Abstract
PreBötzinger complex inspiratory rhythm-generating networks are excited by metabotropic purinergic receptor subtype 1 (P2Y1R) activation. Despite this, and the fact that inspiratory MNs express P2Y1Rs, the role of P2Y1Rs in modulating motor output is not known for any MN pool. We used rhythmically active brainstem–spinal cord and medullary slice preparations from neonatal rats to investigate the effects of P2Y1R signalling on inspiratory output of phrenic and XII MNs that innervate diaphragm and airway muscles, respectively. MRS2365 (P2Y1R agonist, 0.1 mm) potentiated XII inspiratory burst amplitude by 60 ± 9%; 10-fold higher concentrations potentiated C4 burst amplitude by 25 ± 7%. In whole-cell voltage-clamped XII MNs, MRS2365 evoked small inward currents and potentiated spontaneous EPSCs and inspiratory synaptic currents, but these effects were absent in TTX at resting membrane potential. Voltage ramps revealed a persistent inward current (PIC) that was attenuated by: flufenamic acid (FFA), a blocker of the Ca2+-dependent non-selective cation current ICAN; high intracellular concentrations of BAPTA, which buffers Ca2+ increases necessary for activation of ICAN; and 9-phenanthrol, a selective blocker of TRPM4 channels (candidate for ICAN). Real-time PCR analysis of mRNA extracted from XII punches and laser-microdissected XII MNs revealed the transcript for TRPM4. MRS2365 potentiated the PIC and this potentiation was blocked by FFA, which also blocked the MRS2365 potentiation of glutamate currents. These data suggest that XII MNs are more sensitive to P2Y1R modulation than phrenic MNs and that the P2Y1R potentiation of inspiratory output occurs in part via potentiation of TRPM4-mediated ICAN, which amplifies inspiratory inputs.
Key points
The role of metabotropic purinergic receptors (P2YRs) in modulating motor output from the CNS is virtually unknown, despite the fact that many motoneurons, including respiratory motoneurons, express P2YRs.
Using rhythmically active brainstem–spinal cord and medullary slice preparations, we demonstrate that compared to the 4th cervical spinal nerve (C4) inspiratory output controlling the diaphragm, P2YR activation is >10 times more efficacious at potentiating the hypoglossal nerve (XII) inspiratory output controlling airway muscles.
P2YR potentiation of inspiratory output appears largely mediated by P2Y1R.
Whole-cell recordings from XII motoneurons (MNs) suggest that the P2Y1R-mediated potentiation of inspiratory synaptic inputs, glutamate currents, and persistent inward currents, results in part from potentiation of a transient receptor potential cation channel, subfamily M, member 4 (TRPM4)-mediated, calcium-activated, non-specific cation current, ICAN.
The low sensitivity of phrenic output to P2YR activation questions its physiological significance in modulating diaphragm activity. However, the greater sensitivity of XII MNs, combined with observations that ATP is often co-released with noradrenaline and that noradrenergic neuron activity decreases in sleep, makes it tempting to speculate that loss of purinergic modulation contributes to state-dependent reductions in XII MN excitability.
Introduction
ATP acts as a transmitter in the central nervous system (CNS) by binding to two types of purinergic (P2) receptors (Rs). P2XRs are ionotropic, cation-selective, ligand-gated ion channels that comprise seven subtypes (P2X1–7) (North, 2002). P2YRs are metabotropic, G-protein-coupled receptors that signal through slower, second messenger cascades and comprise at least eight subtypes (P2Y1,2,4,6,11–14) (Illes & Alexandre Ribeiro, 2004; Illes & Ribeiro, 2004). P2X and P2Y receptors are distributed throughout the brain, including brainstem regions involved in cardiorespiratory control (Kanjhan 1999; Thomas et al. 2001; Yao et al. 2001; Fong et al. 2002; Gourine et al. 2003; Lorier et al. 2004, 2007; Mulkey et al. 2006; Burnstock, 2007; Funk et al. 2008). ATP is now widely recognized not only as a neurotransmitter in multiple brain regions (Burnstock, 2007), but also as a gliotransmitter released by astrocytes that influences neurons and glia alike (Abbracchio et al. 2009).
A potential role for ATP in motor control was first demonstrated in Xenopus embryos where an interaction between the excitatory actions of ATP via a P2YR-like mechanism and the inhibitory actions of adenosine via P1Rs participate in controlling episodic motor patterns (Dale & Gilday, 1996). A role for ATP signalling in motor control within the mammalian CNS is supported by the sensitivity of brainstem inspiratory rhythm-generating networks to ATP (Lorier et al. 2007, 2008; Huxtable et al. 2010; Zwicker et al. 2011), and the ubiquitous expression of P2Rs on MNs (Collo et al. 1996; Funk et al. 1997; Miles et al. 2002; Kobayashi et al. 2006). Purinergic modulation of MN excitability in the mammalian CNS was first demonstrated in the respiratory network. In XII MNs that innervate muscles of the tongue important for maintaining airway patency (Funk et al. 1997), and to a lesser extent phrenic MNs that innervate the main inspiratory pump muscle, the diaphragm, ATP is excitatory and potentiates inspiratory output (Funk et al. 1997; Miles et al. 2002). ATP excitation is probably mediated in part by a P2X2R mechanism, but a presynaptic P2X7R mechanism may contribute at XII MNs (Ireland et al. 2004). Whether P2YR signalling modulates MN excitability is not known, but preBötzinger complex (preBötC) inspiratory rhythm-generating networks are very sensitive to P2Y1R-mediated excitation (Lorier et al. 2007). XII MNs also show immunolabelling for P2Y1Rs (Fong et al. 2002). Thus, the objectives of this study were to determine, using rhythmically active brainstem–spinal cord and medullary slice preparations from neonatal rats, whether inspiratory motor output of phrenic and XII MNs is sensitive to P2Y1R modulation and to define underlying mechanisms. We specifically tested the hypothesis that potentiation of ICAN is a target through which P2Y1Rs modulate inspiratory MN output based on the observations that: (i) P2Y1Rs activate phospholipase C (Simon et al. 1995; von Kugelgen & Wetter, 2000; Sak & Illes, 2005) which, through phosphatidylinositol 4,5-bisphosphate (PIP2), myo-inositol 1,4,5-trisphosphate (IP3) and possibly also increases in intracellular Ca2+, enhances the TRPM4/5 conductance (Crowder et al. 2007; Pace et al. 2007; Mironov, 2008; Guinamard et al. 2011; Mironov & Skorova, 2011; Mironov, 2013) believed to underlie ICAN (Launay et al. 2002; Hofmann et al. 2003; Nilius et al. 2005; Ullrich et al. 2005); (ii) juvenile XII MNs express TRPM4 (Funk et al. 2011); and (iii) ICAN amplifies inspiratory currents in preBötC inspiratory neurons (Pace et al. 2007), which also express TRPM4/5 (Crowder et al. 2007). We focused on phrenic and XII MNs because in vitro data suggest that phrenic inspiratory activity (Miles et al. 2002) is less sensitive to ATP modulation than XII output (Funk et al. 1997). Understanding the differential modulation of MNs controlling pump and airway muscles by ATP is important because a mismatch in the inspiratory output of these two pools could contribute to the pathology of sleep-related disorders of breathing (Hudgel & Harasick, 1990). ATP is co-released with noradrenaline (norepinephrine) at some central synapses (Poelchen et al. 2001). Thus, state-dependent reductions in noradrenaline release that are implicated in airway muscle atonia in sleep (Chan et al. 2006) may include reductions in ATP.
Methods
Ethical approval
All experimental procedures were approved by the University of Alberta Faculty of Medicine Animal Welfare Committee and performed in accordance with their guidelines for the care, handling and treatment of experimental animals.
Animals
Experiments were performed using neonatal rats ranging in age from postnatal day 0–4 (P0–P4). Both Wistar and Sprague–Dawley (SD) rat pups were used for the nerve recording experiments in which the effects of P2YR activation on C4 and XII inspiratory motor output were examined using the brainstem–spinal cord and rhythmic medullary slice preparations described below. No differences were detected between strains so these data were pooled. All whole-cell recording experiments were performed on SD rats.
Preparations
Brainstem–spinal cord (BSSC) preparations were isolated using methods described previously (Suzue, 1984; Smith & Feldman, 1987; Miles et al. 2002). Briefly, animals were anaesthetized through inhalation of isoflurane and decerebrated. The BSSC was isolated in cold (5–10°C), artificial cerebrospinal fluid (aCSF) containing (in mm): 120 NaCl, 3 KCl, 1 CaCl2, 2 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, and 20 d-glucose, equilibrated with 95% O2–5% CO2. BSSC preparations were transected at the pontomedullary border and between cervical segments 7 and 8 (C7 and C8), pinned with the ventral surface up on Sylgard resin in a recording chamber (volume, 10 ml) and perfused with aCSF that was recirculated at a flow rate of 12 ml min–1. Unless stated otherwise, all experiments (BSSC and slices) were conducted at a temperature of 25–27°C.
Rhythmic slice preparations were produced as described elsewhere (Smith et al. 1991; Ruangkittisakul et al. 2006; Lorier et al. 2007). Briefly, the BSSC was pinned to a wax chuck and serial 100–200 μm sections were cut in the rostral to caudal direction using a vibratome (Leica VT-1000S, Concord, ON, Canada). Sections were transilluminated to identify anatomical landmarks, and referenced against the neonatal rat brainstem atlas (Ruangkittisakul et al. 2006). A single 700 μm slice was cut after the compact division of nucleus ambiguus was no longer evident and the rostral margin of the inferior olive first appeared in the thin sections (−0.35 mm caudal to the caudal aspect of the facial nucleus) (Smith et al. 1991; Ruangkittisakul et al. 2006; Lorier et al. 2007). The caudal boundary of the 700 μm rhythmic slice was just caudal to the obex. Slices contained the preBötC, rostral ventral respiratory group, most of the XII motor nuclei and the rostral XII nerve rootlets. For nerve recording experiments, slices were pinned with the caudal surface up in a recording chamber (volume, 10 ml) and perfused with aCSF that was recirculated at a flow rate of 12 ml min−1.
For whole-cell recording experiments, rhythmic slices were used to examine mechanisms by which P2Y1R modulation affected endogenous glutamatergic synaptic input and inspiratory synaptic drive. These slices were placed caudal surface up in the recording chamber of an upright microscope (Zeiss Axioskop 2 FS Plus, Toronto, ON, Canada) equipped with infrared differential interference contrast optics (IRDIC) and epifluorescence. Slices were held in place with a platinum harp and perfused continuously at a flow rate of 1–2 ml min−1. The concentration of K+ in the aCSF ([K+]o) was raised from 3 to 9 mm at least 30 min prior to the start of data collection. Elevated [K+]o is not a necessary condition for rhythm generation. Medullary slices from neonatal rats that are 700 μm thick generate stable respiratory rhythm in 3 mm [K+]o for 2 h on average, after which rhythm gradually slows over the next 2 h and then ceases (Ruangkittisakul et al. 2006). However, the protocols with rhythmic slices involved multiple interventions, and therefore required slices that produced stable inspiratory-related rhythm for extended periods (i.e. >5 h). Therefore, the [K+]o was raised from 3 to 9 mm. Elevated [K+]o is proposed to compensate for the loss of excitatory/modulatory input (for additional discussions see: Smith et al. 1991; Funk et al. 1993; Ruangkittisakul et al. 2006).
Non-rhythmic slices were used to explore the mechanisms by which P2Y1Rs affected XII MN properties as this increased experimental yield; i.e. only one rhythmic slice can be produced per neonatal rat whereas 2–3 non-rhythmic slices containing the XII nucleus can be produced per rat. Non-rhythmic slices were produced using procedures similar to those described previously (Adachi et al. 2010). The BSSC was pinned to a wax chuck and serial 100–200 μm sections were cut until the rostral margin of the XII nucleus was visible in transilluminated sections. Two to three slices (300 μm) containing the XII nucleus were then collected and transferred to a holding chamber containing standard aCSF at room temperature for at least 1 h prior to recording. Unless stated otherwise, experiments on non-rhythmic slices were performed in aCSF containing 3 mm K+.
Nerve recordings
Inspiratory-related activity was recorded from severed ends of C4 (in BSSC preparations), or XII (in rhythmic slice preparations) nerve roots using glass suction electrodes (80–120 μm i.d.). Signals were amplified, band-pass filtered (300 Hz to 1 kHz) (A-M Systems, Carlsborg, WA, USA), full-wave rectified, integrated using a leaky integrator (τ = 50 ms, Moving Averager, CWE Inc., Ardmore, PA, USA), and displayed on a computer monitor using AxoScope 9.2 software (pCLAMP Suite, Molecular Devices, Sunnyvale, CA, USA). Data were saved to computer using a Digidata 1322 A/D board (Molecular Devices) and AxoScope software for off-line analysis.
