Abstract
Fusobacterium nucleatum is an oral anaerobe associated with periodontal disease, adverse pregnancy outcomes and colorectal carcinoma. A serine endopeptidase of 61–65 kDa capable of damaging host tissue and of inactivating immune effectors was detected previously in F. nucleatum. Here we describe the identification of this serine protease, named fusolisin, in three oral F. nucleatum sub-species. Gel zymogram revealed fusobacterial proteolytic activity with molecular masses ranging from 55–101 kDa. All of the detected proteases were inhibited by the serine protease inhibitor PMSF. analysis revealed that all of the detected proteases are encoded by genes encoding an open reading frame (ORF) with a calculated mass of approximately 115 kDa. Bioinformatics analysis of the identified ORFs demonstrated that they consist of three domains characteristic of autotransporters of the type Va secretion system. Our results suggest that the F. nucleatum fusolisins are derived from a precursor of approximately 115 kDa. After crossing the cytoplasmic membrane and cleavage of the leader sequence, the C-terminal autotransporter domain of the remaining 96–113 kDa protein is embedded in the outer membrane and delivers the N-terminal S8 serine protease passenger domain to the outer cell surface. In most strains the N-terminal catalytic 55–65 kDa domain self cleaves and liberates itself from the autotransporter domain after its transfer across the outer cell membrane. In F. nucleatum ATCC 25586 this autocatalytic activity is less efficient resulting in a full length membrane-anchored serine protease. The mature serine protease was found to cleave after Thr, Gly, Ala and Leu residues at the P1 position. Growth of F. nucleatum in complex medium was inhibited when serine protease inhibitors were used. Additional experiments are needed to determine whether fusolisin might be used as a target for controlling fusobacterial infections.
Introduction
Fusobacterium nucleatum is a ubiquitous oral anaerobic rod classified into five subspecies nucleatum, polymorphum, vincentii, animalis, and fusiforme [1]. Development of periodontal disease has been correlated with a sharp increase in the numbers of F. nucleatum [2], [3]. F. nucleatum has a remarkable ability to attach to a range of early and late colonizing oral species [4], [5], [6], [7], [8], [9] in a process termed coaggregation or coadherence, and has therefore been suggested as a bridging organism that contributes to the structural formation of the multi-species dental biofilm [6], [10].
Virulence mechanisms of F. nucleatum include adhesion to and invasion of host cells [11] and induction of proinflammatory cytokines [12], [13]. F. nucleatum is also the periopathogen most commonly found in systemic infections [2]. It is strongly implicated in preterm deliveries [14], [15], and was also found to be dominant in the microenvironment of colorectal carcinoma [16], [17] and to promote its acceleration [18], [19].
Bacterial pathogens have developed strategies to enable their survival and growth within their specific hosts. Surface and secreted proteases are common virulence factors employed by microorganisms for colonization of new sites within the host, acquisition of growth nutrients and evasion of the host defenses [20]. Serine proteases are the most abundant and functionally diverse group of proteolytic enzymes in eukaryotic and prokaryotic organisms [21]. A family of extracellular serine proteases secreted through the Type V autotransporter secretion pathway, has been described in pathogenic Gram-negative species of Neisseria, Shigella, Escherichia coli, Citrobacter rodentium, Salmonella and Edwarsiella species [22]. These bacterial serine proteases hydrolyze host intracellular and extracellular protein substrates leading to cytoskeleton destruction [23], [24], induction of autophagy [25], [26] or impaired immunity [27].
Oral bacteria found in the subgingival plaque are predominantly anaerobic and rely on the utilization of peptides and amino acids for energy [28], [29]. The proteases of these oral microorganisms are implicated in the degradation of host periodontal tissues while supplying the bacteria’s nutritional requirements [30], [31].
Amino acids and peptides are the preferred substrates for F. nucleatum’s growth [32], [33], [34] and growth of fusobacteria depends on the availability of free glutamate, histidine, serine and lysine [35]. Under natural conditions, the above amino acids are not found in free form but are incorporated in proteins that have to be degraded for the desired amino acids to become accessible.
Previous studies reported a fusobacterial serine protease activity associated with a molecular mass of 65 kDa [36], [37], [38], [39]. This protease was shown to be capable of degrading components of periodontal tissues, and to inactivate host defense effectors [39]. The aim of this study was to identify and characterize the F. nucleatum 65 kDa serine protease which we named fusolisin.
Materials and Methods
Bacteria and growth conditions
F. nucleatum ATCC 10953 (subsp. polymorphum), ATCC 25586 (subsp. nucleatum), ATCC 49256 (subsp. vincentii) and FDC 364 (16S rDNA closest homology to F. nucleatum JCM 6328 subsp. nucleatum, see below) and Porphyromonas gingivalis PK 1924 were a gift from Dr. P. E. Kolenbrander (NIH, Bethesda, MD). F. nucleatum ATCC 23726 (subsp. nucleatum) was a kind gift from Prof. S. K. Haake (UCLA, Los Angeles, CA). Strain 12230 (subsp. polymorphum) was a kind gift from Prof. Y. Han (Case Western Reserve University, Cleveland, OH).
The bacteria were grown under anaerobic conditions (N2:CO2:H2, 85∶5∶10) in a Bactron II anaerobic chamber (Sheldon Manufacturing Inc., Cornelius, OR) at 37°C in Wilkins Chalgren anaerobic broth (Fluka, Spain). Bacterial purity was determined by phase contrast microscopy and Gram staining.
Escherichia coli strain XL1 (Agilent Technologies, CA) used for plasmid construction and E.coli ATCC 25922 were grown in Luria-Bertani (LB) medium or on LB agar plates supplemented with chloramphenicol (35 µg/ml; Sigma-Aldrich, Germany) at 37°C under aerobic conditions.
Culture supernatant and outer membrane vesicle preparation
Four-day-old F. nucleatum cultures were harvested by centrifugation at 10,000×g for 20 min at 4°C. Culture supernatants were collected and filtered through a 0.2 µm filter (Whatman Schleicher & Schuell, Germany). Supernatants were either concentrated×10 using a Centricon microconcentrator (50,000-molecular-weight cutoff; Amicon) or used for outer membrane vesicle preparation.
For vesicle preparation cell-free supernatants were centrifuged at 100,000×g for 2 hrs. The supernatant was discarded and the pellet containing the vesicles was washed twice with TBS by centrifugation at 100,000×g. The pellet was stored at −20°C until further use.
Gel Electrophoresis
For zymogram analysis, samples were dissolved at room temperature in sample buffer (192 mM Tris-HCl [pH 6.8], 30% glycerol, 9% SDS) without β-mercaptoethanol and subjected to SDS-PAGE using 7.5% gels containing 240 µg/ml human fibrinogen (Sigma-Aldrich, Germany). Following electrophoresis, the gels were incubated for 30 min at room temperature in Tris-buffered saline (TBS, 0.05 M Tris-HCl [pH 7.8], 0.1 M NaCl), containing 2% Triton X-100 and then washed three times with TBS. Gels were incubated overnight at 37°C. Proteolytic activity was visualized as a clear band against a blue background after staining with Coomassie brilliant blue R-250 as described before [39].
For denaturing SDS-PAGE, samples were dissolved, boiled at 100°C for 5 min in sample buffer containing 2% β-mercaptoethanol and the gels were stained with Coomassie brilliant blue. Molecular masses of protein bands were calculated by linear regression analysis of molecular mass standards.
Mass spectrometry (MS) identification and database searching
Bands were excised from denaturizing gels and subjected to Qtof2 (Micromass, Manchester, UK) equipped with a nanospray capillary [40], analyzed by electrospray ionization tandem mass spectrometry (ESI-MS/MS) and peptides were identified as described before [40], [41].
DNA isolation
Chromosomal DNA was isolated from F. nucleatum ATCC 25586 using the mini GenElute Bacterial Genomic DNA kit (Sigma-Aldrich, Germany) according to the manufacturer’s instructions. Plasmid DNA was isolated using the Qiagen spin miniprep kit (Qiagen, Germany).
