Abstract
Background
Elevated cardiac markers (CMs) and hyperphosphatemia are commonly encountered in patients with chronic kidney diseases (CKD), but the causal relationship between them has not been established.
Material/Methods
We enrolled 151 patients with different kidney functions in a cross-sectional study to explore the relationship of serum phosphorus with CMs, including cardiac troponin T (cTnT), myoglobin (MYO), creatine kinase-MB (CK-MB), and brain natriuretic peptide (BNP). Then, the effect of reducing phosphorus levels on CMs by taking phosphate binder for 3 months was prospectively observed in 64 hemodialysis patients. Finally, human cardiomyocytes were exposed to different concentrations of inorganic phosphorus to examine its underlying mechanism.
Results
1) Serum phosphorus and CMs gradually increased as the glomerular filtration rate declined in CKD patients (p<0.01). 2) Elevation of CMs was much greater and cardiac structure and function were worse in CKD patients who had higher serum phosphorus concentrations (p<0.05). 3) Serum phosphorus level positively correlated with cTnT, MYO, and BNP in CKD patients (p<0.001). 4) In hemodialysis patients, the reduction of cTnT, MYO, and CK-MB was synchronous with the pharmacologically-induced decline of serum phosphorus level. However, levels of serum Fibroblast growth factor 23 (FGF23) had no statistical decrease. 5) Simulated hyperphosphatemia inhibited proliferation of human cardiomyocytes in a time- and concentration-dependent manner.
Conclusions
Hyperphosphatemia may induce myocardial damage in CKD patients, possibly through triggering apoptosis of human cardiomyocytes, and this could account for the elevated cardiac markers in CKD patients.
MeSH Keywords: Apoptosis; Biological Markers; Fibroblast Growth Factors; Hyperphosphatemia; Renal Insufficiency, Chronic; Troponin T
Background
Chronic kidney disease (CKD) is an independent risk factor for coronary artery disease [1]. Patients with CKD carry a 10–20 times higher cardiovascular disease (CVD) mortality than that in the general population [2]. CVD is the leading cause of mortality in CKD patients [3]. However, the pathogenesis of cardiovascular injury in ESRD remains unclear.
Hyperphosphatemia is a common feature of ESRD. Hemodialysis (HD) patients with serum phosphorus >2.1 mmol/L had 41% greater risk of death resulting from CVD as compared with patients with serum phosphorus levels ranging from 0.7 to 2.1 mmol/L [4]. Hyperphosphatemia in patients with normal renal function is also associated with increased cardiovascular morbidity and mortality [5].
Cardiac markers (CMs), such as cardiac troponin T (cTnT), myoglobin (MYO), creatine kinase-MB (CK-MB), and brain natriuretic peptide (BNP), have been used clinically as sensitive diagnostic markers of myocardial necrosis [6–8]. Interestingly, elevated CMs were observed in patients with various degrees of renal failure [9]. However, the majority of those patients presented without acute coronary syndrome (ACS) [10,11].
Di Marco GS et al. [12] and our previous study [13] proved that phosphorus overload directly led to apoptosis of human vascular endothelial cells; therefore, we hypothesized that serum phosphorus level accounts for the increase of CMs in CKD patients. To confirm this, we conducted in vivo and in vitro studies to explore the role of serum phosphorus in CKD patients with elevated CMs, as well as its underlying mechanism.
Material and Methods
Cross-sectional study design
We recruited patients with different stages of CKD, who were hospitalized from January to August 2013. The race of all those patients was Chinese Han. Inclusion criteria were: 1) met the diagnostic standard of CKD [14]; 2) ranged from 18 to 75 years old; and 3) were on a standard hospital diet. Exclusion criteria included: 1) acute kidney injury [15]; 2) history of myocardial infarction, coronary artery stent implantation, or arteriovenous fistula; 3) history of any of the following in the 6 months prior to recruitment: ACS, malignant hypertension, surgery, trauma, blood infusion, and erythropoietin use; 4) history of malignancy, active tuberculosis, inflammatory diseases, or diabetic ketoacidosis; and 5) loss of appetite, vomiting, or diarrhea.
Glomerular filtration rate (GFR) was assessed by radioisotope nephrography. According to their GFR, patients were divided into 3 groups14: CKD stages 1–2, CKD stage 3, and CKD stages 4–5. Patients were also divided into a high serum phosphorus (HSP) group (serum phosphorus ≥1.45 mmol/L) and a normal serum phosphorus (NSP) group (0.81≤ serum phosphorus <1.45 mmol/L) [16]. We also recruited patients without kidney diseases and with normal GFR levels as a Non-CKD group.
All patients ate 3 standard hospital-based meals per day as regular hospital care, which were offered by the Nutrition Department of our hospital on the day when they were recruited. Meal compositions were adjusted by the nutritionists according to patients’ height, weight, physical strength, and disease. Additional food was forbidden. On the morning of the third day, fasting venous blood was drawn and 24-h urine was collected to examine urinary phosphorus excretion.
A prospective randomized cohort study
To observe the effect of reducing phosphorus absorption on CMs, patients with anuria who were receiving regular HD in the Shanghai 10th People’s Hospital of Tongji University, were enrolled in this non-double blind prospective cohort study. All patients fulfilled the following inclusion criteria: 1) age between 18 to 75 years; 2) time on HD exceeding 1 year; and 3) serum phosphorus ≥1.6mmol/L. Exclusion criteria were: 1) history of coronary artery stent implantation; 2) history of 1 of the following in the 6 months before recruitment: use of any phosphate binder; major acute CVD events, including ACS, stroke, foot ulcer or gangrene, amputation, coronary, carotid, and lower limb revascularization; 3) inability to tolerate adverse effects of phosphate binder or non-compliance with prescription directions; and 4) inadequate dialysis [14]. The flow of participants in the study, protocol for this trial, and Consort checklist are available as supporting information.