MN identification
Neurons were identified as XII MNs based on their location within the XII nucleus and characteristic morphology, both of which are easily established under visualization with IRDIC microscopy (Funk et al. 1993; Stuart et al. 1993; Adachi et al. 2005, 2010). The XII nucleus is relatively homogeneous; less than 5% are interneurons (Viana et al. 1990). Morphological criteria included a large cell soma that was >15 μm along the shortest axis, 20–30 μm in the longest axis (Núñez-Abades et al. 1994; Núñez-Abades & Cameron, 1995). MNs were included in the analysis if they had a resting membrane potential of –40 mV or more hyperpolarized in aCSF at 9 mm K+, or −50 mV or more hyperpolarized in aCSF at 3 mm K+ and Cs+-based intracellular solutions. Cells in which holding current changed or access resistance changed by more than 20% between control and test conditions were excluded from analysis.
Whole-cell recordings
Whole-cell recordings were made from XII MNs in rhythmically-active medullary slices and non-rhythmic slices. Patch pipettes (3–5 MΩ) were pulled on a horizontal puller (P-97, Sutter Instrument, Novato, CA, USA) from filamented borosilicate glass (1.2 mm o.d., Harvard Apparatus, Edenbridge, UK). For experiments on inspiratory XII MNs, pipettes were filled with solution containing (in mm) either: 140 potassium gluconate, 5 NaCl, 1 CaCl2, 1 MgCl2, 10 EGTA, 10 Hepes (liquid junction potential: −12.5 mV), and 1 glucose, or 20 potassium gluconate, 10 NaCl, 10 Hepes, 2 MgCl2, 30 K4-BAPTA (liquid junction potential: −18.8 mV). For experiments examining the putative modulation of ICAN by P2Y1Rs in XII MNs of quiescent slices, three different Cs+-based intracellular solutions were used that contained (in mm): (1) 135 CH3O3SCs, 5 NaCl, 1 MgCl2, 10 Hepes, 0.2 EGTA (liquid junction potential: −8.2 mV); (2) low-BAPTA solution: 135 CH3O3SCs, 5 NaCl, 2 MgCl2, 0.2 CaCl2, 10 Hepes, 2 Cs4-BAPTA (liquid junction potential: −8.7 mV); (3) high-BAPTA solution: 20 CH3O3SCs, 10 NaCl, 2 MgCl2, 10 Hepes, 30 Cs4-BAPTA (liquid junction potential: −18.2 mV). Membrane potentials were not adjusted to correct for liquid junction potentials. High BAPTA was used to produce a time-dependent inhibition of ICAN. With increased time in the whole-cell configuration and progressive dialysis, intracellular BAPTA concentration and calcium buffering capacity increased, progressively attenuating the calcium transients necessary for activation of ICAN. Low BAPTA solution was used as a control for high BAPTA. Osmolarity of all intracellular solutions was adjusted to 290–300 mOsm with sucrose and pH to 7.2–7.3 with either KOH or CsOH. The effects of high BAPTA were assessed by comparing responses evoked immediately after obtaining the whole-cell configuration (first 2 min) with those evoked 15–20 min later.
Unless stated otherwise, the PIC was measured by applying three, consecutive slow depolarizing ramps (−80 mV to 0 mV, 14 mV s−1, 40 s between ramps). The current responses to the voltage ramps were low-pass filtered (Hamm et al. 2010) and then averaged. Leak conductance (gL) was calculated from the line of best fit between current and voltage over the linear range between −80 and −65 mV (i.e. before activation of inward currents) and subtracted from the whole-cell current. PIC amplitude was measured from the leak-subtracted currents, as described previously (Lamanauskas & Nistri, 2008; Bellingham, 2013).
Drugs and their application
All drugs were dissolved in standard aCSF, with the exception of flufenamic acid (FFA) and 9-phenanthrol, which were dissolved in dimethyl sulphoxide (DMSO) and then diluted ×1000 in aCSF to a final concentration of 0.1 mm before use. Drugs used included: γ-aminobutyric acid (GABA), a GABAA and GABAB receptor agonist (1 mm, Sigma, St. Louis, MO, USA); 2-methylthioadenosine 5′-diphosphate (2MeSADP), a P2Y1,12,13R agonist (0.1–10 mm, Sigma); (N)-methanocarba-2MeSADP (MRS2365), a P2Y1R agonist (0.01–1 mm, Tocris, Bristol, UK); l-glutamic acid (glutamate), a general glutamate receptor agonist (0.1 mm, Sigma); FFA, an ICAN antagonist (0.1 mm, Sigma); tetrodotoxin (TTX, 0.5 or 1 μm, Alomone Labs, Jerusalem, Israel); and 9-phenanthrol, a TRPM4 blocker (0.1 mm, Sigma).
Drugs were either applied to the bath or applied locally via pressure ejection from triple-barrelled pipettes pulled from borosilicate glass capillaries (cat no. 3B120F-4, WPI, Sarasota, FL, USA). For nerve recording experiments, drug pipettes were placed at the site at which local application of GABA produced maximum inhibition of inspiratory output (see Results). For whole-cell recording experiments, triple-barrelled drug pipettes were placed superficial to the surface of the slice approximately 25–50 μm upstream of the MN soma. A minimum of 15 min was allowed between consecutive P2R agonist applications because responses are consistent when evoked at this interval (Huxtable et al. 2009, 2010).
Immunohistochemistry
Neonatal rats (P0–P3) were anaesthetized deeply with isoflurane and perfused transcardially with cold phosphate buffer (0.1 m PB) followed by 4% paraformaldehyde in PB. Brainstems were removed, postfixed overnight in 4% paraformaldehyde, and sliced into 50 μm transverse sections on a Leica VT 1000S vibratome. Sections were stored in 0.01% sodium azide in PB until processed.
The pattern of P2Y1R expression in XII MNs in relation to NK1R (receptor for the neuromodulator substance P) and choline acetyl transferase (ChAT, a MN marker) expression was examined via immunohistochemistry using the TSA Fluorescein System protocol (NEL701A, PerkinElmer, Boston, MA, USA). The rabbit anti-P2Y1R antibody (Alomone Labs) was used first, followed by the rabbit anti-NK1R antibody (Advanced Targeting Systems, San Diego, CA, USA) combined with a goat anti-ChAT antibody (Chemicon, Temecula, CA, USA).
In brief, free-floating sections were initially washed three times with 0.1 m phosphate-buffered saline (PBS) for 15 min. Endogenous peroxidase activity was quenched by 20 min incubation with 1% H2O2. All washes mentioned in the following text similarly comprised three washes of 15 min each. All incubations were performed on an oscillating shaker at room temperature. After PBS washes, sections were placed in 0.3% Triton X-100 in TNB (0.1 m Tris-HCl, pH 7.5, 0.15 m NaCl, 0.5% blocking reagent, supplied in kit) buffer for 1 h to decrease non-specific staining and increase antibody penetration. Sections were then incubated in the first primary antibody (rabbit anti-P2Y1R antibody; 1:30,000) and 0.3% Triton-X100 in TNB buffer overnight (14–16 h). After this first primary antibody incubation, sections were washed in TNT (0.1 m Tris-HCl, pH 7.5, 0.15 m NaCl, 0.05% Tween 20) buffer and incubated with biotin-conjugated AffiniPure donkey anti-rabbit IgG (H–L) (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in TNB buffer for 2 h. After washes in TNT buffer, sections were incubated with streptavidin–HRP (1:150) for 1 h, washed in TNT buffer, incubated for 10 min in TSA Fluorescein System Amplification Diluent (1:75), and washed again in TNT buffer.
In preparation for the application of the second and third primary antibodies, sections were again blocked in TNB buffer. Sections were then incubated overnight in the second (rabbit anti-NK1R antibody; 1:1000) and third (goat anti-ChAT; 1:300) primary antibodies, washed in TNT buffer, incubated for 2 h with secondary antibodies conjugated to fluorescent probes for both primary antibodies (NK1R, 1:400, Cy3-conjugated AffiniPure donkey anti-rabbit IgG (H–L), Jackson ImmunoResearch Laboratories; ChAT, 1:400, Cy5-conjugated donkey anti-goat IgG, Jackson ImmunoResearch), washed again in TNT buffer and PBS, and then mounted on slides and coverslipped with Fluorosave Reagent (Calbiochem, San Diego, CA, USA). Control sections were processed in an identical manner with the exception that the primary antibody was omitted from the incubation step. Since both P2Y1 and NK1R antibodies were generated in rabbit, we included an additional control to ensure that there was no cross-reactivity between the NK1R secondary antibody and the P2Y1 primary antibody. Sections were first incubated in the P2Y1R primary antibody, then the P2Y1R secondary antibody, followed by the NK1R secondary antibody. Sections were devoid of NK1R secondary antibody (Cy3) labelling, confirming a lack of cross-reactivity.
Immunofluorescence images (1024 × 1024 pixels) were acquired with a Zeiss (Oberkochen, Germany) LSM510 confocal laser-scanning system using an Axiovert 100M microscope and the following objectives: Fluar ×10 (NA 0.5), Fluar ×20 (NA 0.75), or Plan- Neofluar ×40 (NA 1.3). Images were exported to Adobe Photoshop, version 7.0, and adjusted for contrast and brightness.
Laser-capture microdissection and PCR
Tissue collection and sectioning
Neonatal (P3) and juvenile (P14) rats were anaesthetized with isoflurane and brainstems removed rapidly into ice-cold, sterile aCSF. For tissue punches, the medulla was blocked, glued to the vibratome chuck and 300 μm-thick transverse sections were cut serially on a vibratome. Sections were laid out sequentially, transilluminated and sections containing the XII nuclei or preBötC selected. XII and preBötC tissue was harvested using 19 and 21 gauge tissue punches, respectively (Huxtable et al. 2009). Two punches from each animal were put directly into lysis buffer (Dynabead mRNA DIRECT kit; Invitrogen Molecular Probes, Carlsbad, CA, USA) and stored at −80°C until needed. mRNA was extracted using the Dynabead mRNA DIRECT kit (Invitrogen Molecular Probes). Because preBötC neurons show an ICAN current (Funk et al. 1997; Pace et al. 2007; Mironov, 2008) and preBötC tissue expresses transcripts for TRPM4 and TRPM5 (Crowder et al. 2007), preBötC punches were used as a positive control. mRNA was extracted using the same Dynabead mRNA DIRECT kit (Invitrogen Molecular Probes). mRNA was used as the template to make cDNA using the High Capacity Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA) with oligo d(T) primers. The product of the reverse transcription reaction was then cleaned using MinElute PCR purification kit (Qiagen, Mississauga, Ontario, Canada) to obtain a pure and concentrated sample that was used directly in the real-time PCR reaction (see below).
Laser-capture microdissection (LCM) of XII MNs
The brainstem was blocked with a small segment of spinal cord attached to facilitate tissue manipulation. The tissue was placed in a cryomould (on a bed of dry ice) and oriented for sectioning in the transverse plane. The cryomould was then filled with Tissue-Tek (Sakura Finetek, Torrance, CA, USA), which solidified within 1 min, and stored at −80°C until needed. Brainstems were transversely sectioned in the caudal-to-rostral direction using a cryostat (Leica CM 1850) set at −20°C. Sections were cut at 20 μm thickness (and tissue discarded) until the caudal margin of the XII nucleus was evident. At this point, thickness was reduced to 6 μm and sections were placed on Fisherbrand Superfrost (Fisher Scientific, Ottawa, ON, Canada) microscope slides. Slides were kept in the cryostat (−20°C) for ∼15 min, and then stored at −80°C until LCM was performed. Approximately 60 slides, each containing two or three sections (this minimized freeze–thaw cycles and reduced RNA degradation), were collected from each of two P3 rats (each rat was from a different litter).
Tissue preparation for LCM
Sections were stained and dehydrated according to the Arcturus LCM Histogene Frozen Section Staining Kit protocol (Arcturus Bioscience, Mountain View, CA, USA). Two slides were removed from −80°C, defrosted for 10 s, and placed sequentially in reaction tubes containing 75% ethanol (15 s), water (15 s), cresyl violet (15–30 s), water (quick dip), 75% ethanol (30 s), 95% ethanol (30 s), 100% ethanol (2 × 1 min), xylene (1 min) and finally a second xylene tube where slides were stored until LCM.
