Abstract
We performed a retrospective population-based study using the SEER database to assess survival trends in CBF-AML between 2000 and 2010. Median OS increased from 16 months in 2000-2002 to 25 months in 2006-2008 (p=0.002). The 3-year OS rate for patients with inv(16) was 57.3%, but in t(8;21) was only 35.5%. Patients aged 75-84 had worse survival than patients aged 15-44 (HR 5.61, P=0.0002). Black race was associated with higher mortality (HR 1.50, P=0.03). Compared to clinical trial outcomes, CBFAML survival is poorer in the general population, particularly among African Americans and the elderly, and in t(8;21) compared to inv(16) AML.
Keywords: Acute Myeloid Leukemia, Core Binding Factors, Survival, Population Surveillance, Health Status Disparities
Introduction
Acute myeloid leukemia (AML) with a translocation between chromosomes 8 and 21 (t(8;21)) or a pericentric inversion of chromosome 16 (inv(16)/t(16;16)) is associated with alterations in the core binding factors RUNX1 (also known as CBFA2 or AML1) and CBFB.1–4 Patients harboring these alterations carry a more favorable prognosis when given intensive chemotherapy regimens,5–8 and, excluding acute promyelocytic leukemia, have the best long term survival reported among AML subtypes. Although the group of patients with core-binding factor AML (CBF-AML) is relatively small – only approximately 7-12% of patients with AML carry the t(8;21) or inv(16) abnormality5,9 – their identification is critical as it significantly impacts their subsequent management and prognosis. Nonetheless, despite a favorable prognosis associated with CBF-AML overall, certain small subgroups continue to have poorer outcomes.10–15
Multicenter cooperative group trials report 5-year overall survival (OS) rates approximating 45-66% among patients with CBF-AML;14–17 much less is known about an unselected patient population treated outside of a clinical trial in the current era. The Surveillance, Epidemiology, and End Results (SEER) database is a population-based database maintained by the National Cancer Institute (NCI); it captures 28% of the US population based on the 2010 US Census. Several previous analyses have used SEER data to assess population-level trends in cancer survival and incidence among patients with acute myeloid leukemia in the United States, but none to date have looked specifically at CBF-AML.18–21 It is not currently known whether the favorable outcomes seen in clinical trials for CBF-AML are achieved at the population level.
This analysis presents outcomes of the largest cohort of unselected patients with CBFAML reported to date. We assessed survival trends at the population level in order to determine their uniformity with data from previous clinical trials, and to identify specific subgroups with relatively poor outcomes among all patients with CBF-AML.
Materials and Methods
Patients with a diagnosis of CBF-AML were identified using the 1973-2010 SEER database (Nov 2012 submission) issued on April 29, 2013.22 Starting in 2000, this includes 18 population-based registries: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, San Jose-Monterey, Los Angeles, rural Georgia, Alaska Natives, greater California, Kentucky, Louisiana, New Jersey, and greater Georgia. SEER maintains accurate patient surveillance by recording vital status and date of last contact. It has a case ascertainment standard of 98% and is an accepted standard for population-based study in the United States.23
Patient Selection
CBF-AML diagnoses were identified using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), released in 2000.24 We included patients, aged 15-84 at diagnosis, with a recorded diagnosis of acute myeloid leukemia with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), CBFB-MYH11 (Code 9871) or acute myeloid leukemia with t(8;21)(q22;q22) RUNX1-RUNX1T1 (Code 9896). These codes are confirmed by genetic testing reported to SEER; otherwise, patients who do not meet these specifications are coded in a separate AML category. Patients were diagnosed between January 2000 and December 2010, were actively followed, had a known age, and were cases in the research database.
The SEER database records patient gender, race and ethnicity, state and county of residence, the method of diagnostic confirmation, age at diagnosis, and month and year of diagnosis as well as last follow-up or of death, linked to National Death Index data from the National Center for Health Statistics. Race and ethnicity are recorded according to federal guidelines and those of the North American Association of Central Cancer Registries. Cause of death is recorded according to the International Classification of Diseases, Tenth Edition.25 Each patient was designated as a metro or non-metro dweller according to 2003 rural-urban continuity codes within the SEER dataset.26,27
Incidence
Incidence rates of both AML (all subtypes) and CBF-AML were evaluated, including inv(16) and t(8;21) subtypes, by age cohort (15-44 years old, 45-64 years old, 65-74 years old, and 75-84 years old), gender, race (white, black, other), and ethnicity (non-Hispanic, Hispanic). Incidence rates were age-adjusted to the 2000 US standard population and calculated per 100,000 people. A linear model was used to test for a trend in the incidence rate across the age cohorts, where the age cohort was treated as an ordinal variable. This is the only instance that the age cohort was treated ordinally. Given the format of the population data, race and ethnicity could not be combined into one variable for this analysis; however, race and ethnicity were combined for subsequent analyses.
Overall Survival
Overall survival was calculated as the time from diagnosis to death of any cause and censored at the earliest of the date of last contact or the study cutoff (December 31, 2010). Time was measured as complete months of survival. Covariates of interest include CBF-AML subtype, gender, race/ethnicity (white, black, Hispanic of any race, other), age cohort at diagnosis, year of diagnosis (2000-2002, 2003-2005, 2006-2008, 2009-2010), and resident county (metro, not metro). OS was compared between groups for each covariate using the log-rank test and estimated using the method of Kaplan and Meier. Cox proportional hazards models were used to conduct multivariable analyses. A full model was fit with all the covariates of interest and the reduced model was selected using the backwards elimination method. A subset analysis evaluating the overall survival of those alive at one month and followed at least one month was also conducted.
Early Death Rate
The early death rate was defined as death within one month of CBF-AML diagnosis, and excludes patients alive at one month or greater. The probability of early death was calculated by subtracting the survival rate at one month from 1.28 Survival up to one month was estimated using the method of Kaplan and Meier. A chi-square test was used to test the early death rate difference among groups for each variable of interest. All analyses were performed using SAS 9.2 statistical software (Cary, NC), R version 2.11.1 statistical software, and SEER*Stat software (Ver. 8.0.4; Surveillance Research Program, NCI). All p-values are considered significant at the two-sided 0.05 level.
