Abstract
Abundant data indicate that pathogenesis in allergic airways disease is orchestrated by an aberrant T-helper 2 (Th2) inflammatory response. CD4+ T cells have been localized to airway smooth muscle (ASM) in both human asthmatics and in rodent models of allergic airways disease, where they have been implicated in proliferative responses of ASM. Whether CD4+ T cells also alter ASM contractility has not been addressed. We established an in vitro system to assess the ability of antigen-stimulated CD4+ T cells to modify contractile responses of the Brown Norway rat trachealis muscle. Our data demonstrated that the unloaded velocity of shortening (Vmax) of ASM was significantly increased upon 24 h co-incubation with antigen-stimulated CD4+ T cells, while stress did not change. Enhanced Vmax was dependent upon contact between the CD4+ T cells and the ASM and correlated with increased levels of the fast (+)insert smooth muscle myosin heavy chain isoform. The levels of myosin light chain kinase and myosin light chain phosphorylation were also increased within the muscle. The alterations in mechanics and in the levels of contractile proteins were transient, both declining to control levels after 48 h of co-incubation. More permanent alterations in muscle phenotype might be attainable when several inflammatory cells and mediators interact together or after repeated antigenic challenges. Further studies will await new tissue culture methodologies that preserve the muscle properties over longer periods of time. In conclusion, our data suggest that inflammatory cells promote ASM hypercontractility in airway hyper-responsiveness and asthma.
Key points
Activated CD4+ T cells enhance the contractility of airway smooth muscle.
In order to enhance contractility, contact between CD4+ T cells and smooth muscle is required.
The enhanced contractility is correlated with increased levels of fast myosin isoform.
Our data suggest that inflammatory cells promote airway smooth muscle hypercontractility in airway hyper-responsiveness and asthma.
Introduction
Asthma is an inflammatory disease of the airways characterized in part by airway hyper-responsiveness (AHR), an exaggerated response of airway smooth muscle (ASM) to contractile stimuli (Bateman et al. 2008). The contribution of T-helper 2 (Th2)-biased airway inflammation to pathogenesis in asthma has been the focus of abundant research and still receives a lot of attention (Lloyd & Hessel, 2010), whereas the role of ASM remains elusive (Ozier et al. 2011). It is generally agreed, however, that airway narrowing mediated by ASM contraction is the culminating point in asthma (Brusasco & Pellegrino, 2003; Bossé, 2012; Chin et al. 2012).
Chronic airway inflammation is thought to contribute to most clinical manifestations of AHR in asthma (Berend et al. 2008), presumably by enhancing ASM contractility. Supporting this notion is the fact that mast cells, T cells, eosinophils and macrophages are found within the ASM layers of human asthmatic subjects (Brightling et al. 2002; Begueret et al. 2007; Ramos-Barbon et al. 2010). Airway hyper-responsiveness was initially reported to be proportional to the degree of mast cell infiltration within the ASM layers (Brightling et al. 2002), but more recently, among inflammatory cells, T cells were the only cells found to increase significantly in the smooth muscle of asthmatic subjects (Begueret et al. 2007). The number of activated CD4+ T cells in the airway wall of mice with experimental asthma is also associated with AHR (Zosky et al. 2009). Moreover, asthmatic ASM cells express ligands that interact with and activate associated T cells (Lazaar et al. 1994; Burgess et al. 2005), supporting the notion that ASM also displays pro-inflammatory and immunomodulatory functions (Ozier et al. 2011).
Adoptively transferred T cells have also been found in apparent contact with ASM in Brown Norway (BN) rats with experimental asthma (Ramos-Barbon et al. 2005). Significantly, CD4+ T cells from sensitized BN rats adoptively transferred to naive rats induced an increase in ASM mass after antigen challenge, demonstrating an in vivo modulation of ASM function by CD4+ T cells (Ramos-Barbon et al. 2005). This interaction resulted in cross-activation and increased survival of both cell types (Ramos-Barbon et al. 2005) and is consistent with previous data from human cells, where T cell activation by human ASM cells increased the release of interleukin-13 (Veler et al. 2007) as well as other Th2 cytokines (Lloyd & Hessel, 2010). Together, these data provide evidence that smooth muscle cells can promote survival, activation and/or cytokine production from target T cells. However, the possibility that CD4+ T cells alter contractile protein expression and/or ASM function, either directly or via cytokine release, has not been assessed.
The aim of this study was to determine whether exposure of ASM cells to total splenocytes or purified CD4+ T cells, activated in vitro with antigen, altered contractile protein expression and ASM function. Thus, we investigated the mechanical properties and contractile protein expression of BN rat trachealis after incubation with ovalbumin (OVA)-stimulated total splenocytes or OVA-stimulated CD4+ T cells.
Methods
All procedures were approved by the McGill University Animal Care Committee and complied with the guidelines of the Canadian Council on Animal Care.
Harvesting and culture of splenocytes and isolation of CD4+ T cells
Male BN rats (ssNOlaHsd, Harlan UK Ltd, Shardlow, Derbyshire, England; 8–10 weeks old) were injected i.p. with 200 μg ml−1 OVA (grade V; Sigma-Aldrich, Oakville, Ontario, Canada) in Imject alum (Thermo-Scientific, Ottawa, Ontario, Canada). Two weeks later, the rats were killed by i.p. injection of 150 mg kg−1 of sodium pentobarbital. Spleens were harvested and placed in 50 ml centrifuge tubes containing sterile phosphate buffer solution (PBS). A single-cell suspension of total splenocytes was prepared after lysis of red blood cells with ammonium chloride–potassium buffer and cultured with OVA to induce re-activation and proliferation of the OVA-specific T cells generated in vivo by i.p. OVA administration. Cells were cultured at a concentration of 5 × 106 cells ml−1 for 4 days in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS), 100 units ml−1 interleukin-2, 100 μg ml−1 OVA and 1% penicillin–streptomycin. After 4 days of culture, the splenocytes were either harvested for co-culture with the ASM directly or further processed using EasySep negative selection kit (Stemcell Technologies, Vancouver, British Columbia, Canada) to purify CD4+ T cells. The purity of the CD4+ T cells was assessed by flow cytometry using a CD4 APC antibody (BD Pharmingen, San Diego, CA, USA) and was reliably >95% (Fig. 1A), consistent with images obtained using both bright field (Fig. 1B) and immunofluorescence microscopy using a CD4 Alexa488 antibody (Biolegend, San Diego, CA, USA; Fig. 1C).
Figure 1. CD4+ T cell purity.
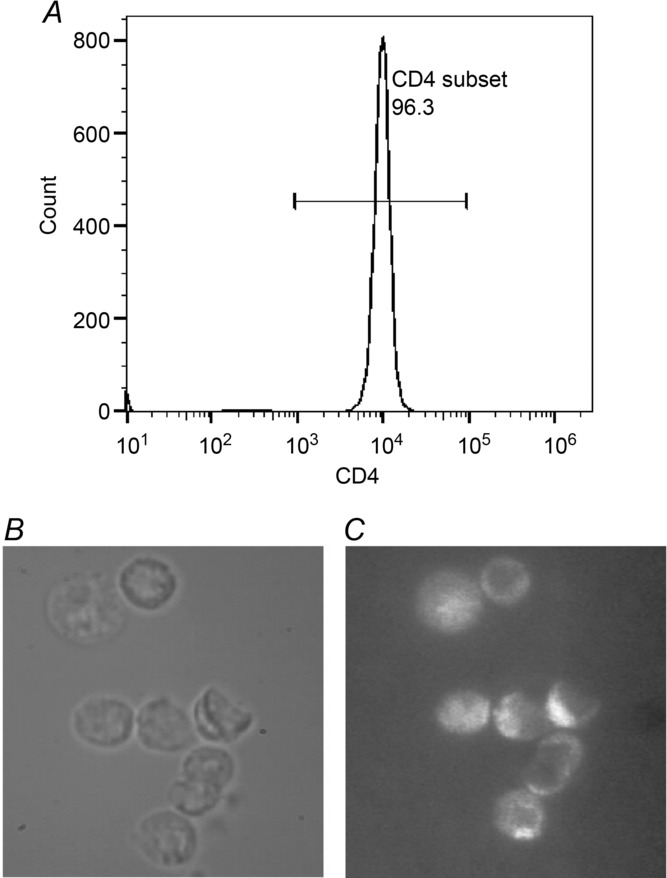
A, flow cytometric quantification of the purity of the CD4+ T cells (>95%) after magnetic selection. CD4+ T cells viewed by bright field imaging (B) and by fluorescence microscopy after treatment with CD4 Alexa488 antibody (C).