Laser-capture microdissection
Microscope slides were placed one at a time on the stage of the Arcturus Autopix LCM (Arcturus Bioscience). A roadmap image of the entire slice was first taken at ×4 magnification, followed by higher magnification (×20) images of the left and right XII nuclei that were used to select MNs and guide LCM. An HS LCM cap was then placed over the tissue and laser settings adjusted to produce a laser spot size of 15 μm (power 80–85 mW, 200 mV per target, pulse duration 1800–1950 μs, automatic double-pulse), which fell completely inside the MN borders. MNs were selected all at once based on size and morphology. Automated dissection was then initiated. All MNs were captured within 1.5 h from starting the dehydration process. MNs surrounded by fractured tissue were not selected because dissecting such sites would often capture tissue outside the boundaries of the MN. Images of the dissected field were taken before and after dissection and also of the cap containing dissected cells (the Arcturus phototriad, Fig. 9B). The number of targeted MNs was compared with the number captured. Twenty to 100 MNs were captured per section and approximately 500 MNs captured from two P3 animals. In all successful runs, laser capture efficiency was 100%; i.e. every targeted MN was captured. Noteworthy is that unsuccessful runs were always 100% unsuccessful. Use of a new cap on the same tissue slice typically resolved the problem.
Figure 9. XII MNs express the transcript for TRPM4.
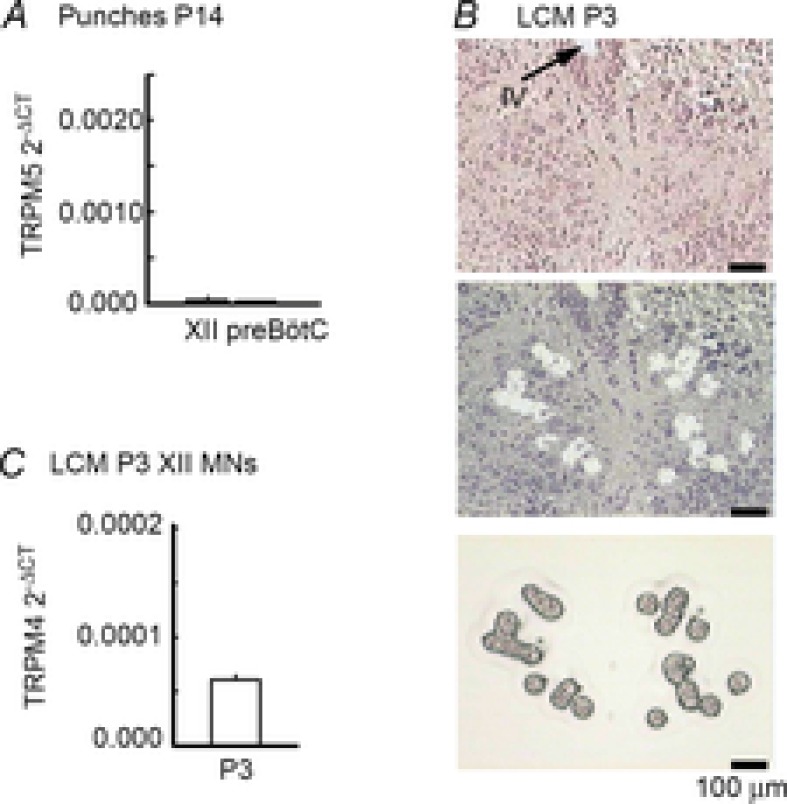
A, mRNA extracted from XII and preBötC tissue punches was subject to real-time PCR analysis for the TRPM5 transcripts. Expression levels are reported relative to cyclophilin A (ΔCT = cycle threshold number for TRPMx – cycle threshold number for cyclophilin A). B, phototriad showing the XII nucleus in a stained tissue section (6 μm) before and after laser-capture microdissection (LCM; Arcturus AutoPIX II) of XII MNs. The image at the bottom is of captured neurons. C, mRNA extracted from laser-captured XII MNs of P3 rats was subject to real-time PCR analysis for the TRPM4 transcripts. Expression levels are reported as described above.
Following laser dissection and capture, each HS LCM cap was examined under a microscope (×20) and discarded if cellular debris or foreign particles were evident. mRNA was extracted using the Arcturus PicoPure RNA Isolation kit. mRNA from each animal was pooled from multiple caps (∼100 neurons) and reverse transcription (RT) performed with an Applied Biosystems (ABI, Burlington, ON, Canada) High Capacity Reverse Transcription kit using oligo d(T) primers. cDNA was stored at −80°C. Samples were not treated with DNase due to small amounts of mRNA and the risk of degradation (Nolan et al. 2006). A no-RT control was used to test for cross-contamination of genomic DNA, while a no-template control, in which water was added in place of cDNA, was used to test for contamination.
Real-time PCR
The Bio-Rad (Hercules, CA, USA) iCycler and Applied Biosystems 2x SYBR Green Master Mix were used to run the PCR. Genes of interest were TRPM4 and TRPM5. The housekeeping gene encoding cyclophilin A was used to control for variations in quality and quantity of cDNA between different samples. For TRPM4, the forward and reverse primer codes were 5′-AGTTGAGTTCCCCCTGGACT-3′ and the 5′-AATTCCAGTCCCTCCCACTC-3′, respectively, making a 148 bp amplicon. For TRPM5, forward and reverse primer sequences were 5′-CATCTCCTTCAGTGAGGATGC-3′ and 5′-CTTC TCCAATT GGCCACCAT-3′, making a 115 bp amplicon. Standard primers were used for cyclophilin A. Primers were designed to span an exon–exon junction to further reduce the possibility of amplifying genomic DNA. Real-time PCR reaction conditions were as follows: 12 μl of ABI 2x SYBR Green buffer, 0.25 μl of Amp Erase, 0.05 μl of forward primer, 0.05 μl of reverse primer and 1 μl of cDNA (50 ng μl−1) for a total reaction volume of 25 μl. Temperature curves were generated for each gene of interest to test for primer dimers. Primer efficiencies were all verified at greater than 98%; each sample was run in triplicate, including standards for each transcript, to obtain validated concentration curves (cycle number vs. cDNA copy number).
Data analysis
Parameters are reported in absolute terms, or relative to control (pre-drug or prestimulus) levels, as mean ± standard error of the mean (SEM). Comparisons between groups were performed on raw data. Student's t test (paired or unpaired as dictated by the data) was used to compare two groups, while analysis of variance (ANOVA) was used in conjunction with Bonferroni correction for multiple comparisons or Newman–Keuls post hoc test (Prism 4.2, GraphPad Software, San Diego, CA, USA) to compare three or more groups. P values less than 0.05 were considered significant.
Results
P2YR activation in phrenic and XII nuclei
To test the hypothesis that phrenic and XII nuclei are sensitive to P2YR modulation, we compared the effects on inspiratory burst activity of locally applying P2YR agonists over the C4 spinal cord of the BSSC preparation and the XII nucleus of the rhythmic slice preparation. Valid comparison required verification that locally applied drugs had equal access to the two MN pools. To address this issue, we compared the effects of GABA (1 mm, 30 s) on C4 (Parkis et al. 1999) and XII inspiratory burst amplitudes (Marchetti et al. 2002; van Brederode et al. 2011). For the XII applications, the drug pipette was placed just below the slice surface in the ventromedial portion of the nucleus. GABA was applied. The pipette was then systematically moved in the transverse plane and the GABA application repeated at 5 min intervals to identify the site of maximum effect. For injections over the phrenic MN pool at C4, the pia mater was gently removed while monitoring C4 output for reductions in burst amplitude indicative of damage to the MNs. Data were excluded from analysis if removal of the pia caused amplitude to fall more than 10%. Note that GABA had very little effect on C4 burst amplitude prior to removing the pia mater. Then, as with XII injections, pipette position was systematically changed to identify the site of maximum GABA sensitivity. Once identified, all subsequent injections were made at the same site. Only two concentrations of 2MeSADP were used in any single experiment because one of the three pipette barrels contained GABA, leaving two barrels for the P2YR agonists.
As shown in the nerve recording traces for a single BSSC and slice preparation (Fig. 1A and B) and group data (Fig. 1C), GABA caused similar reductions in C4 and XII inspiratory burst amplitudes to 22 ± 3 (n = 11, P < 0.05) and 14 ± 5% (n = 9, P < 0.05) of control. Only those preparations in which GABA reduced burst amplitude to at least 35% of control were used.
Figure 1. GABA is equally effective at inhibiting inspiratory burst amplitude in C4 and XII MN pools.
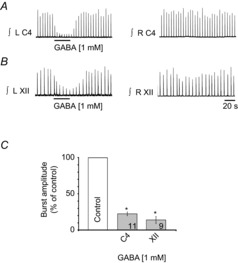
Rectified, integrated recordings from left (L) and right (R) C4 (A) and XII (B) nerve rootlets showing the ipsilateral burst amplitude depression produced by a 30 s injection of 1 mm GABA over each MN pool that was used to identify the site for P2Y agonist application. C, group data; numbers for each group are in the bottom of each column. *Significant difference from control; P < 0.05 (post hoc analysis, Bonferroni method).
We first tested the effects of P2YR activation on inspiratory motor output by locally applying the P2YR agonist 2MeSADP (Chhatriwala et al. 2004; Carrasquero et al. 2005; León et al. 2006; Kahlert et al. 2007; Ortega et al. 2008). The response of C4 and XII nuclei to 2MeSADP comprised a tonic excitation, apparent as a thickening and upward shift in the baseline of the integrated nerve recordings, and a superimposed increase of the inspiratory burst amplitude (Fig. 2A and B). The response was relatively slow in onset compared to that evoked by the general P2R agonist ATP (Funk et al. 1997; Miles et al. 2002), peaking in the second half of the 60 s injection. The effects of 2MeSADP on the C4 nerve activity were exclusively ipsilateral, whereas a small contralateral effect was observed during XII injections in 2 of 30 preparations, probably reflecting the proximity of the contralateral XII nucleus and minimal diffusion barrier compared to the phrenic nucleus. While the responses of C4 and XII MN pools to 2MeSADP were qualitatively similar, the XII MN pool was significantly more sensitive (Fig. 2C). At the phrenic MN pool, 10 mm 2MeSADP evoked minor tonic discharge and a 25 ± 5% potentiation of inspiratory burst amplitude (Fig. 2A and C, n = 11, P < 0.05). Lower concentrations had no effect.
Figure 2. P2YR activation potentiates inspiratory burst amplitude in C4 and XII MN pools.
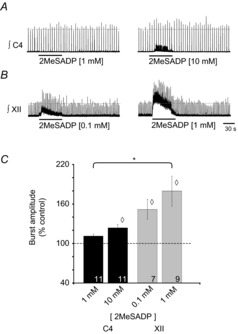
Rectified, integrated recordings from C4 (A) and XII (B) nerve rootlets, illustrating the burst amplitude potentiation produced by a 60 s injection of 2MeSADP, a P2Y agonist, over the C4 and XII MN pools. C, group data showing increase in burst amplitude evoked by 2MeSADP. Numbers for each group are in the bottom of each column. Dotted line indicates control levels (100%); ◊ indicates significant difference from control; * indicates significant difference between 2MeSADP (1 mm) in C4 and XII pools; P < 0.05 (post hoc analysis, Bonferroni method).
In contrast, in the XII nucleus, 0.1 mm and 1 mm 2MeSADP generated robust, dose-dependent increases in tonic activity and burst amplitude. At 0.1 mm 2MeSADP, the tonic discharge was accompanied by a 52 ± 13% (n = 7) increase in XII inspiratory burst amplitude (Fig. 2C). After correcting for the baseline shift associated with the tonic activity, the amplitude potentiation of 80 ± 21% (n = 9) evoked by 1.0 mm 2MeSADP in the XII nucleus was not statistically greater than the effect of 2MeSADP at 0.1 mm, but it was significantly greater than the response evoked in the phrenic nucleus by 1.0 mm 2MeSADP. At 10 mm, the increase in XII tonic activity was so large that it obscured inspiratory burst activity (data not shown).
P2Y1R activation in phrenic and XII nuclei
2MeSADP is an agonist at P2Y1, P2Y12 and P2Y13Rs (Chhatriwala et al. 2004; Carrasquero et al. 2005; Kahlert et al. 2007; Ortega et al. 2008). To further delineate receptor subtype, we assessed the sensitivity of phrenic and XII MN pools to the P2Y1R agonist MRS2365, which has a 4-fold greater affinity for P2Y1Rs than 2MeSADP, no agonist or antagonist activity at P2Y12Rs and is >10,000-fold more selective for P2Y1 compared to P2Y13Rs (Chhatriwala et al. 2004; Jacobson et al. 2006). We focused on P2Y1Rs due to their dominant role in the ATP-mediated excitation of preBötzinger complex (preBötC) inspiratory rhythm-generating networks (Lorier et al. 2007).
In the C4 nucleus, 0.1 and 1 mm MRS2365 potentiated inspiratory burst amplitude by 12 ± 3% and 25 ± 7% (n = 8), respectively (Fig. 3C, P < 0.05). MRS2365 had a relatively minor effect on C4 tonic discharge; it was observed in only 1 of 8 preparations at 0.1 mm and 2 of 8 preparations at 1.0 mm (Fig. 3A). When applied to XII nuclei, again at 10-fold lower concentrations than at C4, 0.01 and 0.1 mm, MRS2365 increased inspiratory burst amplitude by 45 ± 7% and 60 ± 9% (n = 21), respectively (Fig. 3C, P < 0.05). MRS2365-evoked increases in tonic activity were observed in 13 of 21 preparations at 0.01 mm and 14 of 21 preparations at 0.1 mm (Fig. 3B).
