Abstract
The current study investigated the role of hydrogen sulphide (H2S) in oxygen sensing, intracellular signalling and promotion of ventilatory responses to hypoxia in adult and larval zebrafish (Danio rerio). Both larval and adult zebrafish exhibited a dose-dependent increase in ventilation to sodium sulphide (Na2S), an H2S donor. In vertebrates, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) are enzymes that catalyse the endogenous production of H2S. In adult zebrafish, inhibition of both CBS and CSE with aminooxyacetate (AOA) and propargyl glycine (PPG) blunted or abolished the hypoxic hyperventilation, and the addition of Na2S to the water partially rescued the effects of inhibiting endogenous H2S production. In zebrafish larvae (4 days post-fertilization), gene knockdown of either CBS or CSE using morpholinos attenuated the hypoxic ventilatory response. Furthermore, the intracellular calcium concentration of isolated neuroepithelial cells (NECs), which are putative oxygen chemoreceptors, increased significantly when these cells were exposed to 50 μm Na2S, supporting a role for H2S in Ca2+-evoked neurotransmitter release in these cells. Finally, immunohistochemical labelling showed that NECs dissociated from adult gill contained CBS and CSE, whereas cutaneous NECs in larval zebrafish expressed only CSE. Taken together, these data show that H2S can be produced in the putative oxygen-sensing cells of zebrafish, the NECs, in which it appears to play a pivotal role in promoting the hypoxic ventilatory response.
Key points
Hydrogen sulphide (H2S), a gaseous neurotransmitter, is involved in oxygen sensing in glomus cells, which are oxygen-sensing cells found in the mammalian carotid body.
Neuroepithelial cells (NECs) are oxygen-sensing cells of fish and are thought to be phylogenetic precursors of mammalian glomus cells; however, the oxygen-sensing mechanisms of these cells remain largely unknown.
Both adult and larval zebrafish responded to exogenous H2S by increasing ventilation in a dose-dependent manner; H2S increased intracellular [Ca2+] in NECs.
Inhibiting endogenous H2S production decreased or abolished the ventilatory response to hypoxia in both adult and larval zebrafish.
The results demonstrate an important role for H2S in oxygen sensing in zebrafish.
Introduction
The capacity to monitor and detect changes in ambient and internal oxygen (O2) levels is critical to the survival of vertebrates and in particular to that of species which routinely experience fluctuating O2 levels. Changes in O2 availability to cells can arise from an inadequate O2 supply to the body, increased O2 demand, or changes in the delivery of O2 within the body. The ability to sustain or maximize O2 delivery to tissues in the face of alterations in O2 availability depends on an integrated cascade of cardiorespiratory adjustments that are triggered by O2-sensing cells or chemoreceptors. Thus, O2 chemoreceptors increase their activity in response to low O2 levels (hypoxia) to initiate hyperventilation in an attempt to maintain O2 delivery to cells. In mammals, the main O2-sensing organ is the carotid body, found at the bifurcation of the internal and external carotid arteries. This is an ideal location at which to monitor arterial blood O2 levels because glomus cells receive blood leaving the lungs and can detect hypoxaemia before the blood reaches the brain (Prabhakar, 2006). Despite a wealth of research, the precise nature of the O2-sensing mechanism(s) of the carotid body glomus cells and other peripheral chemoreceptors remains uncertain, although several theories continue to be debated vigorously (Peers et al. 2010; Prabhakar, 2013). For example, some of the prevailing theories of oxygen sensing implicate a change in reactive oxygen species (ROS) production by the mitochondria and/or NADPH oxidase (Dinger et al. 2007), activation of AMP-activated protein kinase (AMPK) (Evans et al. 2005), and the inhibition of calcium sensitive potassium (BKCa) channels by haemoxygenase-2 (HO-2) (Prabhakar et al. 1995; Williams et al. 2004) as steps in the process.
Hydrogen sulphide (H2S), a gaseous neurotransmitter, is involved in a myriad of physiological functions including epithelial Na+ transport (Althaus, 2012), metabolism (Zhang et al. 2013), cardiorespiratory control (Wang, 2009; Olson et al. 2012), catecholamine secretion (Perry et al. 2009a; Zhu et al. 2012) and O2 chemoreception (Olson, 2008; Peng et al. 2010; Telezhkin et al. 2010). With respect to the last of these, there is a growing body of evidence implicating H2S as an O2 sensor (Olson, 2011) or mediator of hypoxic signalling (Peng et al. 2010) within the mammalian carotid body. One proposed mechanism (Olson, 2011; Olson et al. 2012) for H2S-mediated O2 sensing involves alterations in the balance between the production of H2S from cysteine by the enzymes cystathionine β-synthase (CBS) and/or cystathionine γ-lyase (CSE) and its oxidative degradation. Under normoxic conditions, H2S is oxidised in the mitochondria to sulphite (SO32−) and sulphate (SO42−). However, under hypoxic conditions the mitochondria cannot fully oxidise the H2S being produced and H2S accumulates in the cell (Olson, 2011; Olson et al. 2012). An alternative mechanism to explain how H2S mediates O2 sensing involves haemoxygenase (HO) (Peng et al. 2010, 2014; Prabhakar, 2013). In normoxia, HO generates carbon monoxide (CO), which inhibits CSE and thereby lowers H2S production. Under hypoxic conditions, CO generation by HO is reduced, removing the inhibition to CSE and thereby increasing H2S production. Several means by which H2S can influence O2 sensing have been proposed and include the closure of potassium channels, activation of NADPH oxidase and AMPK, increases in ROS and/or mitochondrial uncoupling (Buckler & Vaughan-Jones, 1998; Dinger et al. 2007; Wyatt et al. 2007; Olson, 2008; Buckler, 2012). The aim of the current study was to examine in zebrafish (Danio rerio) the role of H2S in O2 sensing, particularly in the putative cardiorespiratory oxygen chemoreceptors, the neuroepithelial cells (NECs), and its involvement in regulating the magnitude of downstream ventilatory responses to acute hypoxia. The zebrafish is an excellent model organism in which to study O2 sensing in vertebrates. Firstly, as in other fish, O2 rather than CO2 is the principal gas which regulates breathing. Secondly, NECs and carotid body glomus cells are thought to be homologous structures (Milsom & Burleson, 2007). Thirdly, genomic initiatives have yielded a fully sequenced/annotated genome allowing for manipulations such as gene knockdown. Finally, methods for the isolation and primary culture of NECs are well established in zebrafish (Jonz et al. 2004).
To test the hypothesis that H2S is involved in O2 sensing and promoting the hyperventilatory responses to hypoxia in zebrafish, we first examined the effects of exogenous sodium sulphide (Na2S) on ventilation in adults and larvae. We predicted that ventilation would increase in a dose-dependent manner and that this response would resemble the hypoxic ventilatory response (HVR). Secondly, we determined the effects of pharmacologically inhibiting in adults, or removing (by splice blocking gene knockdown) in larvae, two of the biosynthetic enzymes of H2S (CBS and CSE). Based on findings from mammalian studies (Buckler, 2012; Makarenko et al. 2012), we predicted that inhibiting or knocking down these enzymes would abolish or blunt the HVR. Thirdly, ratiometric Ca2+ imaging was performed to determine the in vitro response of isolated NECs to exogenous H2S. In keeping with a previous report of H2S-evoked membrane depolarization in zebrafish NECs (Olson et al. 2008), we predicted that exogenous H2S would cause an increase in intracellular [Ca2+] in these cells. In addition, we used immunohistochemistry to localize CBS and CSE to the NECs of adult gill, and CSE to NECs of larval skin.
