Abstract
Acute Myeloid Leukemia (AML) is more common and more lethal among patients over the age of 60. Increased body mass index (BMI) has been associated with a higher incidence of various malignancies, including AML. We sought to determine whether patient BMI at the time of AML diagnosis is related to overall survival among elderly patients. We identified 97 patients with AML diagnosed after the age of 60 and treated with cytarabine-based induction chemotherapy. The median age was 68 years (range 60-87); 52% of patients were male, and our study population was predominantly white (89% of patients). The median overall survival (OS) for all patients was 316 days (95% CI 246-459). The hazard ratio for mortality was increased among patients with a BMI < 25 compared to BMI ≥ 30 (HR 2.14, P=0.009, 95% CI 1.21-3.77), as well as with older age (HR 1.76, P=0.015, 95% CI 1.12-2.79) and with secondary versus de novo disease (HR 1.95, P=0.006, 95% CI 1.21-3.14). After multivariable analysis, we did not find a significant association between OS and other potential confounders such as coronary artery disease or diabetes among these patients. We conclude that increased BMI was independently associated with improved OS among older AML patients at our institution.
Keywords: Acute myeloid leukemia, Body mass index, obesity, elderly
Introduction
Acute Myeloid Leukemia (AML) is a hematologic malignancy characterized by the proliferation of arrested myeloblasts, often accompanied by a diverse number of characteristic cellular mutations. AML is more common among patients over the age of 60, who also carry a worse prognosis with this disease [1]. Although age has been regarded as a marker of poor clinical outcomes in AML, it is likely a surrogate for other poor prognostic factors such as comorbid disease [2], and even among the elderly, cures can be achieved [3]. It is unclear which patients will benefit most from intensive chemotherapy, and prognostic factors have been identified in an attempt to better risk stratify this heterogeneous population. Although patient comorbidities are commonly cited as reasons elderly patients are not candidates for traditional induction therapy, most prognostic scores focus instead on disease-specific characteristics such as myeloblast cytogenetics [2, 4, 5]. and thus may not account for comorbidity that predates a diagnosis of AML.
A number of studies have noted an association between increased body mass index (BMI) and higher incidence of various hematologic malignancies [6–8], including AML [9–11]. An analysis from the Iowa Women's Health Study, a large prospective cohort study of women aged 55 through 69 followed within the Iowa Cancer Registry, noted an increased relative risk of 2.4 for incident AML in those whose BMI was ≥ 30 compared to those with a BMI between 18.5 and 24.9 [9]. However, there is limited data related to the prognosis of obese patients with AML. Those patients who are obese or overweight appear to carry a poorer prognosis within pediatric leukemia and transplant populations [12–15], but obesity does not appear to worsen outcomes among adults with AML, as shown in two studies assessing cohorts of adults, ranging in age from 17 to 89, treated with conventional induction chemotherapy [16, 17]. Indeed, there is some indication that obese and overweight patients may have better remission rates and lower rates of resistant disease when compared to those with a normal BMI [16]; however, to our knowledge, no significant relationship between obesity and survival among adult patients with AML has yet been reported.
Specifically, there is limited data regarding the effect of obesity on outcomes in elderly patients with AML, where comorbid disease would potentially have a greater impact on patient management. Recently, a study of patients with diffuse large B-cell lymphoma (DLBCL) within the VA healthcare system, of whom 70% were older than age 60, showed decreased mortality among those who were overweight or obese, suggesting that obesity may be a good prognostic marker among older patients with DLBCL [18]. Similarly, for patients undergoing transplantation, several series suggest obese adult patients do not have worsened outcomes [19, 20], and indeed may have decreased overall mortality compared to patients who have a normal BMI [21]. It is not entirely clear whether BMI is an independent prognostic factor, or if it is a surrogate for other patient risk factors such as disease severity and cancer cachexia. The prevalence of overweight and obese adults has been increasing, and comprises two thirds of the adult population in the United States [22], and the prevalence of both obesity and AML increase with advanced age. Given these intriguing trends and recent findings, we performed a retrospective analysis to determine whether patient BMI at time of AML diagnosis is associated with overall survival among patients, older than age 60, who received cytarabine-based induction chemotherapy.