Figure 3. The P2Y1R agonist MRS2365 potentiates inspiratory burst amplitude at C4 and XII nuclei.
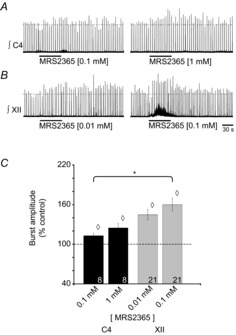
Rectified, integrated recordings from C4 (A) and XII (B) nerve rootlets, showing the burst amplitude potentiation produced by a 60 s injection of MRS2365, a P2Y1R agonist, over the phrenic and XII MN pools. C, group data showing the effect of MRS2365 on inspiratory burst amplitude. Numbers for each group are in the bottom of each column. Dotted line indicates control levels (100%); ◊ indicates significant difference from control; * indicates significant difference between MRS2365 (0.1 mm) in C4 and XII pools; P < 0.05 (post hoc analysis, Bonferroni method).
In summary, the inspiratory and tonic activity of both phrenic and XII MN pools are sensitive to P2YR modulation. The P2Y1R agonist MRS2365 appears to preferentially potentiate inspiratory over tonic activity, and XII MN activity is significantly more sensitive to P2YR modulation than phrenic MN activity.
Mechanisms underlying the P2YR-mediated modulation of XII MN activity
Site of action: pre- vs. postsynaptic mechanisms
C4 activity was only sensitive to P2YR agonists at very high concentrations. We therefore focused on XII MNs to elucidate the mechanisms by which P2YR signalling modulates MN excitability. We began with a generic analysis of P2YR effects due to the greater sensitivity of whole-cell recording methods to detect small, subthreshold effects that may be mediated by other P2YR subtypes. We locally applied the endogenous agonists ADP, which binds to P2Y1,6,12,13Rs, and uridine triphosphate (UTP), which activates P2Y2,4,6Rs (von Kugelgen, 2006). Importantly, aside from weak actions of UTP on P2X3Rs (Rae et al. 1998), and ADP on P2X7R, neither ADP nor UTP activate P2XRs. Under voltage-clamp recording conditions (Vh = −60 mV), UTP (0.1 mm, n = 4; 1.0 mm, n = 3) had no effect on membrane current of inspiratory XII MNs (Fig. 4A). Similarly, 1.0 mm ADP had no effect on baseline membrane current of XII MNs recorded in non-rhythmic slices (Fig. 4B, left panel; n = 3). Given the suggestion from nerve recording experiments (Fig. 3) that P2Y1R activation may potentiate synaptic inputs and that degradation of ADP by ectonucleotidases may obscure its action (Zwicker et al. 2011), we increased ADP concentration to 10 mm in two experiments (Fig. 4B, right panel). In both MNs, 10 mm ADP caused an increase in the frequency and amplitude of the inward synaptic currents. This increased synaptic activity was superimposed on a slow, inward DC current that averaged −18 ± 8 pA (Fig. 4B, right panel, and D, n = 2). Neither 1.0 (n = 6) nor 10 mm (n = 4) ADP had a significant effect on membrane current following bath application of 0.5 μm TTX (Fig. 4C and D).
Figure 4. XII MNs are insensitive to P2YR agonists UTP and ADP.
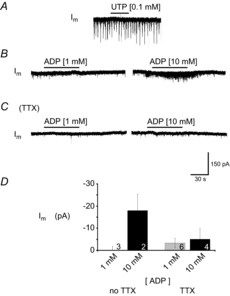
Whole-cell voltage-clamp recordings from XII MNs held at –60 mV demonstrating the effects produced by a 60 s UTP (A) and ADP application in the absence (B) and in the presence (C) of TTX (0.5 μm). D, group data showing effects of ADP on membrane current (Im). Numbers for each group are in the bottom of each column; results from 1 mm and 10 mm ADP injections were not significantly different, in either absence or presence of TTX (0.5 μm); P > 0.05 (one-way ANOVA).
We next used rhythmic medullary slices and the specific P2Y1R agonist MRS2365 to test the hypothesis that P2Y1R activation potentiates inspiratory and non-inspiratory synaptic inputs to XII MNs. Local application of MRS2365 at 0.1 and 1.0 mm over the XII nucleus increased the peak amplitude of inspiratory synaptic currents by 19 ± 5% (n = 8) and 19 ± 3% (n = 8), respectively (Fig. 5, P < 0.05). The similarity of response at both concentrations suggests that the effect was saturated at the lower concentration. Similarly, the effect of MRS2365 on the charge transfer per inspiratory current (i.e. area under the current vs. time plot), was a 20 ± 10% and 22 ± 7% potentiation at 0.1 and 1.0 mm MRS2365, respectively. We also examined the effects of MRS2365 on the non-inspiratory synaptic activity (i.e. that occurring during the interval between inspiratory bursts) that was superimposed on a slow inward current (see also Fig. 6A and B). Postsynaptic current (PSC) activity in the 2 min prior to MRS2365 application was compared to that during the 1 min encompassing the peak of the response. PSC amplitude was minimally affected by 0.1 mm MRS2365 (−37 ± 4 pA in control vs. −40 ± 4 pA in MRS2365; an insignificant 6 ± 3% change, n = 8). At 1.0 mm, MRS2365 increased PSC amplitude from −37 ± 3 to −53 ± 7 pA (39 ± 10%, n = 8, Fig. 6A–C). PSC frequency increased by 133 ± 52% and 466 ± 171% of control in response to 0.1 and 1.0 mm MRS2365, respectively (Fig. 6A, B and D, P < 0.05). Finally, the DC current evoked by 0.1 and 1.0 mm MRS2365 averaged −9 ± 3 pA and −25 ± 7 pA (Fig. 6A, B and E; n = 8).
Figure 5. P2Y1R activation potentiates inspiratory synaptic currents.
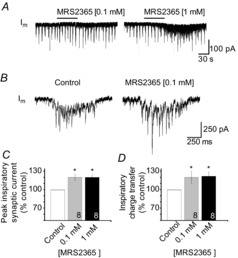
A, long time-series whole-cell voltage-clamp recordings from an inspiratory XII MN held at –60 mV illustrating the effects on inspiratory synaptic currents of MRS2365 locally applied at 0.1 and 1 mm (60 s). B, inspiratory synaptic current averaged from six consecutive inspiratory cycles in control (left) and during the peak of the response to MRS2365 (60 s, 0.1 mm). C, group data showing the MRS2365-mediated potentiation of inspiratory synaptic currents. Data are reported relative to control as the peak current and charge transfer per inspiratory burst. Numbers for each group are in the bottom of each column; * indicates significant difference from control, P < 0.05 (post hoc analysis, Bonferroni method).
Figure 6. MRS2365 potentiates synaptic activity before but not after application of TTX.
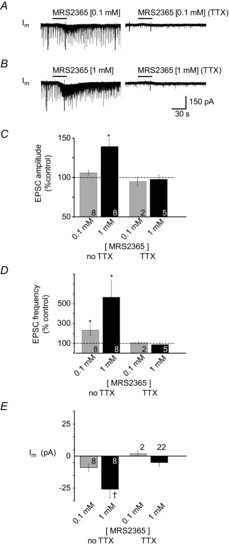
Whole-cell voltage-clamp recordings from an inspiratory XII MN showing the effects of MRS2365 locally applied at 0.1 mm (A) and 1 mm (B) before (left) and after (right) bath application of 0.5 μm TTX. Group data show changes in EPSC or mEPSC (in TTX) amplitude (C), EPSC or mEPSC frequency (D) and membrane current (E) evoked by MRS2365 injections depicted in A and B. Numbers for each group are in the bottom of each column; * indicates significant difference from control; † indicates significant difference from MRS2365 (0.1 mm); P < 0.05 (post hoc analysis, Bonferroni method).
The effects of MRS2365 on PSC amplitude and frequency suggested pre- and postsynaptic actions. To determine the potential site(s) of action, we examined the effects of MRS2365 on the amplitude and frequency of miniature postsynaptic currents (mPSCs) 2 min prior to drug application and at minutes 2–4 when the control response to MRS2365 (i.e. that observed prior to TTX application) was maximal. mPSCs were analysed in five XII MNs. As seen in response to ADP (Fig. 4), activation of P2Y1Rs in the presence of bath-applied TTX had no significant effect on PSC amplitude or frequency (Fig. 6). MRS2365 was also without effect on membrane current. In TTX, only 3 of 22 XII MNs showed a postsynaptic inward current in response to 1 mm MRS2365 (average of −39 ± 6 pA, n = 3). Overall, in TTX, MRS2365 had no significant effect on synaptic inputs or membrane current in XII MNs held at −60 mV.
P2Y1R immunolabelling
Due to the lack of an obvious pre- or postsynaptic effect of MRS2365 on XII MNs in TTX, we used an immunohistochemical approach to determine if XII MNs express P2Y1Rs. Tissue was simultaneously processed for ChAT immunoreactivity to positively identify MNs and NK1R immunoreactivity to provide an approximate reference for receptor labelling intensity. In neonatal rats, NK1R expression is strong in XII MNs and substance P is a potent modulator of XII MN activity (Yasuda et al. 2001). Tissue processed from four rats revealed that while immunolabelling for P2Y1R appeared weaker than for NK1R, the majority of ChAT-labelled neurons in the XII nucleus showed P2Y1R immunoreactivity (Fig. 7).
Figure 7. XII MNs show P2Y1R immunolabelling.
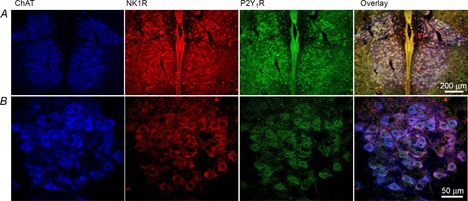
Low- (A) and high-power (B) images of the XII nucleus illustrating XII MN immunolabelling for ChAT (blue), NK1R (red), P2Y1R (green) and the overlays of all three images (right panels).
Presence of ICAN
Block of apparent pre- and postsynaptic actions of MRS2365 by TTX led us to hypothesize that MRS2365 potentiates synaptic currents through an indirect mechanism that cannot be detected under voltage-clamp conditions in TTX. ICAN is a non-inactivating current (Partridge et al. 1994) that contributes to plateau potentials in lumbar MNs (Perrier & Hounsgaard, 1999) and rostral ambiguus MNs (Rekling & Feldman, 1997), as well as persistent inward currents (PICs) in dorsal gastric MNs (Zhang et al. 1995) and spinal interneurons (Dai & Jordan, 2010), and amplifies synaptic currents, including inspiratory synaptic currents, in preBötC inspiratory neurons (Pace et al. 2007). This ICAN-mediated amplification requires three components: (i) a second-messenger-mediated potentiation of ICAN; (ii) an inspiratory current sufficient to depolarize the membrane to the threshold for high-voltage activated Ca2+ channels, and; (iii) Ca2+ influx through voltage-gated Ca2+ channels to activate the potentiated ICAN. We speculated that the MRS2365 potentiation of EPSC activity (including inspiratory synaptic currents) in XII MNs reflects a similar mechanism and that there is no effect in TTX because the level of depolarization associated with miniature excitatory postsynaptic currents (mEPSCs) is insufficient to activate voltage-gated Ca2+ channels and ICAN.
As an initial step in exploring this hypothesis, we tested electrophysiologically for evidence of a PIC in XII MNs using caesium-based intracellular solution (to minimize delayed rectifier potassium conductance) and a voltage-clamp ramp protocol commonly used to reveal PICs (Powers & Binder, 2003; Lamanauskas & Nistri, 2008). At the PIC threshold, a negative slope conductance was apparent where inward current increased as membrane potential depolarized and driving forces for Na+ and Ca2+ currents decreased. PIC magnitude was measured from the leak-subtracted current (Fig. 8A, bottom panel) (Lamanauskas & Nistri, 2008; Bellingham, 2013). PICs have been observed in many MN populations (e.g. lumbar flexor MNs: Schwindt & Crill, 1977; Hounsgaard et al. 1988; Hounsgaard & Kiehn, 1989; Hultborn & Kiehn, 1992; dorsal gastric MNs: Zhang et al. 1995; rostral ambiguous MNs: Rekling & Feldman, 1997; trigeminal MNs: Hsiao et al. 1998; vibrissae MNs: Cramer et al. 2007; cervical MNs: Enríquez Denton et al. 2012), including XII MNs (Powers & Binder, 2003; Koizumi et al. 2008; Lamanauskas & Nistri, 2008; Bellingham, 2013). Forty-seven of 49 XII MNs exhibited a PIC. In a random sample of 6 MNs, the PIC activated at an average membrane potential of −58.3 ± 0.3 mV and reached a peak amplitude of −185 ± 1.0 pA at an average potential of −28.3 ± 0.2 mV.