Results
Patient Characteristics
We identified 777 patients aged 15-84 diagnosed with CBF-AML between 2000 and 2010. Three patients were excluded from the survival and early death rate analyses either because there was no available survival time (n=1) or they were diagnosed by autopsy/death certificate (n=2). Of the 774 remaining patients, 101 died within one month from diagnosis and 9 were not followed for a full month. The subset of cases followed and alive for at least one month after diagnosis was 664 patients.
Incidence of CBF-AML
The annual age-adjusted incidence rate was 0.11 per 100,000 persons (Table 1). This likely underestimates the true CBF-AML incidence, as it represents only those cytogenetic subgroups reported to SEER as CBF-AML and having confirmatory cytogenetic testing. The incidence rates for the inv(16) and t(8;21) subtypes were comparable (0.05 and 0.07 per 100,000, respectively). In the general U.S. population, the incidence of CBF-AML increased with advancing age cohorts (P=0.02), although the proportion of incident AML cases that have CBF alterations decreases with age (Table 1), similar to previously reported data.5,29 The population incidence of CBF-AML roughly doubles moving from 15-44 years of age (0.06 per 100,000) to 45-64 years of age (0.13 per 100,000); the incidence rate doubles again moving to the 65-74 or 75-84 years old age cohorts (0.25 and 0.28 per 100,000). Among patients older than 65, t(8;21) is reported at a higher incidence than inv(16). The incidence rate in males (0.14 per 100,000) is slightly higher than females (0.09 per 100,000). Race and ethnicity appear to be comparable across the groups with the exception that black patients may have a slightly lower overall incidence rate (0.07 per 100,000). Among all patients with CBFAML, male patients were older at diagnosis (p=0.009), as were those of white race (p=0.002), and non-Hispanic ethnicity (p<0.0001). Patients with inv(16) AML were younger at diagnosis compared to those with t(8;21) (p=0.0007).
Table 1.
Incidence of CBF-AML, as well as the cytogenetic subgroups of inv(16) AML, and t(8;21) AML, diagnosed between 2000-2010 in patients 15-84 years old at diagnosis. The incidence of all sub-types of AML reported to SEER is included as a reference.
| AML (all subtypes) | CBF-AML | inv(16) subgroup | t(8;21) subgroup | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Incidence rate† | No. of patients | Incidence rate† | Median age at diagnosis | No. of patients | Incidence rate† | Median age at diagnosis | No. of patients | Incidence rate† | Median age at diagnosis | |
| All patients, 15-84 yr. | 27665 | 4.11 | 777 | 0.11 | 54 | 320 | 0.05 | 50 | 457 | 0.07 | 57 |
| Age Group | |||||||||||
| 15-44 yr. | 4811 | 1.24 | 254 | 0.06 | 31 | 128 | 0.03 | 33 | 126 | 0.03 | 28 |
| 45-64 yr. | 8584 | 3.81 | 285 | 0.13 | 54 | 115 | 0.05 | 55 | 170 | 0.08 | 53 |
| 65-74 yr. | 6659 | 12.35 | 137 | 0.25 | 69 | 46 | 0.08 | 69 | 91 | 0.17 | 69 |
| 75-84 yr. | 7611 | 20.87 | 101 | 0.28 | 79 | 31 | 0.09 | 77 | 70 | 0.19 | 80 |
| Gender | |||||||||||
| Male | 15209 | 4.99 | 441 | 0.14 | 56 | 186 | 0.06 | 51 | 255 | 0.08 | 60 |
| Female | 12456 | 3.43 | 336 | 0.09 | 51 | 134 | 0.04 | 49 | 202 | 0.06 | 52 |
| Race†† | |||||||||||
| White | 23132 | 4.26 | 632 | 0.12 | 56 | 277 | 0.05 | 52 | 355 | 0.06 | 59 |
| Black | 2305 | 3.40 | 57 | 0.07 | 52 | 12 | 0.02 | 46 | 45 | 0.06 | 52 |
| Other | 2102 | 3.23 | 87 | 0.12 | 47 | 31 | 0.04 | 41 | 56 | 0.08 | 49 |
| Ethnicity | |||||||||||
| Non-Hispanic | 24632 | 4.18 | 673 | 0.11 | 56 | 269 | 0.05 | 52 | 404 | 0.07 | 58 |
| Hispanic | 3033 | 3.44 | 104 | 0.09 | 44 | 51 | 0.04 | 39 | 53 | 0.05 | 46 |
Incidence rate was adjusted to the US 2000 standard population and is per 100 000 people
Other includes American Indian, Alaska Native, Asian, or Pacific Islander. The patient in CBF AML (n=1) and overall AML (n=126) with unknown race is not listed.
Early Death Rate
The early death rate, defined as death within one month of diagnosis, was 13% (Table 2). There was no significant difference in early death rate between CBF-AML subtypes (P=0.47), gender (P=0.23), race/ethnicity (P=0.19), metro vs. non-metro residence (P=0.58), or period of diagnosis (P=0.42). The probability of early death clearly increased with older age (P<0.0001). Similar patterns in survival were seen at 3 months (Table 2); of note, over half of patients aged 75-84 were not alive at 3 months.
Table 2.