Methods
We performed a retrospective chart review of patients diagnosed with AML at Massachusetts General Hospital (MGH) between January 1, 1992 and May 1, 2011 using records available in the electronic medical record. Patients were identified using a combination of billing codes and pathology records to first identify possible candidates, and subsequently underwent formal chart review to confirm a diagnosis of AML. We excluded patients whose initial treatment did not include cytarabine chemotherapy, including patients treated initially with hypomethylating agents or other experimental therapies. We thus identified 97 eligible patients aged 60 years or older on the date of pathologic diagnosis that received cytarabine-based induction chemotherapy regimens and had both height and weight or BMI recorded at the time of diagnosis, and this cohort was therefore included in the current analysis.
This study was approved by the institutional review board prior to initiation. The inclusion criteria included patients, older than age 60 at time of diagnosis, with a pathologically-confirmed, new diagnosis of AML, and who were treated with conventional induction chemotherapy. Medical chart review was performed to collect past medical history, presenting labs, leukemia cytopathologic data, as well as height and weight or BMI at diagnosis. Cytogenetic risk was documented as described by the National Cancer Research Institute Adult Leukemia Working Group [23]. Molecular risk, including FLT3 and NPM1 mutational status, was not included in this study because our institution began collecting these markers in 2007, in the midst of the current analysis. BMI was calculated by dividing the patient weight in kilograms (kg) by the square of their height in meters (m2) (BMI = kg/m2). For this analysis, we excluded patients with a diagnosis of acute promyelocytic leukemia (APL).
We then allocated patients to BMI categories as developed by the World Health Organization (WHO): underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30), and obese (BMI ≥ 30). Only one patient was underweight; this patient died from antibiotic-associated renal failure on day 14 following induction. Because this was the only patient in the cohort who was underweight and the death was a complication of supportive care we included this patient in the normal weight category. Chemotherapy dosing was based on calculated body surface area, with the most commonly used method at this institution being that described by Gehan and George [24, 25].
Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC). Patient characteristics were summarized as numbers and percentages. Overall Survival (OS) was defined as the duration from the date of induction to that of death, or censored on the last known alive date if patients were still alive at the time of analysis. OS was estimated using the Kaplan-Meier method, with 95% confidence intervals calculated using Greenwood's formula. We then performed a stepwise multivariable Cox regression analysis, pre-specifying that a variable had to be significant at the 0.3 level before it could be entered into the model, while a variable in the model had to be significant at the 0.05 level for it to remain in the model. We first restricted the stepwise proportional-hazards to complete cases (n=86). We then ran the model again with cases that were complete for the variables that were included in the final model (n=96).
Results
Patient Characteristics
We identified 97 patients who were age 60 or older at the time of diagnosis and who received cytarabine-based induction chemotherapy (Table 1). The median age was 68 years (range 60-87); 52% of patients were male, and 89% were white. Patient disease was identified as de novo in 41%, and secondary in 59%. Cytogenetics, when available (89.7% of patients), were most commonly normal (36.1%) or adverse risk (42.3%); only one case (1%) was characterized as favorable risk, which was a patient with a pericentric inversion of chromosome 16 and rearrangement of CBF beta. Approximately one fifth of this cohort had a recorded past medical history of diabetes (20.6%) or coronary artery disease (17.5%). Baseline laboratories revealed that most patients were anemic and thrombocytopenic on admission, but had, on average, normal creatinine, bilirubin, sodium, and fibrinogen levels.
Table 1. Patient Demographic Information.