Methods
Ethical approval
All procedures were conducted in accordance with the guidelines established by the Canadian Council for Animal Care and were approved by the University of British Columbia Animal Care Committee (Protocol A06-1510) and University of Ottawa Animal Care Committee (Protocol BL-226).
Animals
Adult zebrafish, Danio rerio (Hamilton 1822), were obtained from commercial suppliers (Noah's Pet Ark or Delta Aquatics, Vancouver, BC, Canada; Big Al's Aquarium Services, Ottawa, ON, Canada) and were maintained on a 12 : 12 h light : dark cycle at 28°C in either dechlorinated City of Vancouver tap water [ionic composition of the water: Na+, 0.08 mm; Cl−, 0.06 mm; Ca2+, 0.03 mm; K+, 0.004 mm; pH 7.0 (Metro Vancouver Water Quality Report, 2011)] or dechloraminated City of Ottawa tap water [ionic composition of the water: Na+, 0.78 mm; Cl−, 0.4 mm; Ca2+, 0.25 mm; K+, 0.025 mm; pH 7.0 (Xxxx)]. Embryos were obtained using standard zebrafish breeding techniques (Westerfield, 2000). Fertilized eggs were placed in Petri dishes under conditions identical to those in which the adults were maintained except that 0.05% methylene blue was added to the holding water.
Series I: adult zebrafish
Ventilatory responses of adult zebrafish to Na2S
The ventilation rate in adult zebrafish was measured non-invasively as described previously (Vulesevic et al. 2006). Briefly, adult zebrafish were placed in cylindrical plastic chambers (University of Ottawa). Each chamber was supplied with a continuous water flow (∼1 ml min−1) using gravity. Two electrodes were submerged in the water inside the chamber and mesh was used to prevent the fish from coming into contact with the electrodes. The analog signals from the electrodes, which represented opercular displacements, were amplified (the amplifier was custom-built at the University of Ottawa) and recorded to a computer as digital data using an A/D interfacing system and data acquisition software (AcqKnowledge; BioPac Systems, Inc., Galeta, CA, USA) at a sampling rate of 500 Hz. Although previous studies have used calibration procedures to quantify the magnitude of the linear deflections (in mm) of the opercular movements (Vulesevic & Perry, 2006; Vulesevic et al. 2006), the current study focused exclusively on quantifying breathing frequencies because ventilation amplitude in zebrafish is largely unaffected by hypoxia (Vulesevic & Perry, 2006). Thus, in the current study, each fish was assumed to have a resting breathing amplitude of 0.5 mm and the system was calibrated accordingly. Breathing frequencies were determined by post hoc analysis of the AcqKnowledge files.
Adult zebrafish were placed in the breathing recording chambers for 1–3 h prior to the start of experiments. After breathing had been recorded under control conditions, different groups of zebrafish were exposed to one of five concentrations of Na2S (10 μm, 19 μm, 37 μm, 75 μm and 150 μm) for 5 min; no fish was exposed to more than a single level of Na2S. Measurements were taken during the last minute of the recording period. The fish were allowed to recover for 5 min and breathing frequency was quantified during the final minute of recovery.
Effect of H2S synthesis inhibitors on ventilation in adult zebrafish during acute hypoxia
The CBS and CSE inhibitors aminooxyacetate (AOA) (Sigma-Aldrich Canada Co., Oakville, ON, Canada) and propargyl glycine (PPG) (Sigma-Aldrich Canada Co.) were used to further test the role of H2S in the control of breathing in adult zebrafish. Fish were exposed for 16–20 h to a cocktail of the two conventional inhibitors AOA and PPG (0 μm, 100 μm or 250 μm each) prior to the start of experiments. Ventilation was measured as described above during resting conditions (pre-exposure) and the measurements were continued as fish were exposed to hypoxia (40 mmHg) for 5 min and then allowed to recover under normoxic conditions. Measurements were taken over the final minute of each time period (pre-exposure, hypoxia and recovery).
A separate group of zebrafish that had been exposed to a cocktail of AOA and PPG (250 μm) for 16–20 h were exposed to hypoxia (40 mmHg) for 5 min, and then exposed to both hypoxia (40 mmHg) and Na2S (150 μm) for 5 min.
Dissociation, isolation and primary culture of NECs from adult gills
Adult zebrafish were stunned by a sharp blow to the head and killed by decapitation. NECs were isolated as previously described (Jonz et al. 2004; Qin et al. 2010). All NEC isolation procedures were performed under sterile conditions in a laminar flow hood. Gill baskets were excised and rinsed in 2% penicillin-streptomycin (Sigma-Aldrich Canada Co.) in phosphate-buffered saline (PBS) for 10 min. PBS contained the following: 137 mm NaCl, 15.2 mm Na2HPO4, 2.7 mm KCl, 1.5 mm KH2PO4, at pH 7.8 (Bradford et al. 1994; Jonz et al. 2004). All eight gill arches were separated and thoroughly cleaned of blood and mucous. Distal filaments rich in NECs were selectively removed and placed in 0.25% trypsin/EDTA (Life Technologies, Invitrogen Canada, Inc., Burlington, ON, Canada) for 1 h at 28°C. Tissue was then subjected to mechanical dissociation with fine forceps and triturated in a 15 ml centrifuge tube with a Pasteur pipette for 3 min. The trypsin reaction was stopped with the addition of 10% fetal calf serum (FCS) and undigested tissue was allowed to settle to the bottom of the centrifuge tube. The cell suspension was centrifuged (130 g) for 5 min and the pellet triturated in filter-sterilized PBS. The cell suspension was centrifuged once more with PBS and the pellet resuspended in Leibovitz's L-15 medium with l-glutamine (Life Technologies Inc./Gibco, Grand Island, NY, USA) supplemented with 1% penicillin-streptomycin and 2% FCS. Cells were plated in 0.2 ml L-15 on 35 mm glass-bottomed culture dishes (MatTek Corp., Ashland, MA, USA) coated with 0.1 mg ml−1 poly-l-lysine (Sigma-Aldrich Canada Co.) and Matri-Gel (Becton, Dickenson & Co., Mississauga, ON, Canada). Cells were supplemented with 2 ml L 16–18 h after isolation, and imaging was carried out 4–5 h thereafter.
Immunohistochemistry of dissociated NECs
Immunohistochemistry in dissociated cells was performed as previously described (Qin et al. 2010). Cells were obtained from two separate dissociations, each performed in 10–12 adult zebrafish. Briefly, 24 h after dissociation, cells that were plated on sterile 35 mm glass-bottomed culture dishes (see above) were fixed with 4% Paraformaldehyde (PFA) in PBS for 30 min at room temperature. The cells were incubated with primary antibodies, mouse anti-5-HT, 1 : 250 (catalogue no. ab16007; Abcam, Inc., Toronto, ON, Canada), and rabbit anti-CSE, 1 : 100, commercial polyclonal antibody raised against human CSE (ARP46067_P050; Aviva Systems Biology, San Diego, CA, USA), the epitope of which shares 100% identity with zebrafish CSE (accession no. AAH67624.1), or rabbit anti-CBS, 1 : 100, a commercial polyclonal antibody raised against human CBS (ARP45746_T100; Aviva Systems Biology), the epitope of which is 92% identical to both zebrafish cbsa and cbsb. Primary antibodies were diluted in PBS with 0.5% Triton-X overnight at 4°C. The plates were rinsed (3 × 3 min in PBS) and then cells were incubated with the secondary antibody (1 : 200; AlexaFluor 488 anti-rabbit and AlexaFluor 594 anti-mouse; Molecular Probes, Invitrogen Canada, Inc.) in PBS for 1 h at room temperature. The plates were rinsed (3 × 3 min in PBS) and then mounted with 4′6′-diamidino-2-phenylindole (DAPI) (Vectashield; Vector Laboratories, Inc., Burlingame, CA, USA). Cells were visualized using a fluorescent microscope (Observer A1; Carl Zeiss Jena GmbH, Jena, Germany). Control labelling experiments which excluded incubation with primary antibodies demonstrated lack of non-specific labelling of any of the secondary antibodies. Pre-absorption controls were performed by incubating the CSE antibody for 2 h at room temperature in the presence of CSE blocking peptide (AAP46067; Aviva Systems Biology) at a 1 : 10 antibody to peptide ratio, followed by immunohistochemistry as described above.