Harvesting of tracheae and incubation with inflammatory cells
Two weeks after an i.p. OVA injection, the BN rats were killed and the tracheae dissected and placed in calcium-free Krebs–Henseleit (KH) solution [composition (mm): 118 NaCl, 4.5 KCl, 2.5 MgSO4, 1.2 KH2PO4, 25.5 NaHCO3 and 10.0 glucose, pH 7.4; aerated with 95% O2–5% CO2 for at least 30 min]. Loose connective tissue was then removed and the tracheae were cut transversely into rings, each containing a single ring of cartilage and a strip of smooth muscle from the dorsal side of the trachea. The most caudal ring and the cranial third of each trachea were discarded, so that up to 10 rings were obtained from each trachea. The inner surface of each ring was gently rubbed with a small metal pin or cotton swabs to remove the epithelium.
Given that the ASM properties are known to vary along the length of the trachea (Florio et al. 1996), we standardized the sites from which the rings were obtained (lower part of trachea for the 24 h data points and middle part of trachea for the 48 h data points). Furthermore, adjacent rings were paired as test and control samples to minimize differences due to anatomical origin. After 24 or 48 h of incubation, the rings were either used for mechanics measurements or were snap-frozen in liquid N2 and stored at −80°C for Western blot analysis.
The rings were placed in 24-well cell culture plates (Costar; Corning, Tewksbury, MA, USA) that contained either splenocytes (5 × 106 cells ml−1) or CD4+ T cells (5 × 106 cells ml−1) in complete DMEM containing 0.5% FBS and 100 units ml−1 interleukin-2 + 1% penicillin–streptomycin (test) or in complete DMEM without cells (control) and incubated for 24 or 48 h at 37°C in humidified air containing 5% CO2. The cells in each well were lightly mixed by pipetting up and down several times before adding the tracheal rings. The concentration of cells used was based on the work of Ramos-Barbon et al. (2005), i.e. the total number of lung lymphocytes in naive BN rats adoptively transferred with CD4+ T cells and challenged with OVA ranged from 19 × 106 to 30 × 106 cells (see Fig. 1G of Ramos-Barbon et al. 2005).
Muscle to T-cell contact assay
In some experiments, 12-well Transwell plates (Corning Inc., New York, NY, USA) were used to test whether or not direct contact between ASM cells and T cells is important for the observed responses. The tracheal rings were placed in the bottom chamber in DMEM, while CD4+ T cells were placed in the upper chamber. The T cells were separated from the ASM by a membrane that had 0.4 μm pores, which would allow cytokines released from the CD4+ T cells to reach the ASM cells but prevent direct contact between ASM and T cells. The following three experimental conditions were tested: (1) control ASM incubated in medium alone; (2) ASM co-cultured in direct contact with CD4+ T cells; and (3) ASM co-cultured without direct contact with CD4+ T cells. After 24 h of incubation at 37°C in humidified air containing 5% CO2, the contractile properties of the tracheal rings were assessed.
Muscle strip mechanics measurements
The control and test rings from a matched pair were tested on the same day and in random order. Immediately before testing, the ring was taken from the culture medium and placed in oxygenated calcium-free KH solution for 15–30 min and then the cartilage was cut on both sides of the muscle to yield a muscle strip with ∼1–2 mm of cartilage at each end. T-shaped aluminum foil clips (Ford et al. 1977) were wrapped around the cartilage segments to attach the muscle horizontally in the experimental chamber (Bullimore et al. 2011) at one end to the length controller (model 322C-I; Aurora Scientific Inc., Aurora, Ontario, Canada) and at the other end to the force transducer (model 400A; Aurora Scientific Inc.). The muscle length and force were computer controlled (software# 600A; Aurora Scientific Inc.).
The muscle strips were mounted in calcium-free KH solution to suppress basal tone. Length was set to a reference length (Lref) defined as 1.25 × unstretched length. Defining Lref in this way gave more reproducible results in these small tissue strips than using the in situ length (Bullimore et al. 2011). Muscle strip length and width at the Lref were measured using a microscope eyepiece reticule or using a Hitachi Camera (KP-D20A/B) with ImageJ software imagej.nih.gov/ij/(IJ 1.46r; NIH, USA). After setting the muscle strip at the Lref, the KH solution was changed for one containing Ca2+ (2.5 mm CaCl2). The solution was stored in a reservoir, where it was aerated with 95% O2–5% CO2 and circulated through the experimental chamber at ∼3 ml min−1, except for the periods of methacholine (MCh) stimulation. The chamber temperature was maintained at ∼37°C by a water jacket.
The muscle strips were then equilibrated by stimulating every 10 min for at least 1 h with a KH solution containing KCl (final concentration 80 mm KCl), followed by two periods of supramaximal stimulation (2 min) with MCh (10−4 m, acetyl-β-methylcholine chloride; Sigma Chemical Co., Oakville, Ontario, Canada) separated by washout periods of 15–20 min. The force–velocity measurements were made during a third period of supramaximal MCh stimulation, starting the quick releases 2–5 min after the beginning of activation. Briefly, the shortening velocity at a given force was measured by clamping the force at that level and allowing the muscle strip to shorten for 110 ms. The strip was then stretched back to the Lref, and force was allowed to stabilize before the next force clamp. Force clamps to levels ranging between 5 and 80% isometric force were performed in random order to construct the force–velocity curve.
Phosphorylation analysis of regulatory light chain of smooth muscle myosin (LC20)
To verify whether or not the level of LC20 phosphorylation was altered by the presence of the CD4+ T cells, muscle strips (control and test) were mounted at the Lref in a vertical experimental set-up with a 10 g force transducer (World Precision Instruments, Sarasota, FL, USA), and equilibrated for 1 h in KH solution with Ca2+. The muscle strips were then contracted for 5 min with 10−5 m MCh, and then rapidly frozen using liquid nitrogen-prechilled clamps. The strips were immersed for 2 min in dry ice-cooled acetone containing 10% trichloroacetic acid (w/v) and 20 mm dithiothreitol (Sigma Chemical Co.), washed three times in acetone containing 10 mm dithiothreitol and then dried for 30 min at room temperature.
Western blot analysis
The contractile protein expression in the BN rat trachealis muscle exposed to the total splenocytes, CD4+ T cells or medium was examined by Western blot analysis. After 24 or 48 h of co-culture, the tracheal rings were collected, snap-frozen in liquid nitrogen and kept at −80°C until assayed. Proteins were extracted as described by Gil et al. (2006). Equal quantities, determined by a standard Bradford assay, were resolved on 4–20% SDS-PAGE gradient gels, after which the proteins were electroblotted onto Polyvinylidene flouride (PVDF) membranes (Bio-Rad, Mississauga, Ontario, Canada). The membranes were cut below the 75 kDa molecular marker and sections blocked at room temperature with either 5% non-fat dry milk or 2% BSA in Tris-buffered saline. The upper membranes were probed with the monoclonal antibody that recognizes myosin light chain kinase (MLCK; 1:500 dilution; Sigma-Aldrich) and the polyclonal antibody that recognizes all smooth muscle myosin heavy chain (SMMHC) isoforms (1:2000 dilution; Biomedical Technologies Inc., Stoughton, MA, USA) or the polyclonal antibody that specifically recognizes the (+)insert SMMHC isoform (1:200 dilution) and that was raised against the peptide sequence QGPSFAY (Antibody Resource for Neuroscience Research Core at the Montreal Neurological Institute, Canada). The bottom portions of the membranes were probed by the monoclonal mouse antibody that recognizes glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1000 dilution; Ambion, Burlington, Ontario, Canada), which was used as a loading control, and the monoclonal mouse antibody that recognizes smooth muscle α-actin (1:5000 dilution; Sigma-Aldrich). For detection, secondary antibodies were coupled with horseradish peroxidase (1:10,000 dilution; GE Healthcare, Baie-d’Urfé, Quebec, Canada) except for detection of the (+)insert SMMHC isoform, for which amplification was performed by using biotinylated secondary antibody (1:10,000 dilution; Dakocytomation, Burlington, Ontario, Canada) and streptavidin coupled to horseradish peroxidase (1:40,000 dilution; Thermo Fisher Scientific, Whitby, Ontario, Canada).
To assess the myosin LC20 phosphorylation level, the protein extraction and Phos-tag™ (Wako chemicals, Amagasaki, Japan) SDS-PAGE were performed according to a published protocol (Takeya et al. 2008) with minor modifications. Briefly, dry muscle strips were cut into small pieces and homogenized in SDS-gel sample buffer (4% SDS, 10% glycerol, 100 mm dithiothreitol and 65 mm Tris–HCl, pH 6.8) and 0.04% (w/v) Bromophenol Blue. The samples were incubated for 2 h at 26°C with continuous mixing at 400 r.p.m., sonicated for 3 min and heated at 98°C for 5 min. The proteins were resolved by Phos-tag™ gel electrophoresis and then transferred to PVDF membranes overnight (45 V) at 4°C. The membranes were fixed with 0.5% glutaraldehyde (Fisher Scientific) for 45 min, blocked with 0.3% I-Block (Tropix, New York, NY, USA) for 2 h at room temperature and the LC20 visualized by probing the membrane with polyclonal rabbit anti-LC20 antibody (Santa Cruz Biotechnology, Dallas, TX, USA). The LC20 phosphorylation is reported as the ratio of phosphorylated LC20 to total LC20.
