Abstract
Essential hypertension is linked to an increased sympathetic vasoconstrictor activity and reduced tissue perfusion. We investigated the role of exercise training on functional sympatholysis and postjunctional α-adrenergic responsiveness in individuals with essential hypertension. Leg haemodynamics were measured before and after 8 weeks of aerobic training (3–4 times per week) in eight hypertensive (47 ± 2 years) and eight normotensive untrained individuals (46 ± 1 years) during arterial tyramine infusion, arterial ATP infusion and/or one-legged knee extensions. Before training, exercise hyperaemia and leg vascular conductance (LVC) were lower in the hypertensive individuals (P < 0.05) and tyramine lowered exercise hyperaemia and LVC in both groups (P < 0.05). Training lowered blood pressure in the hypertensive individuals (P < 0.05) and exercise hyperaemia was similar to the normotensive individuals in the trained state. After training, tyramine did not reduce exercise hyperaemia or LVC in either group. When tyramine was infused at rest, the reduction in blood flow and LVC was similar between groups, but exercise training lowered the magnitude of the reduction in blood flow and LVC (P < 0.05). There was no difference in the vasodilatory response to infused ATP or in muscle P2Y2 receptor content between the groups before and after training. However, training lowered the vasodilatory response to ATP and increased skeletal muscle P2Y2 receptor content in both groups (P < 0.05). These results demonstrate that exercise training improves functional sympatholysis and reduces postjunctional α-adrenergic responsiveness in both normo- and hypertensive individuals. The ability for functional sympatholysis and the vasodilator and sympatholytic effect of intravascular ATP appear not to be altered in essential hypertension.
Key points
Essential hypertension is linked to an increased sympathetic vasoconstrictor activity and reduced tissue perfusion.
Exercise training can improve the ability to override sympathetic vasoconstrictor activity.
Here we show that 8 weeks of exercise training reduces the vasoconstrictor response to sympathetic nerve activity (induced by tyramine) and improves the ability to override sympathetic vasoconstrictor activity.
We found no difference in the ability to override sympathetic vasoconstrictor activity during exercise, the reduction in blood flow in response to increases in sympathetic nerve activity or the hyperaemic response to infused ATP between normo- and hypertensive subjects.
These results help us to better understand how exercise training can reduce blood pressure and improve tissue perfusion.
Introduction
Essential hypertension is a leading cause of cardiovascular events and early death and is associated with impaired tissue perfusion and sympathetic nerve activity (SNA) (Levy et al. 2008; Parati & Esler, 2012). In skeletal muscle, perfusion is regulated to match O2 delivery to metabolic demand and is determined by an interaction between local vasodilator and vasoconstrictor substances as well as the degree of sympathetic vasoconstriction (Hellsten et al. 2012a). During exercise, SNA is increased (Alam & Smirk, 1937; Hollander & Bouman, 1975; Mitchell et al. 1983; Seals & Victor, 1991) and it is targeted to both resting and contracting skeletal muscle (Saito et al. 1993; Hansen et al. 1994; Ray & Mark, 1995; Strange, 1999). In inactive tissues, the increase in SNA causes vasoconstriction, as evidenced by a reduction in limb vascular conductance (Bevegard & Shepherd, 1966; Rowell, 1993; Callister et al. 1994), but in contracting muscles the increased sympathetic vasoconstrictor activity can be attenuated or even abolished (termed ‘functional sympatholysis’) (Remensnyder et al. 1962; Thomas et al. 1994; Hansen et al. 1996; Tschakovsky et al. 2002; Saltin & Mortensen, 2012). In the forearm of middle-aged hypertensive individuals, functional sympatholysis has been reported to be impaired and an exaggerated sympathetic response to exercise has been observed (Vongpatanasin et al. 2011). Local adaptations in skeletal muscle in response to exercise training appear to be important for maintaining an intact functional sympatholysis (Mortensen et al. 2012b) and regular physical activity can prevent the age-related impairment in functional sympatholysis (Mortensen et al. 2012a). Exercise training could therefore prove to be a tool to improve functional sympatholysis in individuals with essential hypertension (Saltin & Mortensen, 2012).
