Abstract
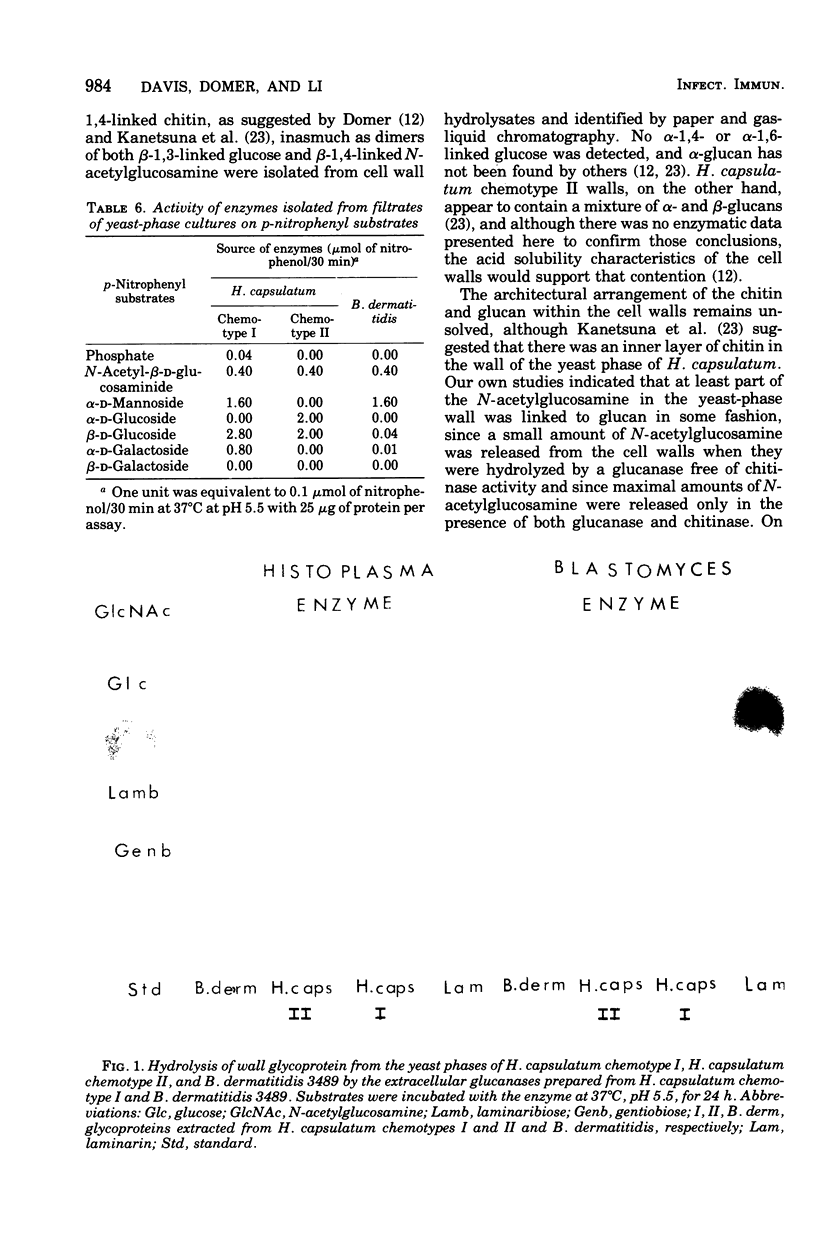
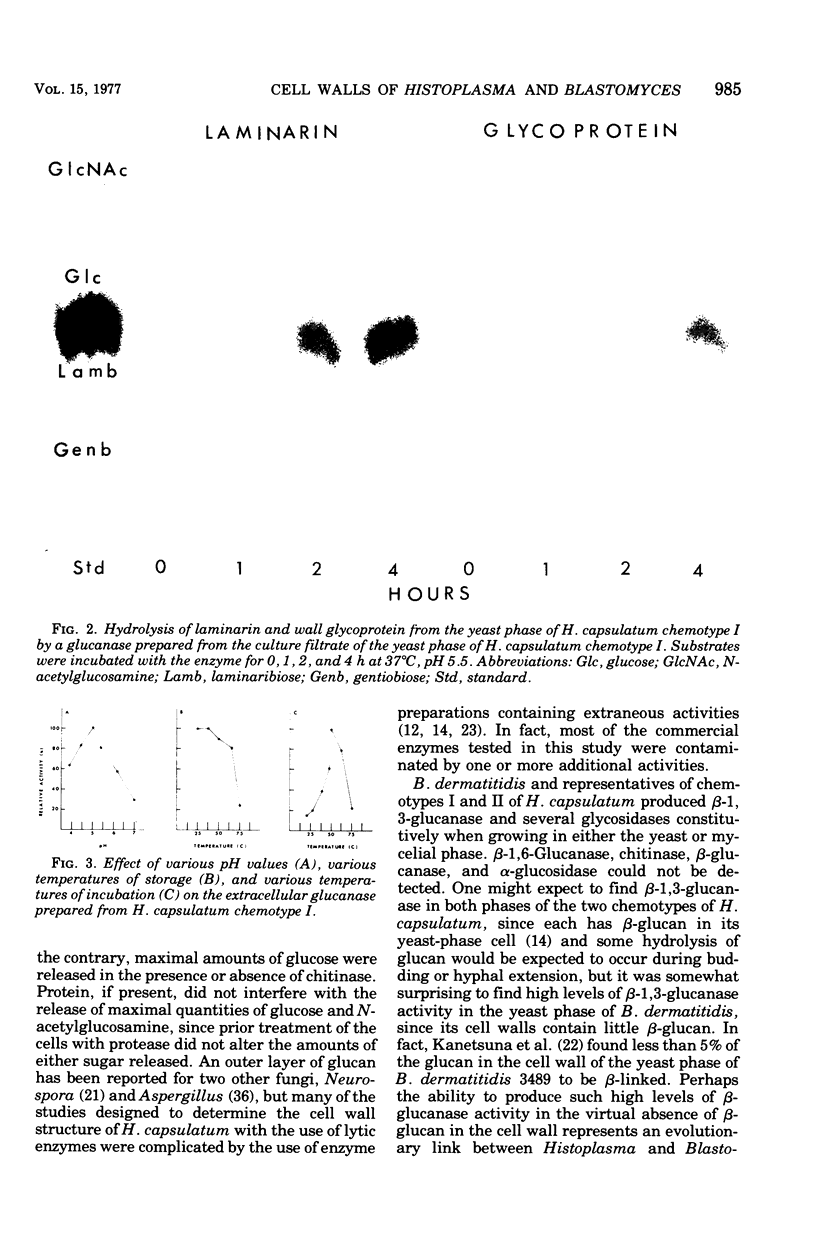
Enzymes capable of hydrolyzing cell walls of Blastomyces dermatitidis and chemotypes I and II of Histoplasma capsulatum were prepared in the laboratory or obtained from commercial sources. They included chitinases, beta-1,3-glucanases, beta-1,6-glucanase, and Pronase. Monosaccharides and disaccharides of glucose released from the cell walls by the enzymes were determined qualitatively by paper and gas-liquid chromatography, and monosaccharides were quantitated by the latter technique as well. An enzyme system isolated from Streptomyces sp. containing both chitinase and glucanase released maximum amounts of glucose and N-acetylglucosamine from the cell walls of H. capsulatum chemotype I. A chitinase preparation, free of glucanase, from Serratia marcescens released only chitobiose and N-acetylglucosamine from chemotype I cell walls, but the total quantity of N-acetylglucosamine released was about 60% less than that released by the Streptomyces system. A beta-1,3-glucanase from Bacillus circulans hydrolyzed the cell walls of H. capsulatum chemotype I, but a beta-1,6-glucanase failed to release glucose from the same walls. Autolytic enzymes, viz., beta-1,3-glucanases and several glycosidases were detected as constitutive enzymes in both yeast and mycelial phases of B. dermatitidis and H. capsulatum chemotypes I and II. No difference in the amount of activity was found between cell sap and culture filtrate preparations. The beta-glucanases prepared from the Histoplasma and Blastomyces strains were active on the cell walls of the yeast phases of H. capsulatum chemotypes I and II, releasing laminaribiose and glucose, but were essentially inactive on the cell walls of B. dermatitidis. Chitinase, beta-1,6-glucanase, alpha-glucanase, and alpha-glucosidase activities were absent from these fungal enzyme preparations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Wheat R. W., Conant N. F., Clingenpeel W. Composition of the cell wall and other fractions of the autolyzed yeast form of Histoplasma capsulatum. Mycopathol Mycol Appl. 1974 Dec 18;54(4):439–451. doi: 10.1007/BF02050050. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Cabib E. Molecular aspects of yeast morphogenesis. Annu Rev Microbiol. 1975;29:191–214. doi: 10.1146/annurev.mi.29.100175.001203. [DOI] [PubMed] [Google Scholar]