Proteins were detected with enhanced chemiluminescence substrate (SuperSignal West Dura; Thermo Fisher Scientific) for all proteins except LC20, which was detected using Luminata Forte (Millipore, Billerica, MA, USA). Quantification was performed with a Fluorchem 8500 imaging system using AlphaEase software (Alpha Innotech, Santa Clara, CA, USA). Western blots from each independent experiment were performed in triplicate.
Data analysis
The muscle strip cross-sectional area was determined by multiplying the measured width by a thickness of 0.1 mm (Bullimore et al. 2011). Maximal stress-generating capacity was calculated by dividing the maximal force (Fmax) by cross-sectional area of ASM and reported in kPa. The velocity of shortening during the force-clamp experiments was determined by fitting a regression line to the relationship of length to time between 60 and 110 ms after the start of the clamp, and normalizing the slope of the line to Lref (length per second). The force during each force clamp was normalized to the isometric force immediately before the clamp. Hill's hyperbolic force–velocity equation (Hill, 1949) was fitted to these normalized forces and velocity data using a non-linear least-squares algorithm in Matlab (The Mathworks Inc., Natick, MA, USA). The intercept of the fitted curve with the velocity axis was taken to be the maximal shortening velocity (Vmax). Results of stress, Vmax, protein expression and phosphorylation levels are reported as means ± SEM, and ‘n’ refers to the number of animals. All data were analysed by Student's paired t test except for the contact assay, which was analysed by a one-way ANOVA with Bonferroni's multiple comparison test. Significance was considered for values of P < 0.05 and is shown in figures by asterisks.
Results
Muscle mechanics after co-culture of ASM with OVA-stimulated splenocytes
To assess whether or not antigen-stimulated inflammatory cells altered ASM mechanical properties, we co-cultured tracheal rings with and without OVA-stimulated splenocytes and measured the muscle strip Vmax and stress. No significant difference was found in Vmax between control and OVA-stimulated splenocytes at 24 h (0.22 ± 0.02 l vs. 0.24 ± 0.02 lengths s−1, respectively, P = 0.26) or 48 h (0.21 ± 0.04 l vs. 0.25 ± 0.03 lengths s−1, respectively, P = 0.23; Fig. 2A). Likewise, no differences were observed for muscle stress at 24 h (83.4 ± 5.6 vs. 99.4 ± 8.2 kPa, P = 0.095; control vs. splenocytes) or 48 h (70.0 ± 9.27 vs. 69.4 ± 10.1 kPa, P = 0.91; control vs. splenocytes; Fig. 2B). Representative data from a control muscle strip and from one incubated with OVA-stimulated splenocytes are presented in Fig. 2C.
Figure 2. Mechanical properties of airway smooth muscle (ASM) incubated with ovalbumin (OVA)-stimulated splenocytes.

A, unloaded velocity of shortening (Vmax) of ASM co-cultured in the absence (open columns) or in the presence of total splenocytes (filled columns) for 24 or 48 h (n = 5 rats per group). B, stress generated by ASM incubated in the absence (open squares) or in the presence of total splenocytes (filled columns) for 24 or 48 h (n = 6 rats per group). C, representative force–velocity curves of ASM co-cultured in the absence (open circles) or in the presence of total splenocytes (filled circles). F/Fmax: force normalized to the maximal force. The force–velocity relationships were accurately fitted by the Hill hyperbolic model, r2 > 0.96.
Western blot analysis of contractile proteins in ASM co-cultured with OVA-stimulated splenocytes
Contractile protein expression of ASM co-cultured with OVA-stimulated splenocytes for 24 h was also assessed. The level of MLCK was significantly increased in the splenocyte-exposed muscle strips compared with control muscle (0.19 ± 0.02 vs. 0.29 ± 0.03 a.u.; control vs. splenocytes, P = 0.034; Fig. 3A). There was no difference observed in the expression of the fast (+)insert SMMHC isoform (0.36 ± 0.03 vs. 0.36 ± 0.02 a.u., P = 0.84), total SMMHC (0.52 ± 0.03 vs. 0.51 ± 0.03 a.u., P = 0.96), α-smooth muscle actin (1.34± 0.03 vs. 1.36 ± 0.13 a.u., P = 0.92) or in the fast (+)insert SMMHC isoform to total SMMHC ratio (0.69 ± 0.03 vs. 0.70 ± 0.1, P = 0.89; Fig. 3B–E).
Figure 3. Western blot analysis of myosin light chain kinase (MLCK; A), 7-amino acid insert in N-terminal part of smooth muscle myosin [(+)insert SMMHC; B], total SMMHC isoform (C), α-smooth muscle actin (SM actin; D) and (+)insert to total SMMHC ratio (E) from ASM strips incubated in the absence (CTR; open columns) or in the presence of OVA-stimulated splenocytes (SPL; filled columns).
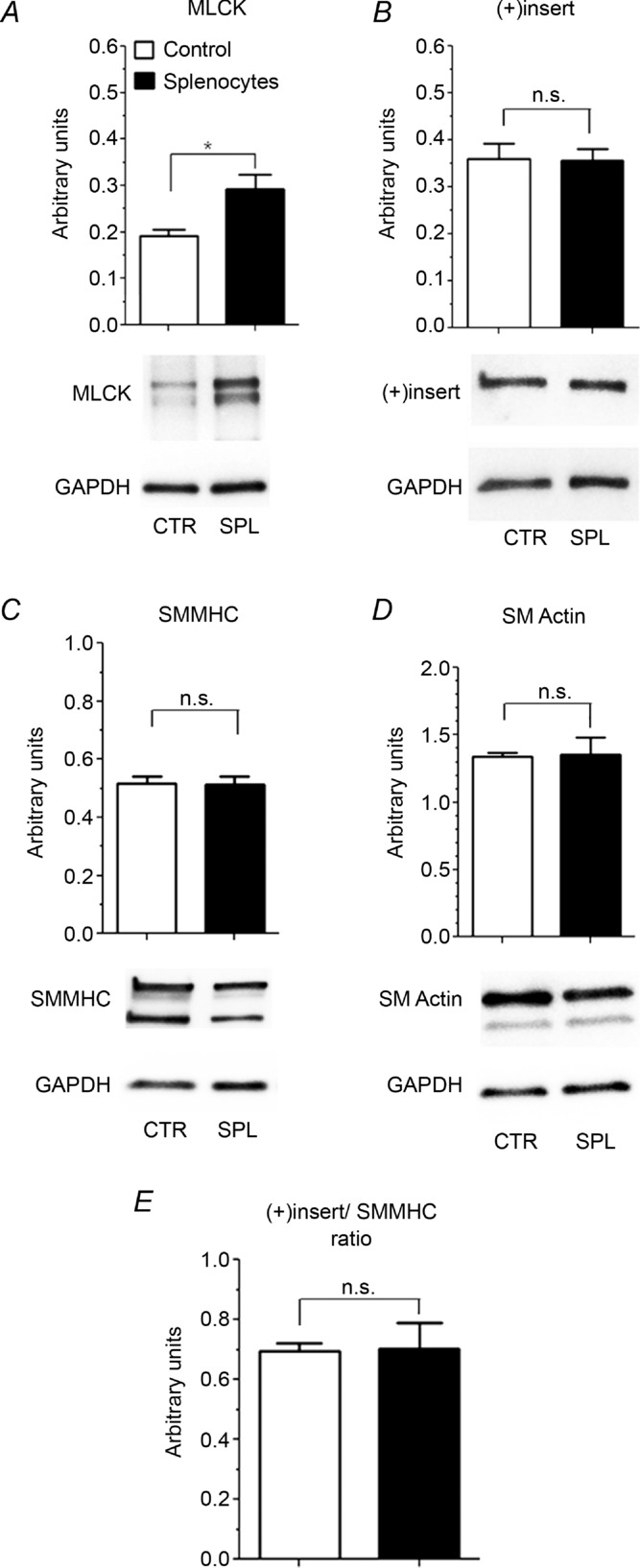
n = 3 rats per group. Representative Western blots are shown. *P < 0.05.
Mechanics of ASM after co-culture with OVA-stimulated CD4+ T cells
To evaluate more directly whether or not CD4+ T cells altered ASM mechanical properties, tracheal rings were co-cultured with or without OVA-stimulated CD4+ T cells, and the muscle strip Vmax and stress were then measured. In contrast to total splenocytes, after 24 h incubation with OVA-stimulated CD4+ T cells, the ASM contractile function was significantly increased in Vmax (0.15 ± 0.01 vs. 0.29 ± 0.02 lengths s−1; control vs. T cells, P = 0.0012; Fig. 4A). However, this alteration in Vmax was transient and disappeared after 48 h of co-incubation (0.21 ± 0.01 vs. 0.25 ± 0.01 lengths s−1, P = 0.099; Fig. 4A). No significant differences in stress were observed between control and CD4+ T cell-exposed muscle strips at 24 h (84.5 ± 13.8 vs. 107.8 ± 4.8 kPa; control vs. T cells, P = 0.1) or 48 h (99.6 ± 5.7 vs. 92.0 ± 5.2 kPa, P = 0.47; Fig. 4B). Representative data from a control muscle strip and from one incubated with OVA-stimulated CD4+ T cells are presented in Fig. 4C.