| Baseline Patient Characteristics | n (%) | |
|---|---|---|
| Age at Diagnosis (median 67, range 60-87) | 97 (100%) | |
|
| ||
| Gender | ||
|
| ||
| Male | 50 (51.5%) | |
| Female | 47 (48.5%) | |
|
| ||
| Race/Ethnicity | ||
|
| ||
| White | 86 (88.7%) | |
| Black | 2 (2.1%) | |
| Hispanic | 3 (3.1%) | |
| Asian | 1 (1%) | |
| Other | 5 (5.2%) | |
|
| ||
| Cardiac Comorbidities | ||
|
| ||
| Coronary Artery Disease | 17 (17.5%) | |
| Diabetes | 20 (20.6%) | |
|
| ||
| Patient Body Mass Index | ||
|
| ||
| Normal (< 25) | 33 (34%) | |
| Overweight (≥ 25 & < 30) | 33 (34%) | |
| Obese (≥ 30) | 31 (32%) | |
|
| ||
| Performance Status (documented at presentation) | ||
|
| ||
| 0 | 14 (14.4%) | |
| 1 | 29 (29.9%) | |
| 2 | 10 (10.3%) | |
| 3 | 2 (2.1%) | |
| Missing | 42 (43.3%) | |
|
| ||
| De novo or Secondary Disease | ||
|
| ||
| De novo | 40 (41.2%) | |
| Secondary | 57 (58.8%) | |
|
| ||
| Patient Cytogenetics | ||
|
| ||
| Favorable | 1 (1.0%) | |
| Intermediate | 10 (10.3%) | |
| Normal karyotype | 35 (36.1%) | |
| Adverse | 41 (42.3%) | |
| Missing | 10 (10.3%) | |
|
| ||
| Presenting Laboratories | Median (range) | |
|
| ||
| Total Bilirubin (mg/dl) | 0.6 (0.2-4.4) | |
| Fibrinogen (mg/dl) | 459 (100-897) | |
| Hematocrit (%) | 26 (15.7-42.3) | |
| Platelet Count (th/cumm) | 57 (9-953) | |
| Sodium (mmol/L) | 138 (125-144) | |
| Creatinine (mg/dl) | 1 (0.5-4.8) | |
| Glucose (mg/dl) | 109 (77-300) | |
| Albumin (g/dl) | 3.8 (2.4-4.6) | |
Dosing information for induction chemotherapeutic agents was available for 86 of 97 (88.8%) patients within the medical record. All of these patients received cytarabine as a part of their initial chemotherapy. The remaining 11 patients had induction regimens described in their notes but specific dosing was not available. The most common regimens were infusional cytarabine dosed at 100 or 200 mg/m2/day over seven days and an intravenous anthracycline for three days (“7+3”) (72/86, 83.7%); a combination of intravenous cytarabine 1 gm/m2/day and topotecan 1.25 mg/m2/day over five days (“TA”) (8/86, 9.3%); or cytarabine 1 gm/m2/day for five days with three days of anthracycline (5/86, 5.8%). In those who received anthracyclines as part of their induction chemotherapy, idarubicin was dosed at 10 or 12 mg/m2 (54/77 patients, 2 patients were dosed at 8 mg/m2), and daunorubicin was dosed at 45 or 60 mg/m2 (23/77 patients). One patient, on a clinical trial, received the experimental agent amonafide over five days along with cytarabine dosed at 200 mg/m2 daily for 7 days. Dosing at our institution is based on calculated BSA, and chemotherapy is not dose-reduced for obese patients.
Survival
The median OS for all patients was 316 days (95% CI 246-459). The 60 day OS for all patients was 91% (95% CI 85-96%). We performed a univariable analysis to determine associations between patient variables and OS using the log-rank test. This analysis showed that a worsened OS was associated with age older than 68 (the cohort median) (P=0.04), BMI < 25 (P=0.04), having secondary rather than de novo disease (P=0.02), and with adverse-risk cytogenetics (P=0.03) (Table 2). We included these variables in the multivariable analysis. We did not find any significant association between OS and total dose of cytarabine (P=0.92), with either idarubicin (P=0.40) or daunorubicin dose (P=0.44), or with topotecan dose (P=0.52). We also failed to find an association between OS and with having a history of diabetes (P=0.52), coronary artery disease (P=0.12), with patient race (P=0.07) or gender (P=0.86), or with presenting hematocrit (P=0.97), sodium (P=0.59), or total bilirubin (P=0.11) levels.
Table 2.