ATP is a potent vasodilator that binds to endothelial purinergic P2 receptors and it is thought to play an important role in skeletal muscle blood flow regulation (Ellsworth et al. 1995; Ellsworth & Sprague, 2012; González-Alonso, 2012). Intravascular ATP can override sympathetic vasoconstrictor activity (Rosenmeier et al. 2004) and induce vasodilation by stimulating nitric oxide (NO) and prostacyclin formation (Mortensen et al. 2009; Crecelius et al. 2011) and activation of inwardly rectifying potassium channels has also been suggested (Crecelius et al. 2012). An impaired sympatholytic effect of ATP, possibly via defect downstream signalling, could therefore be an underlying cause of an impaired functional sympatholysis in essential hypertension.
The aim of the present study was to examine whether functional sympatholysis and ATP signalling is impaired in the leg of hypertensive individuals and to determine the effect of aerobic exercise training on these parameters. We measured leg haemodynamics at rest, during exercise and arterial ATP and/or tyramine (to stimulate local noradrenaline (NA) release) infusion, before and after 8 weeks of high intensity exercise training in individuals with essential hypertension and in normotensive controls. We hypothesized that functional sympatholysis during exercise would be impaired in the hypertensive individuals and that exercise training would improve the ability for functional sympatholysis.
Methods
Eight hypertensive individuals and eight age, weight and height matched, normotensive individuals were studied (Table 1). The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (H-2-2009-096) and conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all individuals before enrolment in the study.
Table 1.
Baseline characteristics before and after 8 weeks of aerobic exercise training
| Normotensive | Hypertensive | |||
|---|---|---|---|---|
| Variable | Before (week 0) | After (week 8) | Before (week 0) | After (week 8) |
| Male/female | 4/4 | 3/5 | ||
| Age (years) | 46 ± 1 | 47 ± 2 | ||
| Systolic blood pressure (mmHg) | 135 ± 4 | 134 ± 5 | 162 ± 4* | 155 ± 6*# |
| Diastolic blood pressure (mmHg) | 81 ± 2 | 82 ± 4 | 102 ± 4* | 91 ± 4*# |
| Body weight (kg) | 78.5 ± 3.2 | 77.9 ± 3.4 | 78.1 ± 6.9 | 77.5 ± 8.4 |
| Body fat (%) | 27.0 ± 2.7 | 26.3 ± 2.7 | 31.8 ± 3.3 | 31.9 ± 3.9 |
| Experimental leg mass (kg) | 12.4 ± 0.9 | 12.0 ± 0.9 | 11.8 ± 1.1 | |
| Femoral arterial diameter (cm) | 0.95 ± 0.03 | 0.98 ± 0.03 | 0.96 ± 0.05 | 0.96 ± 0.05 |
| Femoral arterial blood flow (l min–1) | 0.30 ± 0.04 | 0.33 ± 0.05 | 0.22 ± 0.04* | 0.27 ± 0.04 |
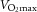 (l min–1) (l min–1) |
2.69 ± 0.27 | 2.87 ± 0.25# | 2.42 ± 0.22 | 2.58 ± 0.14# |
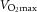 relative to body weight (ml min–1 kg–1) relative to body weight (ml min–1 kg–1) |
33 ± 2 | 37 ± 2# | 32 ± 3 | 36 ± 4# |
| Total cholesterol (mmol l–1) | 4.4 ± 0.3 | 4.7 ± 0.3 | 4.8 ± 0.2 | 5.0 ± 0.2 |
| HDL (mmol l–1) | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.2 |
| LDL (mmol l–1) | 2.4 ± 0.3 | 2.7 ± 0.4 | 3.0 ± 0.3 | 3.0 ± 0.2 |
| Triglycerides (mmol l–1) | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 |
| Noradrenaline (mmol l–1) | 1.84 ± 0.11 | 1.92 ± 0.26 | 2.01 ± 0.36 | 1.92 ± 0.20 |
Values are means ± SEM.
Significantly different from normotensive,
significantly different from before training. Blood pressures are home measurements in supine position (Omron M6 comfort, Kyoto, Japan). Femoral arterial blood flow and diameter were obtained after catheterization, but before the beginning of the experimental protocol.