- Carbonell L. M., Berliner M. D., Gil F. Kinetic and morphological observations on the yeast phase of Histoplasma capsulatum during protoplast formation. J Bacteriol. 1972 May;110(2):731–738. doi: 10.1128/jb.110.2.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries O. M., Wessels J. G. Chemical analysis of cell wall regeneration and reversion of protoplasts from Schizophyllum commune. Arch Microbiol. 1975 Mar 10;102(3):209–218. doi: 10.1007/BF00428371. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G., Harkin J. C. Comparative study of the cell walls of the yeastlike and mycelial phases of Histoplasma capsulatum. J Bacteriol. 1967 Aug;94(2):466–474. doi: 10.1128/jb.94.2.466-474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G. The readily extracted lipids of Histoplasma capsulatum and Blastomyces dermatitidis. Biochim Biophys Acta. 1971 May 4;231(3):465–478. doi: 10.1016/0005-2760(71)90114-7. [DOI] [PubMed] [Google Scholar]
- Domer J. E. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol. 1971 Sep;107(3):870–877. doi: 10.1128/jb.107.3.870-877.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V., Biely P., Bauer S. Extracellular beta-glucanases of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Sep 15;321(1):246–255. doi: 10.1016/0005-2744(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Glucanases in Schizosaccharomyces. Isolation and properties of an exo-beta-glucanase from the cell extracts and culture fluid of Schizosaccharomyces japonicus var. versatilis. Biochim Biophys Acta. 1975 Dec 18;410(2):318–332. doi: 10.1016/0005-2744(75)90234-x. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Glucanases in Schizosaccharomyces. Isolation and properties of the cell wall-associated beta(1 leads to 3)-glucanases. J Biol Chem. 1974 Mar 25;249(6):1717–1728. [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Lysis of yeast cell walls: glucanases from Bacillus circulans WL-12. J Bacteriol. 1974 Jul;119(1):207–219. doi: 10.1128/jb.119.1.207-219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten V. Z., Bartnicki-Garcia S. Intracellular beta-glucanase activity of Phytophthora palmivora. Biochim Biophys Acta. 1972 Jul 13;276(1):221–227. doi: 10.1016/0005-2744(72)90023-x. [DOI] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M. Cell wall composition of the yeastlike and mycelial forms of Blastomyces dermatitidis. J Bacteriol. 1971 Jun;106(3):946–948. doi: 10.1128/jb.106.3.946-948.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Gil F., Azuma I. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol Mycol Appl. 1974 Oct 15;54(1):1–13. doi: 10.1007/BF02055967. [DOI] [PubMed] [Google Scholar]