Expression of Fsp25586 in F. nucleatum ATCC 23726
The DNA fragment containing fsp25586 and 556 bp of its up-stream region was amplified using the F-25586-SP90 (5′-CCgagctcGGAGCTTGATTTACATCCAAG-3′) and R- 25586-SP90 (5′-CCgagctcACTAGTGTTAGTGACGCAA-3′) primers that include a SacI restriction site (small case letters). The 3.9 kb PCR product was restricted with SacI (New England Biolabs Inc. USA), and inserted into the SacI site of the pHS30 E. coli-F. nucleatum shuttle vector [42], [43] to generate pHSPROT. Plasmid electroporation into F. nucleatum ATCC 23726 was performed as described previously [43]. Clones were selected on Columbia agar plates supplemented with 5% sheep blood (Hylabs, Israel) and 5 µg/ml thiamphenicol (Sigma-Alderich, Germany).
Sequencing of FN1426
DNA was isolated from F. nucleatum ATCC 25586 as described above. The following primers were used to amplify and sequence the FN1426 gene:
F-25586-SP90– CCGAGCTCGGAGCTTGATTTACATCCAAG
R-25586-SP90– CCGAGCTCACTAGTGTTAGTGACGCAA
F-IP-25586-SP90– AAGAGCTCGTAACCCTGTTGAGATTACTG
F-2Sq-FnPro – CTGTTGCTGATGTAAAGCCCAT
R-4Sq-FnPro – CCAACTGTAGCTAATCCTTTGG
F-MS-Sq-FnPro – GGTGATGTTTTTACTCTTCTCC
R-MS-Sq-FnPro – CGGAATTAGATGCTAGTCTTGC
R-PE-Sq-FnPro – GCCCAGTATTTGGAGTATATGG
Sequencing of the 16S rDNA of F. nucleatum FDC364
The 16S rRNA gene of F. nucleatum FDC 364 was amplified by PCR using universal primers 4F (CCA GAG TTT GAT YMT GGC) and 1541R (GAA GGA GGT GWT CCA DCC). The resulting product was sequenced (gene bank accession number KM023647) and blasted against the National Center for Biotechnology Information (NCBI) database. Closest alignment was found with the partial sequence of 16S ribosomal RNA gene of F. nucleatum JCM 6328 subsp. nucleatum GI:307219163.
Effect of serine protease inhibitors on growth of F. nucleatum and E. coli
Overnight cultures of F. nucleatum or E. coli ATCC 25922 were diluted to an optical density at 600 nm of 0.02 in the appropriate growth medium. The irreversible serine protease inhibitors Phenylmethanesulfonyl fluoride ((PMSF, Sigma-Aldrich, Germany) and 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF, Sigma-Aldrich, Germany) were prepared to a stock solution of 100 mM in anhydrous ethanol and DDW respectively.
When added, PMSF and AEBSF were used at a final concentration of 1 mM and 2 mM respectively. P. gingivalis supernatants were prepared by centrifugation of four day cultures at 10,000×g for 10 min at 4°C., collection of the supernatants and filtration through a 0.2 µm filter (Whatman Schleicher & Schuell, Germany). When added, P. gingivalis supernatants were diluted 1:10 in the tested reaction. The final reaction contained 180 µl of diluted bacteria in a total volume of 220 µl. Bacterial growth (anaerobic for F. nucleatum and aerobic for E. coli) was monitored using microplate real-time kinetic measurements as described by us in detail previously [44]. Results represent mean and standard deviation of triplicate of an independent representative experiment repeated three times.
Identification of the fusolisin restriction site
Fusolisin was purified from extracellular vesicles by preparative SDS-PAGE followed by electroelution as described before [39]. Briefly, four-day-old F. nucleatum cultures were sedimented by centrifugation at 9000×g for 20 min. The supernatant was collected and filtered through a 0.2 µm filter. The filtrate was aliquoted and outer membrane vesicles were sedimented by centrifugation at 100,000×g for 2 h. The supernatant was discarded, and the precipitate containing the extracellular vesicles was washed twice with 50 mM Tris-HCl (pH 7.8) by centrifugation as described above. For fusolisin purification, the vesicles were subjected to electroelution after separation by SDS-PAGE as follows: the extracellular vesicles were dissolved in sample buffer (without β-mercaptoethanol, see above), centrifuged for 2 min at 10,000×g and submitted to SDS-PAGE (7.5% acrylamide). The protease was electroeluted from the gel using a Bio Trap 1000 electroeluter (Schleicher and Schuell, Germany) with Tris-glycine buffer (25–192 mM) without SDS for 2 h at 200 volts followed by 10 h at 100 volts. The fusolisin enzyme was then stored at –20°C.
Identification of the fusolisin substrate specificity was determined by hydrolysis of fibrinogen and identification of the resulting peptides by mass spectrometry. The purified enzyme (0.25 µg) was incubated with 2.5 µg of fibrinogen in 40 µl TBS pH 8.0 at 37°C for 16 h. A similar reaction mixture with heat inactivated protease (3 min at 100°C) served as control.
The reaction mixture of fusolisin-mediated hydrolysis of fibrinogen was submitted to peptide mapping after N-terminus labeling by reductive dimethylation as follows: the protein sample in 8 M Urea and 50 mM Hepes (pH 8) was modified with 20 mM formaldehyde in the presence of 100 mM NaCBH3 (60°C for 15 min). Neutralization was performed with 500 mM ammonium bicarbonate (final concentration). The protein sample was reduced with 2.8 mM DTT (60°C for 30 min), modified with 9.4 mM iodoacetamide in 100 mM ammonium bicarbonate (room temperature for 30 min in the dark) diluted 4 fold and digested with modified trypsin (Promega) overnight at 37°C in a 1∶50 enzyme-to-substrate ratio.
The resulting peptides were desalted on a stage tip (C18) and resolved by reverse-phase chromatography on 0.075×200-mm fused silica capillaries (J&W) packed with Reprosil reversed phase material (Dr Maisch GmbH, Germany). The peptides were eluted with linear 60 minutes gradients of 5 to 45% and 15 minutes at 95% acetonitrile with 0.1% formic acid in water at flow rates of 0.25 µl/min. On line mass spectrometry was performed by an ion-trap mass spectrometer (OrbitrapXL, Thermo) in a positive mode using repetitively full MS scan followed by collision induced dissociation (CID) of the 7 most dominant ions selected from the first MS scan.
The mass spectrometry data was analyzed using the Thermo Protein Discoverer 1.3 using the Sequest search engine vs a specific sequence or a general database (Uniprot).
The cleavage site of fusolisin was further characterized by hydrolysis of the FRETS-25 Thr fluorescence-quenching substrate library D-A2pr(Nma)- Gly- [Phe/Ala/Val/Glu/Arg] - [Pro/Tyr/Lys/Ile/Asp]- Thr- Ala- Phe- Pro-Lys(Dnp)- D-Arg- D-Arg TRIFLUOROACETATE (PeptaNova GmbH, Germany). The reaction mixture contained 0.1 mM FRETS-25 Thr and 1.2 µg of purified fusolisin in 100 µl TBS pH 8.0 at 37°C. A reaction mixture with heat-inactivated protease served as control. Cleavage was monitored (λex = 340 nm and λem = 440 nm) every 20 min using a GENios Microplate reader (TECAN, Austria). Results represent mean and standard deviation of three independent experiments.
Cleavage of FRETS-25 Thr was analyzed as described above but without the N-terminus labeling.
Fusolisin’s restriction specificity was verified using the FRET substrate CPQ2-Gly-Phe-Ile-Thr-Ala-Phe-Pro-Lys-(5FAM)-Arg-Arg-NH2 that was custom synthesized by CPC scientific (Sunnyvale, CA, USA). The peptide was dissolved in DMSO to a concentration of 1 mM and further diluted with TBS to the desired concentration. The reaction was performed and monitored as described above with the λex = 485 nm and the λem = 535 nm.