Figure 4. Mechanical properties of ASM incubated with OVA-stimulated CD4+ T cells.
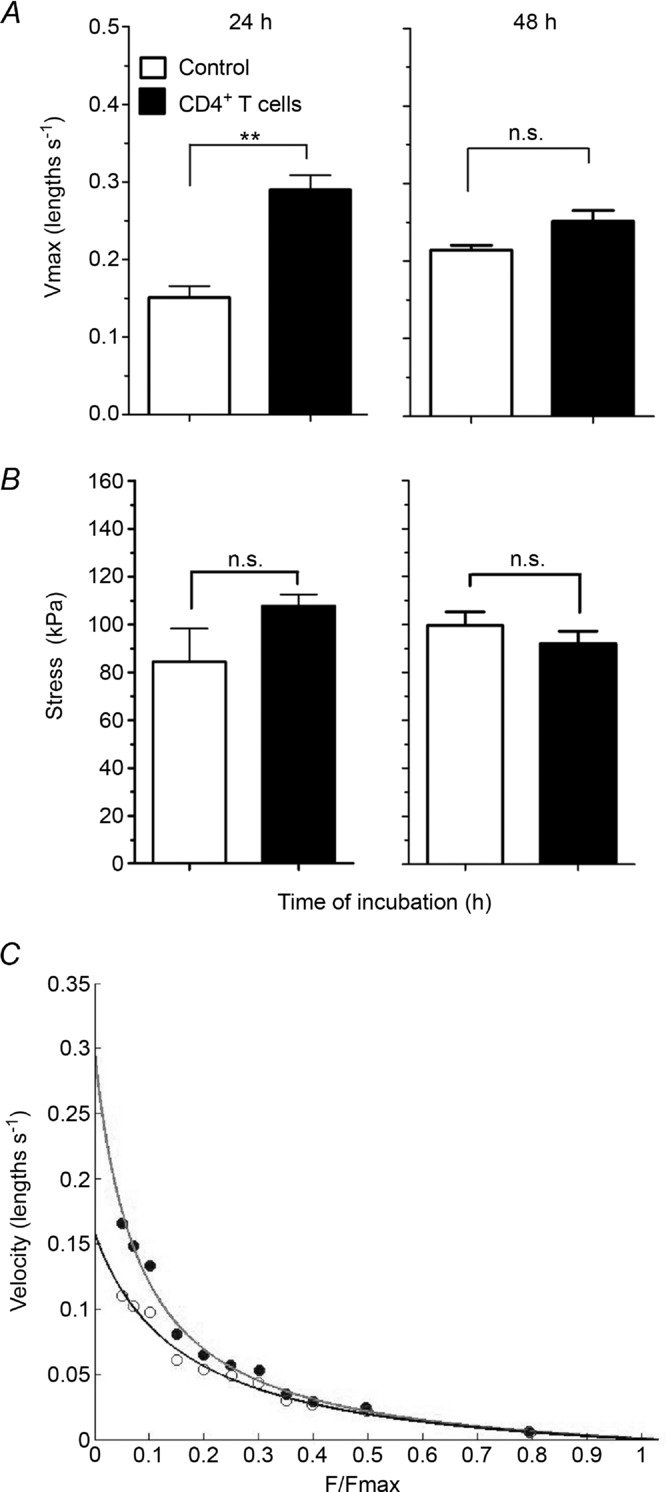
A, Vmax of ASM incubated in the absence (open columns) or presence of OVA-stimulated CD4+ T cells (filled columns) for 24 or 48 h. B, stress generated by ASM incubated in the absence (open columns) or presence of OVA-stimulated CD4+ T cells (filled columns) for 24 or 48 h. C, representative force–velocity curves of BN ASM co-incubated in the absence (open circles) or in the presence of CD4+ T cells (filled circles) for 24 h. F/Fmax: Force normalized to the maximal Force. Force –velocity relationships were accurately fitted by the Hill hyperbolic model, r2 > 0.96. n = 5 rats per group. *P < 0.05.
Western blot analysis of contractile protein expression in ASM co-cultured with OVA-stimulated CD4+ T cells
After 24 h incubation with OVA-stimulated CD4+ T cells, significant increases in levels of both MLCK (0.2 ± 0.03 vs. 0.41 ± 0.09 a.u.; control vs. T cells, P = 0.018; Fig. 5A) and the (+)insert myosin isoform (0.55 ± 0.04 vs. 0.81± 0.05 a.u.; control vs. T cells, P = 0.0023; Fig. 5B) were observed. There was no difference in total SMMHC (0.69 ± 0.04 vs. 0.70 ± 0.07 a.u.; control vs. T cells, P = 0.77; Fig. 5C) or of α-smooth muscle actin (1.2 ± 0.06 vs. 1.3 ± 0.1 a.u.; control vs. T cells, P = 0.36; Fig. 5D). The ratio of (+)insert to total SMMHC was, however, significantly higher in ASM co-cultured with OVA-stimulated CD4+ T cells (0.83 ± 0.11 vs. 1.18 ± 0.16; control vs. T cells, P = 0.024; Fig. 5E). These alterations in contractile protein levels were transient, because at 48 h, levels of MLCK (0.16 ± 0.02 vs. 0.13 ± 0.02 a.u.; control vs. T cells, P = 0.43; Fig. 5A) and (+)insert isoform (0.54 ± 0.003 vs. 0.51± 0.03 a.u.; control vs. T cells, P = 0.42; Fig. 5B) were not statistically different. Furthermore, no differences were observed at 48 h in total SMMHC (0.70 ± 0.07 vs. 0.66 ± 0.04 a.u.; control vs. T cells, P = 0.72; Fig. 5C), α-smooth muscle actin (1.34 ± 0.03 vs. 1.36 ± 0.05 a.u.; control vs. T cells, P = 0.83; Fig. 5D) and in the ratio of (+)insert to total SMMHC (0.79 ± 0.08 vs. 0.77 ± 0.05; control vs. T cells, P = 0.7; Fig. 5E).
Figure 5. Western blot analysis of MLCK (A; n = 7 rats per group for 24 h and n = 3 rats per group for 48 h), (+)insert SMMHC isoform (B; n = 6 rats per group for 24 h and n = 3 rats per group for 48 h), SMMHC (C; n = 7 rats per group for 24 h and n = 3 rats per group for 48 h), α-smooth muscle actin (D; n = 6 rats per group for 24 h and n = 3 rats per group for 48 h) and ratio of (+)insert SMMHC to total SMMHC (E; n = 6 rats per group for 24 h and n = 3 rats per group for 48 h) for ASM incubated in the absence (open columns) or in the presence of OVA-stimulated CD4+ T cells (filled columns).
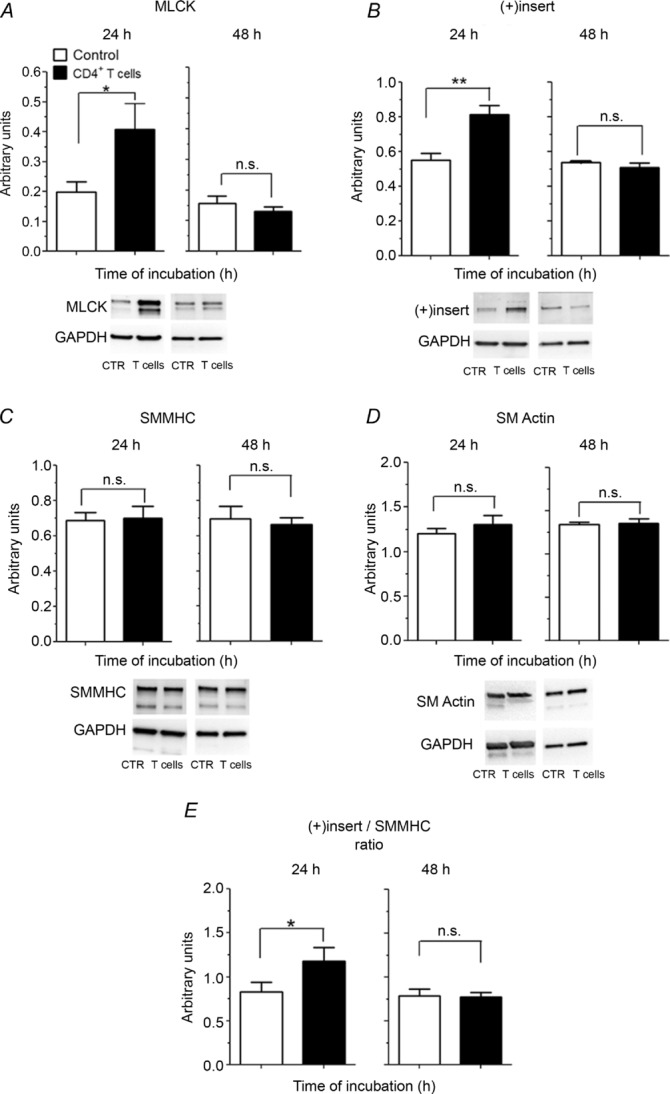
Representative Western blots are shown. *P < 0.05; **P < 0.01.