Univariable analyses of OS by log-rank testing. The model parameters presented are given in the form of median survival in days, along with 95% Lower Confidence Limits (LCL) and 95% Upper Confidence Limits (UCL) and p-values
| Variable | Stratum | Median (days) | Log-rank P | 95% LCL | 95% UCL |
|---|---|---|---|---|---|
| Age | <68 | 425.0 | 0.04 | 293 | 611 |
| ≥68 | 244.0 | 177 | 350 | ||
| Race | Asian | . | 0.07 | . | . |
| Black | 242.0 | 139 | 345 | ||
| Hispanic | 95.0 | 36 | 246 | ||
| Other | 229.0 | 203 | . | ||
| White | 350.0 | 268 | 471 | ||
| Gender | Female | 345.0 | 0.86 | 293 | 494 |
| Male | 257.0 | 155 | 587 | ||
| De novo/Secondary | De novo | 587.0 | 0.02 | 269 | 1062 |
| Secondary | 268.5 | 215 | 350 | ||
| Hematocrit (%) | <30 | 345.0 | 0.97 | 229 | 494 |
| ≥30 | 299.5 | 205 | 394 | ||
| Sodium (mmol/L) | <130 | 335.0 | 0.59 | 95 | 459 |
| ≥130 | 316.0 | 244 | 471 | ||
| Total bilirubin (mg/dl) | ≤1 | 327.0 | 0.11 | 268 | 471 |
| >1 | 178.5 | 46 | 494 | ||
| BMI | < 25 | 215.0 | 0.04 | 141 | 311 |
| ≥ 25 and < 30 | 327.0 | 164 | 494 | ||
| ≥ 30 | 587.0 | 302 | 1065 | ||
| Coronary artery disease | No | 327.0 | 0.12 | 244 | 494 |
| Yes | 269.0 | 70 | 471 | ||
| Diabetes | No | 321.5 | 0.52 | 246 | 471 |
| Yes | 268.0 | 111 | 910 | ||
| Cytogenetics | Missing | 647.0 | 0.03 | 12 | 2698 |
| Favorable | |||||
| Intermediate | 368.0 | 10 | 2990 | ||
| Normal | 471.0 | 269 | 801 | ||
| Adverse | 257.0 | 159 | 311 | ||
| Chemotherapy Dosing | |||||
| Cytarabine | < 2464 | 339.5 | 0.92 | 209 | 471 |
| (total dose in mg) | ≥ 2464 | 316.0 | 229 | 531 | |
| Idarubicin | < 65 | 304.0 | 0.40 | 141 | 494 |
| (total dose in mg) | ≥ 65 | 432.0 | 215 | 1062 | |
| Daunorubicin | < 306 | 293.0 | 0.44 | 115 | |
| (total dose in mg) | ≥ 306 | 312.5 | 94 | 718 | |
| Topotecan | < 12 | 364.0 | 0.52 | 244 | 531 |
| (total dose in mg) | ≥ 12 | 229.0 | 111 | 801 | |
We next performed a multivariable analysis of the Cox proportional-hazards model as described. The hazard ratio for mortality was increased with age > 68 (HR 1.76, P=0.015, 95% CI 1.12-2.79), a lower BMI < 25 compared to a BMI ≥ 30 (HR 2.14, P=0.009, 95% CI 1.21-3.77), and having secondary rather than de novo disease (HR 1.95, P=0.006, 95% CI 1.21-3.14) (Table 3). A BMI of ≥ 25 and < 30 was not significantly worse than having a BMI ≥ 30 (HR 1.55, P=0.127, 95% CI 0.883-2.73). Having higher-risk cytogenetics was no longer significantly associated with an increased hazard ratio for mortality in the multivariable analysis. Kaplan-Meier analysis confirmed an improvement in median survival as BMI increased (Figure 1). After multivariable analysis, we did not find any significant association between OS and the presence of coronary artery disease or diabetes, the patient gender or race, AML cytogenetics, or the presenting hematocrit, sodium, or total bilirubin levels (Table 3).
Table 3.
Multivariable Analysis of OS by Cox Regression. The multivariable analysis of the Cox proportional-hazards model indicates that an increased hazard rate for mortality is positively associated with decreased BMI <25, increased age, and having secondary rather than de novo disease (P<0.05). A history of CAD or diabetes, patient gender and race, patient cytogenetics, and presenting HCT, Na, or Bil were not statistically significant following multivariable analysis.
| Variable | OS (Median Days) | HR for mortality | 95% CI Lower Limit | 95% CI Upper Limit | P-value | |
|---|---|---|---|---|---|---|
| Age | ≥ 68 | 244 (177-356) | 1.76 | 1.12 | 2.79 | 0.015 |
| < 68 | 425 (293-611) | 1 | ||||
|
| ||||||
| BMI | < 25 | 215 (141-311) | 2.14 | 1.21 | 3.77 | 0.009 |
| ≥ 25 & < 30 | 336 (164-494) | 1.55 | 0.883 | 2.73 | 0.127 | |
| ≥ 30 | 587 (302-1065) | 1 | ||||
|
| ||||||
| De novo / secondary | Secondary | 268.5 (215-350) | 1.95 | 1.21 | 3.14 | 0.006 |
| De novo | 557 (302-910) | 1 | ||||
Figure 1.