The 64 enrolled HD patients were randomized to either treatment or control group by drawing numbers according to their ID numbers. Two patients in the treatment group quit the study due to the onset of angina pectoris (n=1) and severe diarrhea n=1). One patient from the control group was lost owing to his relocation to another dialysis center.
Patients in the treatment group were advised to take a 4.002 g total dose of oral calcium acetate (Kunming BANGYU Pharmaceutical Co., Ltd., China), a phosphate binder, split into 3 daily doses continuously for 3 months. If the patient still refused to receive this treatment, he or she would be excluded from the study. Finally, all those in the treatment group agreed to take the medicine. A designated nurse confirmed they had taken the medicine on time. Patients in the control group did not take any phosphate binder, but all other medications remained unchanged. Fasting venous blood was taken before HD at 2 time-points: the start of the study and after 3 months. Dietary intake was carefully recorded to calculate average meal compositions per day over the last HD treatment day and the 2 subsequent non-dialysis days according to Block food frequency questionnaires, supplemented by a person-to-person dietary interview to recall their home meals [17].
Laboratory measurements were recorded in supporting information.
This study was approved by the Institutional Review Board of Shanghai Tenth People’s Hospital of Tongji University (IRB: 2012RES047) and was registered on www.ClinicalTrials.gov (number: NCT01781156). The research was conducted with informed written consent, according to the principles expressed in the Declaration of Helsinki.
Cell culture
Adult HCMs (Cat. No.6210, ScienCell, USA) were cultured in a humidified incubator at 37°C and 5% CO2. HCMs were first incubated in Cardiac Myocyte Medium (Cat. No.6201, ScienCell, USA) at about 85% confluence and then incubated in medium added with inorganic phosphorus to achieve final phosphorus concentrations of 1.0, 1.5, 2.0, and 3.0 mmol/L, according to our previous study [13]. The physiologic range of serum phosphate concentration is approximately 0.9–1.5 mmol/L. Those incubated with 3 mmol/L inorganic phosphorus were also treated with 1 mmol/L phosphonoformic acid (PFA, Sigma, USA), a specific inhibitor of the sodium-phosphate transporter, to block phosphate transport into HCMs.
Cell proliferation and apoptosis studies
After treating HCMs with different concentrations of phosphate for 24 h, cell growth was monitored for 7 days using a Cell Proliferation MTT Kit (Sigma, USA). The absorbance at 570 nm was read on a microplate reader. Apoptosis was determined by staining with Annexin V-FITC/PI apoptosis Kit (BD, Pharmingen). Attached cells were harvested at 12, 24, and 36 h and incubated with Annexin V-FITC/PI for 15 min at room temperature. Cell samples were analyzed by flow cytometry. Live cells are Annexin-V-ve, 7-AAD-ve; necrotic cells are Annexin-V-ve, 7-AAD+ve; apoptotic cells are Annexin-V+ve, 7-AAD-ve or Annexin-V+ve, 7-AAD+ve.
Western blot
Western blot was performed following the kits’ standard protocols. The following primary antibodies were used: Caspase-3 antibody (diluted 1:1000, Cell Signaling, USA) and apoptosis-inducing factor (AIF) antibody (diluted 1:1000, Cell Signaling, USA); both of the 2 secondary antibodies used were diluted 1:2000.
Statistical analysis
Values are expressed as mean ±SD or median (interquartile range) as appropriate unless otherwise indicated. All analyses were carried out using SPSS 18.0 (SPSS Inc., USA) with 2-tailed tests and p<0.05 was regarded as statistically significant. In this cross-sectional study, variables were compared by Analysis of Variance (ANOVA) or Wilcoxon rank sum or Kruskal-Wallis test, as appropriate. The Pearson’s correlation for continuous variables or the Spearman’s correlation for categorical variables was used in univariate analyses to determine the association between CMs and biochemical parameters, LVMI and left ventricular ejection fraction (LVEF) respectively. Multivariate linear regression analysis was used to compare cTnT with other parameters.
Data from HD patients were analyzed by paired t-test or non-parametric test, as appropriate. The significance of the difference of parameters among HCMs cultured in different concentrations of phosphate was analyzed by ANOVA.
Results
Analysis of data collected in the cross-sectional study
We recruited 151 patients in the cross-sectional study. All CMs showed a tendency toward elevation that increased with progression of CKD stages (p<0.01). Comparing CMs in non-CKD with those in CKD stages, we found a statistically significant rise of cTnT and MYO beginning with CKD stage 3 and of CK-MB and BNP beginning with CKD stages 4–5 (Table 1).
Table 1.
Clinical Characteristics of patients in the cross-sectional study.