All individuals were habitually inactive (less than 1 h of moderate intensity exercise per week), had normal resting electrocardiogram (ECG) and no signs of ischaemia or arrhythmias during exercise (ECG), were non-smokers, and none of the individuals in either group had been diagnosed with cardiovascular disease, renal dysfunction, insulin resistance, diabetes or hypercholesterolaemia, and none of the individuals was taking β-blockers. Six of the hypertensive individuals were under treatment with angiotensin-converting enzyme nhibitors (n = 2), calcium channel blockers (n = 4) and/or diuretics (n = 3). Medication was gradually withdrawn from the hypertensive subjects for a period prior to experimental days; angiotensin-converting enzyme inhibitors and Ca2+ antagonist were withdrawn 2 weeks and diuretics 1 week prior to the experimental days. All women were premenopausal and were tested at the same time point during their menstrual cycle before and after training.
Study design
Before the first experimental day the individuals visited the laboratory to become accustomed to the one-leg knee-extensor model and to perform an incremental bicycle ergometer exercise test in which pulmonary maximal oxygen uptake (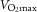 ) was determined with a metabolic cart (CPET system, Cosmed, Rome, Italy).
) was determined with a metabolic cart (CPET system, Cosmed, Rome, Italy). 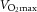 was also measured following training.
was also measured following training.
The subjects were studied on two experimental days separated by 7 days both before and after an 8 week training period. Data presented here are from the experimental day 1, whereas data obtained from experimental day 2, control exercise bout (day 1), acetylcholine infusion (day 1) and muscle biopsy (day 1) have been published previously (Nyberg et al. 2012b; Hellsten et al. 2012b). Subjects refrained from caffeine, alcohol and exercise for 24 h before the experimental days and were asked to record their food intake such that the diet was similar before the experimental days. On the day of the experiment the subjects ingested a standardized breakfast 2 h before reporting to the laboratory at 08.00 am. After local anaesthesia, catheters were placed in the femoral artery and vein of the experimental leg (right) and in the femoral artery of the non-experimental leg (left). A muscle biopsy was obtained from m. vastus lateralis of the non-experimental leg during resting conditions.
Experimental protocol
Following 30 min of rest, the individuals received femoral arterial infusion of: (1) tyramine (11.5 ± 0.5 μmol min−1, i.e. 1.0 μmol min−1 kg−1 leg mass; Sigma Aldrich, St Louis, MO, USA), (2) ATP (0.5 ± 0.0, 2.3 ± 0.1, 4.6 ± 0.2 and 23.1 ± 0.8 μmol min−1, i.e. 0.04, 0.2, 0.4 and 2.0 μmol min−1 kg−1 leg mass, respectively: A7699, Sigma Aldrich), (3) ATP + tyramine (11.5 ± 0.5 μmol min−1), (4) one-legged knee extensor exercise (10, 20 and 30 W) and (5) one-legged knee extensor exercise (10, 20 and 30 W) + tyramine (11.5 ± 0.5 μmol min−1). Each level of exercise and dose of ATP or tyramine lasted 2.5 min and measurements (blood flow, pressures and arterial and venous blood samples (1–3 ml) were obtained after 2 min of exercise/infusion. During the ATP + tyramine and exercise + tyramine trials, tyramine was infused for 3 min before the start of ATP infusion/exercise. To counteract the tyramine-induced reduction in leg blood flow (LBF) before the onset of exercise/ATP infusion, a low dose of sodium nitroprusside (SNP; 5–20 μg min–1; Nitropress, Hospira, Lake Forrest, IL, USA) was co-infused with tyramine until 30 s before the start of exercise/ATP infusion such that LBF was similar to baseline levels at the onset of exercise/ATP infusion. The order of the infusion and exercise trials were randomized and separated by 30 min, but the exercise trials were always performed after the infusion trials.
Exercise training programme
The subjects performed supervised aerobic exercise training (cycling ergometer) for 1 h, two to three times a week. On one additional day per week the individuals performed an independent training session (cycling or running). During all training sessions individuals wore a TEAM2 WearLink+ (Polar, Helsinki, Finland) to record heart rate. Exercise intensity during the training sessions was >80% of maximal heart rate for 41–43% of the training duration and <60% of maximal heart rate for 8–9% of the training duration (see Nyberg et al. 2012a for details).