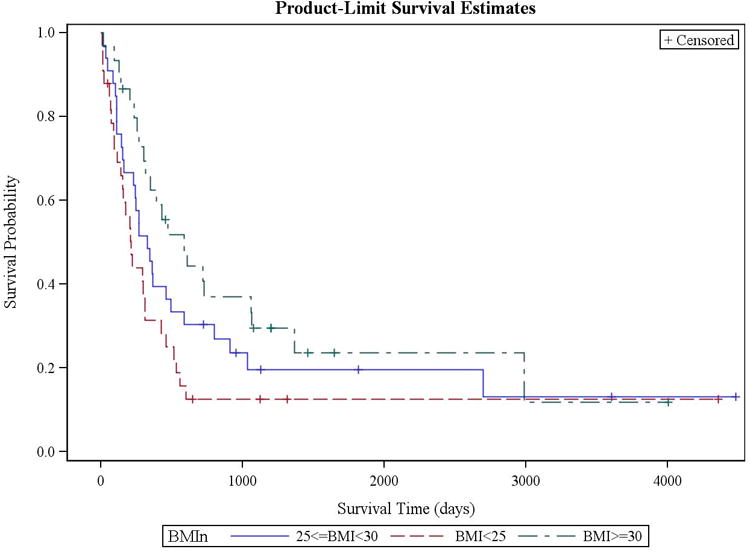
Kaplan-Meier estimation of overall survival by patient BMI among patients over 60 years old. Overall survival differed between patients based on BMI. BMI < 25 (red dashed line) was associated with a worsened OS compared to BMI between 25 and 30 (blue solid line), and with BMI of ≥ 30 (green dashed line) (p=0.039).
Discussion
Patients over the age of 60 with a new diagnosis of AML carry a poor prognosis; comorbidity at the time of presentation may assist clinicians in risk stratification in this population. Obesity is a comorbid condition with a high prevalence in the United States, including among those with malignancy, and predicts for worse outcomes and increased mortality [10]. Although obesity is increasingly prevalent among the general population, its significance may be misrepresented in current risk stratification models for hematologic malignancies; for instance, the Sorror HCT-CI risk stratification tool only includes patients with a BMI greater than 35 [26]. Indeed, a recent study in patients with DLBCL found better outcomes associated with a higher BMI [18]. In this modestly sized single institution retrospective analysis, we found that among AML patients over age 60, a BMI under 25 at presentation was associated with an increased hazard ratio for mortality compared to obese patients with a BMI of 30 or greater.
Previous studies have suggested that elevated BMI should not preclude patients with AML from receiving aggressive chemotherapy regimens. One analysis assessed 329 adult patients with AML, across age groups, treated with high-dose cytarabine and idarubicin induction, and found no difference in patient survival or adverse events based on patient obesity [17]. The Southwest Oncology Group evaluated a much larger cohort of 1,974 AML patients, aged 17 through 89 years, treated with induction chemotherapy, and also found no significant difference in patient survival or adverse outcomes based on BMI; intriguingly, remission rates were higher and resistant disease was lower among obese patients [16]. An important distinction between these studies and ours is that neither of the former focused specifically on the older population of patients, in whom comorbid conditions are thought to impact outcomes to a significantly greater degree.
The relationship between increased BMI and AML does not have a clear etiologic explanation [9, 11]. The incidence of AML is higher among the obese, possibly due to altered immune function [27], as well as altered hormone signaling, such as by insulin-like growth factor-1 (IGF-1) [28, 29] or leptin [30, 31]. Multiple studies suggest that BMI alone should not alter treatment approaches in AML, consistent with other data in hematologic malignancies, and with recent recommendations to avoid dose reduction of chemotherapy in obese patients [32]. A study of 2,534 patients with DLBCL, from the Veteran's Health Administration hospital system, found improved survival among overweight and obese patients when compared to patients of normal weight [18]. Several potential etiologic mechanisms were proposed, including improved tolerance of chemotherapy and differences in chemotherapy metabolism, particularly in relation to anthracycline fat solubility [33, 34]. This phenomenon has been seen in the transplant population as well. Several series out of the Center for International Blood and Marrow Transplant Research have shown that patients who are obese or overweight at the time of transplantation, compared to patients of normal BMI, may have decreased relapse rates [19]; in one analysis, both overweight (RR, 0.87; P = .004) and obese (RR, 0.76; P < .0001) transplant patients had improved overall survival compared to normal BMI patients [21].