Figure 8. ICAN contributes to the XII MN persistent inward current (PIC).
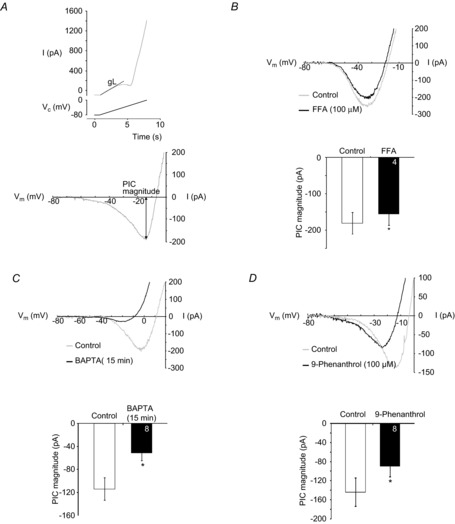
A, top panel shows filtered current response of a XII MN (top trace) evoked in response to the depolarizing phase of a voltage ramp (from –80 mV to 0 mV, bottom trace). Leak conductance (gL) (calculated between −80 and −65 mV) is illustrated. Bottom panel illustrates leak-subtracted current and measurement of PIC magnitude. B, top: leak-subtracted current responses evoked by the voltage ramp described in A during local application of vehicle (control) or FFA, using caesium-based intracellular solution. Bottom: group data showing the peak PIC amplitude in control (open) and FFA (100 μm, filled column). Numbers for each group are in the bottom of each column. *P < 0.05 (one-tailed paired t-test). C, top: leak-subtracted current responses evoked by voltage ramps during bath application of TTX (1 μm) at the start of the whole-cell recording with caesium-based, high BAPTA intracellular solution (control) and 15 min later (BAPTA). Bottom: group data showing the peak PIC amplitude in control (open) and after 15 min with high BAPTA (filled column). Numbers for each group are in the bottom of each column. ◊, P < 0.05 (paired t test). D, top: leak-subtracted current responses evoked by voltage ramps during control or during bath application of 9-phenanthrol (100 μm), using caesium-based, low-BAPTA intracellular solution. Bottom: group data showing the peak PIC amplitude during bath application of TTX (1 μm) in control (open) and during bath application of 9-phenanthrol (filled column). Numbers for each group are in the bottom of each column. *P < 0.05 (paired t test).
To test whether ICAN contributed to this PIC, responses to slow voltage ramps applied before and during local application of the ICAN blocker FFA (100 μm) were compared. Control voltage ramps were applied during vehicle injection. Test ramps were applied 40 s after the onset of an FFA application that lasted until the ramps were complete. FFA significantly reduced PIC magnitude to 85 ± 7% of control (Fig. 8B, P < 0.05, n = 4) without affecting input resistance (vehicle vs. FFA, P > 0.05, paired t test).
PICs in XII MNs consist of a Ca2+ and Na+ component (Powers & Binder, 2003; Lamanauskas & Nistri, 2008; Bellingham, 2013). Since our objective was to explore modulation of a Ca2+-dependent current, ICAN, by P2Y1Rs, we isolated the Ca2+-dependent component of the PIC in all subsequent experiments by blocking the Na+ component with 1 μm TTX in the bath (Hsiao et al. 1997; Lee & Heckman, 2001; Li & Bennett, 2003; Powers & Binder, 2003; Koizumi et al. 2008). In TTX, local application of FFA caused a much greater 40 ± 11% decrease in PIC magnitude from −155 ± 14 to −99 ± 25 pA (Fig. 10B, P < 0.05).
Figure 10. P2Y1R activation potentiates the XII MN PIC through an FFA-sensitive mechanism.
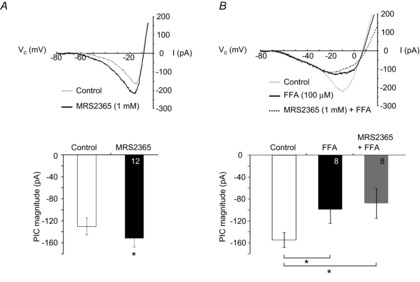
A, top: leak-subtracted (filtered) current response showing PIC evoked by slow voltage-ramp in control and during local application of MRS2365 (1 mm), in TTX. Bottom: group data showing peak PIC amplitude evoked during control (open) and after local application of MRS2365 (1 mm, 15 s, black column; n = 12. *P < 0.05, two-tailed paired t test. B, pre-application of 100 μm FFA prevents MRS2365 potentiation of PIC magnitude (in TTX). Top panel: leak-subtracted (filtered) current response showing PIC evoked by slow voltage-ramp in control, after local application of FFA (black line), or after local application of MRS2365 and FFA (dashed black line). Bottom panel: group data showing peak PIC amplitude evoked in control (open), after 2.5 min of locally applied FFA (2.5 min, black column) and after MRS 2365 (1 mm, 15 s) applied at the end of a 2.5 min application of FFA (grey column). *P < 0.05 (post hoc analysis, Newman–Keuls multiple comparison test).
While FFA at 100 μm is reasonably selective for ICAN, it is difficult to exclude the possibility that some of its actions are through inhibition of voltage-gated Ca2+ currents (Shimamura et al. 2002). We therefore assessed the effects of high intracellular BAPTA concentration on PIC amplitude. Chelation of intracellular Ca2+, if anything, will increase voltage-gated Ca2+ currents by increasing the concentration gradient for Ca2+. In contrast, ICAN will be attenuated or blocked by elevated BAPTA since this will attenuate the increase in intracellular Ca2+ that is required for ICAN activation. BAPTA effects were assessed by comparing responses obtained immediately after achieving whole-cell configuration with those obtained 15 min later. Note that in this case the PIC was measured based on only one ramp due to the potential reduction in PIC amplitude with progressive dialysis. High intracellular BAPTA had no effect on input resistance (183 ± 28 vs. 205 ± 26 MΩ, control vs. control 15 min later, P > 0.05, two-way paired t test). However, PIC magnitude significantly decreased by 47 ± 12% of control values after 15 min (Fig. 8C, P < 0.05).
We then tested the effects of the more selective TRPM4 channel blocker, 9-phenanthrol, on the XII MN PIC, again using the low-BAPTA intracellular solution. TRPM4 is a member of the transient receptor potential family that requires depolarization as well as increased levels of intracellular Ca2+ for activation (Ullrich et al. 2005). In eight cells, we measured PIC magnitude during slow voltage ramps and then again after 10 min of 9-phenanthrol (100 μm) bath application. 9-Phenanthrol caused a 33 ± 6% reduction in PIC magnitude, from −144 ± 40 to −90 ± 22 pA (Fig. 8D, P < 0.05), but had no effect on input resistance (223 ± 48 vs. 189 ± 30 MΩ, control vs. 9-phenanthrol, P > 0.05, paired t test). Based on the sensitivity of the PIC to FFA, high concentrations of intracellular Ca2+ buffer, and the TRPM4 blocker, we conclude that a TRPM4-mediated ICAN contributes to the PIC in XII MNs of neonatal rat pups. We also noticed in some recordings that high BAPTA or 9-phenanthrol caused an apparent increase in the peak outward K+ current. The underlying mechanism was not explored but it has been noted in hippocampal CA1 neurons that BAPTA may induce outward currents (Lancaster & Batchelor, 2000). This may simply reflect that BAPTA can cause neurons to become more electrotonically compact, increasing input resistance and improving space-clamp.
Finally, we asked if XII MNs express the transcript for TRPM4. We also assessed expression of TRPM5 transcripts which is another candidate for ICAN (Launay et al. 2002; Hofmann et al. 2003; Montell, 2005; Nilius et al. 2005; Ullrich et al. 2005). XII MNs from juvenile SD rats (P14) express the transcript for TRPM4 (Funk et al. 2011). Whether neonatal XII MNs also express TRPM4 is not known. Similarly, the expression of TRMP5 in XII MNs is not known at any age. We first tested for the presence of TRPM5 transcripts via real-time PCR analysis of mRNA extracted from punches of XII nuclei from juvenile rats. Punches of preBötC were included as a positive control (Crowder et al. 2007; Pace et al. 2007; Funk et al. 2011). Real-time PCR analysis of mRNA isolated from punches of the XII nucleus and preBötC of juvenile rats revealed signals for TRPM5 that were ∼10-fold lower than previously published levels for TRPM4 (Crowder et al. 2007; Pace et al. 2007; Funk et al. 2011). Transcript expression levels are reported relative to cyclophilin A (ΔCT = cycle threshold number for TRPM4 – cycle threshold number for cyclophilin A) (Fig. 9A). Since punches are not pure MN samples, we next laser-capture-microdissected ∼500 XII MNs from two P3 animals. We did not examine TRPM5 expression in laser-captured MNs due to the low level of expression found in tissue punches. The phototriad (Fig. 9B) shows the XII nucleus in a counter-stained, 6 μm tissue section from a P3 neonatal rat before (top) and after (centre) laser-capture microdissection as well as the captured MNs (bottom). Real-time PCR confirmed the punch data, revealing TRPM4 transcript in neonatal XII MN samples (Fig. 9C). These data provide molecular evidence that the mRNA from TRPM4, a putative molecular substrate for ICAN, is expressed in neonatal XII MNs.
P2Y1R modulation of ICAN
We next addressed whether activation of P2Y1R-mediated signalling pathways potentiates the PIC, and then specifically whether these pathways potentiate the ICAN portion of the PIC. Voltage ramps were applied in control and then again during the local application of MRS2365 that began 10 s prior to the first ramp. MRS2365 significantly potentiated PIC magnitude by 14 ± 4% above control from −131 ± 15 to −152 ± 16 pA (Fig. 10A, n = 12, P < 0.05). This analysis is based on the average of all 12 MNs. MRS2365, however, modulated PIC magnitude in only 8 of these 12 MNs. If these 8 MNs are analysed separately, MRS2365 potentiated PIC magnitude by 21 ± 4% (−117.6 ± 0.9 to −150.7 ± 1.0 pA, n = 8, P < 0.05, paired t test). MRS2365 had no effect on input resistance (177 ± 17 MΩ in control vs. 174 ± 16 MΩ in MRS2365, P > 0.05). In a separate series of control experiments, local application of aCSF was associated with an insignificant 5 ± 7% change in PIC amplitude (n = 7, P > 0.05, paired t test).
To determine whether P2Y1R activation potentiates ICAN specifically, we tested whether the P2Y1R-mediated PIC potentiation was sensitive to FFA. After the control voltage ramps, FFA was locally applied for 2.5 min and additional voltage ramps applied to assess the PIC in FFA. MRS2365 was then applied locally for 15 s (in the continued presence of FFA) prior to a final series of voltage ramps to assess the PIC in MRS2365/FFA. As described above, bath-application of FFA caused a significant, 40 ± 11%, decrease in PIC magnitude (Fig. 10B, P < 0.05). Unlike its potentiating actions in control, MRS2365 had no effect on PIC magnitude in the presence of FFA (−99 ± 25 pA in FFA vs. −88 ± 27 pA in MRS2365 and FFA, Fig. 10B, P > 0.05). Input resistance increased 11 ± 3% from control to FFA (169 ± 34 vs. 186 ± 38 MΩ, P < 0.05), but there was no effect of MRS2365 on input resistance (186 ± 38 vs. 194 ± 37 MΩ, P > 0.05).
Finally, we obtained whole-cell recordings from inspiratory-modulated XII MNs, identified by the presence of rhythmic inward drive currents in phase with bursts of inspiratory-related activity recorded from the XII nerve. We then bath-applied TTX (0.5 μm) to focus on postsynaptic mechanisms. To test whether P2Y1R activation potentiates postsynaptic glutamate currents, we measured the current responses from a holding potential of –60 mV evoked by brief puffs of glutamate (500 ms, 100 μm) during the last 30 s of a 2 min local application of aCSF (control) and then during the last 30 s of a 2 min MRS2365 application. Fifteen minutes later, glutamate puffs were reapplied during the last 30 s of a coapplication of MRS2365 and FFA. Local application of 1 mm MRS2365 potentiated glutamate current amplitude by 27 ± 7% from −160 ± 29 pA to −207 ± 44 pA (n = 7) (Fig. 11A, left panel; and B, P < 0.05). After washout of MRS2365 (15 min), the glutamate currents returned toward control (−176 ± 55 pA; n = 5). In the presence of FFA (100 μm), MRS2365 was associated with an insignificant 4 ± 5% change in glutamate current amplitude (−154 ± 30 pA in control and −163 ± 38 pA in MRS2365 and FFA, P > 0.05) (Fig. 11A, right panel; and B). These data suggest in XII MNs that postsynaptic responses to exogenous glutamate are amplified by a P2Y1R-mediated potentiation of an FFA-sensitive PIC such as ICAN. An important caveat is that even though these experiments were performed in voltage-clamp at a holding potential of –60 mV, the data do not indicate that ICAN was activated solely by a P2Y1R-mediated, intracellular store-derived increase in intracellular Ca2+. Both depolarization and increased intracellular Ca2+ are required to activate ICAN (Ullrich et al. 2005). Given the morphological complexity of XII MNs, voltage control of the dendrites is unlikely (Spruston et al. 1993). ICAN activation during glutamate application therefore most likely reflects glutamate-mediated depolarization of unclamped regions of the dendritic tree and increases in intracellular Ca2+ that derive either via activation of voltage-activated Ca2+ channels on unclamped membrane or P2Y1R-evoked release of Ca2+ from intracellular stores.