Calcium imaging
Calcium imaging was performed as described previously (Zhang et al. 2011). The fluorescent indicators Fura-2-LeakRes-AM (Teflabs, Inc., Austin, TX, USA), hereafter referred to as Fura-2, was used to monitor [Ca2+]i. Cells were obtained from two separate cell dissociations. Cells that adhered to the culture substrate were incubated in a solution of 5 μm Fura-2 in normal Ringer solution containing 135 mm NaCl, 5 mm KCl, 2 mm Mg2Cl, 2 mm Ca2Cl, 10 mm glucose and 10 mm Hepes (pH 7.8) and 0.1% v/v of 10% w/v Pluoronic F-127 (Invitrogen Canada, Inc.) at 28°C for 1 h. Afterward, cells were washed in indicator-free Ringer solution for an additional 30 min at 28°C. NECs were identified using 4 mg ml−1 Neutral Red (NR) (Sigma-Aldrich Canada Co.), a vital marker used to identify neurosecretory cells (Stuart et al. 1974; Youngson et al. 1993), including NECs (Jonz et al. 2004).
Ratiometric imaging was performed using a Nikon Eclipse microscope (Nikon Instruments, Inc., Melville, NY, USA) equipped with a Lamda DG-5 high-speed wavelength changer (Sutter Instrument Co., Novato, CA, USA), a Nikon 40× water-dipping objective lens with a numerical aperture of 0.8 and a digital CCD camera (QImaging Corp., Surrey, BC, Canada). Dual images at 340 nm and 380 nm excitation (510 nm emission) for Ca2+ were acquired every 2 s and ratiometric data were obtained using Northern Eclipse software (Empix Imaging, Inc., Mississauga, ON, Canada). Data are represented as raw ratios (R340/380) and thus indicate relative changes in [Ca2+]i.
Dishes containing NR/Fura-2 positive cells were fitted with a perfusion chamber insert (Warner Instruments, Inc., Hamden, CT, USA) and mounted on the microscope stage. The chamber was perfused continuously under gravity (3–4 ml min−1) with Ringer solution at room temperature (22–24°C). Multiple perfusion reservoirs (50 ml syringes) were connected to a single gas-impermeable tube that was used to deliver the perfusate. A three-way valve was used to switch between control (Ringer solution bubbled with air) and experimental treatments. Stock solutions of Na2S were prepared daily and diluted in Ringer solution to achieve a final concentration of 50 μmol l−1. This concentration was chosen as it has been shown previously to produce membrane depolarization in dissociated NECs of zebrafish (Olson et al. 2008) and to produce a robust ventilatory response in adult zebrafish (this study). The solution within the reservoir was continuously pumped through the outflow opening of the perfusion chamber via a variable flow mini-pump (Fisher Scientific, Inc., Pittsburgh, PA, USA). The volume of Ringer solution within the perfusion chamber never exceeded 400 μl.
Series II: larval zebrafish
Ventilatory responses
Ventilatory responses of zebrafish larvae were obtained as described previously (Jonz & Nurse, 2005; Coccimiglio & Jonz, 2012). Briefly, 4 day post-fertilization (dpf) larvae were anaesthetized with 0.05 mg ml−1 Tris buffered MS-222 (Westerfield, 2000) to minimize gross movements. The larvae were placed in a groove in a plastic chamber and were confined to a small area of the well using fine mesh. The dish was perfused with solutions using gravity. The experimental chamber was placed on the water jacket stage of a dissecting microscope (model SZX10; Olympus Canada, Inc., Richmond Hill, ON, Canada), which was maintained at 28°C using a water bath (model RC 6; Lauda-Brinkmann LP, Delran, NJ, USA). Larvae were left undisturbed in the chamber for 20 min before the experiments were started. Hypoxia was achieved by bubbling a mixture of air and nitrogen to the perfusion chamber using a gas mixer (model GF-3/MP; Cameron Instruments, Inc., Port Aransas, TX, USA). After a pre-exposure period of 3 min, the larvae were exposed for 5 min to either hypoxia (25 mmHg) or Na2S (37 μm, 75 μm or 150 μm made fresh several times per day). The larvae were then allowed to recover for 5 min; breathing rate was determined during the last minute of each of these time periods. Depending on the position and orientation of the larvae, breathing frequency was determined by counting either buccal or opercular movements. For larvae experiencing gene knockdown, the protocol was modified slightly so that the hypoxia and recovery periods were extended to 10 min. In these experiments, breathing rate was measured after 2 min, 4 min, 6 min and 9 min of hypoxia and after 4 min and 9 min of recovery from hypoxia.
Knockdown of CBS and CSE
To provide additional evidence that H2S is involved in the control of breathing, H2S biosynthetic enzymes were knocked down by injecting fertilized embryos with antisense oligonucleotide morpholinos (Gene Tools LLC, Philomath, OR, USA) targeting CSE (5′-CGCACAAGAGTGAACAGCTCTCTGT-3′) or CBS (5′-TTGTCCTGTGAGAAAAAGCTGCATT-3′). In the zebrafish there are two paralogues of CBS: CBSa and CBSb. Based on its more widespread distribution at 4 dpf (Thisse et al. 2001), we chose to focus on CBSb rather than CBSa, which appears to be restricted to the liver and pancreas of larvae at 4–5 dpf (Thisse & Thisse, 2004). The CBSb morpholino was designed to splice out exon 3 (385–491 bp of NM_001014345.2|), and the CSE morpholino was designed to splice out exon 1 (57–219 bp of NC_007117.5).
The morpholinos, which were designed by GeneTools (http://www.gene-tools.com/), were prepared to a final concentration of 4 ng nl−1 in 1× Danieau buffer (58 mm NaCl, 0.7 mm KCl, 0.4 mm MgSO4, 0.6 mm Ca(NO3)2, and 5.0 mm Hepes; pH 7.6) and 0.05% phenol red. Injections were performed using a microinjector system (model IM 300; Narishige International USA, Inc., New York, NY, USA). Control groups were injected with a standard control morpholino (5′-CCTCTTACCTCAGTTACAATTTATA-3′) from GeneTools prepared in the same way as the CSE and CBS morpholinos. The morpholino was injected at a dose of 4 ng embryo−1 at the 1–2 cell stages. No significant mortality or deformities were observed up to 4 dpf. Morpholinos were conjugated with fluorescein isothiocyanate (FITC) and 1 day after injection, embryos were screened using a microscope (model SMZ1500; Nikon Instruments, Inc.) for the presence of widely distributed carboxyfluorescein. Embryos that were FITC-positive were raised to 4 dpf in dechloraminated University of Ottawa tap water (see above) supplemented with 0.05% methylene blue.