Effect of OVA-stimulated CD4+ T cell incubation on the phosphorylation level of LC20
Knowing that the incubation of ASM with OVA-stimulated CD4+ T cells for 24 h increased the expression levels of MLCK, we tested whether or not this led to a corresponding increase in myosin LC20 phosphorylation. Indeed, the LC20 phosphorylation level was significantly increased in muscle strips co-cultured with OVA-stimulated CD4+ T cells compared with control conditions (31.7% ± 4.1 vs. 40.5% ± 2.4; control vs. T cells, P = 0.022; Fig. 6).
Figure 6. Phosphorylation of myosin LC20.
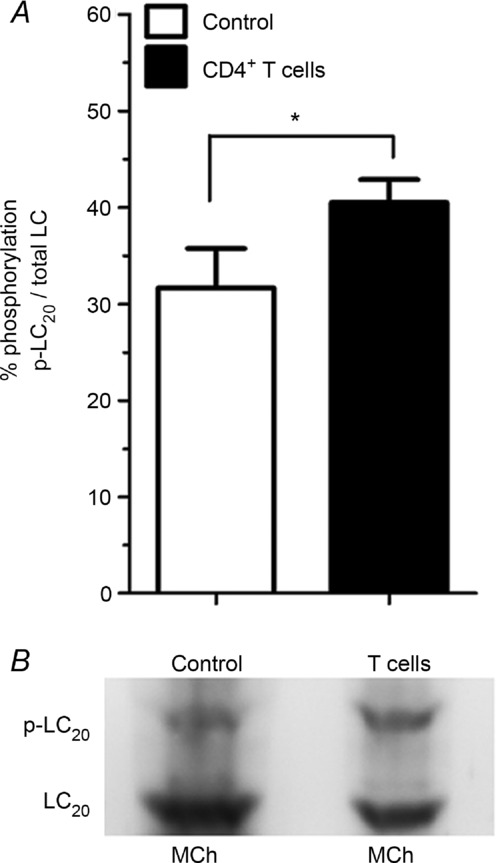
A, response to acetyl-β-methylcholine chloride (MCh) stimulation in ASM incubated for 24 h in the absence (open columns) or in the presence of OVA-stimulated CD4+ T cells (filled columns). B, a representative Western blot of phos-tag gel of phosphorylated (p-LC20) and total LC20 (LC20) after MCh stimulation of a muscle strip incubated in the absence (control) or in the presence of OVA-stimulated CD4+ T cells (T cells). *P < 0.05. n = 6 rats per group.
Mechanics of ASM co-cultured with or without contact with OVA-stimulated CD4+ T cells
To assess whether or not direct contact between OVA-stimulated CD4+ T cells and the ASM was required to enhance muscle mechanics, CD4+ T cells and ASM were co-incubated in a Transwell chamber, separated by a permeable membrane, that allows transfer of small molecules (such as cytokines) but not cells. For ASM that was in contact with co-cultured CD4+ T cells, a significant increase in Vmax was observed (0.31 ± 0.02 lengths s−1) compared with that obtained when tracheal rings were co-cultured with CD4+ T cells in the absence of contact (0.25 ± 0.01 lengths s−1) or compared with tracheal rings incubated in medium alone (0.24 ± 0.01 lengths s−1; P = 0.01; Fig. 7A). Again, stress did not change significantly between the ASM co-cultured with the OVA-stimulated CD4+ T cells in direct contact (85.8 ± 7.7 kPa), without contact (95.2 ± 3.4 kPa) or incubated in medium alone (81.4 ± 7.2 kPa; P = 0.33; Fig. 7B).
Figure 7. Mechanical effect of contact between OVA-stimulated CD4+ T cells and ASM strips.
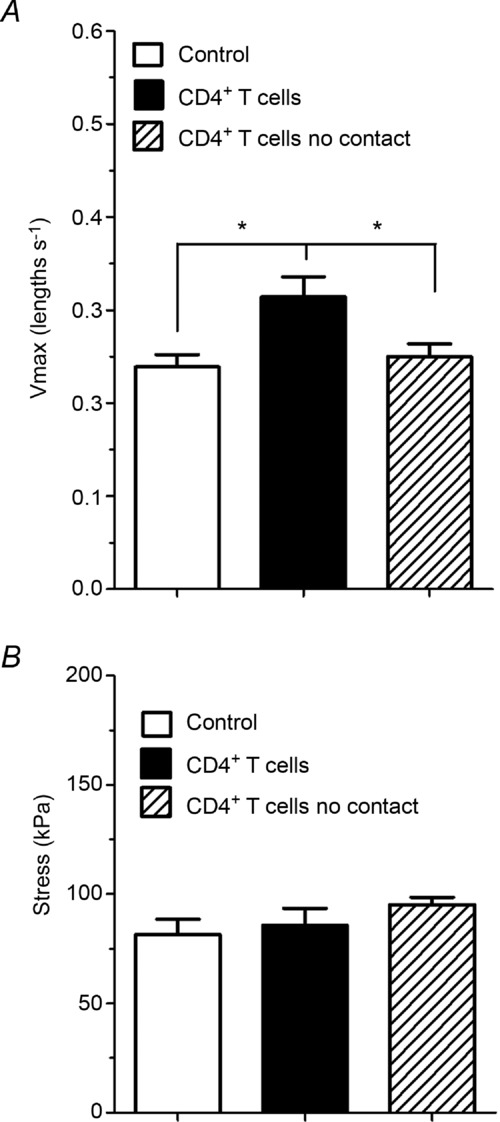
The Vmax (A) and stress (B) measured for ASM strips incubated for 24 h in the absence of CD4+ T cells (open columns) or in the presence of CD4+ T cells either in contact (filled columns) or in Transwells (hatched columns). n = 7 rats per group. *P < 0.05.
Discussion
The major finding of this study is that incubation of BN rat tracheal rings with OVA-stimulated CD4+ T cells enhanced the unloaded Vmax. This increase in Vmax was accompanied by increased levels of the (+)insert SMMHC isoform and MLCK. Furthermore, these changes in mechanics were dependent on contact between the CD4+ T cells and the ASM cells.
As seen in the literature, it has been difficult to define the precise role of ASM in AHR and asthma. A number of studies have reported increases in Vmax in ASM strips from animals with experimentally induced or innate AHR (Antonissen et al. 1979; Jiang et al. 1992b; Fan et al. 1997; Gil et al. 2006). An increase in Vmax was also reported in human asthmatic ASM cells (Ma et al. 2002) and bronchial rings sensitized with IgE from asthmatic subjects (Mitchell et al. 1994), while others did not observe such differences (Chin et al. 2012). An increase in force-generating capacity has also been reported in mice (Chiba et al. 2009) and rabbit ASM bundles (Grunstein et al. 2002). However, the literature on human ASM force-generating capacity is controversial. The force normalized to mass generated by asthmatic ASM strips from the second to the fourth airway generations has been reported to be greater than that of non-asthmatic ASM by Thomson et al. (1996), whereas Chin et al. (2012) did not show any difference in tracheal ASM stress. Taken together, these studies suggest that ASM might be hypercontractile in AHR and asthma, but which mechanical property or properties is/are altered and/or which airways are affected have remained elusive. Irrespective of these issues, the question addressed in the present study is: can inflammation, as encountered in asthma, lead to ASM hypercontractility and, if so, by which mechanism?
Twenty-four hours of incubation of tracheal rings with purified OVA-stimulated CD4+ T cells led to significant increases in Vmax (Fig. 4A) and in levels of MLCK (Fig. 5A) and the (+)insert SMMHC isoform (Fig. 5B). Myosin light chain kinase is the enzyme responsible for myosin activation by phosphorylation of myosin light chain, LC20 (Small & Sobieszek, 1977), whereas myosin light chain phosphatase deactivates it (Sobieszek et al. 1997). Thus, LC20 phosphorylation levels depend on the amount and activity of MLCK and myosin light chain phosphatase. The interaction between all these factors is complex (Jiang et al. 1992a, 1995). Nevertheless, in our rat tissues, exposure to OVA-stimulated CD4+ T cells resulted in a significant increase in myosin LC20 phosphorylation levels (Fig. 6). A direct relationship between Vmax and LC20 phosphorylation has, however, not been established (Moreland et al. 1986; Miller-Hance & Kamm, 1991; Gunst et al. 1994), but an increase in force (or stress) would have been expected (Silver & Stull, 1982). While there was a trend towards an increase in stress, it did not reach statistical significance (Fig. 4B). Importantly, the ragweed-sensitized canine model of AHR (Becker et al. 1989) also showed increases in MLCK levels, but not in activity (Jiang et al. 1992b), and increases in LC20 phosphorylation levels and in Vmax, but no changes in stress (Jiang et al. 1992a, 1995). Thus, our in vitro experiments of muscle stimulation by inflammatory cells produced data consistent with this whole-animal model of allergen sensitization (Becker et al. 1989).