Bioinformatics analysis
Public databases were searched for similar sequences with the BLASTN, BLASTP, and BLASTP/PSI algorithms using default parameters. The features of the predicted proteins were examined by the Pfam programs (http://www.sanger.ac.uk/Software/Pfam/search.shtml). The ExPASy server was used to predict the proteins' molecular weights [45]. Multiple alignment was performed using CLUSTAL W [46], [47]. Structure prediction was generated using the Protein Homology/analogY Recognition Engine (Phyre) [48]. Rare Codon Caltor (http://www.doe-mbi.ucla.edu/~sumchan/caltor.html) was used before cloning in E. coli.
Results
Identification of fusolisin
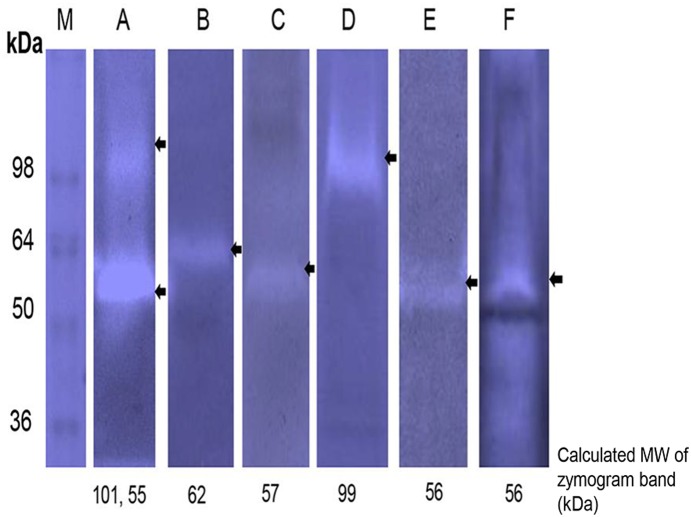
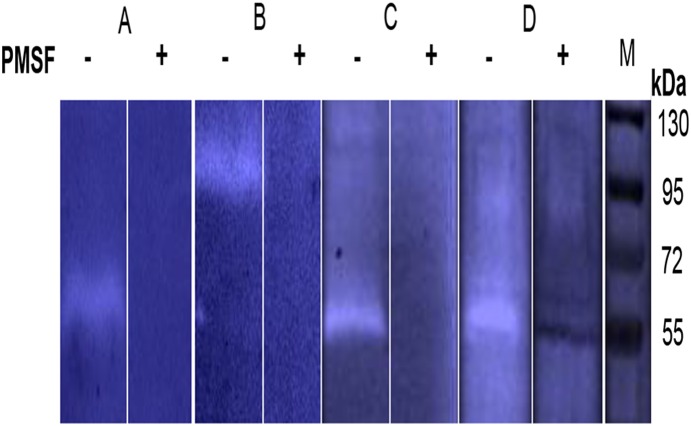
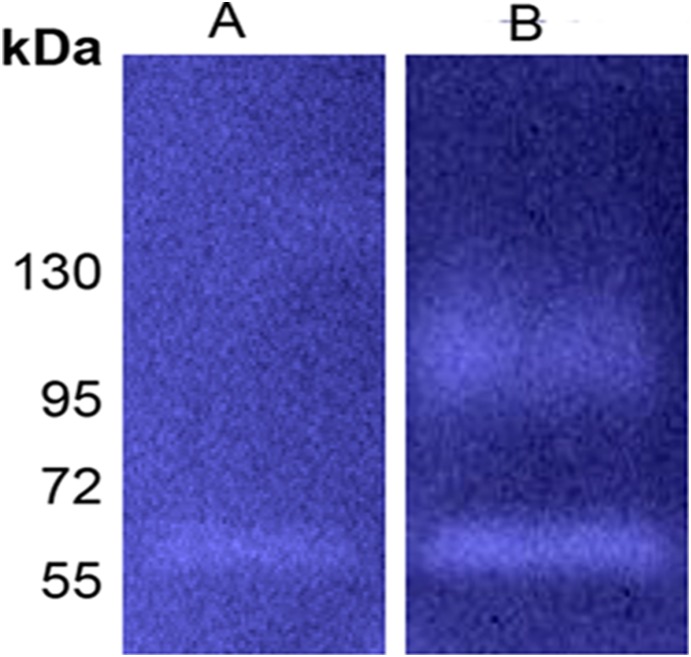
Gel zymograms using human fibrinogen as a substrate, revealed proteolytic activity in the growth media supernatant (Fig. 1) and in outer membrane vesicles prepared from all of the tested F. nucleatum strains that represent three F. nucleatum subspecies: nucleatum, polymorphum and vincentii (Table 1). The molecular weight of the detected proteases varied from 55 to 101 kDa as estimated by gel migration (Fig. 1, Table 1). All the detected proteases were inhibited by the serine protease inhibitor PMSF (presented for F. nucleatum strains FDC 364, ATCC 25586, 12230 and ATCC 23726 in Fig. 2).
Figure 1. Protease profiles of F. nucleatum growth medium supernatants on fibrinogen containing zymograms.
M, Molecular weight markers. A, F. nucleatum ATCC 49256. B, F. nucleatum FDC 364. C, F. nucleatum ATCC 10953. D, F. nucleatum ATCC 25586. E, F. nucleatum ATCC 23726. F, F. nucleatum 12230. Arrows indicate proteolytic bands. Presented data are of representative zymograms.
Table 1. Estimated molecular mass of F. nucleatum fusolisin detected in outer membrane vesicles or in growth medium.
| F. nucleatum strain | Name (GeneBank) | Estimatedmolecular mass ofproteolytic bands |
| ATCC 49256(subsp. vincentii) | Fsp49256 (FNV0835) | 55, 101 |
| FDC 364(subsp. nucleatum) | Fsp364 | 62, 96 a |
| ATCC 10953(subsp. polymorphum) | Fsp10953 (FNP_2077) | 57, 96 a |
| ATCC 25586(subsp. nucleatum) | Fsp25586 (KJ634469, suggested annotation for FN1426) | 99 |
| ATCC 23726(subsp. nucleatum) | Fsp23726 (03970469 | 56, 96 a |
| 12230(subsp. polymorphum) | Fsp12230 | 56 |
Activity detected only in samples prepared from outer membrane vesicles.
Figure 2. PMSF inhibits the proteolytic activity of F. nucleatum.
A, F. nucleatum FDC 364. B, F. nucleatum ATCC 25586. C, F. nucleatum 12230. D, F. nucleatum ATCC 23726. M, Molecular weight markers. Presented data are of representative zymograms.
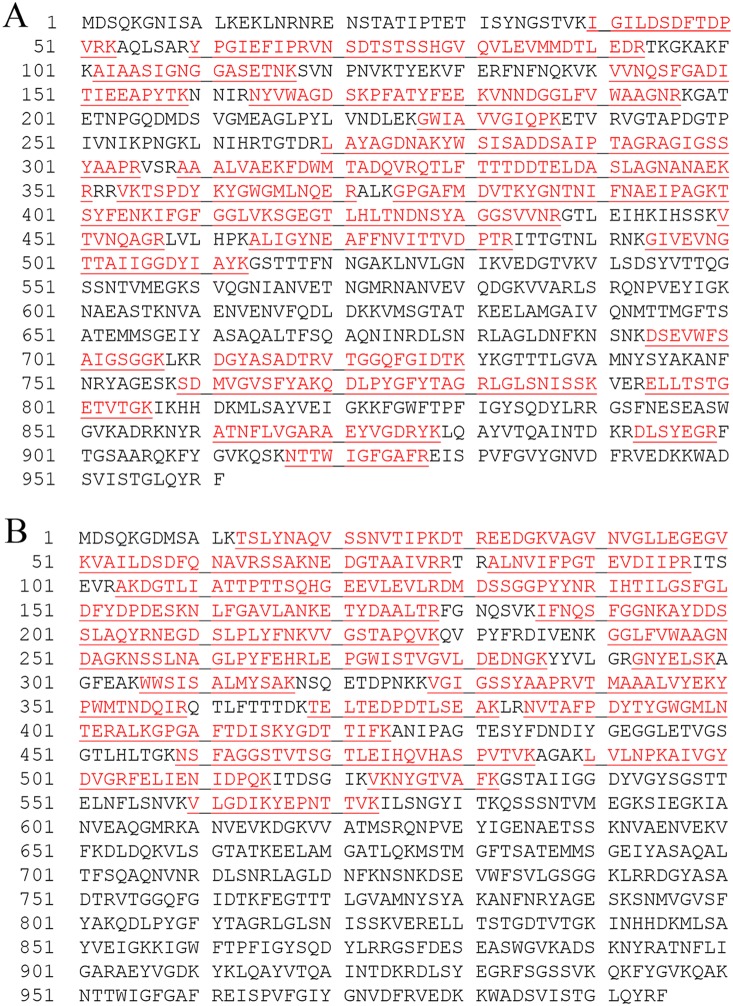
Gel-purified proteases of outer membrane vesicles prepared from the genome-sequenced F. nucleatum strains ATCC 25586 and ATCC 49256 were identified using mass spectrometry (MS). Tryptic fragments of the 99 kDa proteolytic protein of F. nucleatum ATCC 25586 matched those of the entire putative 115 kDa serine proteases designated FN1426 [Genbank Index number (GI):19704758]. Tryptic peptides of both the 55 kDa and 101 kDa serine endopeptidases partially purified from F. nucleatum ATCC 49256 were found to match those of the putative 108 kDa serine protease designated FNV0835 (GI:34763535). However, while the peptide sequences generated from the 99 kDa proteolytic protein extracted from F. nucleatum ATCC 25586 corresponded to the entire FN1426 protein sequence (Fig. 3A), those generated from the 55 kDa proteolytic band of F. nucleatum ATCC 49256 matched only the N-terminal domain of FNV0835 (Fig. 3B) suggesting that the 55 kDa protease of F. nucleatum ATCC 49256 also originated from a larger precursor. The 62 kDa protease of the non-sequenced strain FDC 364 [39] was found to be most homologous to the N-terminal domain of FN1426 of F. nucleatum ATCC 25586 (data not shown).