The probability of early death and median overall survival in patients with CBF-AML diagnosed between 2000 and 2010 and aged 15-84 yrs. at diagnosis
| Early Death Rate | Overall Survival | Survival Rate (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | No. Early Events | P(Early death) | p-value | No. Events | †Median OS Months (95% CI) | p-value | 3 Months | 1 Year | 3 Year | 5 Year†† | |
| CBF-AML | 774 | 101 | 0.13 | - | 406 | 22 (19, 28) | - | 0.77 (0.74,0.80) | 61.5 (57.9,64.9) | 44.3 (40.5,48.1) | 35.7 (30.8,40.7) |
| CBF Type | |||||||||||
| inv(16) | 317 | 38 | 0.12 | 0.47 | 279 | NA | < 0.0001 | 0.81 (0.77,0.85) | 71.9 (66.4,76.6) | 57.3 (51.1,62.9) | 46.9 (38.4, 55.0) |
| t(8;21) | 457 | 63 | 0.14 | 127 | 16 (12, 18) | 0.75 (0.70,0.78) | 54.4 (49.6,59.0) | 35.5 (30.8,40.3) | 28.7 (22.8,34.8) | ||
| Gender | |||||||||||
| Male | 440 | 63 | 0.14 | 0.23 | 246 | 20 (17, 27) | 0.07 | 0.75 (0.71,0.79) | 59.7 (54.8,64.2) | 42.2 (37.2,47.1) | 34.2 (27.9,40.6) |
| Female | 334 | 38 | 0.11 | 160 | 25 (NA) | 0.80 (0.75,0.84) | 63.9 (58.3,69.0) | 47.2 (41.2,53.0) | 38.0 (30.1,45.9) | ||
| Race/Ethnicity | |||||||||||
| White | 529 | 78 | 0.15 | 0.19 | 291 | 21 (17, 24) | 0.003 | 0.74 (0.70,0.77) | 59.7 (55.3,63.9) | 42.1 (37.5,46.6) | 33.7 (27.9,39.6) |
| Black | 56 | 6 | 0.11 | 36 | 14 (8, 24) | 0.84 (0.71,0.91) | 54.3 (39.8,66.6) | 28.8 (15.9,43.1) | 17.4 (5.4,35.0) | ||
| Hispanic | 104 | 11 | 0.11 | 49 | 38 (NA) | 0.85 (0.76,0.90) | 66.6 (56.3, 75.0) | 50.8 (40.0,60.6) | 49.0 (34.7,61.8) | ||
| Other | 84 | 6 | 0.07 | 30 | NA | 0.87 (0.77,0.92) | 71.0 (59.7,79.8) | 60.2 (48.0,70.5) | 43.8 (26.5,60.1) | ||
| Age at DX | |||||||||||
| 15-44 yr. | 254 | 13 | 0.05 | <0.0001 | 74 | NA | < 0.0001 | 0.94 (0.90,0.96) | 82.8 (77.2,87.1) | 68.7 (61.8,74.5) | 55.2 (45.7,63.8) |
| 45-64 yr. | 283 | 28 | 0.10 | 153 | 22 (18, 36) | 0.79 (0.74,0.83) | 64.6 (58.6,70.0) | 43.9 (37.6,50.0) | 37.4 (29.2,45.6) | ||
| 65-74 yr. | 137 | 27 | 0.20 | 94 | 9 (6, 14) | 0.68 (0.59,0.75) | 42.3 (33.7,50.5) | 26.4 (18.6,34.8) | 16.4 (8.4,26.7) | ||
| 75-84 yr. | 100 | 33 | 0.33 | 85 | 2 (1, 4) | 0.44 (0.34,0.53) | 26.4 (18.0, 35.5) | 9.3 (4.0, 17.2) | 6.7 (1.7,16.4) | ||
| Year of DX | |||||||||||
| 2000-2002 | 157 | 18 | 0.12 | 0.42 | 110 | 16 (10, 19) | 0.002 | 0.75 (0.67,0.81) | 52.9 (44.8,60.3) | 32.5 (25.3,39.8) | 31.9 (24.7,39.2) |
| 2003-2005 | 198 | 32 | 0.16 | 123 | 21 (14, 38) | 0.74 (0.67,0.79) | 58.4 (51.2,64.9) | 43.5 (36.5,50.3) | 38.9 (32.1,45.7) | ||
| 2006-2008 | 251 | 33 | 0.13 | 130 | 25 (NA) | 0.78 (0.73,0.83) | 63.6 (57.3, 69.3) | 48.1 (41.7,54.2) | - | ||
| 2009-2010 | 168 | 18 | 0.11 | 43 | NA | 0.83 (0.76,0.88) | 73.0 (64.5,79.7) | - | - | ||
| Resident County | |||||||||||
| Metro | 687 | 88 | 0.13 | 0.58 | 360 | 22 (19, 28) | 0.74 | 0.78 (0.75,0.81) | 61.9 (58.0,65.5) | 44.5 (40.4, 48.5) | 36.7 (31.4,42.1) |
| Not Metro | 87 | 13 | 0.15 | 46 | 21 (NA) | 0.71 (0.60,0.79) | 58.5 (47.2,68.3) | 43.1 (31.6,54.1) | 28.6 (16.0,42.5) | ||
NA = not available due to number of events recorded
5 year survival only looked at those diagnosed in 2000-2005, n=355
Overall Survival
The median OS for all CBF-AML patients was 22 months (95% CI, 19-28 months; Table 2). 61.5% (95% CI, 57.9-64.9%) of patients were alive at 1 year, and 44.3% (95% CI, 40.5-48.1%) of patients were alive at 3 years. The 5-year OS rate for the 355 evaluable patients diagnosed between 2000 and 2005 was 35.7% (95% CI, 30.8-40.7%). Median OS improved from 16 months in 2000-2002 to 21 months in 2003-2005 and to 25 months in 2006-2008 (P=0.002); this point has not been reached in patients diagnosed in 2009-2010 (Figure 1). Because early mortality in certain cohorts, such as elderly patients, may relate to lack of treatment, we performed a survival analysis using only those patients who were alive at one month. These results were no different than those of all patients.
Figure 1. Overall survival by period of diagnosis.