Immunohistochemistry of larvae
Larvae were killed by an overdose of MS-222 and placed in 4% paraformaldehyde prepared in PBS and left overnight at 4°C. Larvae were rinsed with PBS and placed in a permeabilizing solution containing 2% Triton-X in PBS overnight at 4°C. The larvae were then blocked using 10% normal donkey serum (NDS) in PBS for 1 h and incubated in primary antibodies (goat anti-5-HT, 1 : 500, ImmunoStar and rabbit anti-CSE, 1 : 250, or rabbit anti-CBS, 1 : 250; see above) diluted in PBS containing 3% NDS overnight at room temperature. Next, the larvae were rinsed in PBS and incubated with the secondary antibodies (1 : 500, AlexaFluor 488 and 1 : 1000, AlexaFluor 568; Molecular Probes, Invitrogen Canada, Inc.) diluted in PBS containing 3% NDS for 2 h at room temperature. Following another rinse in PBS, the larvae were mounted in water onto glass slides. The larvae were observed and images were captured using a confocal microscope (Fluoview FV10i; Olympus Corp., Tokyo, Japan) equipped with a solid state laser emitting at 405 nm, 473 nm and 559 nm. Z-stacks of 9–19 optical sections taken 1.0 μm apart were captured using the 60× objective of this microscope.
Immunohistochemistry and knockdown validation experiments
In addition to pre-absorption controls (see above), the effectiveness of the CSE antibody and the CSE knockdown were confirmed by Western blotting using the same polyclonal antibody used for immunohistochemistry (see above). Total protein was extracted from morpholino- and sham-injected larvae using Tris buffer (10 mm Tris-HCl with 2% Triton X-100; pH adjusted to 7.4) supplemented with protease inhibitor tablet (Complete Mini; Roche, Mississauga, ON, Canada). Ten larvae were pooled to prepare one sample (n = 1). Extracted samples were loaded onto a 10% SDS-PAGE, size-fractionated at 200 V and transferred onto a polyvinylidene difluoride (PVDF) membrane [Bio-Rad Laboratories (Canada) Ltd, Mississauga, ON, Canada]. After transfer, membranes were blocked with 5% BSA in 0.2% Tween 20 in Tris-buffered saline (TBST) for 2 h at room temperature. Membranes were incubated overnight with gentle shaking with anti-CSE antibody (1 : 750 in 2% BSA in TBST) at 4°C. After that, membranes were washed (3 × 5 min) with TBST and incubated with horseradish peroxidase-conjugated secondary antibody against rabbit IgG (Life Technologies, Invitrogen Canada, Inc.) (1 : 15,000 in 2% BSA in TBST) for 2 h at room temperature. The membranes were then washed (4 × 10 min) and the immunoreactive bands were detected using enhanced chemi-luminescence (Millipore Corp., Billerica, MA, USA) with a ChemiDoc system [Bio-Rad Laboratories (Canada) Ltd]. Afterwards, the membrane was re-probed with β-actin antibody (1 : 4000; Sigma-Aldrich Canada Co.) after stripping with Re-Blot Plus solution (Millipore Corp.).
The effectiveness of the CBS knockdown was confirmed by RT-PCR using a Bio-Rad S1000 Thermal Cycler. Whole-body RNA extraction was performed in 100 embryos per treatment using Trizol (Life Technologies, Invitrogen Canada, Inc.) according to the manufacturer's specifications and cDNA was prepared by treating 1 μg of extracted RNA with DNase (Life Technologies, Invitrogen Canada, Inc.) and RevertAid M-MNuLV reverse transcriptase (Fermentas International, Inc., Burlington, ON, Canada) according to the manufacturers’ instructions. Four sets of primers were used: set 1: forward 5′-ATAGACATGCTGGTCGCAGG-3′ and reverse 5′-CCTCCGGATCCACTCCAATG-3′; set 2: forward 5′-TCAGAATCAGTTGGCACTGG-3′ and reverse 5′-AATCATACCCGATGCCTTCA-3′; set 3: forward 5′-TCAGAATCAGTTGGCACTGG-3′ and reverse 5′-ATCATACCCGATGCCTTCAA-3′, and set 4: forward 5′-AACCATGCCAGCAGTACCAT-3′ and reverse 5′-AATCATACCCGATGCCTTCA-3′. Additionally, 18 S primers (forward 5′-GGCGGCGTTATTCCCATGACC-3′ and reverse 5′-GGTGGTGCCCTTCCGTCAATTC-3′) were used as controls. The PCR conditions were: 94°C for 4 min; 21 cycles of 94°C for 30 s, 56°C for 30 s and 72°C for 1 min, and a final step of 72°C for 5 min.
Statistics
Data are routinely presented as the mean ± 1 s.e.m. All statistical analyses were performed using SigmaPlot Version 10.0 (Systat Software, Inc., San Jose, CA, USA). A significance level of 0.05 was used throughout. One-way repeated measures (RM) ANOVA was used to determine the effects of individual concentrations of Na2S, followed by a Holm–Sidak post hoc test. A one-way ANOVA was performed to compare the magnitude of responses to the various concentrations of Na2S, followed by a post hoc test on ranks (Dunn's test). To evaluate the responses to hypoxia with and without exposure to AOA/PPG cocktails, a two-way RM ANOVA was used to test for differences between treatments (control, 100 μmol l−1 and 250 μmol l−1) and between normoxia and hypoxia within a treatment. This analysis was followed by a Holm–Sidak post hoc multiple comparison test. To test for differences in the ventilatory responses of morphant larvae, a two-way RM ANOVA was used, followed by a Holm–Sidak post hoc comparison. A two-way RM ANOVA was used to test for differences within and among different concentrations of Na2S in larvae and was followed by a Holm–Sidak post hoc comparison. A paired t test was used to determine if Na2S produced a significant increase in [Ca2+]i in isolated NECs.
Results
Series I: adult zebrafish
Ventilatory responses of adult zebrafish to Na2S
Many adult zebrafish exhibited episodic breathing under control conditions, but switched to continuous breathing when exposed to Na2S or hypoxia (Fig. 1A). Adult zebrafish significantly increased gill breathing frequency by 11%, 36%, 69%, 152% and 72% compared with pre-exposure conditions when exposed to 10 μm, 19 μm, 37 μm, 75 μm and 150 μm Na2S, respectively (Fig. 1B and C).
Figure 1. Effects of exogenous hydrogen sulphide (H2S) on ventilation in adult zebrafish (Danio rerio).
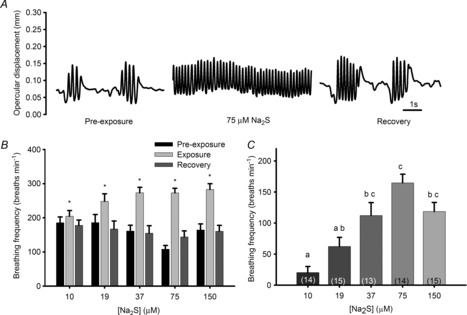
A, representative ventilation traces from one zebrafish before (pre-exposure), during and after (recovery) exposure to 75 μm sodium sulphide (Na2S), an H2S donor. B, average breathing frequency (breaths min−1) of adult zebrafish exposed to various concentrations of Na2S (μm). C, changes in breathing frequency of adult zebrafish exposed to increasing concentrations of Na2S (μm). *, significant (P < 0.05) difference from pre-exposure values. Bars not sharing the same letters are significantly different from one another. Values are means ± s.e.m.; sample sizes are indicated in parentheses.
Effect of inhibiting endogenous H2S production on ventilation
Zebrafish exposed to a cocktail of AOA and PPG exhibited a dose-dependent blunting of the hypoxic ventilatory response (P < 0.05) (Fig. 2A–C). When fish treated with 250 μm of each of AOA and PPG were exposed to hypoxia, the addition of Na2S partially rescued the hypoxic ventilatory response (P < 0.05) (Fig. 2D).
Figure 2. Effects of simultaneously inhibiting both cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) on ventilatory responses of adult zebrafish (Danio rerio) to acute hypoxia.