Also unclear are the mechanisms by which obesity or adiposity may contribute to patient survival in AML. It is not known whether patient BMI has an etiologic role in leukemogenesis and prognosis, or whether it reflects the severity of patient disease on presentation. Patients with lower or normal BMI at presentation may in fact be more cachectic or have more advanced disease. A limitation of our study is that a large proportion of patients (43%) did not have initial ECOG performance status documented, and thus we could not assess for a possible association between lower BMI and illness severity. Alternatively, BMI itself may have prognostic value; one mechanism may be through providing additional physiologic reserve to tolerate the intensity of chemotherapy dosing [18]. Another possibility is that leukemia in the setting of obesity is under an endogenous stimulus related to adiposity, with possible candidates being leptin [30, 31, 35] or through the IGF-1 signaling pathway [29]. Leptin is expressed in a number of hematologic malignancies; in chronic myelogenous leukemia, for instance, leukemic cells in “blast” phase have higher leptin levels [35]. Endogenous driver signals may increase proliferation of leukemic cells, making them more susceptible to chemotherapy.
In addition, anthracycline fat solubility [34, 36] may contribute to survival differences, either the result of an effective increased dose to more obese patients, or by a relative underdosing of those patients with normal BMI who have lesser fat stores. Intensified anthracycline dosing has been recently shown to improve outcomes among elderly patients with AML [37]. In our cohort there was no relationship between the total chemotherapy dose (e.g. accumulated over the duration of induction) and OS; however, this analysis is limited by the variety of chemotherapy regimens used in our small patient cohort. In addition, we found that idarubicin was employed preferentially over daunorubicin at our institution, and when used, daunorubicin was most commonly dosed at 60 mg/m2/day in the “7+3” regimen. Idarubicin differs from daunorubicin in that it lacks a methoxyl group at position 4 of the chromophore ring, resulting in greater lipophilicity [38, 39]. Although we did not find a difference in outcomes between anthracyclines used, this may have been due to our modest patient cohort size. Indeed, we cannot draw further conclusions but can only affirm that chemotherapy dosing did not appear to significantly impact patient survival.
There are a number of limitations to our study, including the relatively small number of patients included in this single-institution analysis. Because of the retrospective nature of our methodology, it is possible that several patients were unintentionally excluded from the study. Additionally, the patient population seen at our institution was predominantly white, and it is unclear whether findings can be extrapolated to other races or ethnicities. In addition, it will be important to validate our findings in larger patient cohorts, looking specifically at patients over the age of 60. In order to maximize our study power, we chose to include patients over a course of 20 years and who had received various forms of conventional cytarabine-based induction chemotherapy. The current analysis also does not include molecular risk, such as FLT3 or NPM1 status, because these assays were not available during the entire time period of investigation. Importantly, we excluded patients who initially received non-standard induction regimens which did not include cytarabine; this excluded a large number of patients, which could limit the generalizability of our findings. Changes in the prevalence of patient comorbidity, as well as recent trends in treatment approaches and supportive care, could potentially bias our conclusions. This is an important consideration, especially as both AML survival and the prevalence of obese and overweight persons continue to increase.
We attempted to control for several known risk factors for poor survival that are associated with obesity by including documented coronary artery disease or diabetes mellitus in our analysis. The extent to which this captures the actual burden of comorbid disease in our population is unknown because we were limited to information available in the medical record. For example, we could not include important variables such as smoking, an established risk factor for developing leukemia and more common among obese persons [40], since this history was not accurately recorded. In addition, we did not have data on metformin use among patients with obesity; metformin, a biguanide molecule commonly used in diabetes management, has been shown to have activity against AML in vitro, possibly by activating the LKB1/AMPK tumor suppressor pathway [41, 42]. Both of these variables should be considered in future prospective study of BMI in this patient population.