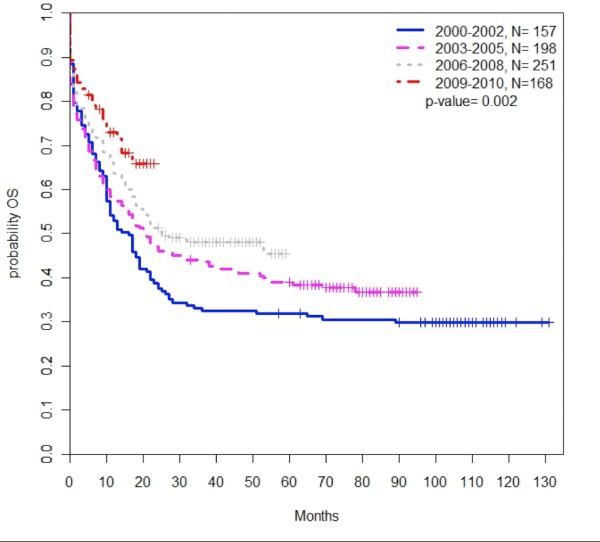
Overall survival among all patients improved with each successive period of diagnosis (P=0.002). Survival is shown in months.
Across the SEER population, each successive age cohort had worse OS compared to patients aged 15-44; particularly patients aged 65-74 (HR for mortality 3.51; 95% CI: 2.57-4.79; P<0.0001) and aged 75-84 (HR 5.86; 95% CI, 4.24-8.11; P<0.0001) (Table 3, Figure 2A). The 3-year OS decreased from 68.7% (95% CI, 61.8-74.5%) among patients aged 15-44 to 43.9% (95% CI, 37.6-50.0%) among patients aged 45-64, and was only 26.4% (95% CI, 18.6-34.8%) and 9.3% (95% CI, 4.0-17.2%) for patients aged 65-74 and 75-84, respectively. Median OS was 9 months for patients aged 65-74 and 2 months for those aged 75-84. OS among older patients remained poor after adjusting the model to only include patients alive at one month (Table 3, Figure 2B).
Table 3.
Cox proportional hazard regression for death for the full analysis set, and for the subset of those patients alive at 1 month.
| Analysis Set | Subset | |||||||
|---|---|---|---|---|---|---|---|---|
| Full Model | Reduced Model | Full Model | Reduced Model | |||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Gender | ||||||||
| Male (ref) | 1 | 1 | ||||||
| Female | 0.90 (0.73, 1.10) | 0.29 | 0.89 (0.71, 1.13) | 0.33 | ||||
| Race/Ethnicity | ||||||||
| White (ref) | 1 | 1 | 1 | 1 | ||||
| Black | 1.50 (1.05, 2.13) | 0.03 | 1.52 (1.07, 2.16) | 0.02 | 1.67 (1.13, 2.47) | 0.01 | 1.70 (1.15, 2.50) | 0.008 |
| Hispanic | 0.96 (0.71, 1.32) | 0.81 | 0.97 (0.72, 1.32) | 0.87 | 0.93 (0.65, 1.33) | 0.69 | 0.94 (0.67, 1.34) | 0.74 |
| Other | 0.71 (0.48, 1.03) | 0.07 | 0.70 (0.48, 1.03) | 0.07 | 0.71 (0.47, 1.10) | 0.12 | 0.71 (0.46, 1.09) | 0.11 |
| Age at DX | ||||||||
| 15-44 yr. (ref) | 1 | 1 | 1 | 1 | ||||
| 45-64 yr. | 2.04 (1.54, 2.71) | <0.0001 | 2.09 (1.58, 2.77) | <0.0001 | 2.10 (1.53, 2.87) | <0.0001 | 2.16 (1.59, 2.94) | <0.0001 |
| 65-74 yr. | 3.34 (2.42, 4.61) | <0.0001 | 3.51 (2.57, 4.79) | <0.0001 | 3.32 (2.30, 4.79) | <0.0001 | 3.52 (2.46, 5.02) | <0.0001 |
| 75-84 yr. | 5.61 (4.02, 7.83) | <0.0001 | 5.86 (4.24, 8.11) | <0.0001 | 5.36 (3.63, 7.94) | <0.0001 | 5.64 (3.85, 8.26) | <0.0001 |
| Year of DX | ||||||||
| 2000-2002 (ref) | 1 | 1 | 1 | 1 | ||||
| 2003-2005 | 0.79 (0.61, 1.03) | 0.08 | 0.80 (0.61, 1.03) | 0.08 | 0.68 (0.51, 0.91) | 0.01 | 0.68 (0.51, 0.91) | 0.01 |
| 2006-2008 | 0.66 (0.51, 0.85) | 0.002 | 0.66 (0.51, 0.86) | 0.002 | 0.58 (0.43, 0.78) | 0.0003 | 0.58 (0.44, 0.78) | 0.0003 |
| 2009-2010 | 0.51 (0.35, 0.73) | 0.0002 | 0.66 (0.53, 0.82) | 0.0002 | 0.40 (0.26, 0.63) | <0.0001 | 0.40 (0.26, 0.63) | <0.0001 |
| CBF Type | ||||||||
| inv(16) | 0.65 (0.53, 0.81) | <0.0001 | 0.66 (0.53, 0.82) | 0.0001 | 0.55 (0.43, 0.71) | <0.0001 | 0.56 (0.44, 0.72) | <0.0001 |
| t(8;21) (ref) | 1 | 1 | 1 | 1 | ||||
| Resident County | ||||||||
| Metro | 1.06 (0.78, 1.45) | 0.71 | 1.08 (0.75, 1.56) | 0.69 | ||||
| Not Metro (ref) | 1 | 1 | ||||||
Of note, the estimated median survival is not available (NA in the tables) for some factors because the number of events recorded is limited and the median of the Kaplan-Meier survival function has not yet been reached.
Figure 2. Overall survival by age of diagnosis among all patients (2A) and of those alive/followed at 1 month (2B).