Measurements and analysis
Femoral arterial blood flow (LBF) was measured with an ultrasound machine (Logic E9, GE Healthcare, Milwaukee, WI, USA) equipped with a probe operating at an imaging frequency of 9 MHz and Doppler frequency of 4.3–5.0 MHz as previously described (Nyberg et al. 2012b). Arterial pressures were monitored with transducers positioned at the level of the heart (Pressure Monitoring Kit, Baxter, Deerfield, IL, USA). Leg mass was calculated from whole-body dual-energy X-ray absorptiometry scanning (Prodigy, GE Medical Systems, Milwaukee, WI, USA). Blood gases, haemoglobin, lactate and glucose concentrations were measured using an ABL725 analyser (Radiometer, Copenhagen, Denmark). Plasma [NA] and [adrenaline] were determined with a radioimmunoassay (LDN, Nordhorn, Germany).
Quantification of purinergic P2Y2 and P2X1 receptor expression
Quantification of protein expression was performed as previously described (Nyberg et al. 2013). Briefly, approximately 25 mg wet weight of the biopsy was freeze dried and then homogenized in homogenization buffer and the protein concentration of the lysate samples was determined. Lysate proteins were separated using 10% SDS gels (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to PVDF membranes (Immobilion Transfer Membrane, Billerica, MA, USA). The membranes were incubated with primary polyclonal antibodies (Alomone Laboratories, Jerusalem, Israel) against the purinergic P2Y2 (1:200) and P2X1 (1:200) receptors. Secondary antibody horseradish-peroxidase (HRP)-conjugated goat anti-rabbit (P-0448, Dako, Glostrup, Denmark) was used for detection. The protein content was expressed in arbitrary units relative to standard samples run on each gel.
Statistical analysis
A two-way repeated measures analysis of variance (ANOVA) was performed to test significance within and between trials and before and after training. A two-way ANOVA was performed to test significance between groups. After a significant F-test, pair-wise differences were identified using a Holm–Sidak test. The significance level was set at P < 0.05 and data are given as means ± standard error of the mean (SEM). Due to technical problems, 5 out of the 21 reqruited subjects did not complete the protocol and 16 subjects are therefore included.
Results
Leg haemodynamics during resting conditions
Femoral arterial blood flow was lower in the hypertensive subjects than the normotensive subjects before the training intervention (P < 0.05) whereas there was no difference after training (Table 1). Plasma [NA] tended (P = 0.083) to be increased in the hypertensive subjects before training.
Leg haemodynamics during arterial tyramine infusion
Tyramine infusion reduced LBF and leg vascular conductance (LVC) (P < 0.05) whereas mean arterial pressure (MAP) remained unchanged in both groups (Fig. 1). There was no difference in the tyramine-induced change in LBF, LVC and [NA] between the hypertensive and normotensive subjects before or after the training period, but the tyramine-induced reduction in LBF and LVC was lower after the training period (P < 0.05). There was no difference in the increase in [NA] before and after training. Heart rate and MAP responses to tyramine were similar in the hypertensive and normotensive subjects both before and after the training period.
Figure 1. Changes in leg blood flow, leg vascular conductance and femoral venous noradrenaline concentration during arterial infusion of tyramine (1.0 μmol min−1 kg−1 leg mass) before and after 8 weeks of exercise training in normotensive and hypertensive individuals.
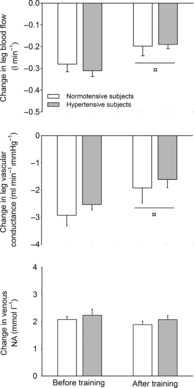
¤Different from before training, P < 0.05.
Leg haemodynamics during one-legged knee extensor exercise
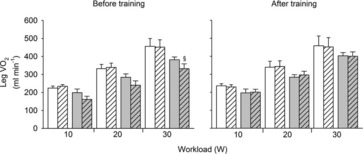
During exercise, LBF was lower at 30 W (P < 0.05), whereas LVC was lower and MAP higher (P < 0.05) at all workloads in the hypertensive subjects compared to the normotensive subjects (Fig. 2). When tyramine was infused during exercise, LBF and LVC were lower compared to without tyramine in both the hypertensive and the normotensive subjects (P < 0.05). The leg arterial 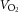 difference increased similarly in both groups during exercise and there was no difference in leg
difference increased similarly in both groups during exercise and there was no difference in leg 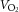 between groups in the control trial (Fig. 3). When tyramine was infused during exercise, the leg arterial
between groups in the control trial (Fig. 3). When tyramine was infused during exercise, the leg arterial 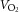 difference increased in both groups, but leg
difference increased in both groups, but leg 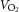 was lower in the hypertensive subjects at 30 W (P < 0.05).
was lower in the hypertensive subjects at 30 W (P < 0.05).