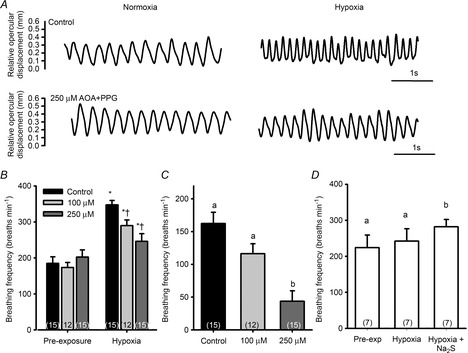
A, representative ventilation traces from one zebrafish during exposure to normoxia and hypoxia (40 mmHg) before (control) and after exposure to 250 μm of both aminooxyacetate (AOA) and propargyl glycine (PPG). B, average ventilatory responses (breaths min−1) of adult zebrafish exposed to hypoxia after treatment with 100 μm or 250 μm of both AOA and PPG. C, average changes in breathing frequency of fish exposed to hypoxia (40 mmHg) under control conditions or after exposure to either 100 μm or 250 μm of both AOA and PPG. D, fish pretreated with 250 μm AOA and PPG exhibited no hypoxic ventilatory response. However, adding sodium sulphide (Na2S) [a hydrogen sulphide (H2S) donor] to the water partially rescued (restored) the response. *, significant (P < 0.05) difference from pre-exposure values; †, significant difference from control. Bars not sharing the same letters are significantly different from one another. Values are means ± s.e.m.; sample sizes are indicated in parentheses.
Calcium imaging
Four out of six cells tested responded to 50 μm Na2S with an increase in [Ca2+]i; this rate of response (66%) was similar to response rates (60%; based on membrane potential measurements) reported previously for zebrafish NECs exposed to acute hypoxia (Jonz & Nurse, 2003). On average, the responsive NECs exhibited a small (22%) but significant (P < 0.05) increase in [Ca2+]i (Fig. 3) when exposed to 50 μm Na2S.
Figure 3. Effects of sodium sulphide (Na2S) on intracellular calcium levels (as estimated by Fura-2 340/380 nm excitation ratios) in dissociated neuroepithelial cells (NECs) from gills of adult zebrafish (Danio rerio).
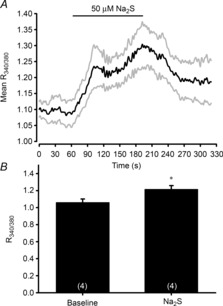
A, mean normalized trace of all dissociated NECs responding to 50 μm Na2S. An increase in the R340/380 represents a rise in intracellular Ca2+. The grey lines represent continuous standard errors. B, average response of dissociated NECs to 50 μm hydrogen sulphide (H2S). *, significant (P < 0.05) difference from the pre-exposure value. Values are means ± s.e.m.; numbers are indicated in parentheses.
Immunohistochemistry of dissociated NECs
Triple labelling of dissociated cells labelled with markers for serotonin (5-HT), CBS and cell nuclei (DAPI) revealed that NECs, identified via 5-HT-immunoreactivity (5-HT-IR) expressed CBS (Fig. 4A, arrows). In addition, other unidentified cells in the culture (non 5-HT-IR cells) were positive for CBS-IR but not all cells (based on DAPI staining) expressed CBS (Fig. 4A, arrowheads). Triple labelling of dissociated cells labelled with markers for 5-HT, CSE and cell nuclei revealed that all dissociated NECs that were 5-HT-IR contained CSE (Fig. 4B, arrows); other cells in culture were only CSE-IR (Fig. 4B, arrowheads), and others were negative for both 5-HT and CSE. CSE peptide pre-absorption resulted in the absence of CSE labelling of 5-HT positive cells (Fig. 4B, insets).
Figure 4. Immunohistochemical labelling of dissociated neuroepithelial cells (NECs) from gills of adult zebrafish (Danio rerio).
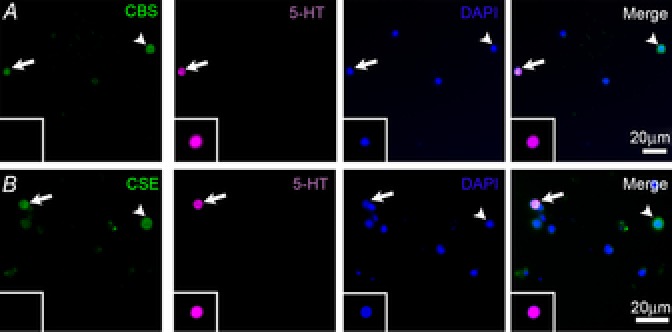
A, NECs were labelled using antibodies against cystathionine β-synthase (CBS) and serotonin (5-HT). B, NECs were labelled using antibodies against cystathionine γ-lyase (CSE) and 5-HT. In both panels cell nuclei were labelled with DAPI. The merged image is an overlay of all three channels. Arrows indicate cells labelled with either CBS or CSE and 5-HT. The inset shows CSE pre-absorption controls showing a 5-HT positive NEC without any CSE labelling. Arrowheads show cells labelled with either CBS or CSE, but not 5-HT. Minor adjustments to the brightness and contrast of the whole image were made using Adobe Photoshop Version 7.0.1.
Series II: larval zebrafish
Ventilatory responses of zebrafish larvae to Na2S
Ventilatory frequency was low (14.4 ± 6.8 breaths min−1) in larval zebrafish exposed to control conditions (aerated water), but increased by 37%, 160% and 520% when exposed to 37 μm, 75 μm and 150 μm Na2S, respectively (Fig. 5). These changes were statistically significant for the two highest concentrations (P < 0.05) and differed significantly from one another, indicating a dose-dependent response (P < 0.05).
Figure 5. Ventilatory responses (breaths min−1) of zebrafish larvae (4 days post-fertilization) exposed to various concentrations of sodium sulphide (Na2S).
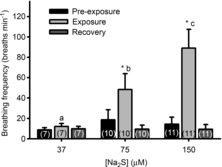
*, significant (P < 0.05) difference from pre-exposure value. Bars not sharing the same letters are significantly different from one another. Values are means ± s.e.m.; sample sizes are indicated in parentheses.
Effect of CBS/CSE knockdown on ventilatory responses to hypoxia
Double knockdown of CBS and CSE was lethal; the embryos did not survive past 24 h of development. Larvae experiencing CBS or CSE knockdown developed normally to 4 dpf and appeared similar to sham-injected larvae. Sham-injected larvae exhibited a robust increase in ventilation frequency in response to hypoxia (P < 0.001) (Fig. 6A and B). Larvae experiencing CSE (Fig. 6A) or CBS (Fig. 6B) knockdown failed to exhibit a statistically significant hypoxic ventilatory response. At all measurement periods during hypoxia, ventilation frequency in the CSE/CBS morphants was significantly lower than in the sham-injected larvae (P < 0.05) (Fig. 6A and B). CSE knockdown was confirmed by Western blotting (Fig. 6C), which showed a 98% reduction in CSE protein expression level (data not shown). CBS knockdown was confirmed using RT-PCR (Fig. 6D).
Figure 6. Effects of knockdown on ventilatory responses in larval zebrafish (Danio rerio) hypoxia.