The expression of MLCK in asthma has been studied extensively. Some studies showed that its expression was not altered in asthma (Woodruff 2008; Matsumoto et al. 2007), whereas others showed an increase at the mRNA level (Ma et al. 2002; Léguillette et al. 2009) and at the protein level using immunoblotting (Ammit et al. 2000) and immunohistochemistry (Benayoun et al. 2003). Benayoun et al. (2003) even reported a positive correlation between MLCK content and asthma severity, whereas the expression pattern of α-actin and total SMMHC was similar in asthmatic and non-asthmatic subjects. We also report a similar content in α-actin and total SMMHC between OVA-activated, T-cell-exposed and control ASM strips (Fig. 5C and D), suggesting that there is no change in muscle mass. The fact that there is no change in total myosin normalized to the total protein loaded (Fig. 5C) may contribute to the fact that we did not observe a significant increase in stress (Fig. 4B).
Incubation of the ASM strips with the OVA-activated CD4+ T cells also led to an increase in the (+)insert SMMHC isoform. In mammalian smooth muscle, four SMMHC isoforms are generated by alternative mRNA splicing. Two of these isoforms (White et al. 1993) differ in the N-terminus by the presence or absence of a 7-amino acid insert near the ATPase site. They are referred to as the (+)insert or SMB and the (−)insert or SMA isoforms, respectively. The (+)insert isoform is preferentially expressed in phasic, rapidly contracting smooth muscle, whereas the (−)insert isoform is found predominantly in tonic, slowly contracting smooth muscle (Kelley et al. 1993). Both isoforms are found in ASM, but the expression of the (+)insert isoform has been shown to be greater in the trachea of an animal model of innate AHR (mRNA and protein levels; Gil et al. 2006) as well as in asthmatic endobronchial biopsies (mRNA level studied only; Leguillette et al. 2009). The (+)insert SMMHC isoform has a 2-fold greater ATPase rate (Kelley & Adelstein, 1994) and velocity of propulsion of actin in in vitro motility assays compared with the (−)insert isoform (Rovner et al. 1997; Lauzon et al. 1998). Thus, the (+)insert SMMHC isoform leads to a greater velocity of shortening. Its overexpression after exposure of the rat trachealis to the CD4+ T cells is likely to play a major role in the increase in Vmax measured.
Incubation of the ASM strips with the CD4+ T cells led to an increase in MLCK (Fig. 5A), (+)insert SMMHC isoform (Fig. 5B) and Vmax (Fig. 4A), whereas incubation with total splenocytes increased only MLCK levels (Fig. 3A) and did not alter the mechanics (Fig. 2). The CD4+ T cells comprise ∼25% of the total splenocyte population after 4 days of incubation with OVA and thus, it is possible that total splenocytes did not contain a large enough number of CD4+ T cells to alter the contractile protein expression significantly and thus, increase Vmax. Alternatively, the presence of other cells, such as CD8+ cells or B cells, might have suppressed the effect of the CD4+ T cells (Mishima et al. 1998).
In order to understand better the mechanism by which CD4+ T cells altered ASM contractility, we examined whether the observed effects required contact between the ASM and the CD4+ T cells or simply involved the release of mediators. Our results (Fig. 7) clearly demonstrate that contact is necessary to alter Vmax and suggest that CD4+ T cell cytokines alone are not sufficient in the absence of direct contact. These interactions between T cells and ASM may be mediated by the intercellular cell adhesion molecule 1, vascular cell adhesion molecule 1 or CD40 and OX40 ligand, all of which are expressed by ASM cells, and by CD44, lymphocyte function-associated antigen 1 and very late antigen 4 expressed by the T cells (Lazaar et al. 1994; Burgess et al. 2005; Al Heialy et al. 2013). Further studies will be required to examine the contribution of these (and potentially other) molecules to the enhanced contractile responses we have observed.
The changes in mechanics (increased Vmax) observed after 24 h of incubation with the CD4+ T cells were only transient (Fig. 4); differences in Vmax between control and the CD4+ T cells were no longer present after 48 h of co-incubation (Fig. 4A). Again, these results were correlated with the changes in levels of the contractile proteins; that is, both MLCK (Fig. 5A) and the (+)insert SMMHC isoform (Fig. 5B) had returned to control levels after 48 h of exposure to the CD4+ T cells. Several factors may have contributed to the transient aspect of the responses of our muscle strips to the CD4+ T cells. Firstly, models of allergic asthma usually require multiple allergen exposures (Becker et al. 1989). Our muscle strips were exposed only once to the CD4+ T cells because of technical limitations of in vitro tissue studies; that is, in preliminary experiments we cultured ASM strips for longer periods of time and observed changes in mechanics after 72 h of incubation even in the absence of inflammatory cells. The most likely explanation for these alterations is the presence of DMEM containing 0.5% FBS, a necessary component of the culture medium for the survival of the tissues (Hsieh & Farley, 2002). Indeed, Hsieh & Farley (2002) showed that swine tracheal muscle strips cultured in DMEM with serum concentrations from 1 to 10% exhibited a decreased M2 muscarinic expression after 48 h of culture. Given that the M2 receptors limit the release of acetylcholine, a decrease in their expression due to the presence of FBS leads to increases in ASM contractility (Fryer et al. 1999). Our FBS concentration was lower (0.5%), so this effect may have taken longer to come into play. Thus, to limit the artifacts due to tissue culture per se, we performed our measurements over a short period of time and so did not address the effect of repeated stimulation. Another possible reason that would explain why our responses were transient is the fact that we studied the CD4+ T cells in isolation from other inflammatory cells. The CD4+ T-cell-induced AHR is associated with eosinophilia in the BN rat (Mishima et al. 1998). A direct interaction between activated human CD4+ T cells and eosinophils occurs through the intercellular cell adhesion molecule 3 receptor (Douglas et al. 2000). Moreover, cytokines released by human CD4+ T cells regulate the priming, activation and survival of eosinophils (Rothenberg et al. 1988). Thus, interactions between these two cell types might be necessary for long-term effects. In addition, Th2-cytokines (interleukin-4 and interleukin-5) are known to prime human mast cells (Ochi et al. 2000), and the mast cells can interact with ASM through CD44 and CD51 receptors (Girodet et al. 2010). Thus, a whole array of interactions between inflammatory cells is likely to be necessary to orchestrate the ASM mechanical alterations and their maintenance.
Contrary to what might have been expected, the mechanical parameter that was readily affected by the CD4+ T cells was Vmax and not stress. As mentioned above, this was also the case in the canine ragweed allergic model of AHR (Jiang et al. 1992a, 1995). Intuitively, one might expect that asthmatic airways would have stronger ASM, thus leading to a greater bronchoconstriction upon simulation. This has not been demonstrated convincingly in asthmatic tissues and remains a topic under debate (Thomson et al. 1996; Chin et al. 2012). Nonetheless, it is conceivable that faster ASM would also lead to greater airway resistance by counteracting the relaxing effect of tidal breathing (Gunst, 1983; Solway & Fredberg, 1997). Indeed, ASM stretching by tidal or deep breaths decreases airway resistance in normal lungs but not in asthmatic patients (Fish et al. 1981; Skloot et al. 1995). Also, imaging techniques have demonstrated that the airways of asthmatic patients dilate upon stretching but that this is brief because they quickly narrow back to their initial diameter (Brown et al. 2001). This suggests that asthmatic ASM can shorten faster than normal ASM after a stretch. This rapid recontraction might maintain asthmatic airways in a more constricted state on average because the ASM has time to shorten significantly between each breath, thus counteracting the relaxing effect of tidal breaths. Despite this, a number of animal model studies have reported that incubation of ASM with Th2 cytokines enhances their isometric force and impairs their relaxation (Hakonarson et al. 1999; Grunstein et al. 2002; Whelan et al. 2004; Chiba et al. 2009). None of those studies addressed the velocity of shortening. As noted above, the stress that we reported in Fig. 4B showed only a tendency to increase after incubation with the CD4+ T cells but did not reach statistical significance. It is likely that the concentrations of cytokines used in the other studies were higher than those obtained from the CD4+ T cells used in our experiments. Furthermore, we report stress (force per cross-sectional area; Chin et al. 2010), whereas these other studies reported force per gram of muscle, which can be erroneously affected by muscle length (Mehta et al. 1996).