Figure 3. Identification of the fusobacterial serine protease.
Amino acid sequences of the putative serine protease open reading frames FN1426 (Fsp25586) (A), and FNV0835 (Fsp49256) (B). Red highlight indicates sequences identified by mass spectrometry of the 99 kDa serine protease of F. nucleatum ATCC 25586 (A), and of the 55 kDa serine protease of F. nucleatum ATCC 49256 (B).
Sequence analysis of fusolisin
Previous annotation of the FN1426 sequence of F. nucleatum ATCC 25586 [49], showed that the start codon proposed earlier by Kapatral and colleagues [50] has been misannotated. Upon detailed analysis, we found that the reading frame proposed by Kapatral and colleagues [50] was indeed truncated. However, we found that the reading frame extends even further than corrected by Desvaux and colleagues [49] which is missing 291 base pairs at the beginning of the gene. The FN1426 gene bank sequence begins with a TTG codon (an alternate start codon in bacteria), our proposed new open reading frame begins with ATG which is located 291 base pairs upstream. Furthermore, analysis of the sequence taken from the gene bank using the SignalP 4.0 server [51], found no signal peptide while analysis of our proposed sequence revealed a putative signal peptide cleavage site between amino acids 58 and 59. This observation was verified by PCR and sequencing of the proposed ORF and the 400 bases upstream to it. The corrected predicted 1,058 amino acid ORF, was named Fsp25586 (for Fusobacterial Serine Protease of F. nucleatum 25586) and was deposited in the gene bank accession number KJ634469. Henceforth, all of the following analysis will refer to the new sequence of Fsp25586. The orthologous sequences FNV0835 will be referred to as Fsp49256, FNP_2077 will be referred to as Fsp10953 and HMPREF0397_0469 will be referred to as Fsp23726.
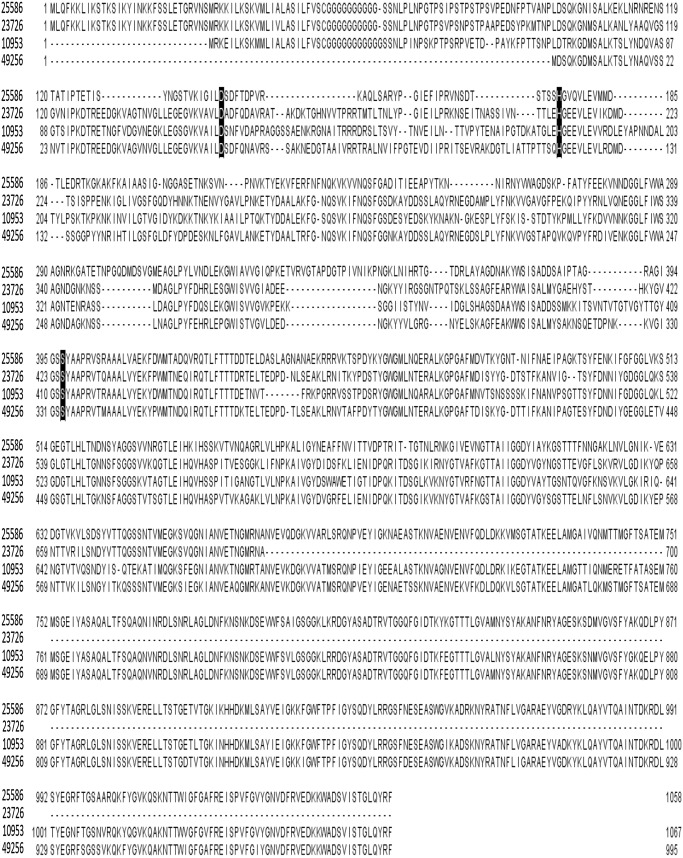
Amino acid sequence alignment (Fig. 4) of Fsp25586 revealed a high homology (71% similarity and 61% identity) with that of Fsp49256, 71% similarity and 60% identity with that of Fsp10953 and 63% similarity and 57% identity with the available partial sequence of the homologous serine protease Fsp23726. Previous annotation of the FN1426 (Fsp25586) and the FNV0835 (Fsp49256) open reading frames revealed a signal peptide and three other functional domains [49]. The N-terminal, peptidase domain [amino acids 131–471 in Fsp25586 and 1–406 in Fsp49256] were found to belong to the peptidase S8 domain family. The C-terminal domain (amino acids 788–1047 in Fsp25586 and 690–995 in Fsp49256) belong to the autotransporter superfamily. While the C-terminal autotransporter domain of Fsp25586 and Fsp49256 were highly conserved (93% identity and 98% similarity), a higher divergence was found between the catalytic domains of the proteases of the two species (37% identity, 47% similarity). As a member of the S8 family of subtilisins, the amino acid sequence analysis of fusolisin revealed that the arrangement of the active site catalytic triad is Asp-His-Ser [52], [53] that was identified using NCBI's conserved domain database (CDD) [54] in the amino acid sequences of Fsp49256, Fsp10953, Fsp23726 and Fsp25586, and can be seen in Fig. 4.
Figure 4. Sequence alignment of fusolisin.
ClustalW alignment of Fsp25586, the available partial sequence of the homologues serine protease Fsp23726, Fsp10953 and Fsp49256. The predicted catalytic triad Asp, His and Ser are highlighted.
Processing of fusobacterial serine protease
Autocatalytic processing is common in type Va secretion systems [55], [56], [57] and in subtilisins [58]. Although F. nucleatum ATCC 25586 remains refractory to plasmid transformation, others and us were previously successful with plasmid expression in F. nucleatum ATCC 23726 [43], [59]. As can be seen in figures 1E and 5A, the serine protease detected in the growth medium of F. nucleatum ATCC 23726 (Fsp23726) is approximately 56 kDa. Zymogram analysis of culture supernatants prepared from F. nucleatum ATCC 23726 expressing Fsp25586 of strain ATCC 25586 revealed the presence of the 99 kDa Fsp25586 protease in addition to the typical 56 kDa protease of ATCC 23726 (Fig. 5B). The fact that Fsp25586 was not cleaved when expressed in F. nucleatum ATCC 23726 suggests that the processing of Fsp25586 is not efficient compared to that in Fsp23726 (and the orthologs in the other tested F. nucleatum strains, Table 1). It is possible that Fsp25586 lacks the restriction site that is cleaved to release the catalytic domain from the autotransporter domain, or that this cleavage site is not exposed for cleavage.