| GFR (ml/min/1.73 m2) | Non-CKD >90 | CKD1-2 ≥60 | CKD3 30~59 | CKD4-5 <30 | P-value |
|---|---|---|---|---|---|
| Demographic data | |||||
| Number (N=151) | 26 | 40 | 58 | 27 | |
| Age (y) | 65.45±8.58 | 66.51±10.02 | 67.71±9.82 | 69.67±12.91 | 0.31 |
| Male n (n%) | 10 (38.46) | 15 (37.50) | 25 (43.10) | 16 (59.26) | 0.74 |
| Diabetes n(n%) | 10 (38.46) | 24 (60.00) | 34 (58.62) | 11 (40.74) | 0.07 |
| MAP (mmHg) | 96.63±14.26 | 96.27±12.33 | 97.74±12.29 | 97.13±12.68 | 0.89 |
| Diet composition (per day) | |||||
| Total calorie (kilocalorie) | 1957.04±167.51 | 1781.70±246.81** | 1694.59 (1500~1800)** | 1716.67±209.40** | <0.001 |
| Fat (g) | 59.88±9.99 | 56.48±9.21 | 56.49 (55~60) | 57.22±6.98 | 0.38 |
| Protein (g) | 53.27 (39~66) | 47.98±14.99 | 39.00±7.34** | 37.11±8.02** | <0.001 |
| Carbohydrate (g) | 306.00±41.40 | 271.35±44.97** | 257.05±36.42** | 263.00±30.71** | <0.001 |
| Phosphorus (mg) | 827.88 (608~991) | 746.60±209.61 | 617.65±106.45** | 591.52±114.62** | <0.001 |
| Calcium (mg) | 502.19±18.52 | 491.93±26.41 | 484.59 (464~501)** | 485.37±23.01* | 0.01 |
| Laboratory measurements | |||||
| Serum phosphorus (mmol/L) | 1.12±0.19 | 1.13±0.16* | 1.25±0.27** | 1.74±0.65** | <0.001 |
| Serum calcium (mmol/L) | 2.27±0.11 | 2.26±0.12 | 2.24±0.16 | 2.11±0.28** | 0.002 |
| Ca×Pi product | 2.50±0.41 | 2.55±0.39 | 2.96±0.53** | 3.57±1.06** | <0.001 |
| 24hs phosphorus excretion in urine (mmol/L) | 15.45±5.40 | 13.40±5.87 | 13.10±5.97 | 10.67±4.17 | 0.17 |
| n=24 | n=36 | n=40 | n=24 | ||
| PTH (pmol/L) | 4.53±0.42 | 4.94±1.95 | 8.34 (5.02~8.18) | 27.28±29.08* | <0.001 |
| n=20 | n=32 | n=46 | n=24 | ||
| Serum creatinine (μmol/L) | 58.96±8.71 | 74.80±10.92 | 112.35±26.13 | 535.78±365.41** | <0.001 |
| Cys-C (mmol/L) | 1.00±0.23 | 0.98±0.27 | 1.63±0.44 | 3.98±1.41** | <0.001 |
| n=23 | n=37 | n=48 | n=27 | ||
| Hemoglobin (g/L) | 128.19±15.78 | 127.47±10.65 | 114.18±15.15** | 97.74±20.99** | <0.001 |
| Serum potassium (mmol/L) | 3.91±0.45 | 3.82±0.45 | 4.23±0.50** | 4.57±0.63** | <0.001 |
| Cardiac markers | |||||
| cTnT (ng/mL) | 0.004 (0.003~0.0032) | 0.008 (0.003~0.009) | 0.019 (0.01~0.022)* | 0.07±0.055** | <0.001 |
| MYO (ng/mL) | 24.47±3.85 | 34.79 (21.23~36.03) | 77.66 (40.18~91.50)** | 174.85±143.69** | <0.001 |
| CK-MB (U/L) | 1.85±0.46 | 2.29±0.65 | 3.98 (2.02~3.33) | 9.75 (2.33~8.92)** | 0.005 |
| BNP (pg/mL) | 121.12 (44.53~165.58) | 266.06 (56.03~238.51) | 709.48 (109.18~508.18) | 6125.00 (209~9677)** | <0.001 |
GFR – glomerular filtration rate; MAP – mean arterial pressure; PTH – parathyroid hormone; Cys-C – cystatin C; cTnT – cardiac troponin T; MYO – myoglobin; CK-MB – MB isoenzyme of creatine phosphokinases; BNP – brain natriuretic peptide. All values mean ±SD or IQR. p-values are analyzed between CKD groups by ANOVA.
p<0.05;
p<0.01.
These represented paired-comparisons results between each CKD group and Non-CKD group respectively.
From non-CKD to CKD stages 4–5, serum phosphorus, Ca×Pi product, and PTH gradually increased. Patients in CKD stage 4–5 had distinctly low phosphorus excretion in 24-h urine compared to patients with normal renal function, but no statistical difference was observed (Table 1).
In univariate correlation analysis, serum phosphorus and Ca×Pi product were all positively associated with cTnT, MYO, and BNP. GFR was negatively correlated to cTnT, MYO, and BNP. However, no correlation was present between CMs and body mass index (BMI), hemoglobin, serum calcium, and serum uric acid (Table 2 and Figure 1). Serum phosphorus, Ca×Pi product, PTH, cTnT, and MYO were all positively correlated to LVMI and negatively correlated to LVEF, while GFR was exactly the opposite (Table 2).
Table 2.
Correlation analysis of cardiac markers in CKD patients.
| cTnT | MYO | CK-MB | BNP | LVMI | LVEF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | |
| Serum Pi | 0.35 | <0.001 | 0.58 | <0.001 | 0.12 | 0.19 | 0.33 | <0.001 | 0.4 | 0.00 | −0.33 | 0.01 |
| Serum Ca | 0.09 | 0.32 | 0.01 | 0.89 | 0.10 | 0.28 | −0.09 | 0.33 | −0.12 | 0.28 | 0.02 | 0.86 |
| Ca×Pi | 0.41 | <0.001 | 0.62 | <0.001 | 0.12 | 0.21 | 0.34 | <0.001 | 0.45 | 0.00 | −0.36 | <0.001 |
| PTH | 0.10 | 0.42 | 0.001 | 0.92 | 0.13 | 0.31 | 0.38 | <0.001 | 0.38 | 0.01 | −0.28 | 0.02 |
| Age | 0.16 | 0.08 | 0.19 | 0.09 | 0.27 | 0.00 | 0.04 | 0.69 | 0.05 | 0.65 | −0.11 | 0.39 |
| BMI | −0.14 | 0.19 | 0.07 | 0.55 | −0.17 | 0.12 | −0.02 | 0.86 | −0.04 | 0.77 | 0.13 | 0.23 |
| Hb | −0.17 | 0.21 | −0.14 | 0.17 | −0.07 | 0.48 | −0.14 | 0.13 | −0.27 | 0.02 | 0.06 | 0.63 |
| SCR | 0.35 | 0.00 | 0.14 | 0.13 | 0.03 | 0.74 | 0.20 | 0.03 | 0.63 | 0.00 | −0.15 | 0.24 |
| SUA | 0.15 | 0.13 | 0.13 | 0.19 | 0.05 | 0.59 | −0.05 | 0.59 | −0.02 | 0.88 | −0.09 | 0.46 |
| GFR | −0.59 | <0.001 | −0.56 | <0.001 | −0.19 | 0.02 | −0.4 | <0.001 | −0.39 | <0.001 | 0.37 | <0.001 |
| LVMI | 0.46 | <0.001 | 0.32 | 0.00 | 0.1 | 0.39 | 0.35 | <0.001 | – | – | – | – |
| LVEF | −0.42 | <0.001 | −0.27 | 0.02 | −0.16 | 0.18 | −0.28 | 0.01 | – | – | – | – |
Abbreviations seen in Table 1. Pi – phosphorus; Ca – calcium; BMI – body mass index; Hb – hemoglobin; SCR – serum creatinine; SUA – serum uric acid; LVMI – left ventricular mass index; LVEF – left ventricle eject fraction.