In conclusion, our data demonstrate that contact between CD4+ T cells and ASM enhances the mechanics of ASM, but in a transient manner. More work will be required to delineate whether repeated challenges and/or interactions between several inflammatory cell candidates and their cytokines can lead to more permanent alterations in ASM mechanical properties, as observed in asthma.
Acknowledgments
We thank H. N. Roman for his help with bright field imaging and fluorescence microscopy of CD4+ T cells and C. McCusker for useful discussions. The polyclonal antibody recognizing the (+)insert SMMHC isoform was produced by the Antibody Resource for Neuroscience Research Core at the Montreal Neurological Institute. The Antibody Core is Directed by Dr Peter S. McPherson, James McGill Professor.
Glossary
- AHR
airway hyper-responsiveness
- ASM
airway smooth muscle
- BN
Brown Norway
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- KH solution
Krebs–Henseleit solution
- LC20
regulatory light chain of smooth muscle myosin
- Lref
reference length
- MCh
acetyl-β-methylcholine chloride
- MLCK
myosin light chain kinase
- OVA
ovalbumin
- SMMHC
smooth muscle myosin heavy chain
- Th2
T-helper 2
- Vmax
unloaded velocity of shortening; (+)insert fast SMMHC isoform, 7-amino acid insert in N-terminal part of smooth muscle myosin
Additional information
Competing interests
None declared.
Author contributions
O.S.M., E.M.N. and L.K. performed the experiments; O.S.M., E.D.F. and A.-M.L. wrote the manuscript; all co-authors were involved in the design of the experiments, the analysis and interpretation of the data and approval of the final version of the manuscript.
Funding
This work was supported by the Canadian Institute of Health Research (CIHR), the National Heart, Lung, and Blood Institute (NHLBI) grant RO1-HL 103405-02, and the Costello Fund. The Meakins-Christie Laboratories (McGill University Health Centre Research Institute) are supported in part by a centre grant from Le Fonds de la Recherche en Santé du Québec (FRSQ). O.S.M. is also partly supported by the Russian Foundation for Basic Research (12-04-33076).
References
- Al Heialy S, Risse PA, Zeroual MA, Roman HN, Tsuchiya K, Siddiqui S, Laporte SA, Martin JG. T cell-induced airway smooth muscle cell proliferation via the epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2013;49:563–570. doi: 10.1165/rcmb.2012-0356OC. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. Am J Respir Crit Care Med. 2000;161:257–263. doi: 10.1164/ajrccm.161.1.9901005. [DOI] [PubMed] [Google Scholar]
- Antonissen LA, Mitchell RW, Kroeger EA, Kepron W, Tse KS, Stephens NL. Mechanical alterations of airway smooth muscle in a canine asthmatic model. J Appl Physiol. 1979;46:681–687. doi: 10.1152/jappl.1979.46.4.681. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- Becker AB, Hershkovich J, Simons FER, Simons KJ, Lilley MK, Kepron MW. Development of chronic airway hyperresponsiveness in ragweed-sensitized dogs. J Appl Physiol. 1989;66:2691–2697. doi: 10.1152/jappl.1989.66.6.2691. [DOI] [PubMed] [Google Scholar]
- Begueret H, Berger P, Vernejoux JM, Dubuisson L, Marthan R, Tunon-de-Lara JM. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62:8–15. doi: 10.1136/thx.2006.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- Berend N, Salome CM, King GG. Mechanisms of airway hyperresponsiveness in asthma. Respirology. 2008;13:624–631. doi: 10.1111/j.1440-1843.2008.01330.x. [DOI] [PubMed] [Google Scholar]
- Bossé Y. Asthmatic airway hyperresponsivness: the ants in the tree. Trends Mol Med. 2012;18:627–633. doi: 10.1016/j.molmed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med. 2001;163:994–1001. doi: 10.1164/ajrccm.163.4.2007119. [DOI] [PubMed] [Google Scholar]
- Brusasco V, Pellegrino R. Complexity of factors modulating airway narrowing in vivo: relevance to assessment of airway hyperresponsiveness. J Appl Physiol. 2003;95:1305–1313. doi: 10.1152/japplphysiol.00001.2003. [DOI] [PubMed] [Google Scholar]
- Bullimore SR, Siddiqui S, Donovan GM, Martin JG, Sneyd J, Bates JH, Lauzon AM. Could an increase in airway smooth muscle shortening velocity cause airway hyperresponsiveness? Am J Physiol Lung Cell Mol Physiol. 2011;300:L121–L131. doi: 10.1152/ajplung.00228.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JK, Blake AE, Boustany S, Johnson PR, Armour CL, Black JL, Hunt NH, Hughes JM. CD40 and OX40 ligand are increased on stimulated asthmatic airway smooth muscle. J Allergy Clin Immunol. 2005;115:302–308. doi: 10.1016/j.jaci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M. Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol. 2009;40:159–167. doi: 10.1165/rcmb.2008-0162OC. [DOI] [PubMed] [Google Scholar]
- Chin LY, Bossé Y, Jiao Y, Solomon D, Hackett TL, Paré PD, Seow CY. Human airway smooth muscle is structurally and mechanically similar to that of other species. Eur Respir J. 2010;36:170–177. doi: 10.1183/09031936.00136709. [DOI] [PubMed] [Google Scholar]
- Chin LY, Bossé Y, Pascoe C, Hackett TL, Seow CY, Paré PD. Mechanical properties of asthmatic airway smooth muscle. Eur Respir J. 2012;40:45–54. doi: 10.1183/09031936.00065411. [DOI] [PubMed] [Google Scholar]
- Douglas IS, Leff AR, Sperling AI. CD4+ T cell and eosinophil adhesion is mediated by specific ICAM-3 ligation and results in eosinophil activation. J Immunol. 2000;164:3385–3391. doi: 10.4049/jimmunol.164.6.3385. [DOI] [PubMed] [Google Scholar]
- Fan T, Yang M, Halayko A, Mohapatra SS, Stephens NL. Airway responsiveness in two inbred strains of mouse disparate in IgE and IL-4 production. Am J Respir Cell Mol Biol. 1997;17:156–163. doi: 10.1165/ajrcmb.17.2.2628. [DOI] [PubMed] [Google Scholar]
- Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. J Appl Physiol. 1981;50:1079–1086. doi: 10.1152/jappl.1981.50.5.1079. [DOI] [PubMed] [Google Scholar]
- Florio C, Styhler A, Heisler S, Martin JG. Mechanical responses of tracheal tissue in vitro: dependence on the tissue preparation employed and relationship to smooth muscle content. Pulm Pharmacol. 1996;9:157–166. doi: 10.1006/pulp.1996.0018. [DOI] [PubMed] [Google Scholar]
- Ford LE, Huxley AF, Simmons RM. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977;269:441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer AD, Adamko DJ, Yost BL, Jacoby DB. Effects of inflammatory cells on neuronal M2 muscarinic receptor function in the lung. Life Sci. 1999;64:449–455. doi: 10.1016/s0024-3205(98)00587-6. [DOI] [PubMed] [Google Scholar]
- Gil FR, Zitouni NB, Azoulay E, Maghni K, Lauzon AM. Smooth muscle myosin isoform expression and LC20 phosphorylation in innate rat airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2006;291:L932–L940. doi: 10.1152/ajplung.00339.2004. [DOI] [PubMed] [Google Scholar]
- Girodet P-O, Ozier A, Trian T, Begueret H, Ousova O, Vernejoux JM, Chanez P, Marthan R, Berger P, Tunon de Lara JM. Mast cell adhesion to bronchial smooth muscle in asthma specifically depends on CD51 and CD44 variant 6. Allergy. 2010;65:1004–1012. doi: 10.1111/j.1398-9995.2009.02308.x. [DOI] [PubMed] [Google Scholar]
- Grunstein MM, Hakonarson H, Leiter J, Chen M, Whelan R, Grunstein JS, Chuang S. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;282:L520–L528. doi: 10.1152/ajplung.00343.2001. [DOI] [PubMed] [Google Scholar]
- Gunst SJ. Contractile force of canine airway smooth muscle during cyclical length changes. J Appl Physiol. 1983;55:759–769. doi: 10.1152/jappl.1983.55.3.759. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Al-Hassani MH, Adam LP. Regulation of isotonic shortening velocity by second messengers in tracheal smooth muscle. Am J Physiol Cell Physiol. 1994;266:C684–C691. doi: 10.1152/ajpcell.1994.266.3.C684. [DOI] [PubMed] [Google Scholar]
- Hakonarson H, Maskeri N, Carter C, Chuang S, Grunstein MM. Autocrine interaction between IL-5 and IL-1β mediates altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1999;104:657–667. doi: 10.1172/JCI7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV. The heat of activation and the heat of shortening in a muscle twitch. Proc R Soc Lond B Biol Sci. 1949;136:195–211. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Hsieh JT, Farley JM. Characterization of contractile function and expression of muscarinic receptors, G proteins and adenylate cyclase in cultured tracheal smooth muscle of swine. J Biomed Sci. 2002;9:339–347. doi: 10.1007/BF02256590. [DOI] [PubMed] [Google Scholar]