Figure 5. Self-restriction of Fsp25586 is not efficient.
Zymogram analysis of cell culture supernatant prepared from F. nucleatum ATCC 23726 carrying the pHS30 vector (A), or the pHSPROT plasmid expressing Fsp25586 (B).
Fusolisin’s cleavage site
Previous work in our laboratory failed to determine the substrate specificity of the fusobacterial proteolytic activity using a large variety of synthetic chromogenic substrates [39]. We now determined the cleavage sites of fusolisin by MS analysis of the peptides resulting from hydrolysis of fibrinogen. Fusolisin cleaved fibrinogen preferentially at the C- terminal side of small residues (Thr, Gly, Ala and Ser), though Leu and Asp were also cleaved (Table 2). However, the major peak of fusolisin mediated fibrinogen hydrolysis resulted from cleavage of Thr at the P1 position.
Table 2. Substrate specificity of fusolisin.
| A | |
| Fibrinogen | Peptide Intensity |
| GTAWT/A | 4.5×109 |
| SGSSG/P | 9.0×108 |
| TAWTA/D | 4.0×108 |
| LGGWL/L | 3.8×108 |
| NFNRT/W | 3.8×108 |
| AWTAD/S | 1.6×108 |
| PRNPS/S | 2.6×107 |
| B | |
| FRETS-25 Thr | Peptide Intensity |
| T/AFPKRR | 3.6×108 |
| GFIT/A | 8.6×107 |
| GVIT/A | 4×107 |
| GEIT/A | 2.4×107 |
| GFPT/A | 1.6×107 |
A) Major peptides obtained by fusolisin hydrolysis of fibrinogen.
B) Major peptides obtained by fusolisin hydrolysis of FRETS-25 Thr.
D-A2pr(Nma)- Gly- [Phe/Ala/Val/Glu/Arg] - [Pro/Tyr/Lys/Ile/Asp]- Thr- Ala- Phe- Pro- Lys(Dnp)- D-Arg- D-Arg TRIFLUOROACETATE.
Mass spectrometry was performed as described in Materials and Methods. The peptide intensity based on the peak area was analyzed by LC-MS and ordered by decreasing abundance.
To verify that fusolisin preferentially cleaves Thr at the P1 position, hydrolysis of the FRETS-25 Thr substrate (PeptaNova) by fusolisin was examined. FRETS-25 Thr, is a protease substrate library that contains a highly fluorescent 2-(N-methylamino)benzoyl (Nma) group linked to the side chain of the amino-terminal D-2,3-diamino propionic acid (D-A2pr) residue, along with a 2,4-dinitrophenyl (Dnp) group that acts as a quencher, linked to the ε–amino group of a Lys residue. The fluorophore and quencher are connected by the peptide Gly- [Phe/Ala/Val/Glu/Arg] - [Pro/Tyr/Lys/Ile/Asp]- Thr- Ala- Phe- Pro. Mass spectrometry analysis of the Fsp23726-mediated FRETS-25 Thr hydrolysis products revealed that similar to fibrinogen cleavage, the major peaks obtained resulted from Thr in the P1 position (Table 2B). Preference for the presence of Ile in the P2 position was also observed (Table 2B).
Based on the FRETS-25 Thr hydrolysis results, the following fusolisin sensitive FRET peptide, (Fu-S-P) was synthesized:
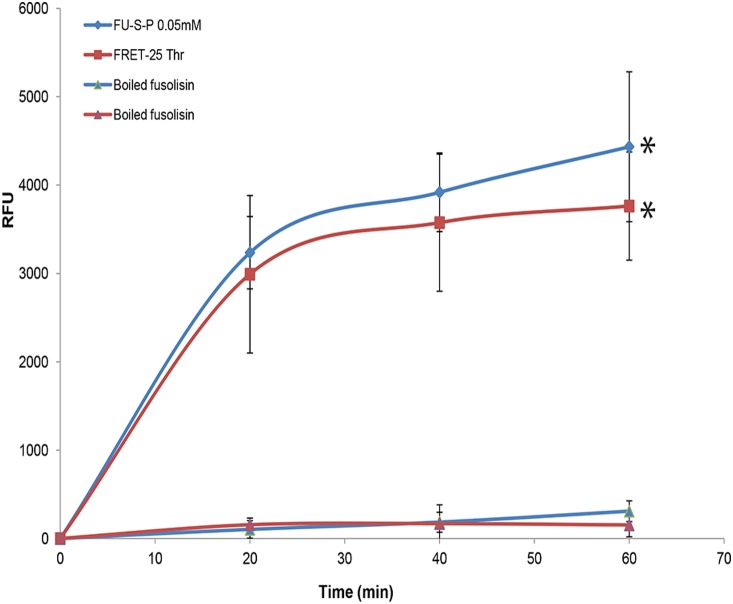
CPQ2-Gly-Phe-Ile-Thr-Ala-Phe-Pro-Lys-(5FAM)-Arg-Arg-NH2. Time course hydrolysis of FRETS-25 Thr and Fu-S-P by purified fusolisin is shown in Fig. 6.
Figure 6. Time course hydrolysis of FRETS-25-Thr and Fu-S-P by fusolisin.
Purified fusolisin (1.2 µg) was incubated with 0.05 mM of Fu-S-P or 0.1 mM (blue) of FRETS-25-Thr (red) in TBS pH 8.0. Relative Fluorescent Units (RFU) were determined as described in materials and methods. *P<0.05 compared to control with heat inactivated fusolisin, determined with Bonferroni test for multiple comparisons using the SPSS 15.0 software.
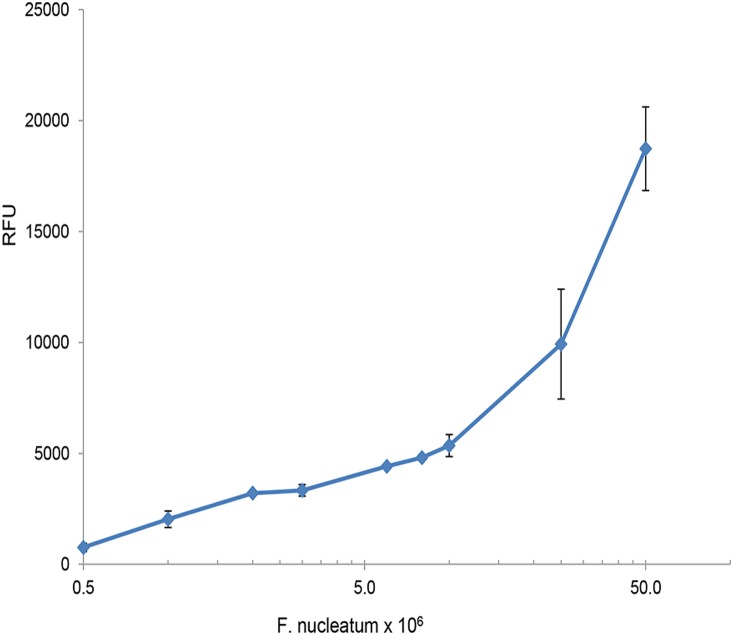
Fu-S-P was found to be a useful biomarker for detecting F. nucleatum ATCC 25586 (not shown) and ATCC 23726. As shown in Fig. 7, 5×105–1×106 fusobacterial cells were sufficient to produce measurable activity after 2 hrs.
Figure 7. Fu-S-P activity correlates with the number of F. nucleatum cells.
Fu-S-P (0.03 mM) was incubated for 2 hrs with increasing numbers of washed F. nucleatum cells. Relative Fluorescent Units (RFU) were determined as described in Materials and Methods. No activity was observed with boiled cells.
