. 2013). However, it has been also reported that chronic gastrointestinal inflammation can induce anxiety-like behaviour and alter central nervous system biochemistry (Bercik et al. 2010, 2011a). Therefore, it is likely that both situations coexist in a self-perpetuating loop, and that the initial trigger can arise centrally or in the periphery. Additional research is needed to solve this intriguing concept, and an interaction between clinical and basic research using gnotobiotic technology will probably help to provide mechanistic insight.
Stress and the microbiota–gut–brain axis
Stress is defined as an organism's total response to environmental demands or pressures. Several different types of stressors can be distinguished, such as acute or chronic, some of which may occur only once, while others are repetitive and can be anticipated. However, stress can be unpredictable and uncontrollable, mild or severe, and occur in or out of context (Lucassen et al. 2014). Moreover, the perception of stress is variable between individuals, and so is the persistence of its consequences (Lucassen et al. 2014). Exposure to stressors has long been known to increase susceptibility to disease, including GI disorders. Stress contributes to many disabilities worldwide and, as such, represents a severe economic burden.
Chronic and acute stress models are widely employed in GI research, because stress has been identified as a risk factor or modulator of the expression of several GI disorders (Collins, 2001; Söderholm & Perdue, 2001; Konturek et al. 2011). Tannock and Savage demonstrated, 40 years ago, that environmental and dietary stress markedly altered the gut microbiota in mice, affecting factors that regulate the localization and population levels of micro-organisms along the GI tract (Tannock & Savage, 1974), possibly favouring the establishment of pathogenic bacterial species (Tannock & Smith, 1972; Tannock & Savage, 1974). More recently, Bailey et al. (2011) demonstrated that exposure to a social disruption stressor affects the gut microbiota and circulating levels of cytokines, particularly interleukin-6 and monocyte chemotactic protein-1. In fact, social stress has been reported to increase the risk of inflammation-related diseases, promoting pro-inflammatory gene expression and monocyte differentiation (Powell et al. 2013). Thus, stressor-induced changes in the microbiota may enhance the ability of enteric pathogens (such as Citrobacter rodentium) to colonize the intestine (Bailey et al. 2010). Accordingly, it has been reported that acute and repeated stress affect levels of intestinal secretory IgA, impacting intestinal homeostasis and probably resulting in inflammation (Campos-Rodríguez et al. 2013). Altered levels of intestinal secretory IgA might cause shifts in commensals and possibly result in dysbiosis.
Psychological and physical stressors activate the HPA axis, resulting in the release of corticotrophin-releasing hormone, the principal regulator of the HPA axis, which is synthesized and secreted by hypophysiotrophic neurons localized in the medial parvocellular subdivision of the paraventricular nucleus (Smith & Vale, 2006). Corticotrophin-releasing hormone induces the release of adrenocorticotrophic hormone into the systemic circulation, which will, in turn, stimulate glucocorticoid synthesis in the adrenal cortex. Glucocorticoids, such as corticosterone or cortisol in humans, are the downstream effectors of the HPA axis, and their biological effects are usually adaptive (Smith & Vale, 2006). Together with glucocorticoids, catecholamines (noradrenaline and adrenaline) are also released into the circulatory system after psychological and physical stressors (Lyte et al. 2011), and it is well known that glucocorticoids can potentiate some of the actions of catecholamines (Sapolsky et al. 2000).
The gastrointestinal tract has long been known to be sensitive to stress and stress mediators, including catecholamines, but the notion that stress, and stress mediators, can influence the composition and function of the gut microbiota is a relatively new concept (Lyte et al. 2011). In fact, stress can influence the outcome of bacterial infection, because enteric bacteria can respond to the release of stress-related neurochemical mediators by the host (Lyte et al. 2011). Moreover, it has been hypothesized recently that bacteria act essentially as neuroactive compound delivery vehicles, affecting host physiology through the provision of neurochemicals. Specifically, the presence of a stress-related neuroendocrine hormone family of catecholamines has been demonstrated in bacteria (Lyte, 2011).