Figure 1.
Bivariate correlation analysis between serum phosphorus and cardiac markers, LVMI, and LVEF. Serum phosphorus was positively correlated with cTnT, MYO, BNP, and LVMI and negatively correlated with LVEF. However, there was no correlation between serum phosphorus and CK-MB.
In a multivariate linear regression analysis model, serum phosphorus was positively associated with cTnT after adjustment for age, sex, GFR, BMI, diabetes, mean artery pressure (MAP), serum albumin, PTH, LVMI, LVEF, phosphate, and calcium in diet in these CKD patients (Table 3).
Table 3.
Multiple linear regression analysis for cTnT in CKD patients
| Model | Unstandardized coefficients | Standardized coefficients | |||
|---|---|---|---|---|---|
| B | Std. error | Beta | t | p-value | |
| Serum phosphorus | 0.05 | 0.01 | 0.52 | 3.61 | <0.00 |
| GFR | 0.00 | 0.00 | −0.27 | −1.63 | 0.11 |
| Serum calcium | 0.11 | 0.03 | 0.52 | 4.24 | <0.001 |
| Sex | 0.02 | 0.01 | 0.27 | 2.35 | 0.05 |
| Age | −0.00 | 0.00 | −0.12 | −1.11 | 0.28 |
| HbA1c | 0.00 | 0.00 | −0.02 | −0.16 | 0.88 |
| Diabetes | −0.02 | 0.01 | −0.17 | −1.56 | 0.13 |
| BMI | 0.00 | 0.00 | 0.08 | 0.75 | 0.46 |
| MAP | −0.00 | 0.00 | −0.20 | −1.88 | 0.07 |
| PTH | 0.00 | 0.00 | 0.15 | 1.22 | 0.23 |
| Serum albumin | −0.01 | 0.00 | −0.64 | −5.42 | <0.001 |
| Phosphorus in diets | 0.00 | 0.00 | 0.25 | 1.40 | 0.17 |
| Calcium in diets | 0.00 | 0.00 | −0.30 | −1.79 | 0.08 |
CMs and parameters of myocardial function, including left atrial dimensions (LAD), left ventricular end-systolic dimension (LVDs), left ventricular end-diastolic dimension (LVDd), inter ventricular septal thickness (IVST), and left ventricular posterior wall thickness (LVPWT), in the HSP group were consistently higher than those in he NSP group, while LVEF showed the opposite trend. To avoid any potential effect of different distribution of patients’ renal function in the NSP and HSP groups, we also focused on patients in CKD stage 3 alone, and then repeated the above comparisons. The results were consistent with that of the comparisons in all CKD patients, as described above (Table 4).
Table 4.
Comparison between high serum phosphorus (HSP) group and normal serum phosphorus (NSP) group.
| CKD stage 1–5 | CKD stage 3 | |||||
|---|---|---|---|---|---|---|
| HSP | NSP | P-value | HSP | NSP | P-value | |
| Number | 47 | 66 | 25 | 33 | ||
| Age (y) | 67.64±11.31 | 68.78±10.28 | 0.56 | 69.20±9.82 | 71.85±9.80 | 0.31 |
| MAP (mmHg) | 95.61±12.95 | 97.41±12.75 | 0.44 | 97.39±13.79 | 98.00±11.30 | 0.86 |
| GFR (ml/min/1.73 m2) | 40.34±27.43 | 57.17±26.58 | 0.43 | 57.01±12.98 | 49.45±10.57 | 0.38 |
| Ingredients of diets | ||||||
| Total calorie (kilocalorie) | 1708.93±224.87 | 1779.18 (1650~1950) | 0.18 | 1681.03±224.57 | 1743.75 (1500~1800) | 0.51 |
| Fat (g) | 56.64±7.77 | 56.89 (50~65) | 0.89 | 56.03±7.49 | 58.13 (50~60) | 0.51 |
| Protein (g) | 39.54±9.56 | 45.26 (33~52) | 0.06 | 37.79±6.99 | 43.38±7.35 | 0.06 |
| Carbohydrate (g) | 259.89±34.19 | 273.19±45.18 | 0.36 | 255.90±35.51 | 261.25±41.86 | 0.72 |
| Phosphorus (mg) | 624.86±132.87 | 708.67 (531~806) | 0.05 | 600.93±102.31 | 678.25±105.12 | 0.07 |
| Calcium (mg) | 490.86±24.19 | 486.07±25.48 | 0.38 | 482.34±21.66 | 492.75±31.27 | 0.28 |
| Cardiac markers | ||||||
| cTnT (ng/mL) | 0.043 (0.015~0.052) | 0.014 (0.003~0.015) | <0.001 | 0.021±0.012 | 0.015 (0.008~0.017) | 0.01 |
| MYO (ng/mL) | 134.01 (60.90~167.10) | 50.76 (23.35~54.13) | <0.001 | 88.60±57.14 | 69.34 (34.10~81.75) | 0.01 |
| CK-MB (U/L) | 6.24 (2.17~4.44) | 3.4 (1.88~2.95) | <0.001 | 5.84 (2.02~3.77) | 2.57±1.01 | 0.23 |
| BNP (pg/mL) | 3507.70 (121~3045) | 583.79 (59.33~251.23) | 0.001 | 995.80 (121~928) | 222.18±242.58 | 0.01 |
| Echocardiography measurements | ||||||
| Number | 39 | 61 | 23 | 29 | ||
| LAD (mm) | 45.77±5.38 | 45.18±5.54 | 0.60 | 46.52±3.92 | 45.34±6.22 | 0.44 |
| LVDs (mm) | 33.10±6.32 | 30.77±4.13 | 0.03 | 31.48±4.01 | 29.31±3.13 | 0.02 |
| LVDd (mm) | 49.92±5.45 | 47.18±4.00 | 0.01 | 49.39±3.49 | 46.66±3.46 | 0.007 |
| IVST (mm) | 10.31 (9.00~11.00) | 9.40 (8.63~10.00) | 0.001 | 10.22±1.28 | 9.45 (9.00~10.00) | 0.02 |
| LVPWT (mm) | 10.08±1.15 | 9.14±0.93 | <0.001 | 10.11±1.07 | 9.29±0.88 | 0.004 |
| LVMI (g/m2) | 139.68±40.93 | 104.39±23.30 | <0.001 | 139.86±40.40 | 105.63±24.19 | 0.002 |