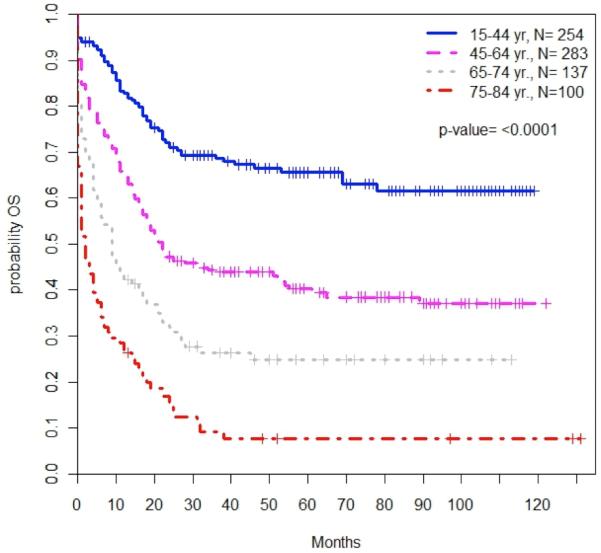
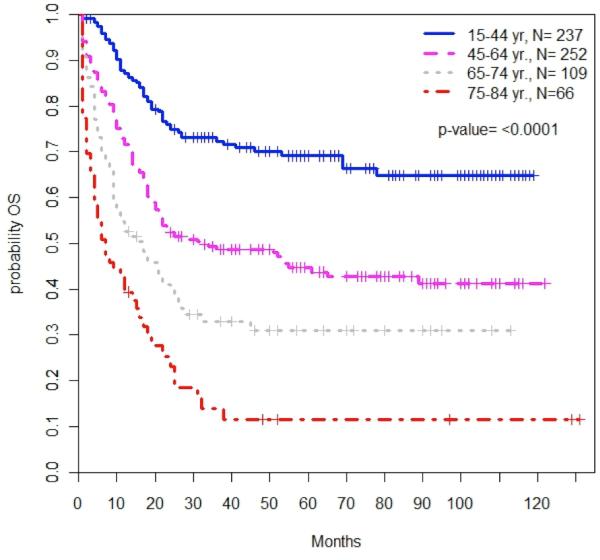
Patient age was significantly associated with overall survival among all patients with CBF-AML. Patient survival decreased with increasing age both at diagnosis (2A, P<0.0001) and among the subset of patients who were alive and followed after 1 month (2B, P<0.0001).
Black patients had a higher mortality than white patients (HR 1.52; 95% CI, 1.07-2.16; P=0.02). There was no difference in survival between white patients and Hispanic patients (P=0.87; Table 3, Figure 3).
Figure 3. Overall survival by race/ethnicity.
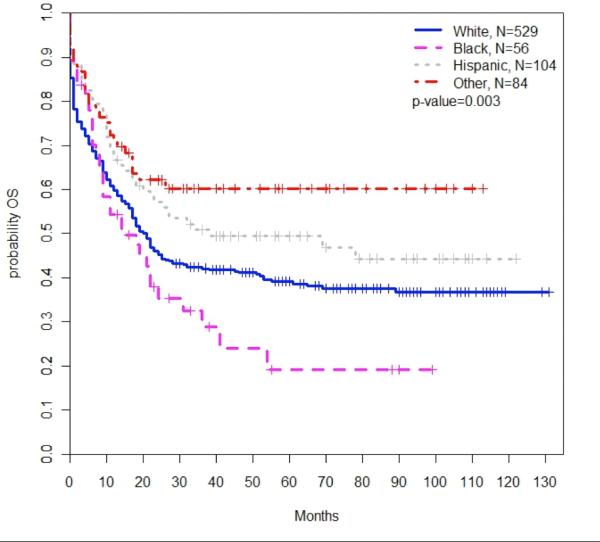
There was a significant association between patient race/ethnicity and survival among all patients with CBF-AML (P=0.003). Black patients had poorer overall survival compared to white non-Hispanic patients.
Patients with inv(16) had improved survival compared to those with t(8;21); 3-year OS was 57.3% (95% CI, 51.1-62.9%) vs. 35.5% (95% CI, 30.8-40.3%), respectively, and for patients diagnosed between 2000 and 2005, 5-year OS was 46.9% (95% CI, 38.4-55.0%) vs. 28.7% (95% CI, 22.8-34.8%). There was a decreased hazard of mortality among patients with inv(16) compared to patients with t(8;21), both at diagnosis (HR 0.66; 95% CI, 0.53-.82; P<0.0001) and among the subset of patients alive and followed after one month (HR 0.56; 95% CI, 0.44-0.72; P<0.0001) (Figure 4).
Figure 4. Overall Survival by CBF-AML type.
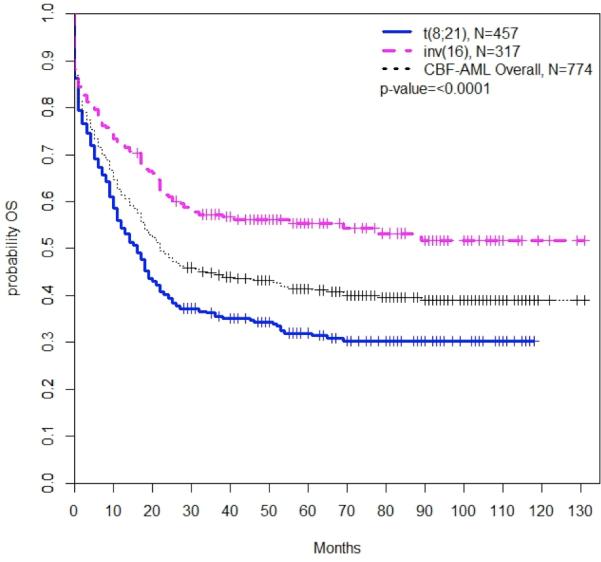
There were differences in overall survival by CBF-AML cytogenetic subtype. Patients with t(8;21) CBF-AML had significantly worse survival compared to those with inv(16) CBF-AML.
Discussion
CBF-AML, characterized by alterations in the RUNX1 and CBFB genes, has been associated with more favorable prognosis compared to other non-APL subtypes of AML, based on data mainly derived from clinical trials. In this analysis, we performed a retrospective cohort study using the SEER database to investigate survival trends for CBF-AML at the population level. This is the largest such study to date, evaluating 777 AML patients with core binding factor alterations.