Today's conceptual framework of the most common entities in gastroenterology, the functional gastrointestinal disorders, such as irritable bowel syndrome and functional dyspepsia, involves the interaction of psychological factors and altered gut physiology via the gut–brain axis, where brain and gut symptoms are reciprocally influencing each other's expression. Psychological, sexual and/or physical abuse in early life has been suggested to play an important role in the pathogenesis of functional gastrointestinal disorders (Heitkemper et al. 2011; Wu, 2012; van Tilburg et al. 2013). This is a time of particular vulnerability, when neurological plasticity as well as establishment of a relatively stable gut microbiota occurs.
Maternal separation in rodents has been widely used as a model of early life stress that induces long-lasting hyperactivity of the HPA axis (Ladd et al. 2000; Barreau et al. 2004b; Daniels et al. 2004; Lippmann et al. 2007; Aisa et al. 2008; Gareau et al. 2008; Oines et al. 2012), anxiety-like behaviour (Varghese et al. 2006; Lippmann et al. 2007; Desbonnet et al. 2010; O'Mahony et al. 2011; Abelaira et al. 2013; Li et al. 2013), visceral hypersensitivity (Eutamene et al. 2007; O'Mahony et al. 2011; Moloney et al. 2012; Felice et al. 2014) and altered cholinergic activity in the gut (Gareau et al. 2007b; O'Malley et al. 2010) accompanied by increased intestinal permeability (Söderholm et al. 2002; Barreau et al. 2004a; García-Ródenas et al. 2006; Eutamene et al. 2007; Gareau et al. 2007b; Oines et al. 2012).
Maternally separated rats show also increased neuronal activation in response to a physical stressor, such as colorectal distension (Felice et al. 2014), probably due to central sensitization to noxious visceral stimuli (Chung et al. 2007), similar to what has been reported for irritable bowel syndrome patients (Tillisch & Labus, 2011; Tillisch et al. 2011; Larsson et al. 2012). Indeed, this model results in a dysfunctional gut–brain axis, mimicking many of the features found in irritable bowel syndrome patients; therefore, it has been widely employed to study the mechanisms behind the dysfunctional communication between the gut and the brain in irritable bowel syndrome (Barreau et al. 2007; Gareau et al. 2008; O'Mahony et al. 2009, 2011). Similar to irritable bowel syndrome (Ringel & Maharshak, 2013), in animal models these alterations at physiological and behavioural levels are often accompanied by altered gut colonization (García-Ródenas et al. 2006; O'Mahony et al. 2009; Barouei et al. 2012), and the use of probiotics appears to improve the detrimental effects of stress (García-Ródenas et al. 2006; Eutamene & Bueno, 2007; Eutamene et al. 2007; Gareau et al. 2007a; Desbonnet et al. 2010; Distrutti et al. 2013).
Our preliminary data show that gut microbiota is essential for the expression of anxiety-like behaviour and behavioural despair in mice, because maternally separated germ-free mice do not show different behaviour when compared with control germ-free mice (De Palma et al. 2012). However, we found that germ-free maternally separated mice have increased levels of basal serum corticosterone and altered cholinergic nerve function (De Palma et al. 2012), similar to previous studies in conventional specific pathogen-free animals (Gareau et al. 2006, 2007a,b; O'Malley et al. 2011), indicating that these alterations occur independently of the presence of gut microbiota.
Acetylcholine is the main excitatory neurotransmitter in the mammalian enteric nervous system and plays an important role in the control of gut motility (Olsson & Holmgren, 2011). Park et al. (2013) demonstrated that central administration of corticotrophin-releasing hormone induces changes in colonic motility in mice, accompanied by altered behaviour in the open field test. Thus, change in the HPA axis may contribute to the development of diverse pathologies; in this case, it altered autonomic control of gut motility (Park et al. 2013). We obtained similar results in germ-free mice subjected to maternal separation, demonstrating that alterations at the level of HPA axis activity disrupt colonic homeostasis and, in turn, alter the gut environment, in a microbiota-independent fashion.
Maternal separation also induces changes in the morphology of the colon of conventional specific pathogen-free maternally separated rats, with an increase in the numbers of goblet cells in the crypts of the proximal colon and a subsequent increase in secretion of mucus, with a thinner mucosal layer (O'Malley et al. 2010). It is therefore plausible that changes to the physiology (Söderholm et al. 2002; Gareau et al. 2007b; O'Malley et al. 2010; De Palma et al. 2012) and morphology (O'Malley et al. 2010) of the gut of maternally separated animals explain the reported changes in gut microbiota composition of maternally separated animals versus control animals (O'Mahony et al. 2009).