| LVEF (%) | 59.68±9.91 | 66.40±10.58 | 0.007 | 62.80±8.99 | 64.60±7.84 | 0.53 |
Abbreviations seen in Table 1. LAD – left atrium dimension; LVDs – left ventricular end-systolic dimension; LVDd – left ventricular end-diastolic dimension; IVST – inter ventricular septal thickness; LVPWT – left ventricular posterior wall thickness.
We recorded total amounts of nutrients in daily meals of enrolled patients. We found: 1) calorie and carbohydrate intake by patients in CKD groups were less than that by patients in non-CKD group; 2) protein, calcium, and phosphorus gradually decreased with the decline of kidney function, while 24-h phosphorus excretion in urine distinctively diminished in the CKD stage 4–5 group (Table 1); and 3) phosphorus intake by patients in the NSP group was much greater in the HSP group (Table 4).
Changes of CMs in HD patients after 3-month oral ingestion of phosphate binder
Basic features of HD patients are presented in supporting information. There was no statistical difference between the 2 groups in age, sex proportion, and number with hypertension or diabetes.
At baseline level, except for serum uric acid, no significant difference existed in other parameters between the 2 groups. Compared with baseline values, there was no significant change of values after 3 months in BMI, Kt/v, serum glucose level, or ingredients of meals in both groups.
After 3 months, irrespective of whether the comparison was with the baseline values or the 3-month levels in the control group, serum phosphorus and Ca×Pi product in the treatment group decreased significantly. Most importantly, cTnT and CK-MB levels in treated patients were both significantly reduced. The levels of PTH, MYO, BNP, and serum FGF23 showed no significant decrease (Table 5).
Table 5.
Effects of reduced phosphorus absorption in cardiac markers and other biochemical parameters in HD patients.
| Treatment group | Control group | |||||
|---|---|---|---|---|---|---|
| Baseline values | Values after 3 months | P-value | Baseline values | Values after 3 months | P-value | |
| BMI | 21.98±3.30 | 21.96±3.25 | 0.64 | 21.93±3.35 | 21.97±3.27 | 0.50 |
| Kt/v | 1.33±0.10 | 1.34±0.18 | 0.28 | 1.35±0.34 | 1.24±0.34 | 0.12 |
| Diet composition | ||||||
| Total calorie (kilocalorie) | 1785.00±224.05 | 1800.5±185.64 | 0.54 | 1720.00±225.37 | 1785.00±224.05 | 0.33 |
| Fat (g) | 59.50±7.47 | 60.07±6.40 | 0.63 | 57.33±7.51 | 59.50±7.47 | 0.33 |
| Protein (g) | 37.93±5.72 | 38.37±6.04 | 0.57 | 37.17±7.40 | 38.33±5.83 | 0.55 |
| Carbohydrate (g) | 274.13±35.98 | 277.73±36.94 | 0.42 | 263.50±34.47 | 274.13±35.98 | 0.30 |
| Phosphorus (mg) | 598.07±79.77 | 598.83±83.58 | 0.94 | 591.60±105.74 | 598.07±79.77 | 0.81 |
| Calcium (mg) | 487.00±19.13 | 489.13±17.79 | 0.61 | 484.73±21.83 | 489.33±16.98 | 0.41 |
| Laboratory measurements | ||||||
| Hemoglobin (g/L) | 108.27±14.92 | 111.33±12.40 | 0.08 | 104.60±13.17 | 106.53±13.82 | 0.14 |
| Serum phosphorus (mmol/L) | 2.54±0.80 | 2.21±0.56 | 0.001 | 2.52±0.53 | 2.47±0.45* | 0.57 |
| Serum calcium (mmol/L) | 2.12±0.28 | 2.15±0.27 | 0.66 | 2.10±0.30 | 2.14±0.33 | 0.32 |
| Ca×Pi product | 5.43±1.91 | 4.77±1.44 | 0.01 | 5.31±1.43 | 5.28±1.28* | 0.90 |
| PTH (pg/mL) | 109.89±73.17 | 91.92±63.03 | 0.01 | 110.88±89.34 | 107.85±86.70 | 0.91 |
| Serum creatinine (μmol/L) | 1051.72±281.79 | 1027.27±221.60 | 0.32 | 934.53±320.79 | 971.17±237.59 | 0.36 |
| Serum uric acid (μmol/L) | 466.90±88.94 | 447.73±101.80 | 0.28 | 417.10±101.59* | 428.26±90.58 | 0.51 |
| Blood glucose(mmol/L) | 6.42±1.83 | 6.53±2.02 | 0.56 | 6.76±3.22 | 6.66±2.22 | 0.85 |
| FGF23 | ||||||
| Intact (pg/mL) | 48.09±19.14 | 42.45±15.28 | 0.24 | 48.36±20.02 | 48.63±21.88 | 0.93 |
| C-terminal (RU/mL) | 807.86±343.20 | 776.14±319.07 | 0.48 | 853.15±316.15 | 859.42±289.62 | 0.86 |
| Cardiac markers | ||||||
| cTnT (ng/mL) | 0.064±0.033 | 0.056±0.032 | <0.001 | 0.068±0.047 | 0.070±0.036* | 0.85 |
| MYO (ng/mL) | 228.76±110.54 | 203.71±76.82 | 0.12 | 212.78±99.19 | 206.48±63.78 | 0.78 |
| CK-MB (U/L) | 2.20±1.03 | 1.90±0.84 | 0.005 | 2.23±0.98 | 2.23±0.98* | 0.97 |
| BNP (pg/mL) | 4114.60±6830.74 | 3726.60±6383.48 | 0.27 | 5899.55±6720.72 | 5185.22±6438.88 | 0.67 |
Abbreviations as in Table 1.