Figure 11. P2Y1R activation potentiates glutamate currents through an FFA-sensitive mechanism.
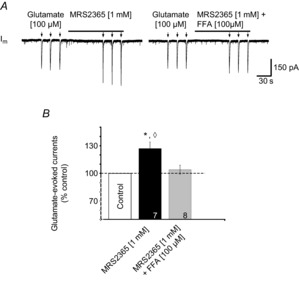
A, voltage-clamp recording of a XII MN held at –60 mV illustrating currents evoked by glutamate puffs (100 μm, 500 ms) in control and in the presence of MRS2365 (left trace), and then again in the presence of MRS2365 and 100 μm FFA (right trace). B, group data showing, relative to control, the change in peak glutamate currents evoked by local application of MRS2365 alone or in combination with FFA. Numbers for each group are in the bottom of each column; * indicates significant difference from control; ◊ indicates significant difference to MRS2365 (1 mm) + FFA (100 μm); P < 0.05 (post hoc analysis, Bonferroni method).
Discussion
The significance of P2R signalling in controlling motor output from the brain is poorly understood. Our previous work in vitro has demonstrated that local application of ATP over XII MNs innervating airway muscles (Funk et al. 1997), and phrenic MNs innervating the major inspiratory pump muscle (Miles et al. 2002) causes a biphasic response comprising an initial P2R-mediated excitatory phase, during which both tonic discharge and inspiratory burst amplitude increase, followed by an inhibitory phase when inspiratory burst amplitude decreases. The inhibitory phase is due, at least in part, to the hydrolysis of ATP by ectonucleotidases into adenosine and the inhibitory actions of adenosine at P1Rs (Funk et al. 1997; Miles et al. 2002). The initial excitatory response was primarily attributed to a P2XR mechanism (Funk et al. 1997; Kanjhan et al. 1999; Miles et al. 2002; Gourine et al. 2003). However, in this study we use brainstem–spinal cord and rhythmically active medullary slice preparations to show the following. (i) Activation of P2YRs, especially P2Y1Rs, evokes tonic discharge from XII and C4 ventral nerve roots and potentiates population inspiratory motor output from XII and phrenic MN pools. (ii) The activity of the XII MN pool is at least 10-fold more sensitive to P2Y1R modulation than the C4 pool of phrenic MNs. (iii) P2Y1R activation enhances non-inspiratory as well as glutamatergic, inspiratory synaptic activity. (iv) In XII MNs, the P2Y1R-mediated potentiation of inspiratory activity appears to occur indirectly through potentiation of ICAN in a majority of XII MNs, which in turn amplifies glutamatergic synaptic inputs. Indeed, the TRPM4 transcript, which is believed to mediate ICAN, is present in XII MNs. Thus, the excitatory actions of ATP on MN excitability will reflect the combined actions of a postsynaptic, P2XR-dependent inward current that causes membrane depolarization, and a P2Y1R-mediated potentiation of ICAN.
Mechanisms underlying the P2YR-mediated modulation of inspiratory root activity
Receptor subtypes
The increase in tonic discharge and potentiation of inspiratory burst amplitude evoked here by P2YR agonists 2MeSADP and MRS2365 are also features of the response evoked by ATP. These effects were initially attributed to P2XRs based on: (i) immunohistochemical and molecular evidence for these receptors on the MNs; (ii) antagonism of the ATP excitation by low concentrations of the P2R antagonist PPADS; and (iii) the observation that in synaptically isolated MNs, ATP evokes postsynaptic inward currents that are associated with increased conductance suggesting a directly-gated ion channel (Funk et al. 1997; Miles et al. 2002). Several observations support a P2YR-specific effect as well. First, 2MeSADP and MRS2365 have very low affinities for P2XRs (Chhatriwala et al. 2004; Carrasquero et al. 2005; León et al. 2006; Kahlert et al. 2007; Ortega et al. 2008). Second, the cellular effects of ATP and 2MeSADP/MRS2365 on XII MN properties are distinct. Whereas ATP evokes a postsynaptic current in synaptically isolated XII MNs by opening an ion channel (Funk et al. 1997), 2MeSADP and MRS2365 had no effect on synaptically isolated XII MNs held at resting potential. A postsynaptic effect was only detected when the membrane was depolarized to reveal a PIC that was potentiated by MRS2365. Third, as confirmed here, XII MNs show immunolabelling for P2Y1Rs (Fong et al. 2002; and present results). Whether XII MNs are sensitive only to P2Y1 or to other P2YRs is not clear. The fact that the selective P2Y1 agonist MRS2365, and the more general P2YR agonist 2MeSADP, produced similar effects on XII burst amplitude suggests that P2Y1Rs are the main contributor, but a role for other receptor P2YR subtypes cannot be excluded.
The relative potencies of P2Y vs. P2XR mechanisms were not compared. Exogenous ATP applied to the XII nucleus of rhythmic slices potentiates XII inspiratory burst amplitude by 40 ± 20% (Funk et al. 1997), similar to that evoked here by P2Y1R agonists. Given that ATP activates both P2X and P2YRs, one would predict a greater effect of ATP. However, it is possible that rapid degradation of ATP by ectonucleotidases will result in submaximal effects compared to the P2YR agonists used here (2MeSADP and MRS2365). These two agonists are substrates for ectonucleoside triphosphate diphosphohydrolases (E-NTPDases), but the monophosphate derivatives are not broken down to adenosine (Ravi et al. 2002; Alvarado-Castillo et al. 2005; Robson et al. 2006). Assessing the relative contribution of P2X and P2Y receptors to the effects of ATP was not our objective and will require assessing the actions of ATP before and during application of selective P2X and P2Y receptor antagonists.
Cellular and synaptic mechanisms
Our mechanistic studies focused on analysis of XII MNs because the high concentration of agonist required to potentiate C4 output raised questions of physiological relevance. At least three mechanisms could contribute to the P2YR-mediated activation of tonic discharge and potentiation of XII (and possibly C4) inspiratory burst amplitude. (i) P2YRs could directly depolarize MNs via modulation of a conductance active at rest, as seen in hippocampal neurons and in Xenopus embryos (Brown & Dale, 2002) where P2YRs block an M-type potassium current (Filippov et al. 2006). Our initial observation that 2MeSADP and MRS2365 evoke small inward currents suggested activation of postsynaptic receptors and direct depolarization. However, the virtual loss of these currents upon bath application of TTX indicates that these actions are unlikely to be due to activation of postsynaptic receptors that modify a current active at rest. (ii) P2YR-mediated potentiation of glutamatergic inspiratory synaptic currents through pre- or postsynaptic mechanisms, as observed in rat medial habenula (Price et al. 2003) and nucleus accumbens (Krügel et al. 2004) where P2YR activation potentiates presynaptic glutamate release, could also contribute. The increase in the frequency of EPSCs and amplitude of inspiratory currents following agonist application suggested both pre- and postsynaptic potentiation of glutamatergic transmission. Loss of these effects in TTX, however, indicates that they were not via direct modulation of glutamatergic transmission. (iii) P2YR activation could also increase MN excitability through modulation of a conductance not active at rest such that a greater output would be produced for the same synaptic input. We hypothesized that the P2YR potentiation of inspiratory activity is due to the potentiation of a Ca2+-activated, non-selective cation current, which then potentiates synaptic inputs. This hypothesis was based on the demonstration in preBötC neurons that metabotropic glutamate receptor (mGluR) activation evokes such a mechanism (Pace et al. 2007), and that mGluRs and P2Y1Rs both signal through phospholipase C which can potentiate ICAN (Simon et al. 1995; von Kugelgen & Wetter, 2000; Sak & Illes, 2005; Crowder et al. 2007; Pace et al. 2007; Guinamard et al. 2011). Several lines of evidence support this hypothesis. First, PCR analysis of XII punches and laser-captured XII MNs revealed the transcript for TRPM4 but very little TRPM5, which is consistent with the limited CNS expression of TRPM5 compared to TRPM4 (Guinamard et al. 2011). TRPM4 and TRPM5 are most commonly considered candidates for ICAN (Guinamard et al. 2011), although a recent paper presented evidence that TRPV2 may be a major contributor to ICAN in spinal MNs (Bouhadfane et al. 2013). However, the fact that the TRPV2 is virtually inactive at the temperatures used in these experiments (Caterina et al. 1997) combined with our demonstration that the TRPM4 blocker 9-phenanthrol attenuates the Ca2+-mediated PIC by ∼33%, indicate that TRPM4 is a significant contributor to ICAN in XII MNs.
Second, sensitivity of the PIC to FFA, high BAPTA and 9-phenanthrol provide electrophysiological and pharmacological evidence that ICAN contributes to the PIC in XII MNs. ICAN is a component of plateau potentials in turtle MNs (Perrier & Hounsgaard, 1999), and PICs in rostral ambigual (Rekling & Feldman, 1997) and dorsal gastric MNs (Zhang et al. 1995). ICAN also contributes to long-lasting excitatory currents in deep dorsal horn neurons (Morisset & Nagy, 1999), locomotor-related spinal interneurons (Dai & Jordan, 2010), and preBötC inspiratory neurons (Pace et al. 2007). In XII MNs, the PIC is primarily attributed to an equal contribution from voltage-gated Ca2+ currents and persistent sodium current (INaP) (Powers & Binder, 2003). A contribution of ICAN to the PIC was proposed in neonatal rat (Lamanauskas & Nistri, 2008), but this possibility was rejected based on analysis of juvenile XII MNs in which substitution of Na+ (a major charge carrier of ICAN) had minimal effect on the PIC (Powers & Binder, 2003). In contrast, our data in neonates showing that FFA reduces the PIC by ∼15% (Fig. 8B) suggest that ICAN contributes a small but significant portion of the total XII MN PIC. Consistent with this, when the Ca2+-dependent component of the PIC was isolated using TTX to exclude INaP, the FFA-sensitive component (presumptive ICAN) contributed ∼40%. In addition, in TTX using high intracellular BAPTA solution, which will block Ca2+-dependent currents like ICAN but not voltage-gated Ca2+ currents, the PIC was reduced by 47%, suggesting that ICAN contributes significantly to the TTX-resistant PIC. Finally, the 33% inhibition of the PIC by 9-phenanthrol, which blocks a TRPM4-mediated ICAN, further supports the conclusion that the XII MN PIC includes an ICAN component.
There are two important caveats regarding the use of FFA to measure ICAN. First, at the concentration of 100 μm used here, FFA does not completely block ICAN (Guinamard et al. 2013). Thus, our measurements with FFA may underestimate the contribution of ICAN to XII MN PICs. Second, sensitivity to 100 μm is not definitive evidence of ICAN. FFA is an aromatic amino acid with a broad spectrum of ion channel targets. Its highest affinity is for non-selective cation channels including TRPM4 and TRPM5. FFA concentration was limited to 100 μm to minimize off-target actions. However, even at 100 μm FFA actions on chloride channels, Ca2+-activated K+ channels, some two-pore domain K+ channels, and L-type Ca2+ channels, cannot be excluded (Shimamura et al. 2002; Guinamard et al. 2013). Nevertheless, off-target actions in our experiments were unlikely. The actual concentration of FFA experienced by the XII MNs in our experiments was more likely to be in the range of 10 μm because FFA was locally applied rather than bath-applied. Drug concentration falls off exponentially from a point source and previous work with this preparation indicates that the pipette concentration must be ∼10-fold higher than the bath concentration to produce similar effects (Liu et al. 1990). Our electrophysiology data further support the selective action of FFA on ICAN. Had FFA activated Ca2+-activated or two-pore domain K+ channels, MN input resistance should have decreased. FFA either had no effect on input resistance or caused a slight increase.