- Jiang H, Rao K, Halayko AJ, Kepron W, Stephens NL. Bronchial smooth muscle mechanics of a canine model of allergic airway hyperresponsiveness. J Appl Physiol. 1992a;72:39–45. doi: 10.1152/jappl.1992.72.1.39. [DOI] [PubMed] [Google Scholar]
- Jiang H, Rao K, Halayko AJ, Liu X, Stephens NL. Ragweed sensitization-induced increase of myosin light chain kinase content in canine airway smooth muscle. Am J Respir Cell Mol Biol. 1992b;7:567–573. doi: 10.1165/ajrcmb/7.6.567. [DOI] [PubMed] [Google Scholar]
- Jiang H, Rao K, Liu X, Liu G, Stephens NL. Increased Ca2+ and myosin phosphorylation, but not calmodulin activity in sensitized airway smooth muscles. Am J Physiol Lung Cell Mol Physiol. 1995;268:L739–L746. doi: 10.1152/ajplung.1995.268.5.L739. [DOI] [PubMed] [Google Scholar]
- Kelley CA, Adelstein RS. Characterization of isoform diversity in smooth muscle myosin heavy chains. Can J Physiol Pharmacol. 1994;72:1351–1360. doi: 10.1139/y94-195. [DOI] [PubMed] [Google Scholar]
- Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem. 1993;268:12848–12854. [PubMed] [Google Scholar]
- Lauzon AM, Tyska MJ, Rovner AS, Freyzon Y, Warshaw DM, Trybus KM. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J Muscle Res Cell Motil. 1998;19:825–837. doi: 10.1023/a:1005489501357. [DOI] [PubMed] [Google Scholar]
- Lazaar AL, Albelda SM, Pilewski JM, Brennan B, Pure E, Panettieri RA., Jr T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J Exp Med. 1994;180:807–816. doi: 10.1084/jem.180.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léguillette R, Laviolette M, Bergeron C, Zitouni N, Kogut P, Solway J, Kachmar L, Hamid Q, Lauzon AM. Myosin, transgelin, and myosin light chain kinase: expression and function in asthma. Am J Respir Crit Care Med. 2009;179:194–204. doi: 10.1164/rccm.200609-1367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just Th2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1181–L1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Moir LM, Oliver BG, Burgess JK, Roth M, Black JL, McParland BE. Comparison of gel contraction mediated by airway smooth muscle cells from patients with and without asthma. Thorax. 2007;62:848–854. doi: 10.1136/thx.2006.070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Wu MF, Gunst S. Role of contractile protein activation in length-dependent modulation of tracheal smooth muscle forse. Am J Physiol Cell Physiol. 1996;270:C243–C252. doi: 10.1152/ajpcell.1996.270.1.C243. [DOI] [PubMed] [Google Scholar]
- Miller-Hance WC, Kamm KE. Force–velocity relation and myosin light chain phosphorylation in bovine coronary arterial smooth muscle. Circ Res. 1991;69:1207–1214. doi: 10.1161/01.res.69.5.1207. [DOI] [PubMed] [Google Scholar]
- Mishima H, Hojo M, Watanabe A, Hamid Q, Martin J. CD4+ T cells can induce airway hyperresponsiveness to allergen challenge in the Brown Norway rat. Am J Res Crit Care Med. 1998;158:1863–1870. doi: 10.1164/ajrccm.158.6.9709123. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Rühlmann E, Magnussen H, Leff AR, Rabe KF. Passive sensitization of human bronchi augments smooth muscle shortening velocity and capacity. Am J Physiol Lung Cell Mol Physiol. 1994;267:L218–L222. doi: 10.1152/ajplung.1994.267.2.L218. [DOI] [PubMed] [Google Scholar]
- Moreland S, Moreland RS, Singer HA. Apparent dissociation between myosin light chain phosphorylation and maximal velocity of shortening in KCl depolarized swine carotid artery: effect of temperature and KCl concentration. Pflugers Arch. 1986;408:139–145. doi: 10.1007/BF00581343. [DOI] [PubMed] [Google Scholar]
- Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Acad Sci U S A. 2000;97:10509–10513. doi: 10.1073/pnas.180318697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozier A, Allard B, Bara I, Girodet PO, Trian T, Marthan R, Berger P. The Pivotal Role of Airway Smooth Muscle in Asthma Pathophysiology. J. Allergy (Cairo) 2011;2011:742710. doi: 10.1155/2011/742710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Barbón D, Fraga-Iriso R, Brienza NS, Montero-Martínez C, Verea-Hernando H, Olivenstein R, Lemiere C, Ernst P, Hamid QA, Martin JG. T Cells localize with proliferating smooth muscle α-actin+ cell compartments in asthma. Am J Respir Crit Care Med. 2010;182:317–324. doi: 10.1164/rccm.200905-0745OC. [DOI] [PubMed] [Google Scholar]
- Ramos-Barbón D, Presley JF, Hamid QA, Fixman ED, Martin JG. Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma. J Clin Invest. 2005;115:1580–1589. doi: 10.1172/JCI19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Owen WF, Jr, Silberstein DS, Woods J, Soberman RJ, Austen KF, Stevens RL. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988;81:1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner AS, Freyzon Y, Trybus TM. An insert in the motor domain determines the functional properties of expressed smooth muscle myosin isoforms. J Muscle Res Cell Motil. 1997;18:103–110. doi: 10.1023/a:1018689102122. [DOI] [PubMed] [Google Scholar]
- Silver PJ, Stull JT. Regulation of myosin light chain and phosphorylase phosphorylation in tracheal smooth muscle. J Biol Chem. 1982;257:6145–6150. [PubMed] [Google Scholar]
- Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Sobieszek A. Ca-regulation of mammalian smooth muscle actomyosin via a kinase-phosphatase-dependent phosphorylation and dephosphorylation of the 20000-Mr light chain of myosin. Eur J Biochem. 1977;76:521–530. doi: 10.1111/j.1432-1033.1977.tb11622.x. [DOI] [PubMed] [Google Scholar]
- Sobieszek A, Babiychuk E, Ortner B, Borkowski J. Purification and characterization of a kinase-associated, myofibrillar smooth muscle myosin light chain phosphatase possessing a calmodulin-targeting subunit. J Biol Chem. 1997;272:7027–7033. doi: 10.1074/jbc.272.11.7027. [DOI] [PubMed] [Google Scholar]
- Solway J, Fredberg JJ. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. Am J Respir Cell Mol Biol. 1997;17:144–146. doi: 10.1165/ajrcmb.17.2.f137. [DOI] [PubMed] [Google Scholar]
- Takeya K, Loutzenhiser K, Shiraishi M, Loutzenhiser R, Walsh MP. A highly sensitive technique to measure myosin regulatory light chain phosphorylation: the first quantification in renal arterioles. Am J Physiol Renal Physiol. 2008;294:F1487–F1492. doi: 10.1152/ajprenal.00060.2008. [DOI] [PubMed] [Google Scholar]
- Thomson RJ, Bramley AM, Schellenberg RR. Airway muscle stereology: implications for increased shortening in asthma. Am J Respir Crit Care Med. 1996;154:749–757. doi: 10.1164/ajrccm.154.3.8810615. [DOI] [PubMed] [Google Scholar]
- Veler H, Hu A, Fatma S, Grunstein JS, DeStephan CM, Campbell D, Orange JS, Grunstein MM. Superantigen presentation by airway smooth muscle to CD4+ T lymphocytes elicits reciprocal proasthmatic changes in airway function. J Immunol. 2007;178:3627–3636. doi: 10.4049/jimmunol.178.6.3627. [DOI] [PubMed] [Google Scholar]
- Whelan R, Kim C, Chen M, Leiter J, Grunstein MM, Hakonarson H. Role and regulation of interleukin-1 molecules in pro-asthmatic sensitised airway smooth muscle. Eur Respir J. 2004;24:559–567. doi: 10.1183/09031936.04.00133803. [DOI] [PubMed] [Google Scholar]
- White S, Martin AF, Perasamy M. Identification of a novel smooth muscle myosin heavy chain cDNA isoform diversity in the S1 head region. Am J Physiol Cell Physiol. 1993;264:C1252–C1258. doi: 10.1152/ajpcell.1993.264.5.C1252. [DOI] [PubMed] [Google Scholar]
- Woodruff PG. Gene expression in asthmatic airway smooth muscle. Proc Am Thorac Soc. 2008;5:113–118. doi: 10.1513/pats.200705-059VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zosky GR, Larcombe AN, White OJ, Burchell JT, von Garnier C, Holt PG, Turner DJ, Wikstrom ME, Sly PD, Stumbles PA. Airway hyperresponsiveness is associated with activated CD4+ T cells in the airways. Am J Physiol Lung Cell Mol Physiol. 2009;297:L373–L379. doi: 10.1152/ajplung.00053.2009. [DOI] [PubMed] [Google Scholar]