Fusolisin is essential for growth of F. nucleatum in a complex medium
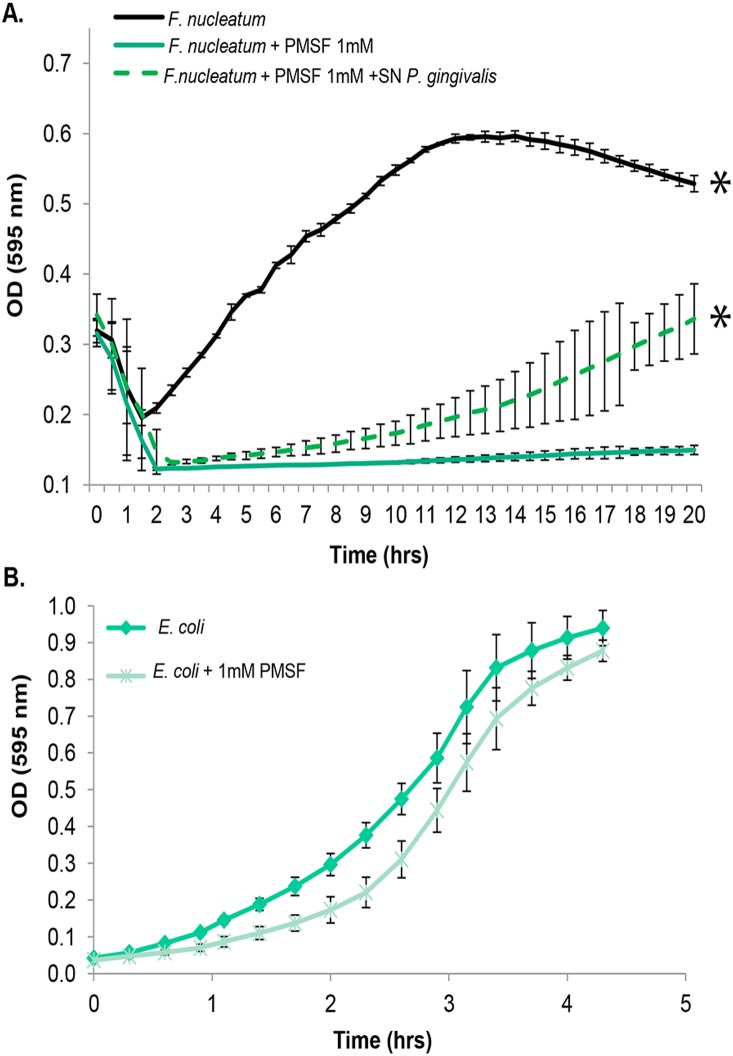
The genes coding for Fsp25586 and Fsp49256 were located in genomic loci involved in metabolic functions suggesting a nutritional role for fusolisin. Indeed, growth of F. nucleatum in a complex medium was inhibited by the AEBSF (not shown) and PMSF serine protease inhibitors (Fig. 8). Both inhibitors did not affect growth of E. coli used as control (Fig. 8), ruling out that growth attenuation of F. nucleatum by both serine-protease inhibitors resulted from non specific toxicity. P. gingivalis is a proteolytic anaerobic periodontal pathogen frequently isolated together with F. nucleatum [3]. The gingipain proteases produced by P. gingivalis are cysteine proteases which are not inhibited by PMSF. Addition of filter-sterilized gingipain-containing supernatant collected from a P. gingivalis culture [60], relieved the PMSF inhibitory effect on F. nucleatum’s growth (Fig. 8). Addition of the P. gingivalis supernatant did not affect the pH of the reaction mixture and did not reduce the inhibitory activity of PMSF as tested on trypsin under similar conditions (data not shown). These results suggest that P. gingivalis can enable fusolisin-independent growth of F. nucleatum.
Figure 8. PMSF inhibits growth of F. nucleatum but not of E. coli.
(A) Growth of F. nucleatum 12230 (black line) is inhibited by PMSF (solid green line), but this inhibition is relieved by P. gingivalis supernatant (SN Pg) containing PMSF-resistant cysteine proteases (broken green line). (B) Growth of E. coli is not affected by PMSF, ruling out PMSF toxicity. *P<0.05 compared to PMSF-treated bacteria, determined with Bonferroni test for multiple comparisons using the SPSS 15.0 software.
Discussion
Obtaining energy by the fermentation of a small number of peptide-derived amino acids was shown to be essential for the growth of F. nucleatum [32], [34], [61]. To date, the only detected endopeptidase activity in F. nucleatum was that of an un-identified serine protease with a molecular weight of 61–65 kDa [36], [37], [38], [39]. In the present work this protease, now named fusolisin, has been identified and characterized at the genetic level. The theoretic isoelectric point of the 55 kDa derivative of Fsp49256 is 5. This calculated isoelectric point is in agreement with that (pH 5) determined for the 65 kDa diisopropylfluorophosphate-binding outer membrane protein of F. nucleatum Fev1 [37]. Mass spectrometry analysis and identification of the high (99 kDa) and the low (55 kDa) molecular weight F. nucleatum serine proteases partially purified from strains ATCC 25586 and ATCC 49256 demonstrated (Fig. 3) that both originate from a precursor with a calculated molecular weight of approximately 115 kDa. Sequence analysis suggested that this precursor contains a signal peptide, a serine protease domain, a linker, and an autotransporter domain. Structure prediction of the serine protease and of the autotransporter domains was generated using the Protein Homology/analogY Recognition Engine (Phyre) [48] and can be seen in Figure 9 and in Movies S1 and S2.
Figure 9. Fusolisin Fsp25586 analysis using the Protein Homology/analogY Recognition Engine (phyre).
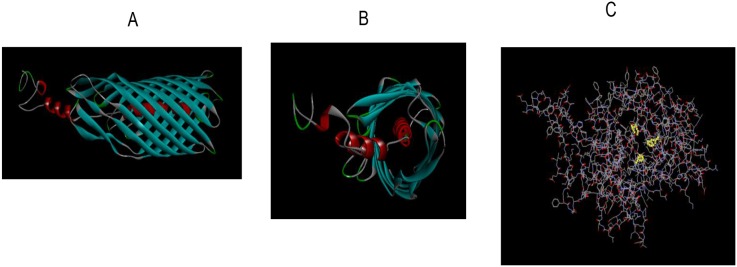
A) Side view of the autotransporter domain. B) Front view of the autotransporter domain. C) The catalytic domain with the characteristic serine protease catalytic triad Asp 141, His 175, and Ser 397 highlighted in yellow.
Our experimental results and in silico predictions suggest that the fusobacterial serine protease belongs to the autotransporter proteins superfamily of the type Va secretion pathway [55], [56], [57]. This conclusion is in agreement with a previous in silico analysis of the F. nucleatum genome [49].
Our bioinformatics results (Fig. 4) suggest that the 101–55 kDa fusolisin proteases (Fig. 1, Table 1) all derive from a precursor of approximately 115 kDa. This precursor crosses the cytoplasmic membrane presumably via the Sec (Secretion) pathway where the signal peptide is removed. The C-terminal domain of the remaining approximately 96–101 kDa poly-peptide then forms a β-barrel pore structure in the outer bacterial membrane (Fig. 9A, Movie S2). The N-terminal serine endopeptidase domain is then most likely threaded through the autotransporter and transported across the outer membrane to the cell’s exterior surface. An alternative model suggests that several autotransporter domains oligomerize and form a wide channel that enables the transfer of the catalytic passenger domain [56].
In the case of Fsp25586, the protease remains intact (99 kDa, Table 1, Fig. 1). In the case of Fsp49256 and its homologs in strains ATCC 10953, FDC 364 and ATCC 23726 the protease can remain intact and cell bound, or the catalytic domain can self cleave the peptide bridge connecting both domains and release itself to form the detected 55–62 kDa protease (Table 1, Fig. 1). Mass spectrometry analysis of the self cleavage product of Fsp49256 suggests that the restriction site is located after amino acid 572 (Fig. 3A). Furthermore, the amino acid sequence GYIT (amino acids 578 to 581 in Fig. 3B) is present in the peptide bridge connecting the catalytic domain to the autotransporter in Fsp49256 and absent in the peptide bridge in Fsp25586 (Fig. 3A). This sequence is similar to the fusolisin P4-P1 cleavage site GFIT in Fu-S-P, indicating that GYIT may be the self cleavage restriction site of Fsp49256.