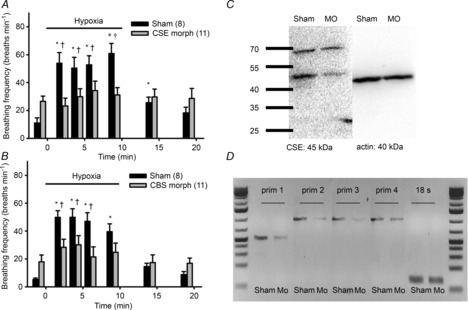
The hypoxic (25 mmHg) ventilatory response of zebrafish larvae (4 days post-fertilization) in which expression of either cystathionine γ-lyase (CSE) (A) or cystathionine β-synthase (CBS) (B) was knocked down using morpholinos. C, representative Western blot of protein derived from morpholino- (MO) and sham-injected zebrafish larvae labelled with the CSE antibody confirming specificity of this antibody. D, confirmation of CBS knockdown using RT-PCR in both CBS- (Mo) and sham morpholino-injected larvae. Four sets of primers were used: set 1 (prim 1): forward 5'-ATAGACATGCTGGTCGCAGG-3' and reverse 5'-CCTCCGGATCCACTCCAATG-3'; set 2 (prim 2): forward 5'-TCAGAATCAGTTGGCACTGG-3' and reverse 5'-AATCATACCCGATGCCTTCA-3'; set 3 (prim 3): forward 5'-TCAGAATCAGTTGGCACTGG-3' and reverse 5'-ATCATACCCGATGCCTTCAA-3'; set 4 (prim 4): 5'-AACCATGCCAGCAGTACCAT-3' and reverse 5'-AATCATACCCGATGCCTTCA-3'. Additionally, 18 S primers (forward 5′-GGCGGCGTTATTCCCATGACC-3′ and reverse 5′-GGTGGTGCCCTTCCGTCAATTC-3′) were used. *, significant difference (P < 0.05) from pre-exposure (normoxia) value; †, significant difference (P < 0.05) from CBS or CSE morphants. Mo, CBS or CSE morphant. Values are means ± s.e.m.; sample sizes are indicated in parentheses.
Whole-mount immunohistochemistry
In all immunohistochemical procedures, omission of the primary antibody eliminated positive labelling. The efficiency of the CSE antibody was confirmed by Western blotting (Fig. 6C). Double labelling of dissociated cells using antibodies against 5-HT and CSE revealed that some 5-HT-IR cells also labelled with CSE (Fig. 7). However, some cells were 5-HT-IR but did not contain CSE, but very few CSE-IR cells did not label for 5-HT. No positive labelling was detected in NECs with the CBS antibody (data not shown).
Figure 7. Immunohistochemical labelling of neuroepithelial cells (NECs) of developing zebrafish (Danio rerio).
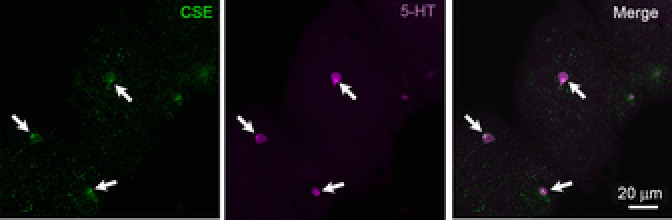
Whole mounts of 4 days post-fertilization larvae were labelled with antibodies against cystathionine γ-lyase (CSE) and serotonin (5-HT). The image shows a section of the body, posterior to the yolk sac projection; the anterior part of the larvae is to the top right and the posterior towards the bottom left. Arrows indicate cells that double-label with CSE and 5-HT. Minor adjustments to the brightness and contrast of the whole image were made using Adobe Photoshop Version 7.0.1.
Discussion
In zebrafish, NECs in culture experience membrane depolarization in response to hypoxia (Jonz et al. 2004) and hypercapnia (Qin et al. 2010). Further, NECs are believed to be phylogenetic precursors to the carotid body glomus cells with which they share several distinct characteristics (Milsom & Burleson, 2007) including K+ conductance, which is inhibited by lowered O2 and increased CO2. In addition, they are innervated and contain synaptic vesicles and neurotransmitters. These findings, when combined with the results of pharmacological treatments used to characterize chemoreceptors in mammals, have led to the theory that NECs are the putative O2 and CO2 chemoreceptors in fish (Jonz et al. 2004; Qin et al. 2010). Both NECs and glomus cells are in close proximity to blood vessels carrying oxygenated blood and thus are ideally located for sensing changes in blood O2 tension. Although several mechanisms have been proposed to explain O2 sensing in mammalian glomus cells (Lόpez-Barneo et al. 2001; Chandel, 2010; Peers et al. 2010; Weir & Archer, 2010), the mechanisms by which NECs detect changes in O2 remain largely unknown.
To determine if exogenous H2S is capable of stimulating breathing in vivo in zebrafish, larvae and adults were exposed to Na2S in order to generate H2S. Although previous studies have used similar approaches to increase exogenous H2S, it is nevertheless challenging to calculate the exact amount of H2S produced by these chemical treatments and therefore the exact dose to which cells, or in this case fish, are being exposed (Olson, 2013a). Moreover, H2S is highly volatile and thus levels of H2S in solution after the addition of Na2S are certain to decrease over time. Despite these issues, both zebrafish adults and larvae showed a dose-dependent increase in breathing frequency in response to H2S produced via dissociation of Na2S (Figs 1 and 5). Similar increases in ventilation to exogenous H2S used at similar concentrations were reported previously in mice (Li et al. 2010), rats (Peng et al. 2010) and trout (Olson et al. 2008; Li et al. 2010; Peng et al. 2010). Unlike in trout (Olson et al. 2008), in zebrafish, breathing frequency rather than breathing amplitude increased in response to Na2S. The differing modes of hyperventilation are likely to reflect species differences because trout respond to hypoxia by predominantly increasing breathing amplitude, whereas zebrafish typically show a much larger increase in frequency (Perry et al. 2009b). Overall, H2S produces an increase in ventilation similar to the HVR in both trout and zebrafish, indicating that exogenous H2S produces effects similar to hypoxia in fish.
To determine if H2S was necessary to initiate the HVR in zebrafish, H2S biosynthesis was impaired. In adult zebrafish, inhibiting CBS and CSE with a cocktail of AOA and PPG blunted or abolished the HVR and exogenous H2S produced from Na2S rescued, at least in part, the response (Fig. 2A–D). Rats and mice treated with PPG showed a blunted or no carotid body response to hypoxia and a blunted or no HVR (Peng et al. 2010; Makarenko et al. 2012), indicating that CSE plays an important role in the HVR in mammals. Similarly, in a separate study, mice treated with AOA, a CBS inhibitor, also showed a blunted HVR and impaired carotid body sensory response (Li et al. 2010). In trout, the addition of a cocktail of both CBS and CSE inhibited H2S production in homogenized gill filaments, but AOA and PPG did not blunt the HVR or hypoxic bradycardia (Olson et al. 2008). It is possible that the discrepancy between the effects of enzyme inhibition on ventilation in these two studies is related to methodological differences. Trout were exposed to a lower dose (5 mg l−1; 46 μm and 44 μm of AOA and PPG, respectively) of the AOA/PPG cocktail for a shorter period of time (30 min), whereas in the current study zebrafish were exposed to higher doses (100–250 μm) of the AOA/PPG cocktail for longer periods of time (16–20 h). Our results suggest that longer incubation periods might be necessary to allow for uptake of these inhibitors from the water and thus inhibition of the enzymes.