In conclusion, obesity is an increasingly prevalent comorbidity in this country, and is closely associated with malignancy, including AML. Whether obesity also impacts response to treatment or outcomes in patients with cancer is an intriguing area of investigation [43, 44]. In this single institution retrospective cohort study of older patients receiving cytarabine-based chemotherapy for AML, we found a survival benefit associated with increased patient BMI. We conclude that obesity alone in the elderly AML patient should not preclude aggressive care, and may in fact be associated with better outcomes. Larger and prospective studies are necessary to confirm these findings and further elucidate the impact of BMI on outcomes in patients undergoing treatment for hematologic malignancies.
Footnotes
Conflict of interest: The authors declare no conflict of interest. This research was performed with departmental support; there are no funding sources to disclose. The authors have no relevant disclosures.
This study was presented in abstract form at the American Society of Hematology 2012 Annual Meeting, Atlanta, GA, December 8-11, 2012
References
- 1.Estey E, Döhner H. Acute myeloid leukaemia. The Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Walter RB, Othus M, Borthakur G, et al. Prediction of Early Death After Induction Therapy for Newly Diagnosed Acute Myeloid Leukemia With Pretreatment Risk Scores: A Novel Paradigm for Treatment Assignment. JCO. 2011;29:4417–4424. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf Å, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 4.Krug U, Röllig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. The Lancet. 2010;376:2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 5.Fröhling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108:3280–3288. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 6.Söderberg KC, Kaprio J, Verkasalo PK, et al. Overweight, obesity and risk of haematological malignancies: A cohort study of Swedish and Finnish twins. European Journal of Cancer. 2009;45:1232–1238. doi: 10.1016/j.ejca.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Lichtman MA. Obesity and the Risk for a Hematological Malignancy: Leukemia, Lymphoma, or Myeloma. The Oncologist. 2010;15:1083–1101. doi: 10.1634/theoncologist.2010-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacInnis RJ, English DR, Hopper JL, et al. Body Size and Composition and the Risk of Lymphohematopoietic Malignancies. JNCI J Natl Cancer Inst. 2005;97:1154–1157. doi: 10.1093/jnci/dji209. [DOI] [PubMed] [Google Scholar]
- 9.Ross JA, Parker E, Blair CK, et al. Body Mass Index and Risk of Leukemia in Older Women. Cancer Epidemiol Biomarkers Prev. 2004;13:1810–1813. [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. New England Journal of Medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: A meta-analysis of cohort studies. International Journal of Cancer. 2008;122:1418–1421. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 12.Inaba H, Surprise HC, Pounds S, et al. Effect of body mass index on the outcome of children with acute myeloid leukemia. Cancer. 2012;118:5989–5996. doi: 10.1002/cncr.27640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange BJ, G R. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293:203–211. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]
- 14.Bulley S, Gassas A, Dupuis LL, et al. Inferior outcomes for overweight children undergoing allogeneic stem cell transplantation. British Journal of Haematology. 2008;140:214–217. doi: 10.1111/j.1365-2141.2007.06900.x. [DOI] [PubMed] [Google Scholar]
- 15.Barker CC, Agovi MA, Logan B, et al. Childhood Obesity and Outcomes after Bone Marrow Transplantation for Patients with Severe Aplastic Anemia. Biol Blood Marrow Transplant. 2011;17:737–744. doi: 10.1016/j.bbmt.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros BC, Othus M, Estey EH, et al. Impact of body-mass index on the outcome of adult patients with acute myeloid leukemia. Haematologica. 2012;97:1401–1404. doi: 10.3324/haematol.2011.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Licht AS, Hyland AJ, et al. Is obesity a prognostic factor for acute myeloid leukemia outcome? Ann Hematol. 2012;91:359–365. doi: 10.1007/s00277-011-1319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carson KR, Bartlett NL, McDonald JR, et al. Increased Body Mass Index Is Associated With Improved Survival in United States Veterans With Diffuse Large B-Cell Lymphoma. JCO. 2012;30:3217–3222. doi: 10.1200/JCO.2011.39.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarro WH, Agovi MA, Logan BR, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myeloid leukemia (AML) in adults. Biol Blood Marrow Transplant. 2010;16:1442–1450. doi: 10.1016/j.bbmt.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro WH. Impact of obesity in the setting of high-dose chemotherapy. Bone Marrow Transplantation. 2003;31:961–966. doi: 10.1038/sj.bmt.1704052. [DOI] [PubMed] [Google Scholar]