BAPTA sensitivity of the PIC potentiation also does not definitively establish ICAN involvement. BAPTA will not affect voltage-gated Ca2+ channels. However, in turtle lumbar MNs BAPTA blocks an ICAN-independent, Ca2+- and calmodulin-dependent PIC potentiation (Perrier et al. 2000). Whether a similar pathway exists in mammalian MNs is not known. If it does, our measurements based on BAPTA sensitivity may overestimate the contribution of ICAN to the XII MN PIC. The fact that FFA and high BAPTA caused similar reductions in the TTX-insensitive PIC, however, suggests that over-estimation is unlikely and therefore that ICAN contributes 40–47% of the Ca2+-dependent PIC in our experiments. Based on estimates that INaP and the Ca2+ component each form ∼50% of the total PIC (Powers & Binder, 2003), the ICAN component probably forms 20–25% of the total PIC in XII MNs. This is smaller than its contribution in deep dorsal horn interneurons (Morisset & Nagy, 1999), but larger than in locomotor-related spinal interneurons (Dai & Jordan, 2010). Whether TRPM4 underlies all of the ICAN in XII MNs is not clear. The fact that the 9-phenanthrol block of the PIC in TTX was only 33% while it was 40% and 47% in FFA and high BAPTA suggests that it may not be exclusively TRPM4.
The second piece of evidence supporting our hypothesis that the P2Y1R potentiation of inspiratory activity is via potentiation of ICAN is that MRS2365 significantly potentiated the XII MN PIC by 21% and that this potentiation was completely blocked by FFA. At the very least these data indicate that MRS2365 is acting via an FFA-sensitive PIC.
The third body of evidence supporting our hypothesized mechanism is that the P2Y1R-mediated potentiation of glutamatergic signalling appears dependent on ICAN. In identified inspiratory neurons synaptically isolated in TTX, MRS2365 potentiation of glutamate-evoked currents was blocked by FFA. Taken together, these data provide strong evidence that P2Y1R signalling potentiates glutamatergic inputs to XII MNs, at least in part through the potentiation of an FFA- and BAPTA-sensitive PIC that is most likely the non-selective cation current ICAN.
Physiological significance of P2YR signalling in modulating inspiratory MN activity
In phrenic and XII nuclei, exogenous ATP evokes a biphasic response comprising an initial, rapid, P2R-mediated excitation followed by a P1R-mediated inhibition (Funk et al. 1997; Miles et al. 2002). Data presented here suggest that P2Y1Rs will contribute to the initial excitatory phase, and possibly offset the magnitude of the secondary inhibition.
Understanding the true physiological significance requires more information about the conditions or stimuli that evoke the release of endogenous ATP. At present, virtually nothing is known of the stimuli that evoke ATP release onto inspiratory MNs in mammals. In Xenopus tadpole, ATP is released in response to tail pinch where it acts via a P2YR-like excitatory process to initiate swimming and a competing adenosinergic inhibitory mechanism to terminate rhythmic activity (Dale & Gilday, 1996). In anaesthetized rats, different compartments of the ventral respiratory network appear to release ATP in response to hypercapnia or hypoxia (Gourine et al. 2005a,b), which in turn may contribute to the respective homeostatic ventilatory responses. Hypercapnia appears to evoke ATP release from astrocytes in the retotrapezoid nucleus, where it excites chemosensitive Phox2b neurons, enhances their excitability and contributes as much as 20% to the ventilatory response (Gourine et al. 2005b). Also in anaesthetized adult rats, hypoxia appears to evoke ATP release from the ventral respiratory group, where it attenuates the secondary hypoxic ventilatory depression (Gourine et al. 2005b), perhaps through P2Y1Rs in the preBötC (Lorier et al. 2007). CO2 or hypoxia-induced release of ATP in motor nuclei would probably increase MN excitability and tidal volume, either by increasing activity of the pump muscles or decreasing airway resistance through increased activity of airway dilator muscles, like those innervated by XII MNs. Of these two possibilities, a reduction in airway resistance is most likely given that XII MNs appear >10 times more sensitive to P2YR modulation than phrenic MNs. The sensitivity of XII MNs to ATP (Funk et al. 1997) was similarly reported as ∼10-fold greater than that of phrenic MNs (Miles et al. 2002). However, direct comparison between these earlier studies was difficult due to potential differences in antagonist access to the two MN pools. Our demonstration that GABA caused equivalent inhibition of XII and C4 inspiratory output suggests equal drug access to both nuclei and therefore greater sensitivity of XII MNs to P2YR activation. In fact the high concentration of P2YR agonists required to potentiate C4 inspiratory nerve output suggests that P2YR signalling has minimal physiological significance in modulating phrenic MN excitability. Spinal MNs do not appear to express P2Y1Rs (Kobayashi et al. 2006), but they do express P2Y4 and P2Y6Rs. A role for P2YRs cannot be excluded, however, because phrenic MNs have not been examined specifically. In addition, P2Y1Rs activate a non-specific cation current in lamina IX neurons, some of which may be MNs (Aoyama et al. 2010).
The source of ATP will also be a critical factor determining the physiological effects of endogenously released ATP, as ATP is often a cotransmitter that has multiple linear and non-linear interactions with other transmitters (Richardson & Brown, 1987; Funk et al. 1997; Matsuka et al. 2001; Mori et al. 2001; Jo & Role, 2002). The potential co-release of ATP with noradrenaline is perhaps most relevant in the context of the differential sensitivity of XII and phrenic MNs to ATP and sleep-disordered breathing. Compared to pump MNs (phrenic), the greater susceptibility of airway MNs (XII) to sleep-related reductions in tone through disfacilitation (loss of excitatory modulatory inputs during sleep) is a hypothesized factor in sleep-disordered breathing (Chan et al. 2006; Horner, 2008, 2011). Locus coeruleus (LC) neurons co-release ATP with noradrenaline (Poelchen et al. 2001). Their activity is state dependent (Aston-Jones & Bloom, 1981), i.e. they are active in wakefulness but virtually silent during rapid-eye-movement (REM) sleep. Thus, loss of noradrenaline and ATP inputs during REM sleep could reduce excitability. LC neurons provide only a small portion of the noradrenergic input to XII MNs. However, if noradrenergic neurons innervating the XII nucleus also co-release ATP and show state-dependent reductions in activity, loss of noradrenaline and purinergic tone could contribute to the greater susceptibility of airway MNs to atonia in REM.
Another potential source of ATP is from glial cells. Evidence obtained in vitro and in vivo suggest that glia on the ventral surface of the medulla at the retrotrapezoid nucleus release ATP in response to acidic pH (Gourine et al. 2010) or elevated CO2 (Huckstepp et al. 2010a,b; Meigh et al. 2013) and that the ATP excites chemosensitive RTN neurons, thereby contributing to central chemosensitivity (Gourine et al. 2010; Huckstepp et al. 2010a,b; Sobrinho et al. 2014). Whether glial cells in motor nuclei respond to hypoxia or other stimuli by releasing ATP is not known but it is possible. In the carotid body, ATP is released from Type-1 glomus cells in hypoxia and this is hypothesized to evoke ATP release from glial-like, type II sustentacular cells (Nurse & Piskuric, 2013). Within the CNS, hippocampal astrocytes release adenosine in response to hypoxia (Martin et al. 2007), while spontaneous Ca2+ waves in cerebellar Bergmann glia increase in hypoxia (Mathiesen et al. 2013).
In summary, our data indicate in mammals that the inspiratory output of XII MNs that control the airway is sensitive to modulation by P2Y1Rs, at least in part via potentiation of an ICAN current that amplifies glutamatergic inputs and is most likely mediated by TRPM4.
Acknowledgments
None declared.
Glossary
- aCSF
artificial cerebrospinal fluid
- BSSC
brainstem–spinal cord
- C4
4th cervical spinal nerve
- ChAT
choline acetyl transferase
- FFA
flufenamic acid
- ICAN
calcium-dependent, non-selective cation current
- INaP
persistent sodium current
- IRDIC
infrared differential interference contrast
- LCM
laser-capture microdissection
- mEPSC
miniature excitatory postsynaptic current
- mGluR
metabotropic glutamate receptor
- MN
motoneuron
- NK1R
neurokinin 1 receptor for the neuromodulator substance P
- P2
purinergic receptor
- P2X
ionotropic P2 receptor
- P2Y
metabotropic P2 receptor
- PIC
persistent inward current
- preBötC
preBötzinger complex
- PSC
postsynaptic current
- SD
Sprague–Dawley
- TRPM4 and TRPM5
transient receptor potential cation channel, subfamily M, member 4 and 5
- UTP
uridine triphosphate
- XII
hypoglossal nerve
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
T.S.A. performed all experiments and data analysis procedures associated with analysis of effects of GABA and P2YR agents on C4 and XII burst amplitude, and whole-cell recording experiments that assessed the effects of P2YR agents on the synaptic and membrane properties of XII MNs, excluding those involved in analysing the PIC. He also contributed to experimental design, article drafting, figure production and revision, and final approval. A.L.R. performed all experiments and data analysis procedures associated with analysis of the PIC in XII MNs. She also contributed to experimental design of this component of the study, article drafting, figure production and revision, and final approval. A.G.H. performed the immunohistochemical analysis of P2Y1R, NK1R and ChAT and expression in XII MNs, as well as the preparation of related figures, and final approval. C.D.L. performed the laser-capture microdissection of XII MNs and real-time PCR analysis of TRPM4 and TRPM5 transcript expression, and final approval. G.D.F. oversaw all aspects of the study, from conception to final publication. All experiments were performed in the laboratory of G.D.F., Dept Physiol, University of Alberta. All authors approved the final version of the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR 53085, RES0006842), the Natural Sciences and Engineering Research Council of Canada (NSERC 402532, RES0012299), Alberta Innovates–Health Solutions (AIHS), the Women and Children's Health Research Institute (Alberta, Canada), the Canada Foundation for Innovation, and the Alberta Science and Research Authority. G.D.F. is an AIHS Scientist.