In zebrafish larvae, double knockdown of CBS and CSE was lethal past 24 h, confirming the importance of H2S in the development of these animals. The efficacy of CSE knockdown was confirmed by Western blotting (Fig. 6C) with a 98% reduction in CSE protein expression level (quantification data not shown), whereas the knockdown of the CBS antibody was confirmed by RT-PCR (Fig. 6D). Knocking down either CBS or CSE abolished the HVR, indicating that H2S is necessary for the HVR of larvae (Fig. 6A and B). Similarly, CSE knockout mice had reduced carotid body sensitivity, a blunted HVR, and reduced H2S generation (Peng et al. 2010; Makarenko et al. 2012). Interestingly, in zebrafish larvae only CSE was localized to NECs; however, larvae experiencing CBS knockdown clearly exhibited a blunted HVR. As CBS is predominantly found in the central nervous system in other vertebrates (Olson, 2008), it is possible that the HVR may have been inhibited in the CNS of these animals, perhaps at the sensory integration sites of the medulla or the central rhythm generators. Indeed, the idea that CSE and CBS may generate H2S at two different sites involved in the regulation of breathing is consistent with our results showing that knockdown or pharmacological inhibition of a single isoform is able to abolish the HVR. At present, we do not have a way of labelling the brain of zebrafish larvae with these antibodies because antibody penetration of this tissue is poor in whole-mount preparations. In the current study, we showed that inhibiting H2S production blunts or abolishes the HVR in adults and larvae, indicating that H2S is necessary and sufficient to produce the HVR in zebrafish.
In fish, NECs are innervated by afferent nerves from cranial nerves IX and X (de Graaf, 1990; Sundin & Nilsson, 2002), which project to the general visceral nucleus, an area thought to be homologous to the nucleus tractus solitarius (NTS) in mammals (Sundin et al. 2003). In the present study, dissociated NECs showed an increase in [Ca2+]i in response to 50 μm Na2S. Although absolute intracellular Ca2+ levels were not measured, the increase in the 340/380 nm excitation ratio was markedly lower than in zebrafish NECs exposed to 5% CO2 (S. Abdallah, M. Jonz and S. F. Perry; unpublished observations, 2013). The changes in 340/380 nm excitation ratios reported here for cells exposed to Na2S were similar to the increases in 340/380 nm excitation ratios reported for dissociated NECs of goldfish (Carassius auratus) exposed to hypoxia (Zachar, 2013). Moreover, in a previous study, dissociated NECs of zebrafish depolarized (∼10 mV) in response to 50 μm Na2S and this depolarization was similar in magnitude to that observed in response to hypoxia (Olson et al. 2008). Taken together, these results show that H2S can depolarize NECs and lead to an increase in Ca2+, steps necessary for the release of neurotransmitters onto afferent nerve fibres and thus a reflex ventilatory response to hypoxia.
Immunohistochemistry was used to determine if the putative O2-sensing cells of zebrafish, the NECs, have the capacity to produce H2S. Similarly to the carotid body glomus cells of mammals (Li et al. 2010; Telezhkin et al. 2010; Fitzgerald et al. 2011; Makarenko et al. 2012), dissociated NECs of adult zebrafish expressed both CBS and CSE. These results are consistent with CBS and CSE mRNA expression in trout gills (Olson et al. 2008) and further demonstrate that the biosynthetic enzymes of H2S are present not only in the gills but specifically in NECs. Interestingly, zebrafish larvae appeared to express only CSE, which is predominantly expressed in peripheral tissues, whereas CBS is predominantly expressed in the CNS in mammals (Olson, 2008). In zebrafish larvae, the HVR develops before NECs form in the gills and innervated NECs on the skin are thought to be responsible for initiating the HVR (Coccimiglio & Jonz, 2012) prior to maturation of gill NECs. Skin NECs are similar to gill NECs, but may represent a different population of cells with similar characteristics. Our results suggest that CBS either does not become expressed in NECs until later in development or that skin NECs never express CBS. Additionally, we cannot exclude the possibility that CBS, although present in skin NECs, was undetectable by immunohistochemistry (as in the adult gill) on whole-mount preparations. Although the scope of this issue is challenging, future studies should attempt to localize CBS mRNA in larval NECs by in situ hybridization or to dissociate NECs from zebrafish larvae in a further attempt to localize CBS in these cells by immunohistochemistry.
There are two proposed mechanisms underlying H2S in O2 sensing based on observations that H2S concentration increases in tissues exposed to hypoxia. In one scenario, H2S production is dependent on O2 availability (Olson, 2013b). If O2 levels are adequate, H2S is oxidised in the nearby mitochondria (Olson, 2013b). When O2 levels in the cell drop, H2S accumulates as the rates of mitochondrial oxidation of H2S fall and vice versa (Olson et al. 2008). An alternative mechanism involves the production of CO by HO in normoxia and the inhibition of CSE by CO, and, therefore, an inhibition of H2S production (Prabhakar, 2013; Peng et al. 2014). In hypoxia, production of CO decreases, releasing the inhibition on CSE and thereby resulting in an increase in H2S production. In mammals, H2S is thought to act as a signalling molecule by inhibiting K+ channels, leading to an increase in [Ca2+]i (Li et al. 2010; Telezhkin et al. 2010; Buckler, 2012). Although further research is necessary to confirm a similar mode of action of H2S on K+ channels in fish, our data clearly revealed an increase in [Ca2+]i in response to exogenous H2S, presumably arising from membrane depolarization (Olson et al. 2008).
Conclusion
In the current study, adult and larval zebrafish exhibited a dose-dependent increase in breathing frequency when exposed to Na2S. Furthermore, adult zebrafish displayed a blunted HVR when pre-exposed to AOA and PPG, inhibitors of the biosynthetic enzymes of H2S; the normal hyperventilatory response was partially restored by the addition of Na2S. In larvae, knockdown of either CBS or CSE inhibited the HVR. Immunohistochemistry of NECs revealed the presence of both CBS and CSE in adults, whereas NECs of larvae appeared to express only CSE. Finally, in Ca2+ imaging experiments, dissociated NECs significantly increased [Ca2+]i in the presence of Na2S. Combined, these results indicate that H2S in NECs is involved in oxygen sensing and that it plays an important role in the HVR.
Acknowledgments
The authors thank Patrick Tamkee, Bill Fletcher and Vishal Saxena for their help with animal husbandry, Matt Pamenter for helpful advice, and the University of British Columbia Bioimaging Facility staff for technical help and equipment.
Glossary
- AOA
aminooxyacetate
- CBS
cystathionine β-synthase
- CO
carbon monoxide
- CSE
cystathionine γ-lyase
- DAPI
4′6′-diamidino-2-phenylindole
- dpf
days post-fertilization
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- HO
haemoxygenase
- H2S
hydrogen sulphide
- 5-HT
5-hydroxytryptamine (serotonin)
- HVR
hypoxic ventilatory response
- Na2S
sodium sulphide
- NEC
neuroepithelial cell
- NR
neutral red
- PPG
propargyl glycine
Additional information
Competing interests
None declared.
Author contributions
S.F.P., C.S.P., Y.K., S.J.A., J.P. and W.K.M. designed and conceptualized the experiments. S.F.P., C.S.P., S.J.A., J.P., R.W.M.K., H.M.Y. and Y.K. carried out the research. C.S.P, S.J.A., J.P., R.W.M.K., H.M.Y. and S.F.P analysed the data; S.F.P. and C.S.P. wrote the paper, and all authors contributed feedback to various drafts of the manuscript.
Funding
This study was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grants to W.K.M. and S.F.P.