Figure 2. Leg blood flow, mean arterial pressure and leg vascular conductance during exercise (10, 20 and 30 W) with and without co-infusion of tyramine before and after 8 weeks of exercise training in normotensive and hypertensive individuals.
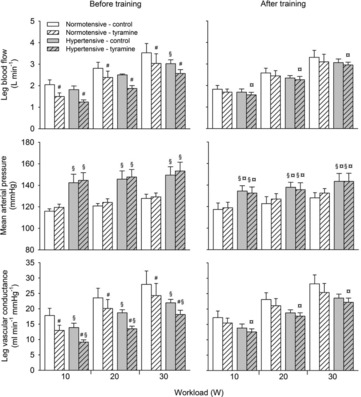
A low dose of SNP was co-infused with tyramine during resting conditions to restore LBF to baseline levels. §Different from normotensive individuals, P < 0.05; #different from control exercise, P < 0.05; ¤different from before training, P < 0.05.
Figure 3. Leg 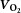 at rest and during exercise (10, 20 and 30 W) with and without co-infusion of tyramine before and after 8 weeks of exercise training in normotensive and hypertensive subjects.
at rest and during exercise (10, 20 and 30 W) with and without co-infusion of tyramine before and after 8 weeks of exercise training in normotensive and hypertensive subjects.
A low dose of SNP was co-infused with tyramine during resting conditions to restore LBF to baseline levels. §Different from normotensive subjects, P < 0.05
After the period of exercise training, LBF and LVC were similar to before training in both groups during exercise whereas MAP was lower in the hypertensive subjects. LBF, LVC and MAP did not change when tyramine was infused during exercise in either group and LBF and LVC were higher compared to before the training period in hypertensive subjects (P < 0.05). There was no difference in leg arterial 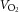 difference and
difference and 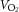 between groups in either the control or the tyramine trial.
between groups in either the control or the tyramine trial.
Femoral venous plasma [NA] increased to similar levels during exercise (Fig. 4) and exercise training did not affect plasma [NA] during exercise in either group.
Figure 4. Femoral venous noradrenaline concentrations during exercise (30 W) with and without co-infusion of tyramine before and after 8 weeks of exercise training in normotensive and hypertensive individuals.
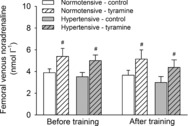
A low dose of SNP was co-infused with tyramine during resting conditions to restore LBF to baseline levels. #Different from control exercise, P < 0.05.
Vasodilatory responsiveness to infused ATP
Arterial ATP infusion resulted in a dose dependent increase in LBF and LVC (Fig. 5). The increase in LBF was higher in the hypertensive subjects than in the normotensive subjects (P < 0.05) due to a higher perfusion pressure (P < 0.05), as indicated by similar LVC between the two groups during ATP infusion. After the training period, the overall increase in LBF and LVC was lower compared to before the training period (P < 0.05), but there was no difference between the two groups. Both before and after the training period, co-infusion of tyramine with ATP did not alter the LBF or LVC response to ATP in either group, but tyramine increased blood pressure during ATP infusion in the hypertensive subjects (P < 0.05), whereas it was unaltered in the normotensive subjects.
Figure 5. Leg blood flow and leg vascular conductance during baseline conditions and arterial ATP infusion with and without co-infusion of tyramine before (left) and after (right) 8 weeks of exercise training in normotensive and hypertensive individuals.
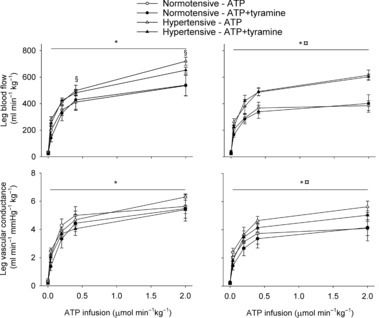
A low dose of SNP was co-infused with tyramine during baseline conditions to restore leg blood flow to baseline levels. *Different from baseline conditions, P < 0.05; §different from normotensive individuals, P < 0.05; ¤different from before training, P < 0.05.
Skeletal muscle purinergic P2X1 and P2Y2 receptor content
Skeletal muscle purinergic P2Y2 and P2X1 receptor content were similar between the hypertensive and normotensive subjects before training (Fig. 6). Exercise training increased the P2X1 receptor content in the normotensive subjects (P < 0.05), but not in the hypertensive subjects, whereas exercise training increased the P2Y2 receptor content in both groups (P < 0.05).