References
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Adachi T, Huxtable AG, Fang X, Funk GD. Substance P modulation of hypoglossal motoneuron excitability during development: changing balance between conductances. J Neurophysiol. 2010;104:854–872. doi: 10.1152/jn.00016.2010. [DOI] [PubMed] [Google Scholar]
- Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve α2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol. 2005;98:1297–1308. doi: 10.1152/japplphysiol.00977.2004. [DOI] [PubMed] [Google Scholar]
- Alvarado-Castillo C, Harden TK, Boyer JL. Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol. 2005;67:114–122. doi: 10.1124/mol.104.006908. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Koga S, Nakatsuka T, Fujita T, Goto M, Kumamoto E. Excitation of rat spinal ventral horn neurons by purinergic P2X and P2Y receptor activation. Brain Res. 2010;1340:10–17. doi: 10.1016/j.brainres.2010.04.053. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. Pre- and postsynaptic mechanisms underlying inhibition of hypoglossal motor neuron excitability by riluzole. J Neurophysiol. 2013;110:1047–1061. doi: 10.1152/jn.00587.2012. [DOI] [PubMed] [Google Scholar]
- Bouhadfane M, Tazerart S, Moqrich A, Vinay L, Brocard F. Sodium-mediated plateau potentials in lumbar motoneurons of neonatal rats. J Neurosci. 2013;33:15626–15641. doi: 10.1523/JNEUROSCI.1483-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Dale N. Modulation of K+ currents in Xenopus spinal neurons by P2Y receptors: a role for ATP and ADP in motor pattern generation. J Physiol. 2002;540:843–850. doi: 10.1113/jphysiol.2001.013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Carrasquero LM, Delicado EG, Jiménez AI, Pérez-Sen R, Miras-Portugal MT. Cerebellar astrocytes co-express several ADP receptors. Presence of functional P2Y13-like receptors. Purinergic Signal. 2005;1:153–159. doi: 10.1007/s11302-005-6211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer NP, Li Y, Keller A. The whisking rhythm generator: a novel mammalian network for the generation of movement. J Neurophysiol. 2007;97:2148–2158. doi: 10.1152/jn.01187.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J Physiol. 2007;582:1047–1058. doi: 10.1113/jphysiol.2007.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Jordan LM. Multiple patterns and components of persistent inward current with serotonergic modulation in locomotor activity-related neurons in Cfos-EGFP mice. J Neurophysiol. 2010;103:1712–1727. doi: 10.1152/jn.01111.2009. [DOI] [PubMed] [Google Scholar]
- Dale N, Gilday D. Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature. 1996;383:259–263. doi: 10.1038/383259a0. [DOI] [PubMed] [Google Scholar]
- Enríquez Denton M, Wienecke J, Zhang M, Hultborn H, Kirkwood PA. Voltage-dependent amplification of synaptic inputs in respiratory motoneurones. J Physiol. 2012;590:3067–3090. doi: 10.1113/jphysiol.2011.225789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Choi RC, Simon J, Barnard EA, Brown DA. Activation of P2Y1 nucleotide receptors induces inhibition of the M-type K+ current in rat hippocampal pyramidal neurons. J Neurosci. 2006;26:9340–9348. doi: 10.1523/JNEUROSCI.2635-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AY, Krstew EV, Barden J, Lawrence AJ. Immunoreactive localisation of P2Y1 receptors within the rat and human nodose ganglia and rat brainstem: comparison with [α33P]deoxyadenosine 5′-triphosphate autoradiography. Neuroscience. 2002;113:809–823. doi: 10.1016/s0306-4522(02)00237-3. [DOI] [PubMed] [Google Scholar]
- Funk GD, Huxtable AG, Lorier AR. ATP in central respiratory control: a three-part signaling system. Respir Physiol Neurobiol. 2008;164:131–142. doi: 10.1016/j.resp.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Funk GD, Kanjhan R, Walsh C, Lipski J, Comer AM, Parkis MA, Housley GD. P2 receptor excitation of rodent hypoglossal motoneuron activity in vitro and in vivo: a molecular physiological analysis. J Neurosci. 1997;17:6325–6337. doi: 10.1523/JNEUROSCI.17-16-06325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Funk GD, Zwicker JD, Selvaratnam R, Robinson DM. Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms. Arch Ital Biol. 2011;149:426–453. doi: 10.4449/aib.v149i4.1271. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signalling in the medullary mechanisms of respiratory control in the rat: respiratory neurones express the P2X2 receptor subunit. J Physiol. 2003;552:197–211. doi: 10.1113/jphysiol.2003.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005a;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci. 2005b;25:1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Salle L, Simard C. The non-selective monovalent cationic channels TRPM4 and TRPM5. Adv Exp Med Biol. 2011;704:147–171. doi: 10.1007/978-94-007-0265-3_8. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Simard C, Del Negro C. Flufenamic acid as an ion channel modulator. Pharmacol Ther. 2013;138:272–284. doi: 10.1016/j.pharmthera.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm TM, Turkin VV, Bandekar NK, O'Neill D, Jung R. Persistent currents and discharge patterns in rat hindlimb motoneurons. J Neurophysiol. 2010;104:1566–1577. doi: 10.1152/jn.00380.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Resp Physiol Neurobiol. 2008;164:179–196. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Horner RL. The tongue and its control by sleep state-dependent modulators. Arch Ital Biol. 2011;149:406–425. doi: 10.4449/aib.v149i4.1256. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Del Negro CA, Trueblood PR, Chandler SH. Ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related β connexins. J Physiol. 2010a;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010b;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J Appl Physiol (1985) 1990;69:443–450. doi: 10.1152/jappl.1990.69.2.443. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Curr Opin Neurobiol. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Alvares TS, Ruangkittisakul A, Fang X, Hahn LB, Posse de Chaves E, Baker GB, Ballanyi K, Funk GD. Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J Neurosci. 2010;30:3947–3958. doi: 10.1523/JNEUROSCI.6027-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vrouwe SQ, Greer JJ, Funk GD. Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites. J Neurosci. 2009;29:14713–14725. doi: 10.1523/JNEUROSCI.2660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Alexandre Ribeiro J. Molecular physiology of P2 receptors in the central nervous system. Eur J Pharmacol. 2004;483:5–17. doi: 10.1016/j.ejphar.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Illes P, Ribeiro JA. Neuronal P2 receptors of the central nervous system. Curr Top Med Chem. 2004;4:831–838. doi: 10.2174/1568026043451032. [DOI] [PubMed] [Google Scholar]
- Ireland MF, Noakes PG, Bellingham MC. P2X7-like receptor subunits enhance excitatory synaptic transmission at central synapses by presynaptic mechanisms. Neuroscience. 2004;128:269–280. doi: 10.1016/j.neuroscience.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Costanzi S, Joshi BV, Besada P, Shin DH, Ko H, Ivanov AA, Mamedova L. Agonists and antagonists for P2 receptors. Novartis Found Symp. 2006;276:58–68. doi: 10.1002/9780470032244.ch6. discussion 68–72, 107–112, 275–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Role LW. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci. 2002;22:4794–4804. doi: 10.1523/JNEUROSCI.22-12-04794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert S, Blaser T, Tulapurkar M, Reiser G. P2Y receptor-activating nucleotides modulate cellular reactive oxygen species production in dissociated hippocampal astrocytes and neurons in culture independent of parallel cytosolic Ca2+ rise and change in mitochondrial potential. J Neurosci Res. 2007;85:3443–3456. doi: 10.1002/jnr.21316. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol. 1999;407:11–32. [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel U, Schraft T, Regenthal R, Illes P, Kittner H. Purinergic modulation of extracellular glutamate levels in the nucleus accumbens in vivo. Int J Dev Neurosci. 2004;22:565–570. doi: 10.1016/j.ijdevneu.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur J Neurosci. 2008;27:2501–2514. doi: 10.1111/j.1460-9568.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Batchelor AM. Novel action of BAPTA series chelators on intrinsic K+ currents in rat hippocampal neurones. J Physiol. 2000;522:231–246. doi: 10.1111/j.1469-7793.2000.t01-1-00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- León D, Hervás C, Miras-Portugal MT. P2Y1 and P2X7 receptors induce calcium/calmodulin-dependent protein kinase II phosphorylation in cerebellar granule neurons. Eur J Neurosci. 2006;23:2999–3013. doi: 10.1111/j.1460-9568.2006.04832.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Bötzinger complex inspiratory rhythm generating network in vitro. J Neurosci. 2007;27:993–1005. doi: 10.1523/JNEUROSCI.3948-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Lipski J, Housley GD, Greer JJ, Funk GD. ATP sensitivity of preBötzinger complex neurones in neonatal rat in vitro: mechanism underlying a P2 receptor-mediated increase in inspiratory frequency. J Physiol. 2008;586:1429–1446. doi: 10.1113/jphysiol.2007.143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Peebles K, Brosenitsch T, Robinson DM, Housley GD, Funk GD. P2 receptors modulate respiratory rhythm but do not contribute to central CO2 sensitivity in vitro. Respir Physiol Neurobiol. 2004;142:27–42. doi: 10.1016/j.resp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Pagnotta S, Donato R, Nistri A. Inhibition of spinal or hypoglossal motoneurons of the newborn rat by glycine or GABA. Eur J Neurosci. 2002;15:975–983. doi: 10.1046/j.1460-9568.2002.01927.x. [DOI] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Brazhe A, Thomsen K, Lauritzen M. Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J Cereb Blood Flow Metab. 2013;33:161–169. doi: 10.1038/jcbfm.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuka Y, Neubert JK, Maidment NT, Spigelman I. Concurrent release of ATP and substance P within guinea pig trigeminal ganglia in vivo. Brain Res. 2001;915:248–255. doi: 10.1016/s0006-8993(01)02888-8. [DOI] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife. 2013;2:e01213. doi: 10.7554/eLife.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Parkis MA, Lipski J, Funk GD. Modulation of phrenic motoneuron excitability by ATP: consequences for respiratory-related output in vitro. J Appl Physiol (1985) 2002;92:1899–1910. doi: 10.1152/japplphysiol.00475.2001. [DOI] [PubMed] [Google Scholar]
- Mironov SL( Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J Physiol. 2008;586:2277–2291. doi: 10.1113/jphysiol.2007.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Skorova EY. Stimulation of bursting in pre-Botzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J Neurochem. 2011;117:295–308. doi: 10.1111/j.1471-4159.2011.07202.x. [DOI] [PubMed] [Google Scholar]
- Mironov SL. Calmodulin and calmodulin kinase II mediate emergent bursting activity in the brainstem respiratory network (preBotzinger complex) J Physiol. 2013;591:1613–1630. doi: 10.1113/jphysiol.2012.237362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol. 2001;535:115–123. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Núñez-Abades PA, Cameron WE. Morphology of developing rat genioglossal motoneurons studied in vitro: relative changes in diameter and surface area of somata and dendrites. J Comp Neurol. 1995;353:129–142. doi: 10.1002/cne.903530112. [DOI] [PubMed] [Google Scholar]
- Núñez-Abades PA, He F, Barrionuevo G, Cameron WE. Morphology of developing rat genioglossal motoneurons studied in vitro: changes in length, branching pattern, and spatial distribution of dendrites. J Comp Neurol. 1994;339:401–420. doi: 10.1002/cne.903390308. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Piskuric NA. Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol. 2013;24:22–30. doi: 10.1016/j.semcdb.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Ortega F, Pérez-Sen R, Miras-Portugal MT. Gi-coupled P2Y-ADP receptor mediates GSK-3 phosphorylation and β-catenin nuclear translocation in granule neurons. J Neurochem. 2008;104:62–73. doi: 10.1111/j.1471-4159.2007.05021.x. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkis MA, Dong X, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Müller TH, Swandulla D. Calcium-activated non-selective channels in the nervous system. Brain Res Brain Res Rev. 1994;19:319–325. doi: 10.1016/0165-0173(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Ca2+-activated nonselective cationic current (ICAN) in turtle motoneurons. J Neurophysiol. 1999;82:730–735. doi: 10.1152/jn.1999.82.2.730. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol. 2000;528:107–113. doi: 10.1111/j.1469-7793.2000.t01-1-00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchen W, Sieler D, Wirkner K, Illes P. Co-transmitter function of ATP in central catecholaminergic neurons of the rat. Neuroscience. 2001;102:593–602. doi: 10.1016/s0306-4522(00)00529-7. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–624. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Price GD, Robertson SJ, Edwards FA. Long-term potentiation of glutamatergic synaptic transmission induced by activation of presynaptic P2Y receptors in the rat medial habenula nucleus. Eur J Neurosci. 2003;17:844–850. doi: 10.1046/j.1460-9568.2003.02501.x. [DOI] [PubMed] [Google Scholar]
- Rae MG, Rowan EG, Kennedy C. Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. Br J Pharmacol. 1998;124:176–180. doi: 10.1038/sj.bjp.0701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Calcium-dependent plateau potentials in rostral ambiguus neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1997;78:2483–2492. doi: 10.1152/jn.1997.78.5.2483. [DOI] [PubMed] [Google Scholar]
- Richardson PJ, Brown SJ. ATP release from affinity-purified rat cholinergic nerve terminals. J Neurochem. 1987;48:622–630. doi: 10.1111/j.1471-4159.1987.tb04138.x. [DOI] [PubMed] [Google Scholar]
- Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak K, Illes P. Neuronal and glial cell lines as model systems for studying P2Y receptor pharmacology. Neurochem Int. 2005;47:401–412. doi: 10.1016/j.neuint.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res. 1977;120:173–178. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Zhou M, Ito Y, Kimura S, Zou LB, Sekiguchi F, Kitramura K, Sunano S. Effects of flufenamic acid on smooth muscle of the carotid artery isolated from spontaneously hypertensive rats. J Smooth Muscle Res. 2002;38:39–50. doi: 10.1540/jsmr.38.39. [DOI] [PubMed] [Google Scholar]
- Simon J, Webb TE, King BF, Burnstock G, Barnard EA. Characterisation of a recombinant P2Y purinoceptor. Eur J Pharmacol. 1995;291:281–289. doi: 10.1016/0922-4106(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Sobrinho CR, Wenker IC, Poss EM, Takakura AC, Moreira TS, Mulkey DK. Purinergic signalling contributes to chemoreception in the retrotrapezoid nucleus but not the nucleus of the solitary tract or medullary raphe. J Physiol. 2014;592:1309–1323. doi: 10.1113/jphysiol.2013.268490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Jaffe DB, Williams SH, Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol. 1993;70:781–802. doi: 10.1152/jn.1993.70.2.781. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Bardini M, Burnstock G, Spyer KM. Evidence for the involvement of purinergic signalling in the control of respiration. Neuroscience. 2001;107:481–490. doi: 10.1016/s0306-4522(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- van Brederode JF, Yanagawa Y, Berger AJ. GAD67-GFP+ neurons in the Nucleus of Roller: a possible source of inhibitory input to hypoglossal motoneurons. I. Morphology and firing properties. J Neurophysiol. 2011;105:235–248. doi: 10.1152/jn.00493.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Gibbs L, Berger AJ. Double- and triple-labeling of functionally characterized central neurons projecting to peripheral targets studied in vitro. Neuroscience. 1990;38:829–841. doi: 10.1016/0306-4522(90)90075-f. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- Yao ST, Barden JA, Lawrence AJ. On the immunohistochemical distribution of ionotropic P2X receptors in the nucleus tractus solitarius of the rat. Neuroscience. 2001;108:673–685. doi: 10.1016/s0306-4522(01)00438-9. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Robinson DM, Selvaratnam SR, Walsh CW, McMorland AJ, Funk GD. Modulation of hypoglossal motoneuron excitability by NK1 receptor activation in neonatal mice in vitro. J Physiol. 2001;534:447–464. doi: 10.1111/j.1469-7793.2001.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wootton JF, Harris-Warrick RM. Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. II. Calcium-activated slow inward current. J Neurophysiol. 1995;74:1938–1946. doi: 10.1152/jn.1995.74.5.1938. [DOI] [PubMed] [Google Scholar]
- Zwicker JD, Rajani V, Hahn LB, Funk GD. Purinergic modulation of preBötzinger complex inspiratory rhythm in rodents: the interaction between ATP and adenosine. J Physiol. 2011;589:4583–4600. doi: 10.1113/jphysiol.2011.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]