There was a marked improvement in median survival from 2000 to 2010 among patients with CBF-AML, but survival at the population level remains significantly lower than that reported in the majority of the published trials.5,13–15,30,31 Several recent analyses have used the SEER database and reported similar improvements in survival over the last 4 decades among all patients with acute myeloid leukemia.18–21,32 None of these analyses looked specifically at patients with core-binding factor leukemia, which is important given the relative chemosensitivity and favorable prognosis of this disease. In spite of these gains in CBF-AML survival, older patients and African Americans nonetheless experienced disproportionally poor outcomes.
Notably, patients harboring a t(8;21) alteration had significantly worse survival compared to those with inv(16), a finding noted in prior studies,13,14 but not to the same degree as in the current analysis. Only 28.7% of patients with t(8;21) were alive at 5 years,30,31 in contrast with previous 5-year survival rates for trial patients with t(8;21) approximating 45% or better.13,14 For instance, the CALGB reported 5-year OS rates of 52% for t(8;21) disease and 57% for inv(16) disease, across all ages.30 More recent clinical trial results, including those incorporating gemtuzumab ozogamicin into treatment regimens, report even greater 5 year survival;34 the MRC recently reported 89% 5-year survival for CBFAML patients under age 60 diagnosed in 2009 or later.35 Like those with inv(16), patients with t(8;21) are felt to be particularly responsive to high-dose cytarabine-based consolidation. However, our findings, similar to other reports, contribute to literature suggesting that t(8;21) does not portend the same favorable risk as inv(16). In contrast to the data from clinical trials, poorer outcomes in the general population may be explained by a hesitancy or inability to consistently treat patients with three or four consolidative cycles of high-dose cytarabine,6,33 or to consider hematopoietic cell transplantation in consolidation or as a salvage therapy. Alternatively, improved survival with inv(16) in the general population could reflect the increased likelihood of successful salvage for these patients after relapse,13 which has not been shown for patients with t(8;21).6,33,36 Unfortunately, SEER does not include chemotherapy regimens, transplant data, or relapse information to confirm these hypotheses, and thus further confirmatory studies are needed.
Although the incidence of CBF-AML comprises a larger percentage of total incident AML cases among younger patients, the incidence of CBF-AML in the entire population actually increases with advancing age. The increase in CBF-AML incidence among the general population with advancing age likely reflects the dramatically increased incidence of all AML subtypes in the general population aged over 60. SEER likely underestimates the actual incidence of the CBF-AML subtype, since it relies on reported cytogenetics. Previous reports have shown that the fraction of all patients with AML who have CBF-AML decreases with age, from 12% of patients ages 16-60 to 7% of those older than age 60.5,9 Our data is consistent with these findings; nonetheless, although a smaller percentage of older AML patients have CBF-AML, the absolute number of patients remains large given the rise in total AML incidence with advancing age and is an important consideration in the evaluation of an older patient.
A number of analyses have previously reported worse outcomes among those over age 60 with CBF-AML.9,10,14,20,37 A retrospective analysis of 370 patients with CBF-AML found that advancing age correlated with resistant disease and worse survival.14 Although only 37 patients were older than 65, their 5-year OS was 22%, significantly lower than other age groups. A separate analysis of 361 AML patients older than 60 years reported 3-year OS rates for inv(16) disease of 26% (n=14) and for t(8;21) disease of 10% (n=12), though these numbers again are small.9 More recently, the French CBF-AML Intergroup reported their experience treating 147 CBF-AML patients over age 60 and reported 5-year OS of 31%.10 Although survival worsened with age, those with t(8;21) who received cytarabine-based consolidation had 3-year leukemia-free survival rates near 70%. Overall, however, very few older patients with CBF-AML received repetitive cycles of intermediate or high-dose cytarabine in these trials. Apart from the greater prevalence of comorbid disease and compromised functional status, which may partially account for survival differences, patients over age 60 typically receive lower doses of cytarabine during consolidation.38 Arguably, consolidation with intermediate or high-dose cytarabine has yet to be adequately tested in those over 60 with CBF-AML, suggesting an important area of future clinical study.
The current analysis represents the largest cohort of elderly patients with CBF-AML (n=238 aged 65-84), reporting 5-year OS of 16.4% for patients aged 65-74, and only 6.7% for those aged 75-84. We did discover a high rate of early mortality in patients aged 75-84, with approximately one-third of patients dying within the first month and over half dying within 3 months. In comparison, the one-month survival is roughly 90% or higher among patients aged 15-64. The elevated early death rate among the elderly may relate in part to a higher prevalence of compromised functional status and comorbidities. Nevertheless, long-term outcome trends did not differ when we excluded those who survived less than one month. Indeed, there are reports that high CR rates can be achieved in older patients with CBF-AML, with one French series reporting a rate of 88%.10 Early results from the CALGB 10801 study adding dasatinib to standard chemotherapy in CBF-AML, which included 15 patients over age 60, report that these older patients had an 80% CR rate, 93% 30-day survival, and 62% 1-year survival, in contrast to our findings.39 It is important to note, however, that such impressive results may contain selection bias, since the elderly on clinical trials are likely to be healthier and more functional. Unfortunately, the SEER database contains no information on chemotherapy and minimal data on comorbidity and functional status, limiting our ability to draw relevant conclusions. A prior study showed that among patients over age 65 in the SEER-Medicare database, those who were treated with chemotherapy had a longer overall survival.18,40 It may be that some of the survival improvement we report is related to increased use of chemotherapy in this population, in addition to improvements in supportive care such as newer antifungal and antiviral strategies.
In our analysis, black patients had a lower incidence of CBF-AML, and demonstrated worse OS, when compared to white non-Hispanic patients. At least one prior report has noted this disparity.13 The reason for this difference is unclear, but remains significant even after accounting for the higher incidence of t(8;21) relative to inv(16) among black patients. A recent study similarly found that black and Hispanic patients with AML, across all cytogenetic groups, were diagnosed at younger age and had worse survival compared to white patients.41 Whether these findings relate to inherent differences in AML between races, to the nature of the disease or response to chemotherapy, or to a number of environmental factors including differential access to care, is unclear and requires further study.