Altogether, these findings suggest that stress, whether acute or chronic, modulates the gut environment to select a dysbiotic microbiota, which in turn can induce anxiety and depression; however, the exact pathways and mediators of this effect are yet to be elucidated. Commensal bacteria might modulate brain biochemistry and behaviour through the production of specific metabolites (Lyte, 2011; Barrett et al. 2012a,b; Hsiao et al. 2013). It has been shown previously that commensal bacteria can modulate behaviour through the vagus nerve (Bercik et al. 2011b; Bravo et al. 2011), affecting neurotransmitter metabolism (Asano et al. 2012), or through alternative pathways, yet to be defined (Bercik et al. 2010, 2011a).
It is plausible to postulate that in the future the manipulation of gut microbiota, through probiotics or symbiotics, might be a valuable adjuvant to traditional medicine in the treatment of irritable bowel syndrome patients with co-morbid anxiety or depression.
Additional information
Competing interests
None declared.
References
- Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Rev Bras Psiquiatr. 2013;35(Suppl 2):S112–S120. doi: 10.1590/1516-4446-2013-1098. [DOI] [PubMed] [Google Scholar]
- Abrams GD, Bishop JE. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med. 1967;126:301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Effects of maternal separation on hypothalamic–pituitary–adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, Vieira LQ, Souza DG, Teixeira MM. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A. 2008;105:2193–2197. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le PD, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le RK, Maguin E, Mérieux A, Melo MR, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- Bäckhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58(Suppl 2):44–52. doi: 10.1159/000328042. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouei J, Moussavi M, Hodgson DM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One. 2012;7:e46051. doi: 10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004a;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004b;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr Res. 2007;62:240–245. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- Barrett E, Fitzgerald P, Dinan TG, Cryan JF, Ross RP, Quigley EM, Shanahan F, Kiely B, Fitzgerald GF, O'Toole PW, Stanton C. Bifidobacterium breve with α-linolenic acid and linoleic acid alters fatty acid metabolism in the maternal separation model of irritable bowel syndrome. PLoS One. 2012a;7:e48159. doi: 10.1371/journal.pone.0048159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012b;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011a;141:599–609. doi: 10.1053/j.gastro.2011.04.052. 609e.1–3. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol Motil. 2011b;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdú EF, Foster JA, Lu J, Scharringa A, Kean I, Wang L, Blennerhassett P, Collins SM. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am J Physiol Regul Integr Comp Physiol. 2009;296:R587–R594. doi: 10.1152/ajpregu.90752.2008. [DOI] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- Bolte ER. Autism and Clostridium tetani. Med Hypotheses. 1998;51:133–144. doi: 10.1016/s0306-9877(98)90107-4. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol. 2011;14:666–683. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- Campos-Rodríguez R, Godínez-Victoria M, Abarca-Rojano E, Pacheco-Yépez J, Reyna-Garfias H, Barbosa-Cabrera RE, Drago-Serrano ME. Stress modulates intestinal secretory immunoglobulin A. Front Integr Neurosci. 2013;7:86. doi: 10.3389/fnint.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res. 2007;1153:68–77. doi: 10.1016/j.brainres.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol. 2009;62:264–269. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- Collins SM. Stress and the gastrointestinal tract IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G315–G318. doi: 10.1152/ajpgi.2001.280.3.G315. [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. 2013;16:240–245. doi: 10.1016/j.mib.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome Med. 2013;5:63. doi: 10.1186/gm467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Blennerhassett P, Lu J, Park AJ, Philip V, Silva MA, Verdu EF, Collins SM, Bercik P. Su1990 The role of microbiota in the maternal separation model of depression. Gastroenterology. 2012;142(5S1):pS554. [Google Scholar]