Figure 6. Purinergic P2X1 and P2Y2 receptor content in vastus lateralis muscle before and after 8 weeks of exercise training in normotensive and hypertensive individuals.
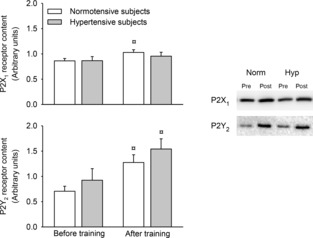
¤Different from before training, P < 0.05.
Discussion
This study investigated the effect of exercise training on functional sympatholysis and ATP-induced vasodilation in hypertensive and healthy middle-aged individuals. The main findings are that exercise training reduces α-adrenergic responsiveness and improves the ability for functional sympatholysis during exercise. In addition, the results show that essential hypertension is not associated with a reduced ability for functional sympatholysis during leg exercise or with an altered vasodilator response to infused ATP.
Resting haemodynamics and α-adrenergic responsiveness in hypertension
The similar haemodynamic response and change in venous [NA] between hypertensive and normotensive individuals suggest that postjunctional α-adrenergic responsiveness is not altered in the leg of hypertensive individuals. This observation is in line with previous findings in the human forearm (Egan et al. 1987). Whereas previous studies have stimulated mainly luminal receptors by exogenous NA administration, the present study extended these findings by demonstrating that the responsiveness to neurally released NA is intact in hypertension. Despite the unaltered α-adrenergic responsiveness and higher perfusion pressure, resting LBF was lower in the hypertensive individuals. Hypertension has been linked to increased levels of SNA (Egan et al. 1987; Parati & Esler, 2012). We did not observe a difference in cathecholamines at rest between the hyper- and normotensive individuals under the present experimental conditions, indicating that SNA was not increased in the individuals with essential hypertension. This may be due to the large individual variations in SNA (Charkoudian et al. 2005), but may also reflect the rather poor correlation between SNA and blood pressure (Joyner et al. 2010). It is therefore possible that an increased sympathetic vasoconstrictor activity contributed to the lower resting LBF in the hypertensive individuals. Moreover, NO formation in response to arterial shear stress appears to be impaired in hypertensive subjects (Pohl et al. 1986), which also could contribute to the lower resting LBF (Nyberg et al. 2012b).
Functional sympatholysis during exercise
The ability to overcome sympathetic vasoconstrictor activity in contracting skeletal muscle is well established in young individuals (Rosenmeier et al. 2004) and this ability is impaired in inactive elderly individuals (Mortensen et al. 2012b). Functional sympatholysis has previously been shown to be impaired in the forearm of middle-aged men and women with essential hypertension compared to activity matched healthy controls (Vongpatanasin et al. 2011). The discrepancies in these results and the present findings may reflect limb specific differences in vascular function (Pawelczyk & Levine, 2002; Thijssen et al. 2011) and/or the method used to increase SNA (cold pressor test versus tyramine). Moreover, certain medications have been suggested to improve the ability for functional sympatholysis (Price et al. 2013) and we cannot exclude that the shorter withdrawal period affected the present findings, although careful evaluation of the individual data did not indicate a connection between the ability for functional sympatholysis and medication. Interestingly, leg 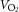 was compromised in the hypertensive individuals during exercise with tyramine before training, which may reflect that hypertensive individuals are more sensitive to reductions in blood flow, because LBF is already impaired compared to healthy individuals.
was compromised in the hypertensive individuals during exercise with tyramine before training, which may reflect that hypertensive individuals are more sensitive to reductions in blood flow, because LBF is already impaired compared to healthy individuals.