There are limitations to the current analysis. The SEER database does not contain certain clinical information known to correlate with poor prognosis in CBF-AML. For instance, there is no information regarding additional genetic alterations with prognostic significance, such as c-KIT mutations.11,42 These mutations have been identified in 20-30% of patients with CBF-AML;11,12 it is possible, though unlikely, that c-KIT mutations are enriched in the general population relative to patients enrolled in clinical trials.
Although concurrent chromosomal abnormalities are not reported through SEER, these do not appear to adversely impact the favorable prognosis of CBF-AML.5 We also lack information regarding which patients subsequently underwent allogeneic stem cell transplantation, and this may have impacted our survival data. Additionally, our findings may not be generalizable to the whole US population. While SEER approximates the US population in measures of poverty and education, it tends to be more urban and has a higher percentage of foreign-born persons (17.9% vs. 12.8%).43 It also includes a larger proportion of the total U.S. American Indian/Alaskan Native (43.8%), Asian (50.4%), and Hawaiian/Pacific Islander (66.5%) populations compared to the total U.S. white (24.9%) and black (25.6%) populations.
In summary, our data lends further support to distinguishing inv(16) and t(8;21) subtypes in assessing AML prognosis,13 and shows an improved survival among patients with inv(16) compared to those with t(8;21) alterations at the population level. Increased early death and worse long-term survival were seen among elderly patients. The poor outcomes seen in the elderly and black non-Hispanic patients may suggest that, in the general population, these populations may have had less access to the highly effective conventional therapies associated with superior outcomes in clinical trials. Nonetheless, survival rates in CBF-AML have slowly improved since 2000, suggesting that advances in supportive care and the increasing incorporation of intensive consolidative chemotherapy have had a profound and growing impact among the US population.
Footnotes
This study has been presented in abstract form at the American Society of Hematology 2013 Annual Meeting, New Orleans, LA, December 7-10, 2013
References
- 1.Le Beau MM, Larson RA, Bitter MA, Vardiman JW, Golomb HM, Rowley JD. Association of an Inversion of Chromosome 16 with Abnormal Marrow Eosinophils in Acute Myelomonocytic Leukemia. N Engl J Med. 1983;309(11):630–6. doi: 10.1056/NEJM198309153091103. [DOI] [PubMed] [Google Scholar]
- 2.Liu P, Tarle SA, Hajra A, Claxton DF, Marlton P, Freedman M, et al. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993 Aug 20;261(5124):1041–4. doi: 10.1126/science.8351518. [DOI] [PubMed] [Google Scholar]
- 3.Erickson P, Gao J, Chang KS, Look T, Whisenant E, Raimondi S, et al. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992 Oct 1;80(7):1825–31. [PubMed] [Google Scholar]
- 4.Look AT. Oncogenic Transcription Factors in the Human Acute Leukemias. Science. 1997 Nov 7;;278(5340):1059–64. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010 Jul 22;116(3):354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Dodge RK, Carroll A, Baer MR, Edwards C, Stamberg J, et al. Patients With t(8;21)(q22;q22) and Acute Myeloid Leukemia Have Superior Failure-Free and Overall Survival When Repetitive Cycles of High-Dose Cytarabine Are Administered. J Clin Oncol. 1999 Dec 1;17(12):3767–75. doi: 10.1200/JCO.1999.17.12.3767. [DOI] [PubMed] [Google Scholar]
- 7.Delaunay J, Vey N, Leblanc T, Fenaux P, Rigal-Huguet F, Witz F, et al. Prognosis of inv(16)/t(16;16) acute myeloid leukemia (AML): a survey of 110 cases from the French AML Intergroup. Blood. 2003 Jul 15;102(2):462–9. doi: 10.1182/blood-2002-11-3527. [DOI] [PubMed] [Google Scholar]
- 8.Dastugue N, Payen C, Lafage-Pochitaloff M, Bernard P, Leroux D, Huguet-Rigal F, et al. Prognostic significance of karyotype in de novo adult acute myeloid leukemia. The BGMT group. Leukemia. 1995 Sep;9(9):1491–8. [PubMed] [Google Scholar]
- 9.Fröhling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Döhner K, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006 Nov 15;108(10):3280–8. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 10.Prébet T, Boissel N, Reutenauer S, Thomas X, Delaunay J, Cahn J-Y, et al. Acute Myeloid Leukemia With Translocation (8;21) or Inversion (16) in Elderly Patients Treated With Conventional Chemotherapy: A Collaborative Study of the French CBF-AML Intergroup. J Clin Oncol. 2009 Oct 1;27(28):4747–53. doi: 10.1200/JCO.2008.21.0674. [DOI] [PubMed] [Google Scholar]
- 11.Schnittger S, Kohl TM, Haferlach T, Kern W, Hiddemann W, Spiekermann K, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006 Mar 1;107(5):1791–9. doi: 10.1182/blood-2005-04-1466. [DOI] [PubMed] [Google Scholar]
- 12.Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, et al. Adverse Prognostic Significance of KIT Mutations in Adult Acute Myeloid Leukemia With inv(16) and t(8;21): A Cancer and Leukemia Group B Study. J Clin Oncol. 2006 Aug 20;24(24):3904–11. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci G, Mrózek K, Ruppert AS, Maharry K, Kolitz JE, Moore JO, et al. Prognostic Factors and Outcome of Core Binding Factor Acute Myeloid Leukemia Patients With t(8;21) Differ From Those of Patients With inv(16): A Cancer and Leukemia Group B Study. J Clin Oncol. 2005 Aug 20;23(24):5705–17. doi: 10.1200/JCO.2005.15.610. [DOI] [PubMed] [Google Scholar]
- 14.Appelbaum FR, Kopecky KJ, Tallman MS, Slovak ML, Gundacker HM, Kim HT, et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol. 2006;135(2):165–73. doi: 10.1111/j.1365-2141.2006.06276.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoyos M, Nomdedeu JF, Esteve J, Duarte R, Ribera JM, Llorente A, et al. Core binding factor acute myeloid leukemia: The impact of age, leukocyte count, molecular findings, and minimal residual disease. Eur J Haematol. 2013 Sep;91(3):209–18. doi: 10.1111/ejh.12130. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt VR, Kantarjian H, Cortes JE, Ravandi F, Borthakur G. Therapy of Core Binding Factor Acute Myeloid Leukemia: Incremental Improvements Toward Better Long-Term Results. Clin Lymphoma Myeloma Leuk. 2013 Apr;13(2):153–8. doi: 10.1016/j.clml.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith ML, Hills RK, Grimwade D. Independent prognostic variables in acute myeloid leukaemia. Blood Rev. 2011 Jan;25(1):39–51. doi: 10.1016/j.blre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012 Dec 1;97(12):1916–24. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013 Apr 11;121(15):2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia. Cancer. 2013;119(15):2720–7. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012 Jan 5;119(1):34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) Program . Surveillance Research Program, Surveillance Systems Branch. National Cancer Institute, DCCPS; Research Data (1973-2010) ( www.seer.cancer.gov) released April 2013, based on the November 2012 submission. [Google Scholar]
- 23.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 24.Percy C, Fritz A, Jack A, Shanmugarathan S, Sobin L, Parkin DM, et al. International classification of diseases for oncology (ICD-O) World Health Organization Geneva; 2000. [Google Scholar]
- 25.World Health Organization . International Statistical Classification of Diseases and Related Health Problems,Tenth Revision. World Health Organization; Geneva: 1992. [PubMed] [Google Scholar]
- 26.United States Department of Agriculture Economic Research Service [2013 Jul 4];2003 Rural- Urban Continuum Codes [Internet] Available from: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx.