- Kobayashi G. S., Guiliacci P. L. Cell wall studies of Histoplasma capsulatum. Sabouraudia. 1967 Feb;5(3):180–188. [PubMed] [Google Scholar]
- Kreger D. R., Kopecká M. On the nature and formation of the fibrillar nets produced by protoplasts of Saccharomyces cerevisiae in liquid media: an electronmicroscopic, x-ray diffraction and chemical study. J Gen Microbiol. 1976 Jan;92(1):207–220. doi: 10.1099/00221287-92-1-207. [DOI] [PubMed] [Google Scholar]
- LI Y. T., SHETLAR M. R. OCCURRENCE OF ALPHA-GALACTOSIDASE IN HIGHER FUNGI: ISOLATION OF ALPHA-GALACTOSIDASE FROM CALVATIA CYATHIFORMIS. Arch Biochem Biophys. 1964 Dec;108:523–530. doi: 10.1016/0003-9861(64)90437-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Monreal J., Reese E. T. The chitinase of Serratia marcescens. Can J Microbiol. 1969 Jul;15(7):689–696. doi: 10.1139/m69-122. [DOI] [PubMed] [Google Scholar]
- Page W. J., Stock J. J. Sequential action of cell wall hydrolases in the germination and outgrowth of Microsporum gypseum macroconidia. Can J Microbiol. 1974 Apr;20(4):483–489. doi: 10.1139/m74-075. [DOI] [PubMed] [Google Scholar]
- REYNOLDS D. M. Exocellular chitinase from a Streptomyces sp. J Gen Microbiol. 1954 Oct;11(2):150–159. doi: 10.1099/00221287-11-2-150. [DOI] [PubMed] [Google Scholar]
- Senft A. W., Maddison S. E. Hypersensitivity to parasite proteolytic enzyme in schistosomiasis. Am J Trop Med Hyg. 1975 Jan;24(1):83–89. doi: 10.4269/ajtmh.1975.24.83. [DOI] [PubMed] [Google Scholar]
- Sentandreu R., Elorza M. V., Villanueva J. R. Synthesis of yeast wall glucan. J Gen Microbiol. 1975 Sep;90(1):13–20. doi: 10.1099/00221287-90-1-13. [DOI] [PubMed] [Google Scholar]
- Skujins J. J., Potgieter H. J., Alexander M. Dissolution of fungal cell walls by a streptomycete chitinase and beta-(1-3) glucanase. Arch Biochem Biophys. 1965 Aug;111(2):358–364. doi: 10.1016/0003-9861(65)90197-9. [DOI] [PubMed] [Google Scholar]
- Skujiņs J., Puķite A., McLaren A. D. Chitinase of Streptomyces sp: purification and properties. Enzymologia. 1970 Dec 30;39(6):353–370. [PubMed] [Google Scholar]
- Stimson W. H. Gas-liquid chromatography of hexosamines. FEBS Lett. 1971 Feb 12;13(1):17–20. doi: 10.1016/0014-5793(71)80654-3. [DOI] [PubMed] [Google Scholar]
- Taylor I. E., Cameron D. S. Preparation and quantitative analysis of fungal cell walls: strategy and tactics. Annu Rev Microbiol. 1973;27:243–259. doi: 10.1146/annurev.mi.27.100173.001331. [DOI] [PubMed] [Google Scholar]