p<0.05 between experimental group and control group.
Effect of inorganic phosphorus on HCMs
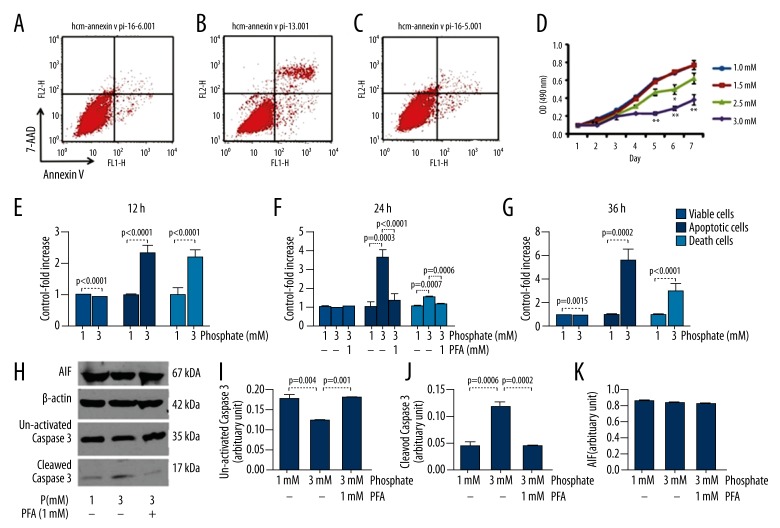
In the in vitro study, a 3-mM concentration of inorganic phosphate induced apoptosis and necrosis of human cardiac myocytes (HCMs). However, the majority death type of HCMs was recognized as apoptosis, based on the analysis of cell numbers (Figure 2) and morphology of dead cells (features of necrosis like cell swelling and plasma membrane damage were observed, data not shown). Phosphorus added to the medium inhibited HCMs proliferation in a time- and concentration-dependant manner. Compared with HCMs cultured in medium with 1mmol/L phosphorus, viable cells decreased, while necrotic and apoptotic cells increased when HCMs were cultured in medium with 3 mmol/L of phosphorus, at all time periods (Figure 2).
Figure 2.
Effect of phosphate on proliferation and apoptosis of human cardiac myocytes (HCMs). After incubation for 24 h, compared to the HCMs cultured in 1mmol/L inorganic phosphorus (A), apoptotic cells increased when cultured in medium with 3 mmol/L phosphorus (B). This effect was inhibited by adding 1 mmol/L of PFA, a specific inhibitor of phosphate transport (C). MTT assay (D) showed phosphorus overload in moderately-inhibited HCMs proliferation in a time- and concentration-dependant manner (* p<0.05, ** p<0.01). Compared to HCMs cultured in 1 mmol/L of phosphorus, apoptotic cells and necrotic cells of HCMs in medium with 3 mmol/L of inorganic phosphate increased after incubation for 12 h (E), 24 h (F), and 36 h (G). When 1 mmol/L of PFA was added, apoptotic and necrotic cells decreased after 24 h of incubation (F). Western blot analysis (H) showed that expression of cleaved Caspase-3 increased in cells cultured in medium with 3 mmol/L of phosphate compared to medium with 1 mmol/L of phosphate. However, this up-regulating effect was inhibited when 1 mmol/L of PFA was added. (I–K) The expression of uncleaved Caspase-3 protein exhibited a reciprocal pattern to that of cleaved Caspase-3, but the expression of apoptosis-inducing factor (AIF) proteins exhibited no significant change.
Western blotting showed a significantly increased expression of cleaved Caspase-3 proteins when cultured in medium with 3 mmol/L of inorganic phosphate. However, this up-regulating effect was inhibited when PFA was added to medium with 3 mmol/L of phosphate. The expression of uncleaved Caspase-3 protein exhibited a reciprocal change to that of cleaved Caspase-3, while the expression of AIF proteins exhibited no significant change (Figure 2). Consistent with these findings, the number of apoptotic HCMs decreased and the numbers of viable HCMs increased in the proliferation study (data not shown).