Effect of exercise training on functional sympatholysis and α-adrenergic responsiveness
An important finding was that 8 weeks of aerobic exercise training lowered the response to arterially infused tyramine. The training-induced lowering of the reduction in LBF and LVC in response to tyramine occurred without alterations in [NA], suggesting that tyramine-induced NA release was not affected by exercise training. α-Adrenergic responsiveness has been shown to be altered by ageing but, to our knowledge, the effect of exercise training on α-adrenergic responsiveness has not previously been examined in humans. In support of the present findings, exercise training has also been shown to attenuate α-adrenergic vasoconstriction in skeletal muscle arterioles of rats (Donato et al. 2007). Exercise training also improved functional sympatholysis during exercise in both groups. Evidence obtained from local limb training and immobilization (Mortensen et al. 2012b) as well as cross-sectional data obtained in active and sedentary elderly individuals (Mortensen et al. 2012a) have demonstrated a clear association between the training status of the skeletal muscle and the ability for functional sympatholysis. Apart from exercise training, β1-adrenergic receptor blockade (Price et al. 2013) and oestrogen treatment (Fadel et al. 2004) have been shown to improve functional sympatholysis, although the mechanisms remain unclear. Functional sympatholysis has been suggested to be mediated by ATP (Saltin & Mortensen, 2012) and NO (Chavoshan et al. 2002) and impaired by reactive oxygen species (ROS) (Fadel et al. 2012), but stimulation of NO availability does not blunt sympathetic vasoconstriction in young men (Rosenmeier et al. 2003) or increase exercise hyperaemia in elderly men with impaired functional sympatholysis (Nyberg et al. 2012a). Taken together, these observations suggest that the training induced improvement in functional sympatholysis could be coupled to the lower α-adrenergic responsiveness with exercise training. Due to increased levels of SNA in hypertension (Parati & Esler, 2012), maintaining an intact functional sympatholysis is likely to be more important in these patients in order to maintain adequate tissue perfusion. This is supported by the similar leg blood flow and 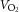 between the normotensive and hypertensive individuals after the training intervention.
between the normotensive and hypertensive individuals after the training intervention.
ATP signalling
The vasodilator response to infused ATP was not different between the hypertensive and normotensive individuals, which is consistent with the similar endothelial function (as evaluated by acetylcholine infusion) in these subjects (Nyberg et al. 2012b; Mortensen et al. 2009). Although exercise training increased P2Y2 receptor content, the increase in LVC with ATP infusion was reduced with exercise training, suggesting that P2Y2 receptor quantity is not important for the hyperaemic response. In line with the lower response to infused ATP in the trained state, we have previously observed an increase in this response after 2 weeks of limb immobilization of recreationally active young individuals (Mortensen et al. 2012b). A potential mechanism underlying these changes is that physical activity may increase membrane-bound (Burnstock & Ralevic, 2014) and/or soluble ectonucleotidases (Yegutkin et al. 2003), such that the infused ATP is more rapidly removed from the circulation. Alternatively, exercise training may cause partial desensitization of P2Y2 receptors. Regardless of the underlying mechanisms, the response was similar in the normo- and hypertensive individuals, demonstrating that the vasodilator response to ATP is not altered in hypertension.
Perspectives
The present data suggest that functional sympatholysis is impaired in both hypertensive and healthy middle-aged individuals and identifies physical activity level as a key player in maintaining the ability for functional sympatholysis. Essential hypertension is associated with increased levels of SNA, and an impaired functional sympatholysis is therefore likely to have a greater consequence for tissue perfusion in hypertensive than in normotensive individuals with a similar impairment in functional sympatholysis. The training-induced lowering in α-adrenergic responsiveness to neurally released NA suggests that altered α-adrenergic sensitivity could play a key role in mediating functional sympatholysis.
Conclusion
The ability for functional sympatholysis during leg exercise and the α-adrenergic responsiveness are closely coupled to the physical activity level, but does not appear to be altered in essential hypertension per se. The vasodilatory and sympatholytic effect of intravascular ATP does not appear to be affected by hypertension in middle-aged individuals.
Glossary
- LBF
leg blood flow
- LVC
leg vascular conductance
- MAP
mean arterial pressure
- NA
noradrenaline
- SNA
sympathetic nerve activity
- SNP
sodium nitroprusside
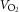
oxygen uptake
Additional information
Competing interests
None.
Author contributions
The experiments were conducted at the Copenhagen Muscle Research Centre, Rigshospitalet, Denmark. Conception and design of the study: S.P.M., B.S. and Y.H.; collection, analysis and interpretation of data: S.P.M., M.N., L.G., P.T., Y.H. and S.P.M.; drafting the article or revising it critically for important intellectual content: S.P.M., M.N., L.G., P.T., B.S. and Y.H. All authors approved the final version.
Funding
This work was supported by a grant from the Lundbeck Foundation and the Danish Council for independent Research – Medical Sciences. S.P.M. was supported by a grant from the Danish Council for Independent Research – Medical Sciences. M.N. was supported by a grant from the P. Carl Petersen Foundation.