The 55–62 kDa form of fusolisin was found in the growth media but also in outer membrane vesicles (Table 1). This indicates that the autotransporter domain is not essential for adherence of the catalytic domain to the bacteria’s outer membrane surface. Hydrophobic interactions between hydrophobic sub-domains (not shown) in the catalytic domain and the membrane, or non-covalent interactions between the catalytic and autotransporter domains can enable such association [62]. In strains ATCC 23726, ATCC 10953 and FDC 364 the full-length (∼96 kDa) protease was detected mainly in outer membrane vesicle preparations but not in growth media where the 56–62 kDa protease was found. This indicates that unlike the secreted mature 55–62 kDa protease, the full length protease in these strains is mostly membrane bound. The release of the mature serine protease that can act on extracellular targets [39] can enable its diffusion to distant locations and presumably increase its effectiveness [63]. Regulation of cleavage and detachment of the passenger from the autotransporter domain has been demonstrated in IcsA of Shigella flexneri [64], and the Hap autotransporter of Haemophilus influenzae [65].
The self cleavage mechanism that determines F. nucleatum serine protease secretion is yet unknown. Possible regulation of this secretion mechanism by the bacteria (in response to nutritional needs or proteinaceous host defense challenges) remains to be determined.
The fusolisin’s autotransporter domain was found to be much more conserved than the catalytic one (Fig. 4). This can result from strict structural-functional requirements. An additional possibility is that being extracellular, the catalytic domain is under much greater immunological stress than the non-exposed intramembrane autotransporter domain. This constant immunological selective pressure might accelerate alterations in the catalytic domain’s amino acid sequence.
Previous attempts to find the substrate specificity of fusolisin using a large number of chromogenic substrates have failed [39]. In the present study we synthesized a fusolisin substrate that allows the detection of low numbers of fusobacterial cells (Fig. 7). Such a substrate might be made useful for detection of fusobacteria associated with colorectal cancer [17], [18].
In order to survive in the host, fusobacteria need to overcome the host immune system and to acquire nutrients. Our previous results demonstrated that the F. nucleatum serine protease is capable of inactivating host immune mediators [39]. Our current study demonstrates the essential nutritional role of fusolisin. This was concluded from the fact that fusolisin inhibition restricted growth of F. nucleatum (Fig. 8A). In the oral plaque, F. nucleatum is always found in a multispecies environment that contains proteolytic members [3] that are likely to relieve the dependency on fusolisin for fusobacterial growth. Indeed, addition of filter-sterilized supernatant collected from a P. gingivalis culture that contains the gingipain cysteine proteases (which are not inhibited by PMSF) relieved the PMSF inhibitory effect on F. nucleatum’s growth (Fig. 8A).
F. nucleatum is frequently isolated or identified by molecular methods in the amniotic fluid of preterm births [15], [66], [67]. In some of these isolations F. nucleatum was found as a sole bacterium [68]. In this extraoral environment, and without the proteolytic oral bacterial neighbors, it is plausible that fusobacterial proliferation in the placenta is fusolisin dependent. It is therefore tempting to speculate that inhibition of fusolisin might affect the ability of F. nucleatum to translocate from the multispecies oral cavity and colonize the placenta.
Supporting Information
Structure prediction of serine protease catalytic domain of Fsp25586 (catalytic triad marked) generated using the Protein Homology/analogY Recognition Engine (Phyre).
(MOV)
Structure prediction of the autotransporter domain of Fsp25586 generated using the Protein Homology/analogY Recognition Engine (Phyre).
(MOV)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Israel Science Foundation (grant 208/10) and in part by the United States-Israel Binational Science Foundation (grant number 2005084). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gharbia SE, Shah HN (1992) Fusobacterium nucleatum subsp. fusiforme subsp. nov. and Fusobacterium nucleatum subsp. animalis subsp. nov. as additional subspecies within Fusobacterium nucleatum . Int J Syst Bacteriol 42: 296–298. [DOI] [PubMed] [Google Scholar]
- 2. Moore WE, Moore LV (1994) The bacteria of periodontal diseases. Periodontol 2000 5: 66–77. [DOI] [PubMed] [Google Scholar]
- 3. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25: 134–144. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan A, Kaplan CW, He X, McHardy I, Shi W, et al. (2014) Characterization of aid1, a Novel Gene Involved in Fusobacterium nucleatum Interspecies Interactions. Microb Ecol. [DOI] [PMC free article] [PubMed]
- 5. Bradshaw DJ, Marsh PD, Watson GK, Allison C (1998) Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun 66: 4729–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kolenbrander PE, Palmer RJ Jr, Rickard AH, Jakubovics NS, Chalmers NI, et al. (2006) Bacterial interactions and successions during plaque development. Periodontol 2000 42: 47–79. [DOI] [PubMed] [Google Scholar]
- 7. Kolenbrander PE, Parrish KD, Andersen RN, Greenberg EP (1995) Intergeneric coaggregation of oral Treponema spp. with Fusobacterium spp. and intrageneric coaggregation among Fusobacterium spp. Infect Immun 63: 4584–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosen G, Nisimov I, Helcer M, Sela MN (2003) Actinobacillus actinomycetemcomitans serotype b lipopolysaccharide mediates coaggregation with Fusobacterium nucleatum . Infect Immun 71: 3652–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosen G, Sela MN (2006) Coaggregation of Porphyromonas gingivalis and Fusobacterium nucleatum PK 1594 is mediated by capsular polysaccharide and lipopolysaccharide. FEMS Microbiol Lett 256: 304–310. [DOI] [PubMed] [Google Scholar]
- 10. Kolenbrander PE, London J (1993) Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol 175: 3247–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han YW, Shi W, Huang GT, Kinder Haake S, Park NH, et al. (2000) Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun 68: 3140–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang GT, Kim D, Lee JK, Kuramitsu HK, Haake SK (2001) Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun 69: 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gursoy UK, Kononen E, Uitto VJ (2008) Stimulation of epithelial cell matrix metalloproteinase (MMP-2, -9, -13) and interleukin-8 secretion by fusobacteria. Oral Microbiol Immunol 23: 432–434. [DOI] [PubMed] [Google Scholar]
- 14. Liu H, Redline RW, Han YW (2007) Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol 179: 2501–2508. [DOI] [PubMed] [Google Scholar]
- 15. Han YW, Redline RW, Li M, Yin L, Hill GB, et al. (2004) Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 72: 2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, et al. (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22: 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, et al. (2013) Fusobacterium is associated with colorectal adenomas. PLoS One 8: e53653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, et al. (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, et al. (2013) Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finlay BB, Falkow S (1997) Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev 61: 136–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page MJ, Di Cera E (2008) Serine peptidases: classification, structure and function. Cell Mol Life Sci 65: 1220–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruiz-Perez F, Nataro JP (2014) Bacterial serine proteases secreted by the autotransporter pathway: classification, specificity, and role in virulence. Cell Mol Life Sci 71: 745–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canizalez-Roman A, Navarro-Garcia F (2003) Fodrin CaM-binding domain cleavage by Pet from enteroaggregative Escherichia coli leads to actin cytoskeletal disruption. Mol Microbiol 48: 947–958. [DOI] [PubMed] [Google Scholar]
- 24. Villaseca JM, Navarro-Garcia F, Mendoza-Hernandez G, Nataro JP, Cravioto A, et al. (2000) Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect Immun 68: 5920–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Z, Klionsky DJ (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 22: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lievin-Le Moal V, Comenge Y, Ruby V, Amsellem R, Nicolas V, et al. (2011) Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell Microbiol 13: 992–1013. [DOI] [PubMed] [Google Scholar]