Discussion
Our study demonstrates that: 1) CMs and serum phosphorus gradually increase with the severity of renal dysfunction in CKD patients; 2) CMs were elevated more in patients with higher serum phosphorus concentration than in those with normal serum phosphorus level; 3) serum phosphorus and Ca×Pi product were positively correlated with cTnT, MYO, and BNP, but only serum phosphorus was independently associated with cTnT; and 4) excluding the effect of phosphorus excretion in urine, the decline of cTnT and CK-MB were synchronous with the descent of serum phosphorus levels in HD patients. All these suggest that the overload of serum phosphorus is responsible for the elevation of CMs in CKD patients.
Studies have demonstrated hyperphosphatemia-induced apoptosis of vascular endothelial cells [12,13]. Our study found that simulated hyperphosphatemia in vitro could induce HCMs apoptosis in both a concentration- and time-dependent manner. Moreover, this effect was inhibited by adding PFA. These data strongly suggest that hyperphosphatemia can trigger apoptosis of cardiomyocytes. Correlation analysis showed that serum phosphorus was positively associated with cTnT. Importantly, the increase of serum phosphorus and Ca×Pi product was accompanied by the elevation of CMs (Table 1). Hence, we propose that the decline in GFR of CKD patients gives rise to serum phosphorus overload, which in turn leads to the elevation of CMs, possibly via inducing apoptosis of HCMs.
As Caspase-3 and AIF are 2 key proteins in the process of HCMs apoptosis [18], we monitored their expression. Our results suggest that hyper-phosphorus amplified the expression of Caspase-3, but the effect was then significantly diminished after PFA blocked phosphate transport into HCMs. In contrast, the expression of AIF remained unchanged under the same condition. These findings suggest that hyper-phosphorus regulates apoptosis of HCMs and leads to elevation of CMs via Caspase-3.
Left ventricular hypertrophy (LVH) is regarded as a common cardiomyopathy in CKD patients and its associated morbidity increases with declining kidney function [19]. LVH is generally considered to be associated with hypertension caused by uremia [3]. Nevertheless, our results demonstrate that serum phosphorus and Ca×Pi product are positively correlated with LVMI and negatively correlated with LVEF. LVDs, LVDd, IVST, LVPWT, and LVMI were much more enhanced, and LVEF was decreased in the HSP group compared to the NSP group. However, there was no difference in MAP between these 2 groups (Table 4). These data indicate that myocardial remodeling, including LVH and the declining LVEF, may be associated with serum phosphorus rather than hypertension. Moreover, patients in the HSP group had higher CMs and more severe damage of cardiac structure and function (Table 4). This supports the relationship of phosphorus overload with myocardial injury.
PTH was elevated concomitantly with the deterioration of kidney function (Table 1) and was also positively correlated with BNP and LVMI and negatively with LVEF (Table 2). Admittedly, hyperphosphatemia and hypocalcemia both could trigger secondary elevation of PTH. It has been reported that pronounced PTH levels were associated with myocardial hypertrophy and fibrosis, as well as higher coronary lesion scores in 5/6 nephrectomy mice [20]. Likewise, a close association between PTH and LVH has also been reported in patients [21]. Thus, in addition to the effect on apoptosis of HCMs, serum phosphorus overload might also participate in myocardial remodeling through stimulating the elevation of PTH.
FGF23 is the prime phosphaturic hormone to maintain serum phosphate homeostasis [22]. It has been known that FGF23 levels rise over time and often reach levels that are >1000-fold above normal in patients with ESRD undergoing dialysis [23]. The elevated FGF23 levels are independently associated with progression of CKD and with greater risk of cardiovascular events and mortality in ESRD [22,24]. In 5/6 nephrectomy rats in a model of CKD, elevated FGF23 directly induced LVH and treatment with an FGF receptor blocker-attenuated LVH [25]. In the present study, we found reduction of serum phosphorus after 3-month treatment with calcium acetate in HD patients. However, there was no consistent reduction in FGF23, which is identical to the results of others [26,27]. This implies that the early reduction of serum CMs levels induced by phosphate binder was not dependent on the circulating FGF23 levels.
Regarding dietary composition, the appearance of hyperphosphatemia was not due to the increased intake of dietary phosphorus, but to the reduced excretion of phosphorus in urine; this is consistent with the results of Block et al. [28]. Moreover, the prospective cohort study showed no statistical difference in BMI, Kt/v, serum glucose, or phosphorus intake after 3-month use of phosphate binder. Therefore, blood glucose level, surplus phosphorus intake from diet, and HD could be excluded from this study as potential factors that affect serum phosphorus and CMs.
This is the first study to investigate the causal relationship of hyperphosphatemia with the elevation of CMs and the underlying mechanisms; most other studies have focused on hyperphosphatemia as a risk factor for the onset of CVD [4,5,29]. Previous studies were mostly observational and prospective. In contrast, we conducted in vivo and in vitro studies to test our hypotheses, including a cross-sectional study, a prospective and interventional study, and a HCMs study. In addition, daily phosphate and calcium intake of included patients were brought into this study, which highlighted this research.
The calcium acetate intervention period was relatively short. Although serum phosphorus levels were reduced with statistical significance, most of them did not return to normal values. Thus, long-term effect of calcium acetate on FGF23 and correlation between FGF23 and LVH in dialysis patients warrant further study. Active vitamin D has been found to be a risk factor for cardiovascular diseases in multiple observational studies [30–32]. However, no sufficient evidence supports that vitamin D supplementation can reduce adverse cardiovascular events [33,34]. In addition, active vitamin D was not a regular parameter measured clinically. Therefore, vitamin D supplementation was not taken into account in this study.