- 27.United States Census Bureau [2013 Jul 4];Census 2000 Gateway [Internet] Available from: http://www.census.gov/main/www/cen2000.html.
- 28.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011 Aug 4;118(5):1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001 Sep 1;98(5):1312–20. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 30.Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB8461.). Blood; Presented in part at the 43rd annual meeting of the American Society of Hematology; Orlando, FL. December 10, 2001; Dec 15, 2002. pp. 4325–36. and published in abstract form.59. [DOI] [PubMed] [Google Scholar]
- 31.Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and Treatment Outcome in Cytogenetically Normal Acute Myeloid Leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 32.Juliusson G, Lazarevic VL, Hörstedt A-S, Hagberg O, Hoglund M. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012 Apr 26;119(17):3890–9. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd JC, Ruppert AS, Mrózek K, Carroll AJ, Edwards CG, Arthur DC, et al. Repetitive Cycles of High-Dose Cytarabine Benefit Patients With Acute Myeloid Leukemia and inv(16)(p13q22) or t(16;16)(p13;q22): Results from CALGB 8461. J Clin Oncol. 2004 Mar 15;22(6):1087–94. doi: 10.1200/JCO.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of Patients With Acute Myeloblastic Leukemia Who Benefit From the Addition of Gemtuzumab Ozogamicin: Results of the MRC AML15 Trial. J Clin Oncol. 2011 Feb 1;29(4):369–77. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 35.Burnett AK, Hills RK, Russell N, Milligan D, Hunter AE, Clark RE, et al. Reasons For Survival Improvement In Core Binding Factor AML: A 25 Year Analysis Of The UK MRC/NCRI AML Trials. Blood. 2013 Nov 15;122(21):358–358. [Google Scholar]
- 36.Schlenk RF, Benner A, Krauter J, Büchner T, Sauerland C, Ehninger G, et al. Individual Patient Data–Based Meta-Analysis of Patients Aged 16 to 60 Years With Core Binding Factor Acute Myeloid Leukemia: A Survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2004 Sep 15;22(18):3741–50. doi: 10.1200/JCO.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Schoch C, Kern W, Schnittger S, Buchner T, Hiddemann W, Haferlach T. The influence of age on prognosis of de novo acute myeloid leukemia differs according to cytogenetic subgroups. Haematologica. 2004 Jan 1;89(9):1082–90. [PubMed] [Google Scholar]
- 38.Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, et al. Frequency of Prolonged Remission Duration after High-Dose Cytarabine Intensification in Acute Myeloid Leukemia Varies by Cytogenetic Subtype. Cancer Res. 1998 Sep 15;58(18):4173–9. [PubMed] [Google Scholar]
- 39.Marcucci G, Geyer S, Zhao J, Caroll AJ, Bucci D, Vij R, et al. Adding The KIT Inhibitor Dasatinib (DAS) To Standard Induction and Consolidation Therapy For Newly Diagnosed Patients (pts) With Core Binding Factor (CBF) Acute Myeloid Leukemia (AML): Initial Results Of The CALGB 10801 (Alliance) Study. Blood. 2013 Nov 15;122(21):357–357. [Google Scholar]
- 40.Lang K, Earle CC, Foster T, Dixon D, Van Gool R, Menzin J. Trends in the treatment of acute myeloid leukaemia in the elderly. Drugs Aging. 2005;22(11):943–55. doi: 10.2165/00002512-200522110-00004. [DOI] [PubMed] [Google Scholar]
- 41.Patel MI, Ma Y, Mitchell BS, Rhoads KF. Age and Genetics: How Do Prognostic Factors at Diagnosis Explain Disparities in Acute Myeloid Leukemia? Am J Clin Oncol. 2013 Apr;:1. doi: 10.1097/COC.0b013e31828d7536. [DOI] [PubMed] [Google Scholar]
- 42.Boissel N, Leroy H, Brethon B, Philippe N, de Botton S, Auvrignon A, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia. 2006 Apr 6;20(6):965–70. doi: 10.1038/sj.leu.2404188. [DOI] [PubMed] [Google Scholar]
- 43. [2012 Oct 21];Population Characteristics: Characteristics of the SEER Population Compared with the Total United States Population [Internet] Available from: http://seer.cancer.gov/registries/characteristics.html.