- 21.Navarro WH, Loberiza FR, Jr, Bajorunaite R, et al. Effect of Body Mass Index on Mortality of Patients with Lymphoma Undergoing Autologous Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2006;12:541–551. doi: 10.1016/j.bbmt.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, C M. Prevalence of overweight and obesity in the united states, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 23.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 24.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–235. [PubMed] [Google Scholar]
- 25.Mosteller R. Simplified Calculation of Body-Surface Area. New England Journal of Medicine. 1987;317:1098–1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 26.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature Reviews Immunology. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 28.Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 29.Chapuis N, Tamburini J, Cornillet-Lefebvre P, et al. Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: therapeutic value of neutralizing anti-IGF-1R antibody. Haematologica. 2010;95:415–423. doi: 10.3324/haematol.2009.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruserud O, Huang TS, Glenjen N, et al. Leptin in human acute myelogenous leukemia: studies of in vivo levels and in vitro effects on native functional leukemia blasts. Haematologica. 2002;87:584–595. [PubMed] [Google Scholar]
- 31.Hino M, Nakao T, Yamane T, et al. Leptin receptor and leukemia. Leuk Lymphoma. 2000;36:457–461. doi: 10.3109/10428190009148392. [DOI] [PubMed] [Google Scholar]
- 32.Griggs JJ, Mangu PB, Anderson H, et al. Appropriate Chemotherapy Dosing for Obese Adult Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline. [cited 2013 Jan 10];JCO. 2012 doi: 10.1200/JOP.2012.000623. Internet. Available from: http://jco.ascopubs.org/content/early/2012/03/27/JCO.2011.39.9436. [DOI] [PMC free article] [PubMed]
- 33.Hunter RJ, Navo MA, Thaker PH, et al. Dosing chemotherapy in obese patients: Actual versus assigned body surface area (BSA) Cancer Treatment Reviews. 2009;35:69–78. doi: 10.1016/j.ctrv.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. JCO. 1988;6:1321–1327. doi: 10.1200/JCO.1988.6.8.1321. [DOI] [PubMed] [Google Scholar]
- 35.Nakao T, Hino M, Yamane T, et al. Expression of the leptin receptor in human leukaemic blast cells. British Journal of Haematology. 1998;102:740–745. doi: 10.1046/j.1365-2141.1998.00843.x. [DOI] [PubMed] [Google Scholar]
- 36.Ritzmo C, Söderhäll S, Karlén J, et al. Pharmacokinetics of doxorubicin and etoposide in a morbidly obese pediatric patient. Pediatric Hematology-Oncology. 2007;24:437–445. doi: 10.1080/08880010701451343. [DOI] [PubMed] [Google Scholar]
- 37.Löwenberg B, Ossenkoppele GJ, Van Putten W, et al. High-Dose Daunorubicin in Older Patients with Acute Myeloid Leukemia. New England Journal of Medicine. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 38.Supino R, Necco A, Dasdia T, et al. Relationship between Effects on Nucleic Acid Synthesis in Cell Cultures and Cytotoxicity of 4-Demethoxy Derivatives of Daunorubicin and Adriamycin. Cancer Res. 1977;37:4523–4528. [PubMed] [Google Scholar]
- 39.Berman E, Heller G, Santorsa J, et al. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666–1674. [PubMed] [Google Scholar]
- 40.Fernberg P, Odenbro Å, Bellocco R, et al. Tobacco Use, Body Mass Index, and the Risk of Leukemia and Multiple Myeloma: A Nationwide Cohort Study in Sweden. Cancer Res. 2007;67:5983–5986. doi: 10.1158/0008-5472.CAN-07-0274. [DOI] [PubMed] [Google Scholar]
- 41.Green AS, Chapuis N, Maciel TT, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116:4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 42.Li D. Metformin as an antitumor agent in cancer prevention and treatment. Journal of Diabetes. 2011;3:320–327. doi: 10.1111/j.1753-0407.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 43.Griggs JJ, S M. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 44.Davies BJ, Walsh TJ, Ross PL, et al. Effect of BMI on Primary Treatment of Prostate Cancer. Urology. 2008;72:406–411. doi: 10.1016/j.urology.2007.11.032. [DOI] [PubMed] [Google Scholar]