- 27. Orth D, Ehrlenbach S, Brockmeyer J, Khan AB, Huber G, et al. (2010) EspP, a serine protease of enterohemorrhagic Escherichia coli, impairs complement activation by cleaving complement factors C3/C3b and C5. Infect Immun 78: 4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah HN, Gharbia E, Al-Jalili TA (1987) Biochemical characteristics and pathogenicity of periodontal pathogens. Chemioterapia 6: 16–17. [PubMed] [Google Scholar]
- 29. Shah HN, Gharbia SE (1989) Ecological events in subgingival dental plaque with reference to Bacteroides and Fusobacterium species. Infection 17: 264–268. [DOI] [PubMed] [Google Scholar]
- 30. Sheets SM, Robles-Price AG, McKenzie RM, Casiano CA, Fletcher HM (2008) Gingipain-dependent interactions with the host are important for survival of Porphyromonas gingivalis . Front Biosci 13: 3215–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bamford CV, Fenno JC, Jenkinson HF, Dymock D (2007) The chymotrypsin-like protease complex of Treponema denticola ATCC 35405 mediates fibrinogen adherence and degradation. Infect Immun 75: 4364–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bakken V, Hogh BT, Jensen HB (1989) Utilization of amino acids and peptides by Fusobacterium nucleatum . Scand J Dent Res 97: 43–53. [DOI] [PubMed] [Google Scholar]
- 33. Gharbia SE, Shah HN (1991) Comparison of the amino acid uptake profile of reference and clinical isolates of Fusobacterium nucleatum subspecies. Oral Microbiol Immunol 6: 264–269. [DOI] [PubMed] [Google Scholar]
- 34. Yoneda S, Loeser B, Feng J, Dmytryk J, Qi F, et al. (2014) Ubiquitous Sialometabolism Present among Oral Fusobacteria. PLoS One 9: e99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rogers AH, Chen J, Zilm PS, Gully NJ (1998) The behaviour of Fusobacterium nucleatum chemostat-grown in glucose- and amino acid-based chemically defined media. Anaerobe 4: 111–116. [DOI] [PubMed] [Google Scholar]
- 36. Brokstad KA, Bakken V, Vasstrand EN, Jensen HB (1990) Diisopropylfluorophosphate-binding proteins in the outer membrane of Fusobacterium nucleatum: strain variations. FEMS Microbiol Lett 54: 235–238. [DOI] [PubMed] [Google Scholar]
- 37. Brokstad KA, Jensen HB (1991) Purification and characterization of a 65-kilodalton diisopropylfluorophosphate-binding protein in the outer membrane of Fusobacterium nucleatum Fev1. Scand J Dent Res 99: 20–29. [DOI] [PubMed] [Google Scholar]
- 38. Ogawa AT, Brasil de Souza Tde A, de Uzeda M, Jankevicius JV, Jankevicius SI (2006) Characterization of proteolytic activities of Fusobacterium nucleatum . J Endod 32: 521–523. [DOI] [PubMed] [Google Scholar]
- 39. Bachrach G, Rosen G, Bellalou M, Naor R, Sela MN (2004) Identification of a Fusobacterium nucleatum 65 kDa serine protease. Oral Microbiol Immunol 19: 155–159. [DOI] [PubMed] [Google Scholar]
- 40. Wilm M, Mann M (1996) Analytical properties of the nanoelectrospray ion source. Anal Chem 68: 1–8. [DOI] [PubMed] [Google Scholar]
- 41. Rosen G, Sela MN, Bachrach G (2012) The antibacterial activity of LL-37 against Treponema denticola is dentilisin protease independent and facilitated by the major outer sheath protein virulence factor. Infect Immun 80: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kinder Haake S, Yoder S, Gerardo SH (2006) Efficient gene transfer and targeted mutagenesis in Fusobacterium nucleatum . Plasmid 55: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bachrach G, Haake SK, Glick A, Hazan R, Naor R, et al. (2004) Characterization of the novel Fusobacterium nucleatum plasmid pKH9 and evidence of an addiction system. Appl Environ Microbiol 70: 6957–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eini A, Sol A, Coppenhagen-Glazer S, Skvirsky Y, Zini A, et al. (2013) Oxygen deprivation affects the antimicrobial action of LL-37 as determined by microplate real-time kinetic measurements under anaerobic conditions. Anaerobe 22: 20–24. [DOI] [PubMed] [Google Scholar]
- 45. Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, et al. (1999) Protein identification and analysis tools in the ExPASy server. Methods Mol Biol 112: 531–552. [DOI] [PubMed] [Google Scholar]
- 46. Higgins DG (1994) CLUSTAL V: multiple alignment of DNA and protein sequences. Methods Mol Biol 25: 307–318. [DOI] [PubMed] [Google Scholar]
- 47. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelley LA, Sternberg MJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4: 363–371. [DOI] [PubMed] [Google Scholar]
- 49. Desvaux M, Khan A, Beatson SA, Scott-Tucker A, Henderson IR (2005) Protein secretion systems in Fusobacterium nucleatum: genomic identification of Type 4 piliation and complete Type V pathways brings new insight into mechanisms of pathogenesis. Biochim Biophys Acta 1713: 92–112. [DOI] [PubMed] [Google Scholar]
- 50. Kapatral V, Anderson I, Ivanova N, Reznik G, Los T, et al. (2002) Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J Bacteriol 184: 2005–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786. [DOI] [PubMed] [Google Scholar]
- 52. Perona JJ, Craik CS (1995) Structural basis of substrate specificity in the serine proteases. Protein Sci 4: 337–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siezen RJ, Renckens B, Boekhorst J (2007) Evolution of prokaryotic subtilases: genome-wide analysis reveals novel subfamilies with different catalytic residues. Proteins 67: 681–694. [DOI] [PubMed] [Google Scholar]
- 54. Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, et al. (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41: D348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pohlner J, Halter R, Beyreuther K, Meyer TF (1987) Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325: 458–462. [DOI] [PubMed] [Google Scholar]
- 56. Dautin N, Bernstein HD (2007) Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol 61: 89–112. [DOI] [PubMed] [Google Scholar]
- 57. Henderson IR, Cappello R, Nataro JP (2000) Autotransporter proteins, evolution and redefining protein secretion: response. Trends Microbiol 8: 534–535. [DOI] [PubMed] [Google Scholar]
- 58. Coutte L, Willery E, Antoine R, Drobecq H, Locht C, et al. (2003) Surface anchoring of bacterial subtilisin important for maturation function. Mol Microbiol 49: 529–539. [DOI] [PubMed] [Google Scholar]
- 59. Haake SK, Yoder SC, Attarian G, Podkaminer K (2000) Native plasmids of Fusobacterium nucleatum: characterization and use in development of genetic systems. J Bacteriol 182: 1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sol A, Skvirsky Y, Nashef R, Zelentsova K, Burstyn-Cohen T, et al.. (2014) Actin enables the antimicrobial action of LL-37 in the presence of microbial proteases. J Biol Chem M114.579672 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 61. Rogers AH, Zilm PS, Gully NJ, Pfennig AL, Marsh PD (1991) Aspects of the growth and metabolism of Fusobacterium nucleatum ATCC 10953 in continuous culture. Oral Microbiol Immunol 6: 250–255. [DOI] [PubMed] [Google Scholar]
- 62. Henderson IR, Navarro-Garcia F, Nataro JP (1998) The great escape: structure and function of the autotransporter proteins. Trends Microbiol 6: 370–378. [DOI] [PubMed] [Google Scholar]
- 63. Holt SC, Bramanti TE (1991) Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med 2: 177–281. [DOI] [PubMed] [Google Scholar]
- 64. Wing HJ, Goldman SR, Ally S, Goldberg MB (2005) Modulation of an outer membrane protease contributes to the virulence defect of Shigella flexneri strains carrying a mutation in the virK locus. Infect Immun 73: 1217–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fink DL, St Geme JW 3rd (2003) Chromosomal expression of the Haemophilus influenzae Hap autotransporter allows fine-tuned regulation of adhesive potential via inhibition of intermolecular autoproteolysis. J Bacteriol 185: 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hill GB (1993) Investigating the source of amniotic fluid isolates of fusobacteria . Clin Infect Dis 16 Suppl 4S423–424. [DOI] [PubMed] [Google Scholar]
- 67. Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, et al. (2010) Term stillbirth caused by oral Fusobacterium nucleatum . Obstet Gynecol 115: 442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS (2009) Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Structure prediction of serine protease catalytic domain of Fsp25586 (catalytic triad marked) generated using the Protein Homology/analogY Recognition Engine (Phyre).
(MOV)
Structure prediction of the autotransporter domain of Fsp25586 generated using the Protein Homology/analogY Recognition Engine (Phyre).
(MOV)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.