Authors’ present addresses
C. S. Porteus: Department of Biosciences, College of Life and Environmental Sciences, University of Exeter, Exeter, UK.
Y. Kumai: School of Medicine, Case Western Reserve University, Cleveland, OH, USA.
References
- Althaus M. Gasotransmitters: novel regulators of epithelial Na+ transport? Front Physiol. 2012;3:83. doi: 10.3389/fphys.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford CS, Sun L, Collodi P, Barnes D. Cell cultures from zebrafish embryos and adult tissues. J Tissue Cult Methods. 1994;16:99–107. [Google Scholar]
- Buckler K. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012;463:743–754. doi: 10.1007/s00424-012-1089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513:819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS. Mitochondrial complex III: an essential component of universal oxygen sensing machinery? Respir Physiol Neurobiol. 2010;174:175–181. doi: 10.1016/j.resp.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccimiglio ML, Jonz MG. Serotonergic neuroepithelial cells of the skin in developing zebrafish: morphology, innervation and oxygen-sensitive properties. J Exp Biol. 2012;215:3881–3894. doi: 10.1242/jeb.074575. [DOI] [PubMed] [Google Scholar]
- de Graaf PJF. Innervation pattern of the gill arches and gills of the carp (Cyprinus carpio. J Morphol. 1990;206:71–78. doi: 10.1002/jmor.1052060108. [DOI] [PubMed] [Google Scholar]
- Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol. 2007;157:45–54. doi: 10.1016/j.resp.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Mustard KJW, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M, Chang I, Kostuk E, Kiihl S. The impact of hydrogen sulfide (H2S) on neurotransmitter release from the cat carotid body. Respir Physiol Neurobiol. 2011;176:80–89. doi: 10.1016/j.resp.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonz MG, Fearon IM, Nurse CA. Neuroepithelial oxygen chemoreceptors of the zebrafish gill. J Physiol. 2004;560:737–752. doi: 10.1113/jphysiol.2004.069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonz MG, Nurse CA. Neuroepithelial cells and associated innervation of the zebrafish gill: a confocal immunofluorescence study. J Comp Neurol. 2003;461:1–17. doi: 10.1002/cne.10680. [DOI] [PubMed] [Google Scholar]
- Jonz MG, Nurse CA. Development of oxygen sensing in the gills of zebrafish. J Exp Biol. 2005;208:1537–1549. doi: 10.1242/jeb.01564. [DOI] [PubMed] [Google Scholar]
- Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang L, Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal. 2010;12:1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- Lόpez-Barneo J, Pardal R, Ortega-Sáenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Makarenko VV, Nanduri J, Raghuraman G, Fox AP, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am J Physiol Cell Physiol. 2012;303:C916–C923. doi: 10.1152/ajpcell.00100.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom WK, Burleson ML. Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir Physiol Neurobiol. 2007;157:4–11. doi: 10.1016/j.resp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Olson KR. Hydrogen sulfide and oxygen sensing: implications in cardiorespiratory control. J Exp Biol. 2008;211:2727–2734. doi: 10.1242/jeb.010066. [DOI] [PubMed] [Google Scholar]
- Olson KR. Hydrogen sulfide is an oxygen sensor in the carotid body. Respir Physiol Neurobiol. 2011;179:103–110. doi: 10.1016/j.resp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Olson KR. Hydrogen sulfide: both feet on the gas and none on the brake? Front Physiol. 2013a;4:1–3. doi: 10.3389/fphys.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR. A Theoretical examination hydrogen sulfide metabolism and its potential in autocrine/paracrine oxygen sensing. Respir Physiol Neurobiol. 2013b;186:173–179. doi: 10.1016/j.resp.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Olson KR, Donald JA, Dombkowski RA, Perry SF. Evolutionary and comparative aspects of nitric oxide, carbon monoxide and hydrogen sulfide. Respir Physiol Neurobiol. 2012;184:117–129. doi: 10.1016/j.resp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, Whitfield NL, Yang G, Wang R, Perry SF. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R669–R680. doi: 10.1152/ajpregu.00807.2007. [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Peng Y-J, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci U S A. 2014;111:1174–1179. doi: 10.1073/pnas.1322172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y-J, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SF, McNeill B, Elia E, Nagpal A, Vulesevic B. Hydrogen sulfide stimulates catecholamine secretion in rainbow trout (Oncorhynchus mykiss. Am J Physiol Regul Integr Comp Physiol. 2009a;296:R133–R140. doi: 10.1152/ajpregu.00185.2008. [DOI] [PubMed] [Google Scholar]
- Perry SF, Jonz MG, Gilmour KM. Oxygen sensing and the hypoxic ventilatory response. In: Richards JG, Brauner CJ, Farrell AP, editors. Hypoxia. San Diego, CA: Academic Press; 2009b. pp. 194–255. [Google Scholar]
- Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol. 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci U S A. 1995;92:1994–1997. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Lewis JE, Perry SF. Zebrafish (Danio rerio) gill neuroepithelial cells are sensitive chemoreceptors for environmental CO2. J Physiol. 2010;588:861–872. doi: 10.1113/jphysiol.2009.184739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart AE, Hudspeth AJ, Hall ZW. Vital staining of specific monoamine-containing cells in the leech nervous system. Cell Tissue Res. 1974;153:55–61. doi: 10.1007/BF00225445. [DOI] [PubMed] [Google Scholar]
- Sundin L, Nilsson S. Branchial innervation. J Exp Zool. 2002;293:232–248. doi: 10.1002/jez.10130. [DOI] [PubMed] [Google Scholar]
- Sundin L, Turesson J, Burleson M. Identification of central mechanisms vital for breathing in the channel catfish, Ictalurus punctatus. Respir Physiol Neurobiol. 2003;138:77–86. doi: 10.1016/s1569-9048(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Telezhkin V, Brazier SP, Cayzac SH, Wilkinson WJ, Riccardi D, Kemp PJ. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir Physiol Neurobiol. 2010;172:169–178. doi: 10.1016/j.resp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Thisse B, Pflumio S, Fürthauer M, Loppin B, Heyer V, Degrave A, Woehl R, Lux A, Steffan T, Charbonnier XQ, Thisse C. 2001. Expression of the zebrafish genome during embryogenesis. ZFIN Direct Data Submission. Available at: http://zfin.org. [Accessed 10 January 2014.]
- Thisse B, Thisse C. 2004. Fast release clones: a high throughput expression analysis. ZFIN Direct Data Submission. Available at: http://zfin.org. [Accessed 10 January 2014.] [DOI] [PubMed]
- Vulesevic B, McNeill B, Perry SF. Chemoreceptor plasticity and respiratory acclimation in the zebrafish Danio rerio. J Exp Biol. 2006;209:1261–1273. doi: 10.1242/jeb.02058. [DOI] [PubMed] [Google Scholar]
- Vulesevic B, Perry SF. Developmental plasticity of ventilatory control in zebrafish, Danio rerio. Resp Physiol Neurbiol. 2006;154:396–405. doi: 10.1016/j.resp.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wang R. Hydrogen sulfide: a new EDRF. Kidney Int. 2009;76:700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol. 2010;174:182–191. doi: 10.1016/j.resp.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- Williams SEJ, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem. 2007;282:8092–8098. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]
- Zachar PC. A Comparative Study of Neuroepithelial Cells and O2. Ottawa, ON: Department of Biology, University of Ottawa; 2013. Sensitive in the Gills of Goldfish (Carrasius auratus) and Zebrafish (Danio rerio) [Google Scholar]
- Zhang L, Nurse CA, Jonz MG, Wood CM. Ammonia sensing by neuroepithelial cells and ventilatory responses to ammonia in rainbow trout. J Exp Biol. 2011;214:2678–2689. doi: 10.1242/jeb.055541. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang G, Untereiner A, Ju Y, Wu L, Wang R. Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology. 2013;154:114–126. doi: 10.1210/en.2012-1658. [DOI] [PubMed] [Google Scholar]
- Zhu D, Yu X, Sun J, Li J, Ma X, Yao W. H2S induces catecholamine secretion in rat adrenal chromaffin cells. Toxicology. 2012;302:40–43. doi: 10.1016/j.tox.2012.07.008. [DOI] [PubMed] [Google Scholar]