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevegard BS, Shepherd JT. Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. J Appl Physiol. 1966;21:123–132. doi: 10.1152/jappl.1966.21.1.123. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol. 1994;77:1403–1410. doi: 10.1152/jappl.1994.77.3.1403. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. ATP-mediated vasodilatation occurs via activation of inwardly-rectifying potassium channels in humans. J Physiol. 2012;590:5349–5359. doi: 10.1113/jphysiol.2012.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol. 2011;301:H1302–H1310. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007;579:115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Panis R, Hinderliter A, Schork N, Julius S. Mechanism of increased alpha adrenergic vasoconstriction in human essential hypertension. J Clin Invest. 1987;80:812–817. doi: 10.1172/JCI113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Sprague RS. Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP. J Physiol. 2012;590:4985–4991. doi: 10.1113/jphysiol.2012.233106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Farias M, Gallagher KM, Wang Z, Thomas GD. Oxidative stress and enhanced sympathetic vasoconstriction in contracting muscles of nitrate tolerant rats and humans. J Physiol. 2012;590:395–407. doi: 10.1113/jphysiol.2011.218917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol. 2004;561:893–901. doi: 10.1113/jphysiol.2004.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J. ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans. J Physiol. 2012;590:5001–5013. doi: 10.1113/jphysiol.2012.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest. 1996;98:584–596. doi: 10.1172/JCI118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Jacobsen TN, Victor RG. Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human skeletal muscle. Am J Physiol. 1994;266:H2508–H2514. doi: 10.1152/ajpheart.1994.266.6.H2508. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol. 2012a;590:6297–6305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Jensen L, Thaning P, Nyberg M, Mortensen S. Impaired formation of vasodilators in peripheral tissue in essential hypertension is normalized by exercise training: role of adenosine and prostacyclin. J Hypertens. 2012b;30:2007–2014. doi: 10.1097/HJH.0b013e328356dd57. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975;38:272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension. 2010;56:10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol. 2012a;590:6227–6236. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Mørkeberg J, Thaning P, Hellsten Y, Saltin B. Two weeks of muscle immobilization impairs functional sympatholysis, but increases exercise hyperemia and the vasodilatory responsiveness to infused ATP. Am J Physiol Heart Circ Physiol. 2012b;302:H2074–H2082. doi: 10.1152/ajpheart.01204.2011. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Hellsten Y. Physical activity opposes the age-related increase in skeletal muscle and plasma endothelin-1 levels and normalizes plasma endothelin-1 levels in individuals with essential hypertension. Acta Physiol (Oxf) 2013;207:524–535. doi: 10.1111/apha.12048. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol. 2012a;590:5361–5370. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol. 2012b;590:1481–1494. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol. 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Price A, Raheja P, Wang Z, Arbique D, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD, Vongpatanasin W. Differential effects of nebivolol versus metoprolol on functional sympatholysis in hypertensive humans. Hypertension. 2013;61:1263–1269. doi: 10.1161/HYPERTENSIONAHA.113.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray CA, Mark AL. Sympathetic nerve activity to nonactive muscle of the exercising and nonexercising limb. Med Sci Sports Exerc. 1995;27:183–187. [PubMed] [Google Scholar]
- Remensnyder J, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Fritzlar SJ, Dinenno FA, Joyner MJ. Exogenous NO administration and α-adrenergic vasoconstriction in human limbs. J Appl Physiol. 2003;95:2370–2374. doi: 10.1152/japplphysiol.00634.2003. [DOI] [PubMed] [Google Scholar]
- Rowell LB. In: Human cardiovascular control. Rowell LB, editor. New York: Oxford University Press; 1993. p. 500. [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Saltin B, Mortensen SP. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J Physiol. 2012;590:6269–6275. doi: 10.1113/jphysiol.2012.241026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev. 1991;19:313–349. [PubMed] [Google Scholar]
- Strange S. Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. J Physiol. 1999;514:283–291. doi: 10.1111/j.1469-7793.1999.283af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen DHJ, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ. Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol. 2011;111:244–250. doi: 10.1152/japplphysiol.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589:1209–1220. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Jalkanen S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 2003;17:1328–1330. doi: 10.1096/fj.02-1136fje. [DOI] [PubMed] [Google Scholar]