- De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. doi: 10.1186/1471-2180-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Diaz–Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoSOne. 2013;8:e63893. doi: 10.1371/journal.pone.0063893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eutamene H, Bueno L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut. 2007;56:1495–1497. doi: 10.1136/gut.2007.124040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthésy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901–1907. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- Felice VD, Gibney SM, Gosselin RD, Dinan TG, O'Mahony SM, Cryan JF. Differential activation of the prefrontal cortex following psychological stress and colorectal distension in the maternally separated rat. Neuroscience. 2014;267:252–262. doi: 10.1016/j.neuroscience.2014.01.064. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA., 3rd Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. 2012;18:260–262. doi: 10.1016/j.anaerobe.2011.12.018. [DOI] [PubMed] [Google Scholar]
- García-Ródenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, Ornstein K, Rochat F, Corthésy-Theulaz I. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr. 2006;43:16–24. doi: 10.1097/01.mpg.0000226376.95623.9f. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007a;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007b;293:G198–G203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res. 2006;59:83–88. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8:274–281. doi: 10.2174/156652408784533760. [DOI] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Gustafsson BE, Midtvedt T, Strandberg K. Effects of microbial contamination on the cecum enlargement of germfree rats. Scand J Gastroenterol. 1970;5:309–314. [PubMed] [Google Scholar]
- Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, Tlaskalova-Hogenova H, Hansen AK. Patterns of early gut colonization shape future immune responses of the host. PLoS One. 2012;7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R, 3rd, McCoy KD, Macpherson AJ. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Cain KC, Burr RL, Jun SE, Jarrett ME. Is childhood abuse or neglect associated with symptom reports and physiological measures in women with irritable bowel syndrome. Biol Res Nurs. 2011;13:399–408. doi: 10.1177/1099800410393274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery IB, Claesson MJ, O'Toole PW, Shanahan F. Categorization of the gut microbiota: enterotypes or gradients. Nat Rev Microbiol. 2012;10:591–592. doi: 10.1038/nrmicro2859. [DOI] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591–599. [PubMed] [Google Scholar]
- Kunii J, Takahashi K, Kasakura K, Tsuda M, Nakano K, Hosono A, Kaminogawa S. Commensal bacteria promote migration of mast cells into the intestine. Immunobiology. 2011;216:692–697. doi: 10.1016/j.imbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lagier JC, Million M, Hugon P, Armougom F, Raoult D. Human gut microbiota: repertoire and variations. Front Cell Infect Microbiol. 2012;2:136. doi: 10.3389/fcimb.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MB, Tillisch K, Craig AD, Engstrom M, Labus J, Naliboff B, Lundberg P, Ström M, Mayer EA, Walter SA. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–472. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledochowski M, Sperner-Unterweger B, Fuchs D. Lactose malabsorption is associated with early signs of mental depression in females: a preliminary report. Dig Dis Sci. 1998a;43:2513–2517. doi: 10.1023/a:1026654820461. [DOI] [PubMed] [Google Scholar]
- Ledochowski M, Sperner-Unterweger B, Widner B, Fuchs D. Fructose malabsorption is associated with early signs of mental depression. Eur J Med Res. 1998b;3:295–298. [PubMed] [Google Scholar]
- Ledochowski M, Widner B, Murr C, Sperner-Unterweger B, Fuchs D. Fructose malabsorption is associated with decreased plasma tryptophan. Scand J Gastroenterol. 2001;36:367–371. doi: 10.1080/003655201300051135. [DOI] [PubMed] [Google Scholar]
- Ledochowski M, Widner B, Sperner-Unterweger B, Propst T, Vogel W, Fuchs D. Carbohydrate malabsorption syndromes and early signs of mental depression in females. Dig Dis Sci. 2000;45:1255–1259. doi: 10.1023/a:1005527230346. [DOI] [PubMed] [Google Scholar]
- Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xue X, Shao S, Shao F, Wang W. Cognitive, emotional and neurochemical effects of repeated maternal separation in adolescent rats. Brain Res. 2013;1518:82–90. doi: 10.1016/j.brainres.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G, Swaab DF, Czéh B. Neuropathology of stress. Acta Neuropathol. 2014;127:109–135. doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33:574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa–bacteria interactions. Cell Tissue Res. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Geuking MB, McCoy KD. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33:160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, Benno Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Savidge T, Shulman RJ. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi S. Antibiotic-induced psychosis: a link to d-alanine? Med Hypotheses. 2010;75:676–677. doi: 10.1016/j.mehy.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Minot S, Bryson A, Chehoud C, Wu GD, Lewis JD, Bushman FD. Rapid evolution of the human gut virome. Proc Natl Acad Sci U S A. 2013;110:12450–12455. doi: 10.1073/pnas.1300833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minot S, Sinha R, Chen J, Li H, Keilbaugh SA, Wu GD, Lewis JD, Bushman FD. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 2011;21:1616–1625. doi: 10.1101/gr.122705.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney RD, O'Leary OF, Felice D, Bettler B, Dinan TG, Cryan JF. Early-life stress induces visceral hypersensitivity in mice. Neurosci Lett. 2012;512:99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep. 2013;15:337. doi: 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007;56:1669–1674. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. e119. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Mielcarz DW, Begum-Haque S, Kasper LH. Gut, bugs, and brain: role of commensal bacteria in the control of central nervous system disease. Ann Neurol. 2011;69:240–247. doi: 10.1002/ana.22344. [DOI] [PubMed] [Google Scholar]
- Oines E, Murison R, Mrdalj J, Grønli J, Milde AM. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol Behav. 2012;105:1058–1066. doi: 10.1016/j.physbeh.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Olsson C, Holmgren S. Autonomic control of gut motility: a comparative view. Auton Neurosci. 2011;165:80–101. doi: 10.1016/j.autneu.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain–gut axis dysfunction. Psychopharmacology (Berl) 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG, Cryan JF. Altered expression and secretion of colonic interleukin-6 in a stress-sensitive animal model of brain-gut axis dysfunction. J Neuroimmunol. 2011;235:48–55. doi: 10.1016/j.jneuroim.2011.04.003. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Julio-Pieper M, Gibney SM, Dinan TG, Cryan JF. Distinct alterations in colonic morphology and physiology in two rat models of enhanced stress-induced anxiety and depression-like behaviour. Stress. 2010;13:114–122. doi: 10.3109/10253890903067418. [DOI] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- Rajilić-Stojanović M, Heilig HG, Tims S, Zoetendal EG, de Vos WM. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol. 2013;15:1146–1159. doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305:G529–G541. doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, Nelson MN, Wexler HM. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2001;280:G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Yates DA, Gareau MG, Yang PC, Macqueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- Sommer F, Bäckhed F. The gut microbiota – masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Sternbach H, State R. Antibiotics: neuropsychiatric effects and psychotropic interactions. Harv Rev Psychiatry. 1997;5:214–226. doi: 10.3109/10673229709000304. [DOI] [PubMed] [Google Scholar]
- Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock GW, Smith JMB. The effect of food and water deprivation (stress) on Salmonella-carrier mice. J Med Microbiol. 1972;5:283–289. doi: 10.1099/00222615-5-3-283. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Labus JS. Advances in imaging the brain–gut axis: functional gastrointestinal disorders. Gastroenterology. 2011;140:407–411. doi: 10.1053/j.gastro.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Track NS. The gastrointestinal endocrine system. Can Med Assoc J. 1980;122:287–292. [PMC free article] [PubMed] [Google Scholar]
- van Tilburg MA, Palsson OS, Whitehead WE. Which psychological factors exacerbate irritable bowel syndrome? Development of a comprehensive model. J Psychosom Res. 2013;74:486–492. doi: 10.1016/j.jpsychores.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese AK, Verdu EF, Bercik P, Khan WI, Blennerhassett PA, Szechtman H, Collins SM. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology. 2006;130:1743–1753. doi: 10.1053/j.gastro.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Victor DW, 3rd, Quigley EMM. Hepatic encephalopathy involves interactions among the microbiota, gut, brain. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.01.022. doi: 10.1016/j.cgh.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011;77:6718–6721. doi: 10.1128/AEM.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57:2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Buie T, Bauman ML, Cho PM, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoSOne. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3(1):e00261-11. doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostmann BS. The germfree animal in nutritional studies. Annu Rev Nutr. 1981;1:257–279. doi: 10.1146/annurev.nu.01.070181.001353. [DOI] [PubMed] [Google Scholar]
- Wu JC. Psychological co-morbidity in functional gastrointestinal disorders: epidemiology, mechanisms and management. J Neurogastroenterol Motil. 2012;18:13–18. doi: 10.5056/jnm.2012.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res. 2010;9:2996–3004. doi: 10.1021/pr901188e. [DOI] [PubMed] [Google Scholar]
- Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605–1615. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]