It would be more appropriate if the same study population was used for the cross-sectional study and the interventional study. However, it is too difficult to enroll the same study population in a clinical study for 2 main objective reasons: 1) the numbers of patients with hyperphosphatemia in groups with normal renal function and early stages of CKD were relatively less compared with those in group of ESRD, especially those on dialysis; thus, statistical bias would inevitably occur in the interventional study due to the large difference in sample size; and 2) clinical factors contributing to the cardiac injury are different in patients receiving dialysis vs. those pre-ESRD (e.g., loss of residual renal function and dialysis-related inflammation). Nevertheless, the results from the 2 study populations supported our main hypothesis.
Conclusions
We propose that elevated serum phosphorus may induce the elevation of CMs following apoptotic injury to cardiomyocytes. Moreover, elevated serum phosphorous may also play inter-related roles in myocardial remodeling by up-regulating PTH levels. The analysis of the complex relationship between serum phosphorus and myocardial remodeling awaits further confirmation.
Acknowledgements
The authors thank study participants for their collaboration and all staff of the Nephrology Department of Shanghai Tenth People’s Hospital for their dedication to the study. The authors also thank Prof. Ai Zisheng for his help with statistical analysis and Prof. Junyan Liu for his advice in editing of this manuscript.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (81100090, 81100527, 81171792, and 81270136), and the High Technology Research and Development Program of China (2009AA02Z416)
References
- 1.Tamura K, Tsurumi-Ikeya Y, Wakui H, et al. Therapeutic potential of low-density lipoprotein apheresis in the management of peripheral artery disease in patients with chronic kidney disease. Ther Apher Dial. 2013;17:185–92. doi: 10.1111/j.1744-9987.2012.01149.x. [DOI] [PubMed] [Google Scholar]
- 2.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–67. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 3.Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16:101–5. doi: 10.1046/j.1525-139x.2003.16025.x. [DOI] [PubMed] [Google Scholar]
- 4.Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–38. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 5.Larsson TE, Olauson H, Hagström E, et al. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and non cardiovascular mortality in the community. Arterioscler Thromb Vasc Biol. 2010;30:333–39. doi: 10.1161/ATVBAHA.109.196675. [DOI] [PubMed] [Google Scholar]
- 6.Apple FS, Voss E, Lund L, et al. Cardiac troponin, CK-MB and myoglobin for the early detection of acute myocardial infarction and monitoring of re-perfusion following thrombolytic therapy. Clin Chim Acta. 1995;237:59–66. doi: 10.1016/0009-8981(95)06064-k. [DOI] [PubMed] [Google Scholar]
- 7.Amodio G, Antonelli G, Di Serio F. Cardiac biomarkers in acute coronary syndromes: a review. Curr Vasc Pharmacol. 2010;8:388–93. doi: 10.2174/157016110791112250. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal SK, Avery CL, Ballantyne CM, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin Chem. 2011;57:891–97. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammann P, Pfisterer M, Fehr T, Rickli H. Raised cardiac troponins. BMJ. 2004;328:1028–29. doi: 10.1136/bmj.328.7447.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas NA, John RI, Webb MC, et al. Cardiac troponins and renal function in nondialysis patients with chronic kidney disease. Clin Chem. 2005;51:2059–66. doi: 10.1373/clinchem.2005.055665. [DOI] [PubMed] [Google Scholar]
- 11.Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2006;106:2941–45. doi: 10.1161/01.cir.0000041254.30637.34. [DOI] [PubMed] [Google Scholar]
- 12.Di Marco GS, Hausberg M, Hillebrand U, et al. Increased inorganic Phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol. 2008;294:F1381–87. doi: 10.1152/ajprenal.00003.2008. [DOI] [PubMed] [Google Scholar]
- 13.Peng A, Wu T, Zeng C, et al. Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability. PLoS One. 2011;6:e23268. doi: 10.1371/journal.pone.0023268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, et al. Acute Dialysis Quality Initiative work group. Acute renal failure definition, outcome measures, animal models, International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 17.Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–47. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiong M, Wang ZV, Pedrozo Z, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Custódio MR, Koike MK, Neves KR, et al. Parathyroid hormone and phosphorus overload in uremia: impact on cardiovascular system. Nephrol Dial Transplant. 2012;27:1437–45. doi: 10.1093/ndt/gfr447. [DOI] [PubMed] [Google Scholar]
- 21.Wright JR, Shurrab AE, Cooper A, et al. Progression of cardiac dysfunction in patients with atherosclerotic renovascular disease. QJM. 2009;102:695–704. doi: 10.1093/qjmed/hcp105. [DOI] [PubMed] [Google Scholar]
- 22.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82(7):737–47. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viaene L, Bammens B, Meijers BK, et al. Residual renal function is an independent determinant of serum FGF-23 levels in dialysis patients. Nephrol Dial Transplant. 2012;27:2017–22. doi: 10.1093/ndt/gfr596. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis. 2012;59:177–85. doi: 10.1053/j.ajkd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23in normo-phosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–91. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 29.McGovern AP, de Lusignan S, van Vlymen J, et al. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: A large community based cohort study. PLoS One. 2013;8:e74996. doi: 10.1371/journal.pone.0074996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg) Heart. 2012;98:920–25. doi: 10.1136/heartjnl-2011-301345. [DOI] [PubMed] [Google Scholar]
- 31.Neuhouser ML. Dietary supplement use by American women: challenges in assessing patterns of use, motives and costs. J Nutr. 2003;133:1992S–6S. doi: 10.1093/jn/133.6.1992S. [DOI] [PubMed] [Google Scholar]
- 32.Neuhouser ML, Patterson RE, Levy L. Motivations for using vitamin and mineral supplements. J Am Diet Assoc. 1999;99:851–54. doi: 10.1016/S0002-8223(99)00202-3. [DOI] [PubMed] [Google Scholar]
- 33.Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567–80. doi: 10.1007/s00198-012-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai CK, Huang J, Lokhandwala A, et al. The role of vitamin supplementation in the prevention of cardiovascular disease events. Clin Cardiol. 2014 doi: 10.1002/clc.22299. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]